INTRODUCTION
Leishmaniasis is a disease caused by protozoa of the Leishmania genus, which are transmitted by the bite of a female sandfly of the genus Phlebotomus (Mitropoulos et al. Reference Mitropoulos, Konidas and Durkin-Konidas2010). In the case of Leishmania (Leishmania) amazonensis, the parasite affects skin causing ulcers characteristic of cutaneous leishmaniasis (Grevelink and Lerner, Reference Grevelink and Lerner1996; Basano and Camargo, Reference Basano and Camargo2004; García-Almargo, Reference García-Almagro2005). The systemic pharmacological treatment of leishmaniasis includes pentavalent antimonials as first-choice drugs (Brasil, 2007). However, when the therapeutic response is unsatisfactory or in cases of inability to use them, second-choice drugs such as amphotericin B and pentamidins can be used for the treatment (Gontijo and Carvalho, Reference Gontijo and Carvalho2003; Brasil, 2007). This current scenario of antileishmanial drugs – characterized by their high toxicity, high cost and, in some cases, parasite resistance – has motivated the search for new therapeutic approaches. In this sense, natural products appear as sources of bioactive molecules, which are important tools for discovering new therapeutic targets.
Snake venom toxins are commonly used as tools for drug design, in view of the wide variety of pharmacological actions attributed to these substances (Tempone et al. Reference Tempone, Andrade, Spencer, Lourenço, Rogero and Nascimento2001). Microbicidal activity described for snake venom has been associated with L-amino acid oxidase and/or the enzymes phospholipase A2 (PLA2) (Barbosa et al. 2005; Samy et al. 2007). Phospholipase A2 (EC 3.1.1.4) is a class of enzymes defined by their ability to catalyse the hydrolysis of the sn-2-acyl bond of sn-3-phospholipids, generating free fatty acids and lysophospholipids as products (van Deenen and de Haas, Reference van Deenen and de Haas1963; Diaz and Arm, Reference Diaz and Arm2003). The action of PLA2s on Leishmania sp. was assessed in cytotoxicity studies by several research groups (Stábeli et al. Reference Stábeli, Amui, Sant'ana, Pires, Nomizo, Monteiro, Romão, Guerra-Sá, Vieira, Giglio, Fontes and Soares2006; Costa et al. Reference Costa, Menaldo, Oliveira, Santos-Filho, Teixeira, Nomizo, Fuly, Monteiro, de Souza, Palma, Stábeli, Sampaio and Soares2008; Passero et al. Reference Passero, Laurenti, Tomokane, Corbett and Toyama2008; Torres et al. Reference Torres, Dantas, Toyama, Filho, Zara, de Queiroz, Nogueira, de Oliveira, Toyama, Monteiro and Martins2010; Peichoto et al. Reference Peichoto, Tavares, Dekrey and Mackessy2011). With the exception of one investigation by Passero et al. (Reference Passero, Laurenti, Tomokane, Corbett and Toyama2008), these studies evaluated the action of the toxin only by the viability of the parasite. Thus, there are few data on the effect of toxins on parasite–host interactions, as well as a shortage of studies seeking to elucidate the mechanism of cytotoxicity or the biochemical and physiological changes observed in the parasite. It should be emphasized that no study, so far, has shown morphological alterations due to snake toxin action on Leishmania sp. Studies on the effects of bioactive molecules against Leishmania sp. are important not only for ascertaining the parasite biology, but also for generating new drugs to treat leishmaniasis since they may serve as tools in the discovery of new therapeutic targets. The present work aimed to elucidate the cytotoxic effects of a PLA2 isolated from the Bothrops pauloensis snake venom, denominated BnSP-7 (Soares et al. Reference Soares, Guerra-Sá, Borja-Oliveira, Rodrigues, Rodrigues-Simioni, Rodrigues, Fontes, Lomonte, Gutiérrez and Giglio2000), on L. (L.) amazonensis, by evaluating the effect of this toxin on proliferation, cell viability, morphology and amastigote–promastigote differentiation, as well as to determine the toxin's effect on the infective capacity of promastigotes in murine peritoneal macrophages.
MATERIALS AND METHODS
Chemicals
Dimethylsulfoxide (DMSO), ethylenediamine tetraacetic acid (EDTA), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), sodium dodecyl sulfate (SDS), RPMI 1640 medium, penicillin and streptomycin were purchased from Sigma Chemical Co. (St Louis, USA), heat-inactivated fetal bovine serum (FBS) from Cultilab (Brazil) and Schneider's insect medium from LGC Biotecnologia (Brazil). All other reagents were analytical grade or superior.
Promastigote culture
Leishmania (L.) amazonensis (IFLA/BR/67/PH8 strain) promastigotes were cultured in Schneider's insect medium, pH 7·0, supplemented with 10% FBS, penicillin (100 UI mL−1) and streptomycin (100 μg mL−1) – complete Schneider's insect medium – at 23 °C. Promastigotes used in all experiments were isolated from the stationary growth phase (metacyclic promastigotes, in 5- to 6-day-old culture).
Preparation of amastigotes
Leishmania (L.) amazonensis amastigotes were obtained by footpad infection of BALB/c mice (6–8 weeks old) with promastigote forms (1 × 107 cells/footpad) for 5 to 6 weeks. Footpad lesions were surgically removed and the tissue homogenized in phosphate-buffered saline (PBS). Debris was eliminated by nylon Nitex filtration (pore size, 80 mm). The cell suspension was centrifuged at 1800 g for 10 min. To lyse erythrocytes, the pellet was re-suspended in ammonium chloride solution (8·29 g of NH4Cl, 1 g of KHCO3 and 37·3 mg of EDTA per litre) for 10 min, and the insoluble material containing infected macrophage cells was homogenized (20 strokes). The resulting suspension was centrifuged 4 times at 1800 g, and the final pellet was re-suspended in RPMI 1640 in the presence of penicillin/streptomycin. The suspension was shaken for 3 h and the amastigotes washed 4 times by centrifugation at 1800 g (Tanaka et al. Reference Tanaka, Valero, Takahashi and Straus2007). All animal experimental procedures were approved by the Institutional Ethics Committee for Animal Use at the Federal University of Uberlândia (CEUA 052/09).
