Introduction
Maize (Zea mays L.) is an important cereal crop and grown widely across the world (Paudyal et al., Reference Paudyal, Opit, Osekre, Arthur, Bingham, Payton, Danso, Manu and Nsiah2017a). Besides being a staple food crop in sub-Saharan Africa, maize is also used for industrial purposes and animal feed (Nwosu, Reference Nwosu2018). However, a substantial amount of maize grain is lost to insect pests during storage in sub-Saharan Africa. The major insect pests of stored maize worldwide include the larger grain borer Prostephanus truncatus (Horn) and the maize weevil, Sitophilus zeamais (Motschulsky) (De Groote et al., Reference De Groote, Kimenju, Likhayo, Kanampiu, Tefera and Hellin2013; Quellhorst et al., Reference Quellhorst, Athanassiou, Bruce, Scully and Morrison2020). The former is the most damaging pest and in endemic areas causes weight loss estimated at 30% while the maize weevils can cause 10–20% weight loss when untreated maize is stored in traditional structures (Boxall, Reference Boxall2002). As a result of insect feeding, damage and contamination, the volume of stored grain, its quality, value and marketability are reduced (Affognon et al., Reference Affognon, Mutingi, Sanginga and Borgmeister2015). This is aggravated by the lack of effective, appropriate and affordable storage devices (Baributsa et al., Reference Baributsa, Djibo, Lowenberg-DeBoer, Moussa and Baoua2014). To avoid the risk of losing the harvested crop to insect pests, some farmers sell their maize early at a low price while others treat it with dilute insecticide dust but satisfactory protection is rarely achieved (Obeng-Ofori, Reference Obeng-Ofori2011). The control of these pests therefore remains a challenge to resource-poor smallholder farmers. Novel effective grain storage technologies that reduce insect activity and preserve grain quality and quantity till the next season are therefore required.
Hermetic storage bag technology offers farmers an effective alternative for the protection of stored maize against insect pests. The technology functions by creating a modified atmosphere around the grains through physical and biological means which results in depletion of oxygen and increased carbon dioxide levels. The depleted oxygen level leads to low insect activity and survival in the stored grain (Anankware et al., Reference Anankware, Fatumbi, Afreh-Nuamah, OBeng-Ofori and Ansah2012). In recent years, the technology has received significant attention from researchers, development agencies, governments and the private sector as a means of safeguarding stored grain (Murdock et al., Reference Murdock, Dago, Ntoukam, Kitch and Shade2003). Currently, there are five evaluated commercially available hermetic bags in Kenya namely, Purdue Improved Crop Storage (PICSTM), SuperGrain IV-RTM bags (GrainPro Inc), AgroZTM and AgroZTM Plus (A-Z Textile Mills Ltd) and Elite bags (Elite Innovations Kenya Limited). Although hermetic storage bags have become increasingly popular, the food grain value chain has several handling points, which expose hermetic bags to increased risk of puncture resulting in reduced performance.
Although hermetic storage bags effectively control storage insect pests of crops such as maize (De Groote et al., Reference De Groote, Kimenju, Likhayo, Kanampiu, Tefera and Hellin2013), cowpeas (Moussa et al., Reference Moussa, Abdoulaye, Coulibaly, Baributsa and Lowenberg-Deboer2014) and beans (Mutungi et al., Reference Mutungi, Affognon, Njoroge, Manono, Baributsa and Murdock2015), the plastic film (liner) has frequently been found perforated (García-Lara et al., Reference García-Lara, Ortíz-Islas and Villers2013; Martin et al., Reference Martin, Williams, Baributsa and Murdock2015; Likhayo et al., Reference Likhayo, Bruce, Tefera and Mueke2018; Mutambuki et al., Reference Mutambuki, Affognon, Likhayo and Baributsa2019) thus compromising the integrity of the bags. To address this challenge, it is imperative to continually evaluate novel and improved hermetic bags. The use of a laminated woven polypropylene (PP) bag provides additional oxygen barrier and moisture resistance properties and adds protection for the hermetic liner to help achieve better control of insect pests in stored grain leading to improved grain quality.