Peritoneal macrophage culture
Resident peritoneal macrophages were harvested from the peritoneal cavity of male BALB/c mice (6–8 weeks old) by washing with cold PBS. Collected cells were washed 3 times with cold PBS by centrifugation at 950 g and re-suspended in RPMI 1640 supplemented with 10% FCS, 10 mm HEPES, penicillin (100 U mL−1) and streptomycin (100 mg mL−1) – complete RPMI medium. Macrophages were placed on 96-well plates (2 × 105 cells/well) for the viability assay or placed on sterile glass coverslips in 24-well plates (5 × 105 cells/well) for the infectivity assay, in both cases for 1 h. Non-adherent cells were removed by several washings with RPMI and the plates were kept at 37 °C in a CO2 incubator. All animal experimental procedures were approved by the Institutional Ethics Committee for Animal Use at the Federal University of Uberlândia (CEUA 052/09). BALB/c mice were supplied by the Centre for Animal Experimentation of the Federal University of Uberlândia. They were maintained in sterilized cages under a controlled environment (25 ± 5 °C, 12 h day/night cycle) and received water and food ad libitum.
Toxin
BnSP-7 toxin was isolated from B. pauloensis snake venom according to the description of Rodrigues et al. (Reference Rodrigues, Soares, Mancin, Fontes, Homsi-Brandeburgo and Giglio1998) and Soares et al. (Reference Soares, Guerra-Sá, Borja-Oliveira, Rodrigues, Rodrigues-Simioni, Rodrigues, Fontes, Lomonte, Gutiérrez and Giglio2000) with minor modifications. Briefly, approximately 200 mg of whole venom was dissolved in 2 mL of 50 mm ammonium bicarbonate ((NH4)HCO3) buffer (pH 7·8) and centrifuged at 3000 g for 10 min at 4 °C. The supernatant was recovered and injected on a CM-Sepharose column previously equilibrated and initially eluted with the same buffer. A convex gradient was then applied until obtaining 0·5 m(NH4)HCO3 buffer, pH 7·8. Fractions of 1 mL/tube were collected at a flow rate of 6·5 mL h−1. The CM5 fraction containing the PLA2 BnSP-7 (1 mg) was dissolved in 500 μL of solvent A (0·1% trifluoroacetic acid) and applied to an HPLC system, using C2–C18 μRPC 4·6/100 (GE Health Care) column, previously equilibrated with solvent A. The elution of the protein was then conducted using a linear gradient of solvent B (80% acetonitrile, 0·1% trifluoroacetic acid) from 0 to 100% at a flow rate of 1 mL min−1 at room temperature. To certify protein homogeneity, polyacrylamide gel electrophoresis (PAGE) was performed in the presence of sodium dodecyl sulfate (SDS-PAGE) according to the method of Laemmli (Reference Laemmli1970).
The BnSP-7 PLA2, derived from some RP-HPLC, was lyophilized and solubilized in DMSO to obtain a stock solution (40 mg mL−1), and maintained at −20 °C. In viability assays, the highest concentration tested in the parasite was 400 μg mL−1, and thus the final DMSO concentration in culture medium never exceeded 1% (v/v). It is well established that DMSO at the concentration of 1% does not affect parasite proliferation (Granthon et al. Reference Granthon, Braga, Rodrigues, Cammerer, Lorente, Gilbert, Urbina and Souza2007; Santos et al. Reference Santos, Ueda-Nakamura, Dias Filho, Veiga Junior, Pinto and Nakamura2008; Neto et al. Reference Neto, Sousa, Dias, Barbosa Filho, Oliveira and Figueiredo2011).
Proliferation and viability assays
In order to evaluate the BnSP-7 toxin effect on cellular proliferation of L. (L.) amazonensis promastigotes, parasites (5 × 106 cells mL−1) were cultured in 25 cm2 cell culture flasks containing complete Schneider's insect medium with progressively higher concentrations of toxin (25·0–200·0 μg mL−1) at 23 °C. At 24, 48, 72 and 96 h after incubation with toxin, the number of promastigotes was determined by counting in a Neubauer chamber.
Viability assays in the presence of BnSP-7 toxin were performed on parasites (promastigote and amastigote forms) and peritoneal macrophages by a colorimetric method based on mitochondrial oxidation of MTT reagent. First, parasites were re-suspended in complete Schneider's insect medium and placed on 96-well culture plates (5 × 105 parasites/well) and incubated at 23 °C (promastigote) or at 37 °C (amastigote) with different toxin concentrations (2-fold serial dilution from 400·0 μg mL−1 of toxin to promastigote and 200·0 μg mL−1 of toxin to amastigote) for 72 h (promastigote) or 24 h (amastigote). To determinate the macrophage viability in the presence of toxin, resident peritoneal macrophages collected from BALB/c mice, as described above, were placed on a 96-well culture plate (2 × 105 cells/well) and incubated with complete RPMI medium containing toxin (2-fold serial dilution from 400 μg mL−1 of toxin) at 37 °C with 5% CO2 for 24 h. After incubation, the parasite and macrophage viabilities were accessed by adding MTT reagent (5 mg mL−1 in Schneider's insect medium, 100 μg/well) to the plates and incubation proceeded for about 4 h at 37 °C protected from light. The reaction was stopped by adding 100 μL of PBS containing 10% SDS and 0·01 m HCl. The absorbances were measured in an EL × 800 microplate reader (BioTek Instruments) at 595 nm. Each assay was carried out in triplicate and independent experiments were performed. The IC50 values with 95% confidence limits were determined by GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, USA).
Amastigote differentiation
The effect of toxin on amastigote differentiation in promastigotes was analysed by the addition of BnSP-7 toxin (100 μg mL−1) in 1 × 106 amastigotes mL−1 cultured in 25 cm2 cell culture flasks containing complete Schneider's insect medium at 23 °C. The normal differentiation profile from amastigotes to promastigotes was evaluated by amastigote culture in the absence of toxin. Aliquots of culture were collected daily for 6 days to determine the cell density of promastigotes and amastigotes.
Promastigote morphology
In order to evaluate morphological changes, promastigotes treated for 72 h with BnSP-7 toxin (100 μg mL−1) were collected by centrifugation, washed 3 times in PBS and fixed with 2% formaldehyde in PBS. Fixed parasites were placed on glass slides, stained with the fast Panótico kit (LB Laborclin, Brazil) and analysed by optical microscopy.
In order to evaluate ultrastructural changes, promastigotes treated for 72 h with BnSP-7 toxin (100 μg mL−1) were analysed by electron transmission microscopy. After treatment with toxin, parasites were fixed for 2 h at 4 °C in a solution containing 2·5% glutaraldehyde in PBS. Fixed parasites were washed in PBS and post-fixed for 1 h in a solution containing 1% osmium tetroxide (OsO4) and 0·8% potassium ferrocyanide in PBS. The cells were washed in PBS, dehydrated in a graded acetone series and embedded in resin. Ultrathin sections were stained with uranyl acetate and lead citrate then observed under a Zeiss EM 109 transmission electron microscope (Zeiss, Oberkochen, Germany). Three independent experiments were performed in triplicate.
Macrophage infectivity by L. (L.) amazonensis promastigotes
Promastigote forms previously incubated for 72 h in the presence of BnSP-7 toxin (25, 100 and 200 μg mL−1) or absence of the toxin (control parasite) were harvested by centrifugation and washed 3 times in PBS. Then, parasites were added onto macrophages placed on glass coverslips in a 24-well plate, obtained as previously described, at a 10 : 1 ratio (parasites : macrophage), and kept at 25 °C for 1 h. Plates were washed with RPMI 1640 medium to remove non-internalized parasites. Complete RPMI medium was added and the plates were incubated at 37 °C in a CO2 incubator for 24 h. Coverslips containing macrophages infected with parasites were fixed with 2% formaldehyde in PBS, stained by Giemsa and analysed through optical microscopy. The infectivity index was determined by multiplying the percentage of macrophages that had at least 1 intracellular parasite by the average number of intracellular parasites per infected macrophage. These data were determined by randomly counting at least 100 cells in each of the triplicate coverslips. Three independent experiments were performed.
Statistical analysis
All experiments were performed at least 3 times. Representative results of experiments developed in triplicate are shown throughout the article. The data were analysed statistically using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, USA). Data are expressed as mean±s.d. of 3 independent experiments performed in triplicate. Comparisons of data for each group were analysed by one-way ANOVA and post-test, when necessary. Differences were considered statistically significant for a P value <0·05.
RESULTS
Effect of BnSP-7 toxin on proliferation and cellular viability
The PLA2 BnSP-7 was shown to be homogeneous by SDS–PAGE after chromatographic isolation (results not shown). Thus, in order to evaluate the effect of toxin on the proliferation of L. (L.) amazonensis promastigotes, BnSP-7 toxin (25–200 μg mL−1) was added to promastigotes from stationary growth phase and monitored for 96 h. The toxin caused a dose-dependent inhibition on promastigote proliferation that was evident 72 h after incubation with toxin. Proliferation inhibitions of between 60 and 70% for toxin concentrations of 50–200 μg mL−1 were observed 96 h after incubation (Fig. 1).
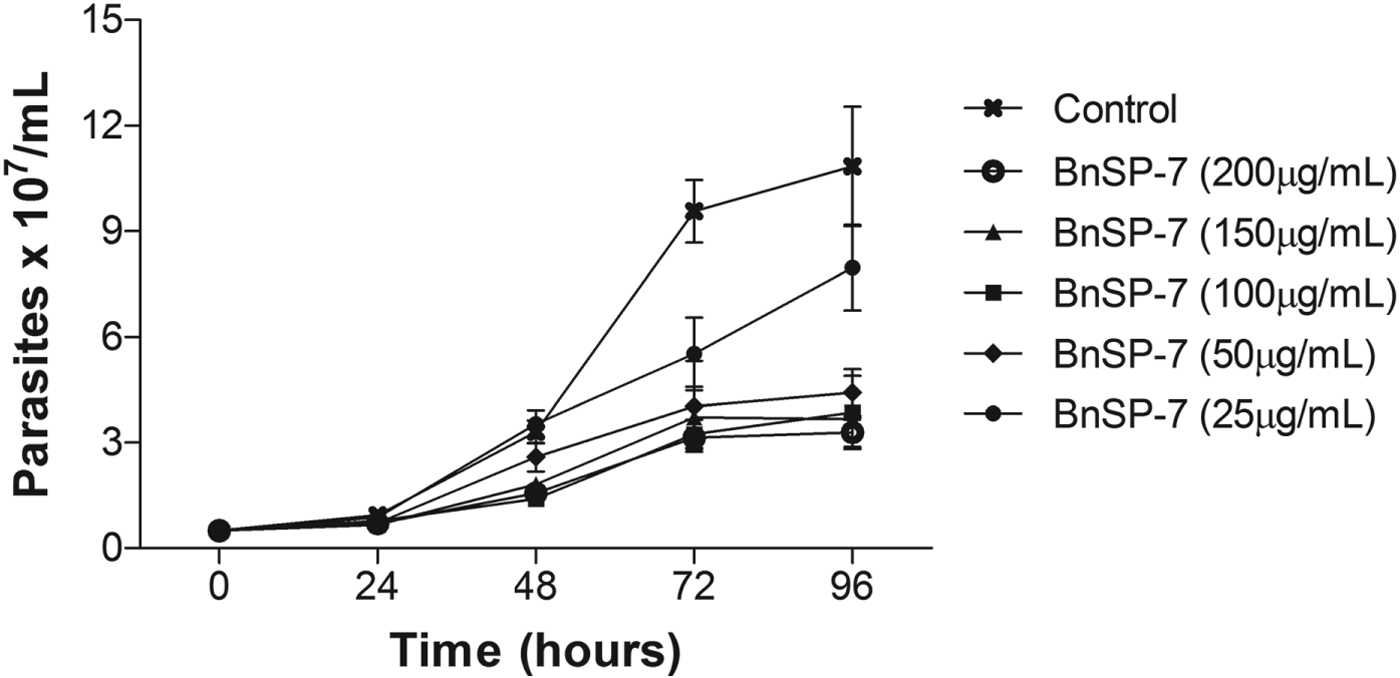
Fig. 1. Effect of BnSP-7 toxin on the proliferation of Leishmania (L.) amazonensis promastigotes: The growth pattern of L. (L.) amazonensis promastigotes was monitored in parasites cultivated in Schneider's insect medium at 23 °C in the absence (control) or presence of increasing doses of BnSP-7 toxin (25–200 μg mL−1) up to 96 h. The toxin was added to the culture at hour 0. The parasite concentration was determined daily. Data are expressed as the mean±s.d. of 3 independent experiments performed in triplicate.