Hermetic grain storage bags of 100 kg capacity offer smallholder farmers the desired bag size flexibility and control of the quality of their produce during storage. ZeroFly® hermetic bag, developed by Vestergaard S.A., aims at protecting cereals and pulses against postharvest insect pests. In an effort to contribute towards more control options, Vestergaard-Kenya submitted samples of ZeroFly® Hermetic bags to Kenya Agricultural and Livestock Research Organisation (KALRO)-Kabete for local evaluation to independently evaluate its effectiveness. The aim of this study was therefore to verify the manufacturer's claims of the efficacy of the ZeroFly® hermetic bag in protecting stored maize grain against the larger grain borer P. truncatus (Horn) and other important storage insect pests. The new product was compared to PICS hermetic bag, and to insecticide dust-treated and untreated grains kept in woven PP bags under simulated field conditions.
Materials and methods
Description of the evaluation site
The evaluation was carried out at KALRO-Kiboko in Makueni County. Kiboko is hot and dry, situated 37.7234oE, 2.2172oS and 975 m above sea level (CIMMYT, 2013). The hottest months are February to March and September to October before the onset of long and short rain seasons, respectively (fig. 1). During the months of June to August, the area experiences cool conditions. The choice of the site was due to the prevalence of P. truncatus in the area, and a barn suitable for simulation of farmers’ storage conditions.

Figure 1. Mean monthly ambient temperature (oC) and relative humidity (%) during the evaluation period.
Storage bag technologies evaluated
Details of the six maize grain storage treatments compared and their capacity, cost and supplier are shown in table 1. The woven PP (farmer) bags of 90 kg holding capacity each were provided by Vestergaard S.A. Woven PP bags are made from synthetic fibre that is similar to plastic but is more degradable when exposed to sun rays. The bags impede the free circulation of air within the grain and are difficult to fumigate (ACDI/VOCA, 2007).
Table 1. Maize storage treatments evaluated during the trial at KALRO-Kiboko, Makueni County, Kenya

a Bags with one or two line liners are hermetic.
b The cost is inclusive of woven polypropylene bag (US$1 = ksh.100).
c Polypropylene bag is impregnated with insecticide Deltamethrin at the rate of 3 g per kg.
d Actellic Gold dust is a combination of pirimiphos-methyl 1.6% and thiamethoxam 0.36%, applied at 50 g per 90 kg grain, and manufactured by Twiga Chemicals Ltd, Nairobi, Kenya. It was bought from Agrovet, Mfangano Street; 50 g sachet of Actellic Gold dust costs US$1.2; Pt, Prostephanus truncatus; Sz, Sitophilus zeamais.
Two types of ZeroFly® hermetic bags made of multi-layered recyclable plastic polyethylene (PE) liner were tested. One type is a laminated PP woven outer bag with one inner PE bag as an oxygen barrier. The lamination provides excellent gas and moisture barrier properties that enhance the gas impermeability of the liner to ensure satisfactory protection of the stored grain from insect attack. The other type is an ordinary woven PP bag and one inner PE bag with a similar oxygen barrier. The liner in both bag types has an oxygen transmission rate (OTR) of ≤50 cc m−2 day−1 and a water transmission rate of ≤1.2 g m−2 day−1. Both the laminated and ordinary PP bags measured 122 cm × 76 cm while the inner plastic liner measured 130 cm × 80 cm. The bags were developed and supplied by Vestergaard S.A., Switzerland.
The insecticide-incorporated woven PP ZeroFly® storage bag is another innovative bag developed by Vestergaard S.A., Switzerland for the storage of grains. The active ingredient, deltamethrin, is incorporated into the PP yarns woven together and released on the surface of the fabric in a sustained manner to continuously protect grains stored in the bags against insect attack for a minimum of 2 years (Okonkwo et al., Reference Okonkwo, Mwambani, Otitodun, Ogundare, Bingham, Odhiambo and Williams2017; Paudyal et al., Reference Paudyal, Opit, Arthur, Bingham, Payton, Gautam and Noden2017b). The insecticide will knockdown and/or kill insects that land on it, thereby preventing their entry into the bags. Additionally, the bags repel insects thereby reducing insect population in the store area.
PICS bag is a triple-layer plastic bag that allows small-scale farmers to protect stored grain from insect damage without the use of insecticides (Murdock and Baoua, Reference Murdock and Baoua2014). The PICS bag consists of an outer ordinary woven PP bag and two inner liners of high-density PE, each 80 μ thick and OTR of 50–150 cc m−2 day−1.
Actellic Gold® Dust (a combination of Pirimiphos-methyl 1.6% + Thiamethoxam 0.3%) was procured from an AgroVet stockist, applied at the recommended rate of 50 g per 90 kg of grain.
Experimental procedure
Prior to filling the bags, the maize was thoroughly mixed on a clean tarpaulin to ensure uniformity. Fifty kg lots of white hybrid maize variety PH3253 that had been fumigated were put into each bag treatment, with four replications. Unsexed adult insects, 50 each of P. truncatus and S. zeamais (based on one adult insect per kg), were introduced into all five treatments except in the non-hermetic ZeroFly® bag to test its effectiveness in repelling pests from outside from entering the bag. In total, 100 adult insects (50 P. truncatus and 50 S. zeamais) were introduced into each bag. For the chemical treatment, grain maize was admixed with Actellic Gold® dust at the recommended rate of 50 g per 90 kg of grain and put in PP bags to serve as a positive control. PICS hermetic bags were included to provide a further comparison. Prior to loading grain into the hermetic bags, they were tested for air tightness or leakage by filling with air to form a pouch before compressing them with both hands, any that leaked were discarded. The PICS bags had two plastic liners, while the ZeroFly® hermetic bags had one liner; these are placed inside PP bags which provided support and handling convenience. Untreated maize grains were put in PP bags to serve as a negative control. The bags were then randomly placed in a barn on pallets (dunnage) in a randomised complete design (fig. 2).

Figure 2. Picture of lay-out of the experiment midway through the trial.
Sampling was done after every 4 weeks up to 36 weeks. A sample of 1 kg of grain was initially taken from each bag for baseline data using a compartmented long spear probe and further 1 kg samples were subsequently taken at intervals of 4, 8, 12, 16, 20, 24 and 36 weeks. Repeated sampling from the same storage device reflected farmer practices of opening the device at regular interval to draw grain for use as household food. The entrapped air was removed according to the manufacturer's instructions and securely tied at onset and after every sampling time to ensure air-tightness. To monitor hermetic conditions, oxygen and carbon dioxide levels were measured in four replicates of each treatment prior to each sampling using a MOCON®portable oxygen/carbon dioxide analyser (Pac Check® 325, Mocon Inc, USA). Measurement of gas composition levels in PP bags was not considered worthwhile as their open weave would allow the free exchange of gas and so it was not expected to accumulate carbon dioxide. Each grain sample was sieved to separate dust from insects and grain. The weight of dust (flour and frass) produced due to insect feeding activity was recorded. Grain moisture content was determined using Foss InfratecTM 1241 Grain Analyzer, which is a Near Infrared Transmission (NIT) instrument that uses transmission absorption. Moisture content was measured at the beginning of the experiment and at every sampling time. Five readings of each sample were taken by the instrument and the average recorded.
The sample was then divided using a riffle divider until four sub-samples of approximately 65 g were obtained. Grains in three of the sub-samples were sorted into undamaged, damaged, discoloured and broken grain categories which were counted and weighed. The damaged grain was expressed as a percentage of the total grain in the sub-sample. The means of grain categories of the sub-samples were recorded for each respective sample. The fourth sub-sample was reserved for reference. Weight of dust, percentage of grain moisture content, number of live adult insects and percentage of grain damage and weight loss were parameters used to judge the efficacy of each treatment. The percentage of insect-damaged grain and weight loss was calculated using the method of Boxall (Reference Boxall1986) as shown below:
where W u = weight of undamaged grains; N u = number of undamaged grains; W d = weight of damaged grains and N d = number of damaged grains.