Viability assays on parasites and macrophage were performed by MTT assay, based on the ability of viable cells to reduce MTT reagent to an insoluble formazan product. The toxin provoked dose-dependent cytotoxicity in both amastigotes and promastigotes. The data were analysed statistically to determine BnSP-7 toxin IC50 values. The IC50 values were 58·7 μg mL−1 72 h after incubation and 28·1 μg mL−1 24 h after incubation for promastigotes and amastigotes, respectively (Table 1). These results indicate that amastigotes were more susceptible than promastigotes to BnSP-7 toxin. Despite the lower IC50 value for amastigotes in relation to promastigotes, 100 μg mL−1 of toxin caused similar cytotoxicity regardless of the parasite form (85 and 88% for amastigote and promastigote forms, respectively). Also, the cytotoxic effect of BnSP-7 toxin on peritoneal murine macrophages was analysed by MTT assay and showed that in the presence of 5·6 μg mL−1 of toxin only 50% of cells remained viable after 24 h when compared with the experimental control.
Table 1. Effects of BnSP-7 toxin on Leishmania (L.) amazonensis viability

* IC50: concentration that produces 50% inhibitory effect on cell viability.
a The viability assay was tested 72 h after BnSP-7 treatment.
b The viability assay was tested 24 h after BnSP-7 treatment.
Effect of BnSP-7 toxin on amastigote differentiation
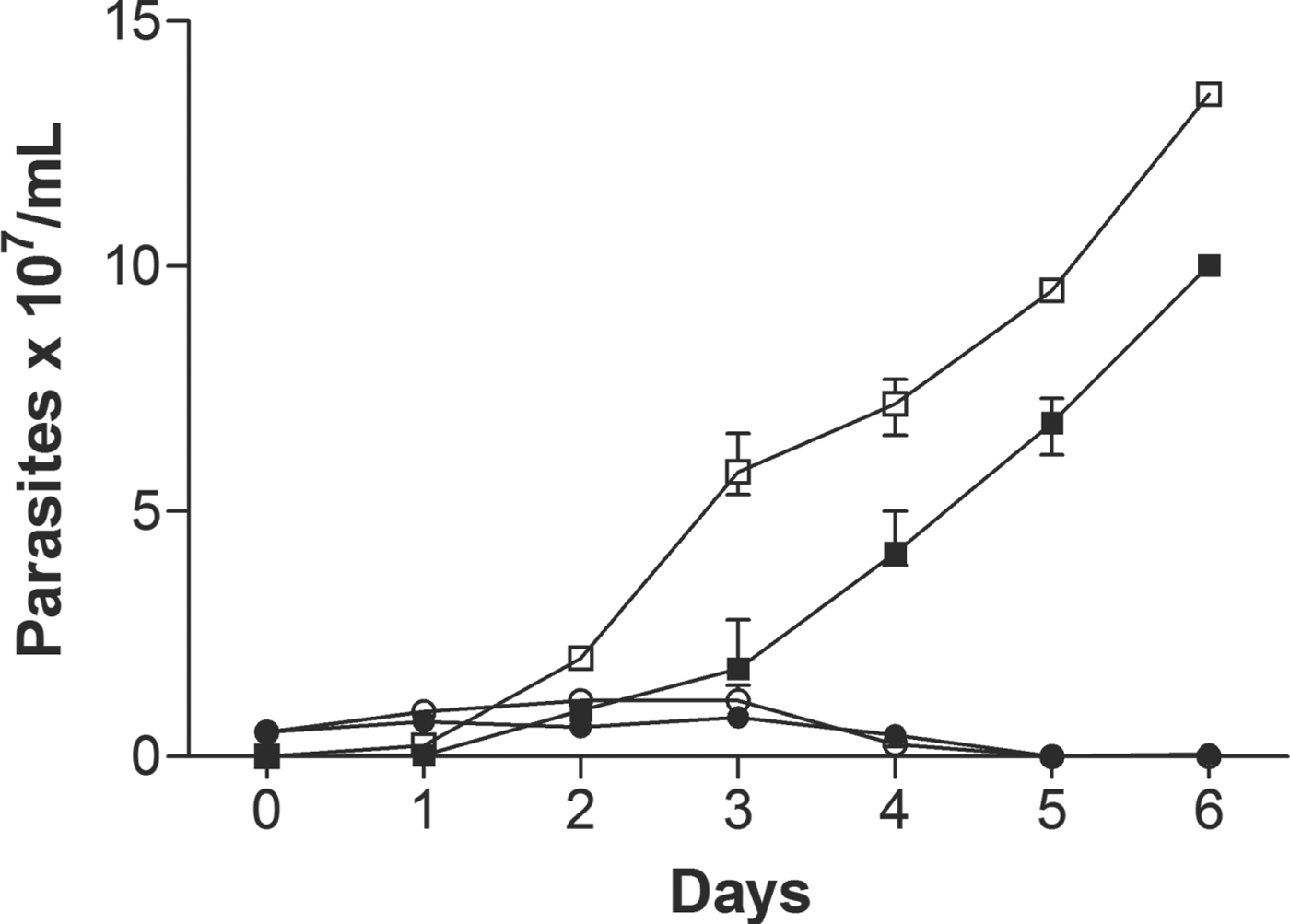
Amastigote forms isolated from BALB/c mice footpad lesions were incubated with BnSP-7 toxin (100 μg mL−1) and maintained at 23 °C for 6 days, in order to evaluate the effect of the toxin on the differentiation of amastigotes into promastigotes, as shown in Fig. 2. After 3 days, the number of promastigotes in culture without BnSP-7 toxin was 6·0 × 107 parasites mL−1 whereas in the presence of toxin the number of promastigotes was 65% less, suggesting a toxic effect on amastigote–promastigote differentiation. After the fourth day of cultivation, the effect of the toxin on differentiation was no longer detectable while the number of promastigotes had also increased in the culture containing the toxin. On the sixth day, the number of promastigotes in control cultures (absence of BnSP-7 toxin) reached approximately 1·35 × 108 parasites mL−1 whereas in the presence of toxin the number of promastigotes was 26% lower. These results indicate that the toxin prevented the differentiation process only during the first 3 days, after which the differentiation process occurred, a delay probably attributable to the initial action exerted by the toxin during the first days.

Fig. 2. Effect of BnSP-7 toxin on Leishmania (L.) amazonensis amastigote–promastigote differentiation. Amastigotes (1 × 106) isolated from BALB/c mice lesions were cultured in Schneider's insect medium at 23 °C in the absence (open circles and squares) or presence of 100 μg mL−1 of BnSP-7 toxin (filled circles and squares) up to 7 days. Aliquots were collected daily to determine the numbers of amastigotes (circles) and promastigotes (squares).