Upon termination of the study, the hermetic bags were inspected for perforation (holes) made by adult P. truncatus as no other insect pest of stored maize is known to bore through the plastic liners, and the number of holes recorded.
Grain germination
The three sub-samples used for insect damage analysis were combined and a small portion is taken from which 100 grains were randomly selected for germination testing. For each sample, 25 grains were placed on moistened filter paper (Whatman No. 1) in each of four 9 cm plastic petri-dishes. The petri-dishes were arranged on a wooden laboratory shelf and moistened every 3 days with 10 ml of distilled water. The number of grains that germinated was recorded after 7 days. The percentage of grain germination was calculated as shown below:
Aflatoxin analysis
Five hundred grams of the sampled maize grains were analysed for total aflatoxin using a competitive enzyme-linked immune-sorbent assay (ELISA) kit (Helica Biosystems Inc, Santa Ana, CA, USA) according to the manufacturer's instructions. A portion of 10 g finely ground maize sample was mixed vigorously with 50 ml methanol:distilled water (70:30, v/v) using a magnetic stirrer for 15 min. The mixture was filtered through Whatman® filter paper No.1 and the filtrate applied to ELISA. Aliquots (100 μl) of the standards or sample filtrate were mixed with 200 μl of the assay diluent and transferred into mixing wells. Portions of 100 μl of the mixture was transferred to the appropriate antibody-coated wells in duplicate and incubated at ambient temperature for 30 min. The wells were washed three times with PBS-T and blotted dry. Aflatoxin HRP-conjugate (100 μl) was added to each antibody coated well and incubated at room temperature for 30 min. Enzyme TMB substrate (100 μl) was added to each microwell and the plate was incubated at ambient temperature for 10 min. A stop solution (100 μl) was added to each well and the optical density of each microwell was read at 450 nm using an ELISA reader (HumaReader HS, Human GBDH, Germany). Total aflatoxin levels were calculated using ELISA software (Rida®Soft, Z9999, R-Biopharm AG, Germany). The aflatoxin ELISA test had a lower limit of detection of 1.75 parts per billion (ppb). Only maize grain sampled up to 24 weeks of storage were analysed for aflatoxin.
Statistical analysis
The number of insects and holes was log10 (count +1) transformed, while percentage data (gas composition levels, moisture content, grain damaged and weight loss, germination) were square root transformed to stabilize the variances. The transformed data were analysed using General Linear Model procedure of GenStat Release 12.1 (VSN International Ltd 2009, Hemel Hempstead, UK), with treatment and storage period as main factors. Gas composition levels, grain moisture content, weight of dust, insect numbers, number of holes, per cent grain damage and weight loss, germination percentage and aflatoxin levels at each time-point post-treatment were the response variables. Significant differences between the means were separated by Tukey test at P < 0.05. However, for ease of understanding, untransformed means are presented.
Results
Gas composition levels (inside the plastic liners)
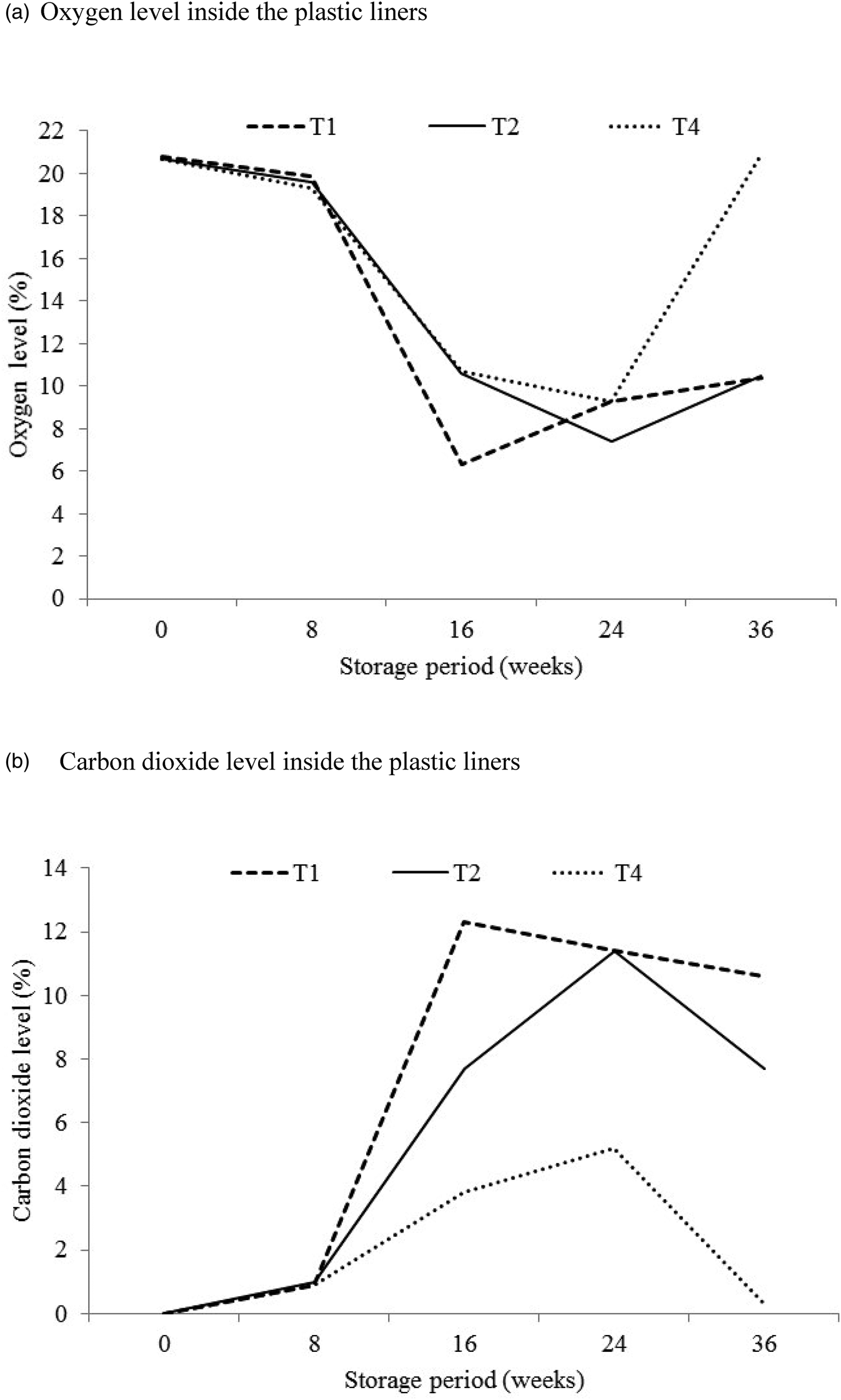
The changes in oxygen and carbon dioxide levels inside the plastic liners as measured in four replicates of each of ZeroFly® non-laminated, ZeroFly® laminated and PICS bags are presented in fig. 3. At the onset of the evaluation, oxygen levels ranged 20.7–20.8% with the highest level recorded in non-laminated two-layer ZeroFly® hermetic bag. There was a significant difference in oxygen levels by treatment (F = 2890.09; df = 2, 42; P < 0.001); storage period (F = 21823.73; df = 4, 42; P < 0.001) and treatment-storage period interaction (F = 1853.77; df = 8, 42; P < 0.001). The oxygen level decreased until 24 weeks of storage (fig. 3a). Eight weeks after onset of the study, oxygen levels in ZeroFly® and PICS bags had decreased to between 19.3 and 19.9%. At 16 weeks, the levels reached 10.7% for PICS and 6.3% for ZeroFly® hermetic bags and further dropped to 9.3% in PICS bags at 24 weeks. A gradual increase in oxygen level to 10.4% was recorded for both laminated single-layer and non-laminated two-layer ZeroFly® hermetic bags between 24 and 36 weeks of storage, while in the PICS bags it increased to 20.9% (fig. 3a). Oxygen depletion was similar in both types of ZeroFly® hermetic bags and the PICS bags for the first 8 weeks of storage and faster thereafter in the non-laminated two-layer ZeroFly® hermetic bag than the laminated single-layer ZeroFly® hermetic bag (fig. 3a).