Effect of BnSP-7 toxin on promastigote morphology
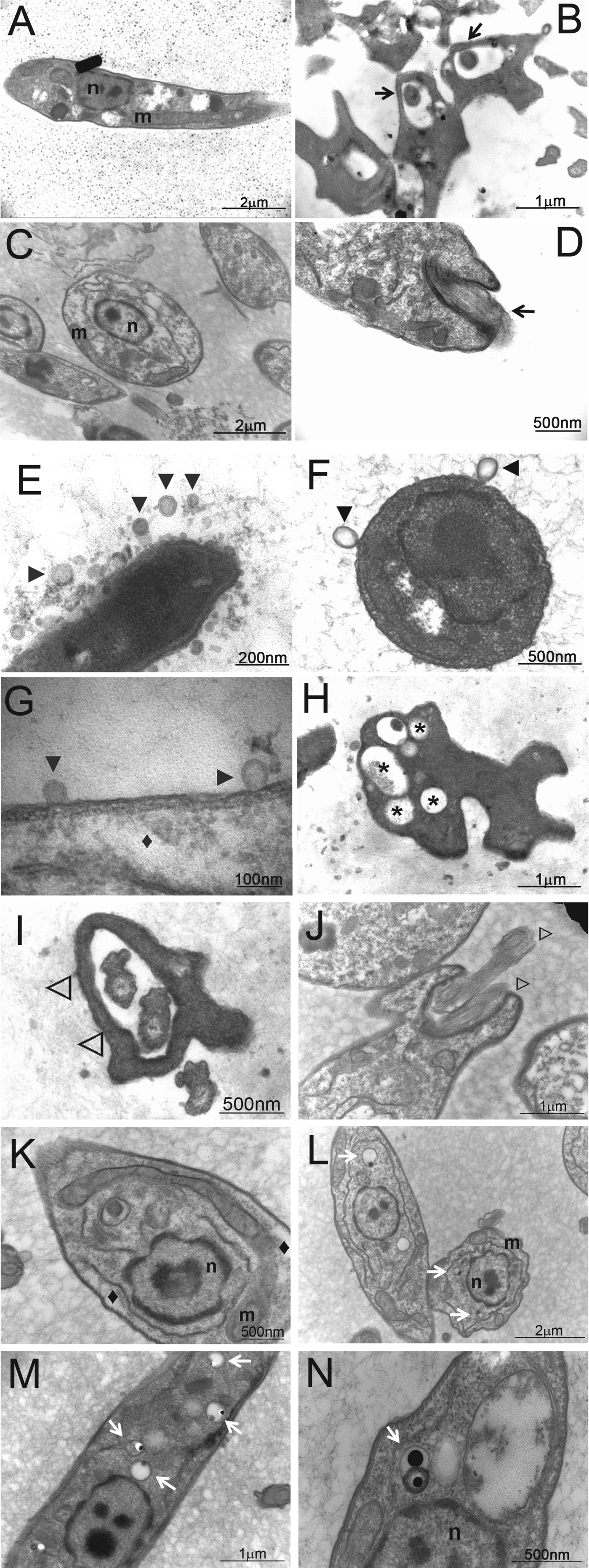
Based on toxic and antiproliferative effects provoked by BnSP-7 toxin on L. (L.) amazonensis, the next step was to investigate the action exerted by the toxin on parasite morphology. Initial studies by optical microscopy showed that parasites cultured with BnSP-7 toxin present relevant alterations in their typical morphologies including an altered shape of the promastigote body and either 2 flagella or a single short flagellum (results not shown). To ascertain the effect of the toxin on parasite ultrastructure, promastigotes cultured with BnSP-7 toxin (100 μg mL−1) for 72 h were analysed by transmission electron microscopy (TEM) (Fig. 3) and compared morphologically with control promastigotes (cultured in the absence of toxin). Analysis by TEM confirmed the presence of parasites containing 2 flagella (Fig. 3I, J, ⊳) as indicated by optical microscopy analysis (results not shown), and revealed additional details. Results showed blebs detaching from the entire cell surface (Fig. 3E–G, ►), the presence of a crenate nucleus and chromatin condensation (Fig. 3K–N, n), a higher number of vacuoles (Fig. 3H, *), acidocalcisomes (Fig. 3L–N, white arrows), mitochondrial swelling (Fig. 3K, L, m) and shrinkage of the cytoplasm (Fig. 3G, K, ♦). These results corroborate the deleterious effects of BnSP-7 toxin on promastigote morphology.

Fig. 3. Ultrastructural changes in Leishmania (L.) amazonensis promastigotes treated with BnSP-7 toxin. Promastigotes were cultured in Schneider's insect medium at 23 °C in the absence (panels A–D) or presence of 100 μg mL−1 of BnSP-7 for 72 h (panels E–N). Subsequently, the parasites were processed for electron transmission microscopy. Single flagellum (panels B, D, black arrows), membrane blebbing (panels E–G, ►), membrane shrinkage (panels G, K, ⧫), vacuoles (panel H, *), presence of 2 flagella in a flagellar pocket (panels I, J, ⊳), acidocalcisomes (panel L-N, white arrows). Lower-case letters indicate: m, mitochondria; n, nucleus.
Effect of BnSP-7 toxin on the infective capacity of promastigote forms
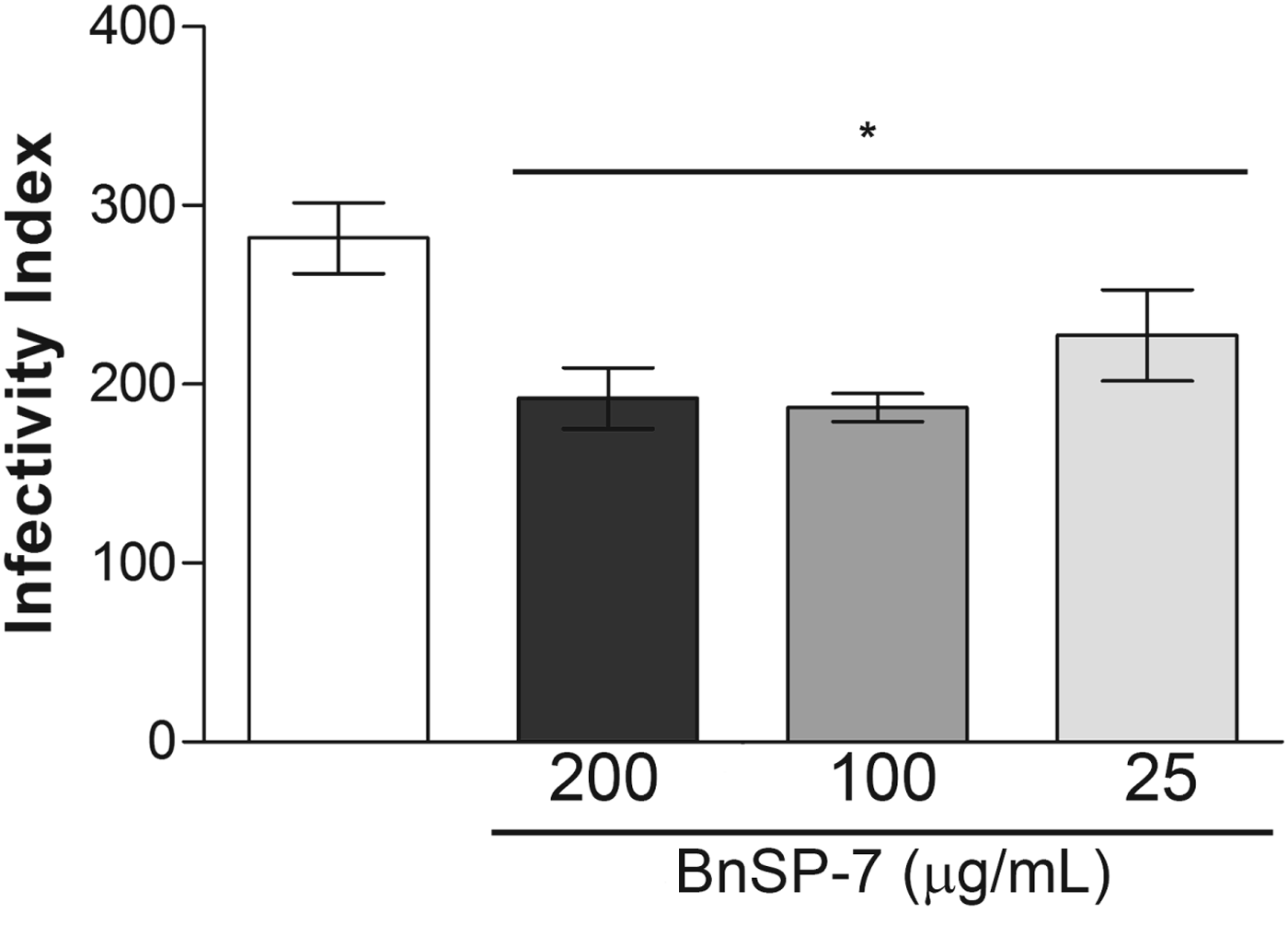
Promastigote forms of L. (L.) amazonensis pre-treated with BnSP-7 toxin (25, 100 and 200 μg mL−1) for 72 h were added to a culture of macrophages, and the infectivity indices were calculated 24 h after infection. The results showed that BnSP-7 toxin reduced the infective capacity of promastigotes in murine peritoneal macrophages, causing statistically significant reductions of approximately 20–35% (Fig. 4) at all toxin concentrations tested after 24 h, in relation to the control (P < 0·05). There was no statistically significant difference between groups (P < 0·05). Approximately 60% of macrophages were infected with control parasites after 24 h. Interestingly, the parasites pre-treated with toxin showed percentages very similar to control parasites, regardless of BnSP-7 toxin concentration. However, the number of parasites per infected macrophage by pre-treated parasites was significantly lower than among macrophages infected with control parasites, in a dose-dependent manner (data not shown).

Fig. 4. Effect of BnSP-7 toxin on the infective capacity of Leishmania (L.) amazonensis promastigotes in murine peritoneal macrophages. Peritoneal macrophages were infected with promastigotes cultured in the absence or presence of BnSP-7 toxin (200, 100 and 25 μg mL−1) for 72 h. Infected macrophages were incubated at 37 °C in a CO2 incubator for 24 h. Infectivity indices were calculated by multiplying the percentage of macrophages that had at least 1 intracellular parasite by the average number of intracellular parasites per infected macrophage, counting 100 macrophages/coverslip (n = 3). *Statistically significant difference (P < 0·05) compared with the control. There is no statistically significant difference between groups (P < 0·05).
DISCUSSION
As mentioned earlier, the effect of the whole snake venoms on Leishmania promastigotes has been well documented by several research groups (Fernandez-Gomez et al. Reference Fernandez-Gomes, Zerrouk, Sebti, Loyens, Benslimane and Ouaissi1994; Tempone et al. Reference Tempone, Andrade, Spencer, Lourenço, Rogero and Nascimento2001; Gonçalves et al. Reference Gonçalves, Soares, Souza, Damatta and Alves2002; Passero et al. Reference Passero, Tomokane, Corbett, Laurenti and Toyama2007), since they are recognized as useful sources of bioactive substances showing a wide range of pharmacological activities. The PLA2 action on Leishmania sp. promastigote growth and/or viability was previously demonstrated by other research groups. MjTX-II, a Lys49 PLA2 isolated from Bothrops moojeni, reduced Leishmania sp. viability by around 50% in the presence of 75 μg mL−1 of toxin (Stábeli et al. Reference Stábeli, Amui, Sant'ana, Pires, Nomizo, Monteiro, Romão, Guerra-Sá, Vieira, Giglio, Fontes and Soares2006). Costa et al. (Reference Costa, Menaldo, Oliveira, Santos-Filho, Teixeira, Nomizo, Fuly, Monteiro, de Souza, Palma, Stábeli, Sampaio and Soares2008) assayed 2 PLA2s (MTX-I and II) from Bothrops brazili and their respective C-terminal region-derived synthetic peptides (pepMTX-I and pepMTX-II). Both MTX-I, an Asp49 PLA2, and MTX-II, a Lys49 PLA2, caused an inhibition of approximately 90% in parasite viability at 100 μg mL−1 concentration, while the peptides killed about 70% of Leishmania sp. at 120 μg mL−1.
In contrast, another study demonstrated that an enzymatically active PLA2 from Crotalus durissus collilineatus venom stimulates the growth of L. (L.) amazonensis promastigotes and amastigotes (Passero et al. Reference Passero, Laurenti, Tomokane, Corbett and Toyama2008). In a more recent study, Peichoto et al. (Reference Peichoto, Tavares, Dekrey and Mackessy2011) found that Trimorphin, an Asp49 PLA2 isolated from Trimorphodon biscutatus lambda, exhibited a dose-dependent biphasic effect on L. (L.) major growth, with potent cytotoxicity at higher concentrations, and stimulation of proliferation at very low concentrations. According to the authors, this finding could be explained by the adaptive response of cells to a moderate stress. Since PLA2s are important mediators of complex intracellular signalling pathways, low concentrations of these enzymes would lead to a stimulus of cell growth, whereas high concentrations of the enzyme would result in direct damage to the cell.