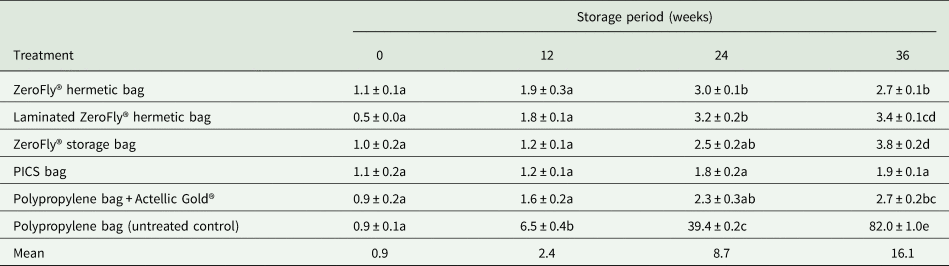
Figure 3. Mean (n = 4) changes in gas composition levels (%) inside the plastic liners. Where T1 = non-laminated two-layer ZeroFly® hermetic bag, T2 = laminated single-layer ZeroFly® hermetic bag and T4 = PICS bag. (a) Oxygen level inside the plastic liners. (b) Carbon dioxide level inside the plastic liners.
Carbon dioxide level at setup was 0.0% and increased thereafter (fig. 3b). There were significant differences between treatments (F = 13275.92; df = 2, 42; P < 0.001), storage periods (F = 31146.35; df = 4, 42; P < 0.001) and treatment-storage period interactions (F = 2882.64; df = 8, 42; P < 0.001). The highest reading was recorded at 16 weeks of storage in the non-laminated two-layer ZeroFly® hermetic bags (12.3%) and at 24 weeks for PICS bags (5.2%). Between 24 and 36 weeks of storage (fig. 3b), carbon dioxide decreased by between 1.7 and 3.7% in the ZeroFly® hermetic bags and by 4.9% in the PICS hermetic bags to reach 0.3%. The order of effectiveness in retaining carbon dioxide was as follows; non-laminated two-layer ZeroFly® hermetic bag > laminated single-layer ZeroFly® hermetic bag >PICS bag. Carbon dioxide evolution was similar in ZeroFly® hermetic and PICS bags for the first 8 weeks of storage and faster thereafter in the ZeroFly® hermetic bags until 24 weeks when levels dropped in all treatments.