Our results showed that BnSP-7 toxin, a Lys49 PLA2, provoked a leishmanicidal effect on L. (L.) amazonensis promastigotes, causing inhibition of both proliferation and mitochondrial dehydrogenases activity of the parasite, as determined by the growth curve and MTT assay, respectively. BnSP-7 toxin showed a dose-dependent inhibitory effect on the proliferation of promastigotes. Parasite growth inhibition started 48 h after culturing and became evident after 72 h. A 60% inhibition of promastigote growth was observed 96 h after incubation with 50 μg mL−1 of BnSP-7 toxin vs a maximum inhibition of about 70% on growth produced by both 150 and 200 μg mL−1 of toxin. These data indicate that 150 μg mL−1 of toxin presents the maximum anti-proliferative effect against L. (L.) amazonensis promastigotes.
The study of the cytotoxic action was supplemented by viability assays showing that 58·7 μg mL−1 of BnSP-7 toxin provoked the death of 50% of promastigotes whereas 28·1 μg mL−1 killed 50% of amastigotes, suggesting that the amastigote is more susceptible to BnSP-7 toxin than the promastigote form. Tempone et al. (Reference Tempone, Andrade, Spencer, Lourenço, Rogero and Nascimento2001) observed that L. (L.) amazonensis amastigotes were not affected by L-amino acid oxidase (LAAO) from B. moojeni, even in the presence of 300 μg mL−1 of toxin, whereas 1·44 μg mL−1 of LAAO killed 50% of promastigote forms. Considering that the cytotoxic mechanism for LAAOs is related to hydrogen peroxide production, the result obtained by incubating toxin with amastigotes is justified because this parasite form provides more efficient protection against free radicals and hydrogen peroxide when compared with promastigotes. In contrast, our results suggest that PLA2s are more effective against free amastigotes. The greater effectiveness of the toxin BnSP-7 against amastigotes is very interesting because it is responsible for the maintenance of the infection in the vertebrate host. In addition to the cytotoxic action on amastigotes, the toxin caused a delay in the differentiation process to the promastigote form.
The actions of PLA2s on Leishmania promastigote viability were described by other research groups and showed variations among IC50 values. For example, the Lys49 PLA2s (catalytically inactive) produced IC50 values of 75 μg mL−1 of MjTX-II toxin to L. (L.) amazonensis, L. (L.) donovani and L. (L.) major (Stábeli et al. Reference Stábeli, Amui, Sant'ana, Pires, Nomizo, Monteiro, Romão, Guerra-Sá, Vieira, Giglio, Fontes and Soares2006); approximately 40 and 60 μg mL−1 of MTX-II toxin to L. (L.) amazonensis and L. (V.) braziliensis, respectively (Costa et al. Reference Costa, Menaldo, Oliveira, Santos-Filho, Teixeira, Nomizo, Fuly, Monteiro, de Souza, Palma, Stábeli, Sampaio and Soares2008). These results are very close to those found in the present study, considering the wide variation in susceptibility observed among different species and strains of the parasite. On the other hand, no cytotoxic effect on L. (L.) amazonensis or L. (L.) chagasi was observed even at 200 μg mL−1 of BmarPLA2, which is also a Lys49 PLA2 (Torres et al. Reference Torres, Dantas, Toyama, Filho, Zara, de Queiroz, Nogueira, de Oliveira, Toyama, Monteiro and Martins2010). For Asp49 PLA2s (catalytically active) the IC50s were approximately 40 and 20 μg mL−1 of MTX-I to L. (L.) amazonensis and L. (V.) braziliensis, respectively (Costa et al. Reference Costa, Menaldo, Oliveira, Santos-Filho, Teixeira, Nomizo, Fuly, Monteiro, de Souza, Palma, Stábeli, Sampaio and Soares2008); and more recently it was demonstrated that Trimorphin toxin presented an IC50 of 3·6 μg mL−1 to L. (L.) major.
Taken together, these findings indicate the possibility that catalytic activity is not necessary to produce anti-Leishmania action. Furthermore, studies have demonstrated that the C-terminal region, comprising amino acids from 115 to 129, is related to the cytotoxic and bactericidal effects of phospholipase A2, independently of catalytic activity (Páramo et al. Reference Páramo, Lomonte, Pizarro-Cerda, Bengoechea, Gorvel and Moreno1998; Stábeli et al. Reference Stábeli, Amui, Sant'ana, Pires, Nomizo, Monteiro, Romão, Guerra-Sá, Vieira, Giglio, Fontes and Soares2006; Nevalainen et al. Reference Nevalainen, Graham and Scott2008).
Besides the cytotoxic and anti-proliferative effects provoked by BnSP-7 toxin, the induction of several morphological changes was verified in the promastigote ultrastructure. Such alterations ranged from discrete to total destruction of the parasite. Control parasites presented normal ultrastructure, showing a typically elongated cell body, anterior flagellum, single mitochondrion containing the kinetoplast and a normal nucleus.
The rounded shape of the promastigote body, the blebbing effect, mitochondrial swelling and cytoplasm shrinking observed in BnSP-7-treated promastigotes indicate an important effect of the toxin on promastigote morphology. Other indications of morphological changes resulting from the toxin action were localized in the nuclear region. These changes appeared as a nuclear crenate aspect (pyknotic effect) and altered chromatin organization, strongly resembling apoptotic cells. Apoptosis, a type of programmed cell death that plays a central role in normal tissue development and homoeostasis (Paris et al. Reference Paris, Loiseau, Bories and Bréard2004; Deolindo et al. Reference Deolindo, Teixeira-Ferreira, Damatta and Alves2010; Paiva et al. Reference Paiva, Figueiredo, Antonucci, Paiva, Bianchi, Rodrigues, Lucarini, Caetano, Pietro, Martins, de Albuquerque and Sampaio2011), involves phosphatidylserine externalization, release of cytochrome c with consequent activation of cellular proteases and DNA cleavage into oligonucleosomal fragments. These biochemical events lead to characteristic changes in cell morphology and subsequent death. In Leishmania sp., cellular alterations during this process include rounding up of the cell, cytoplasmic retraction (pyknosis), chromatin condensation, nuclear fragmentation, plasma membrane blebbing and mitochondrial swelling (Arnoult et al. Reference Arnoult, Akarid, Grodet, Petit, Estaquier and Ameisen2002; Paris et al. Reference Paris, Loiseau, Bories and Bréard2004; Menna-Barreto et al. Reference Menna-Barreto, Salomão, Dantas, Santa-Rita, Soares, Barbosa and de Castro2009; Jiménez-Ruiz et al. Reference Jiménez-Ruiz, Alzate, Macleod, Lüder, Fasel and Hurd2010; Paiva et al. Reference Paiva, Figueiredo, Antonucci, Paiva, Bianchi, Rodrigues, Lucarini, Caetano, Pietro, Martins, de Albuquerque and Sampaio2011). Moreover, nuclear and mitochondrial alterations are probably among the main factors underlying the potent anti-proliferative effect and the loss of viability in Leishmania species (Ledezma et al. Reference Ledezma, Jorquera, Bendezú, Vivas and Pérez2002).
The presence of multiple flagella was also observed by Nakamura et al. (Reference Nakamura, Mendonça-Filho, Morgado-Díaz, Maza, Dias Filho, Cortez, Alviano, Rosa, Lopes and Nakamura2006), when L. (L.) amazonensis promastigotes were treated with Eugenol-rich essential oil from Ocimum gratissimum, and by Havens et al. (Reference Havens, Bryant, Asher, Lamoreaux, Perfetto, Brendle and Werbovetz2000), in L. (L.) donovani exposed to tubulin inhibitors. The emergence of these multiflagellar forms suggests an interference with the process of cell division. In trypanosomatids, microtubules participate in 4 crucial processes: (i) subpellicular microtubules are involved in the protozoan structural maintenance; (ii) the flagella are associated with motility; (iii) the basal body and mitotic spindles are implicated in cell division; and (iv) the cytosome is involved in endocytosis (Menna-Barrreto et al. Reference Menna-Barreto, Salomão, Dantas, Santa-Rita, Soares, Barbosa and de Castro2009).
Also, photomicrographs obtained by electron microscopy of parasites cultured in the presence of BnSP-7 toxin revealed vacuolization and augmentation of the number of acidocalcisomes, which are membrane-bounded organelles with an electron-dense content, characterized by their acidic nature, high electron density and high content of polyphosphates (polyPs), calcium, magnesium and other elements. They appear to be involved in phosphorus (Pi, PPi and poly P) and cation storage, intracellular pH homoeostasis and osmoregulation (Docampo and Moreno, Reference Docampo and Moreno1999, Reference Docampo and Moreno2001; Moreno and Docampo, Reference Moreno and Docampo2009).
Drug-induced ultrastructural damage enables the following interpretations as to the mode of action and cell death pathway in pathogenic protozoa: such changes in myelin figures and autophagosome-like formations are indicative of autophagy; the membrane blebbing effect and chromatin alterations resemble apoptosis; and plasma membrane rupture suggests necrosis (Menna-Barrreto et al. Reference Menna-Barreto, Salomão, Dantas, Santa-Rita, Soares, Barbosa and de Castro2009). In the light of these interpretations and in accordance with observations presented herein, it can be suggested that the cytotoxic mechanism of BnSP-7 toxin involves the activation of apoptosis in L. (L.) amazonensis. However, studies including time-dependent morphology and biochemical assays need to be performed to prove this hypothesis. Moreover, other morphological changes may also suggest an effect of the toxin on the promastigote cell cycle. In any case, the regulation and modulation of death pathways in Leishmania sp. are poorly understood (Jiménez-Ruiz et al. Reference Jiménez-Ruiz, Alzate, Macleod, Lüder, Fasel and Hurd2010) and need to be studied further to enable the development of new therapeutic agents and the elucidation of mechanisms involved in the cytotoxic action of different drugs.
Murine infection is one of the best-characterized experimental models for studying Leishmania-mammalian host cell interactions (Pereira and Alves, Reference Pereira and Alves2008). As to infectivity, it was observed that BnSP-7 toxin interfered with the infective capacity of promastigotes in murine peritoneal macrophages, causing statistically significant reductions at all toxin concentrations tested. Moreover, macrophages infected with BnSP-7-pre-treated promastigotes showed a decreased parasite load, suggesting parasite changes that hampered the intracellular survival in macrophages. In the last 20 years several research groups have studied the mechanism whereby the parasite subverts the host cell response to create an environment favourable for their differentiation into an amastigote form able to persist and divide within acid compartments. In this scope, many molecules are considered to be virulence factors on account of their involvement in the signalling pathways of the macrophage which contribute to influencing macrophage functions and consequently to establishing the infection (Olivier et al. Reference Olivier, Atayde, Isnard, Hassani and Shio2012). Perhaps the BnSP-7 toxin acts on or prevents synthesis of molecules considered to be virulence factors essential for the infectious process.
The present study showed that BnSP-7 toxin, a phospholipase A2 isolated from B. pauloensis snake venom, presents anti-Leishmania activity against promastigote and amastigote forms of L. (L.) amazonensis. The toxin is capable of inhibiting promastigote proliferation and promoting drastic changes in parasite morphology. It can also delay the process of differentiating amastigotes into promastigotes. The high toxicity observed for the toxin in mammalian cells prevented assays for testing the action of the toxin in intracellular amastigotes. Thus the action of the toxin in the infectivity process was only evaluated like pre-treatment of the parasite. This study revealed that the toxin also interferes in the infective capacity of the parasite in host cells during the establishment of infection.
In this sense, the findings reported herein are not intended to propose the use of BnSP-7 toxin as a disease treatment, but rather to show that this toxin is an important tool for studies focused on searching for new targets in the parasite, as well as studies to improve the understanding of Leishmania biology. Additional detailed studies must be conducted to better understand the biological and molecular effects of BnSP-7 toxin on L. (L.) amazonensis, such as investigation of pathways involved in the death of L. (L.) amazonensis promastigotes and proteomic studies aimed at the discovery of new therapeutic targets. Furthermore, future perspectives should include studies of synthetic peptides derived from this toxin in order to ascertain not only targets in the parasite, but also to reveal a possible drug that utilizes the peptide itself or another drug designed from a cytotoxic peptide.
ACKNOWLEDGEMENTS
The authors thank Professors Amélia Hamaguchi and Maria Inês Homsi-Brandeburgo for their valuable instruction, encouragement and confidence, and the laboratory technician Marcelo Arantes Levenhagen for his extensive help with electron microscopy. We declare that there is no potential conflict of interest.