Effect of treatment on grain damage and weight loss
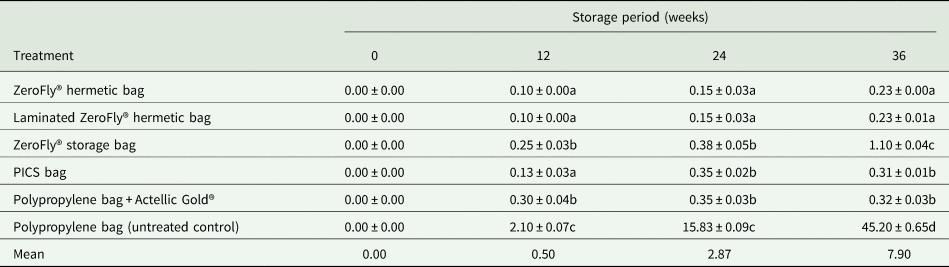
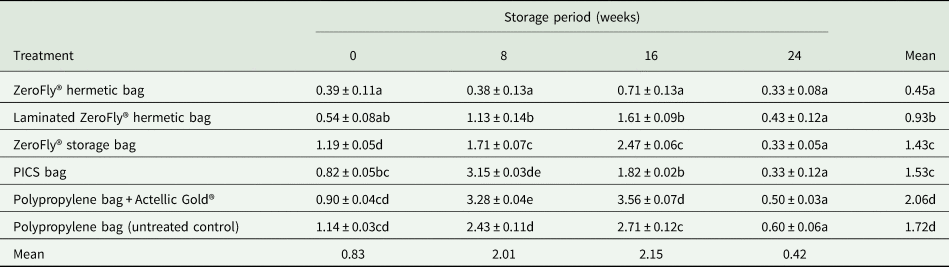
Grain damage levels occurring in the different treatments during the 36-week storage period is presented in table 2. There were significant differences in grain damage between treatments (F = 1687.84; df = 5, 69; P < 0.001) and storage periods (F = 1083.35; df = 3, 69; P < 0.001). Treatment and storage period interaction was also significant (F = 436.35; df = 15, 69; P < 0.001). At the start of the evaluation, the maize had negligible evidence of damage (0.9%). At 12 weeks storage duration, grain damage remained below 2% in the ZeroFly® hermetic bags, ZeroFly® non-hermetic bags, PICS bags and PP bags with Actellic Gold® dust-treated maize grains (positive control). Conversely, grain damage in PP bags containing untreated maize grains (negative control) increased steadily reaching 6.5% at 12 weeks storage, and 82.0% at the end of the study (table 2). Notably, from the onset of storage to 24th week of storage, grain damage in both the laminated and non-laminated ZeroFly® hermetic bags did not significantly differ (table 2), nor did they differ in efficacy from the ZeroFly® storage bag or PP bag with Actellic Gold® dust-treated maize grains at 24 weeks storage. At the termination of evaluation after 36 weeks of storage, of hermetic bags tested, non-laminated (2.7%) and laminated (3.4%) ZeroFly® hermetic bags had higher grain damage than the PICS bags (1.9%). Since no insects were added in non-hermetic ZeroFly® bags, the 3.8% damage incurred by grains kept in it at 36 weeks of storage is not comparative. However, for 36 weeks of storage, all the treatments tested except the negative control kept the grain damage level below 4%, while in the untreated grain stored in a PP bag, grain damage reached 82.0%.
Table 2. The effect of the treatment on the mean (±SEM) percentage of grain damage

Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Weight loss for the treatments during the 36-week storage period is presented in table 3. There were significant differences among the treatments (F = 1499.16; df = 3, 69; P < 0.001); storage periods (F = 1367.79; df = 3, 69; P < 0.001) and the interaction between treatment and storage period (F = 570.64; df = 15, 69; P < 0.001). No significant differences in weight loss among the hermetic bags tested were detected during the first 24 weeks of storage. For the entire storage period of 36 weeks, grain weight loss in the PICS bags did not exceed 1%. At termination, the weight loss incurred in non-laminated ZeroFly® hermetic bags (1.1%) was comparable to that of Actellic Gold® dust treatment (positive control) (table 3) but slightly higher than that of the non-hermetic ZeroFly® bags (0.3%) although that treatment had no insects added. There was an increasing trend in weight loss over the storage period. The hermetic bags evaluated kept maize grain safe, with grain weight loss remaining below 1.3% for 24 weeks storage, and below 3% for 36 weeks storage. A similar pattern was observed for grains treated with Actellic Gold® dust held in PP bag. A sharp rise in weight loss was recorded in untreated grains kept in PP bags, reaching 28% by 36 weeks storage.
Table 3. The effect of the treatment on the mean (±SEM) percentage of grain weight loss

Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Effect of treatment on insect-generated dust weight
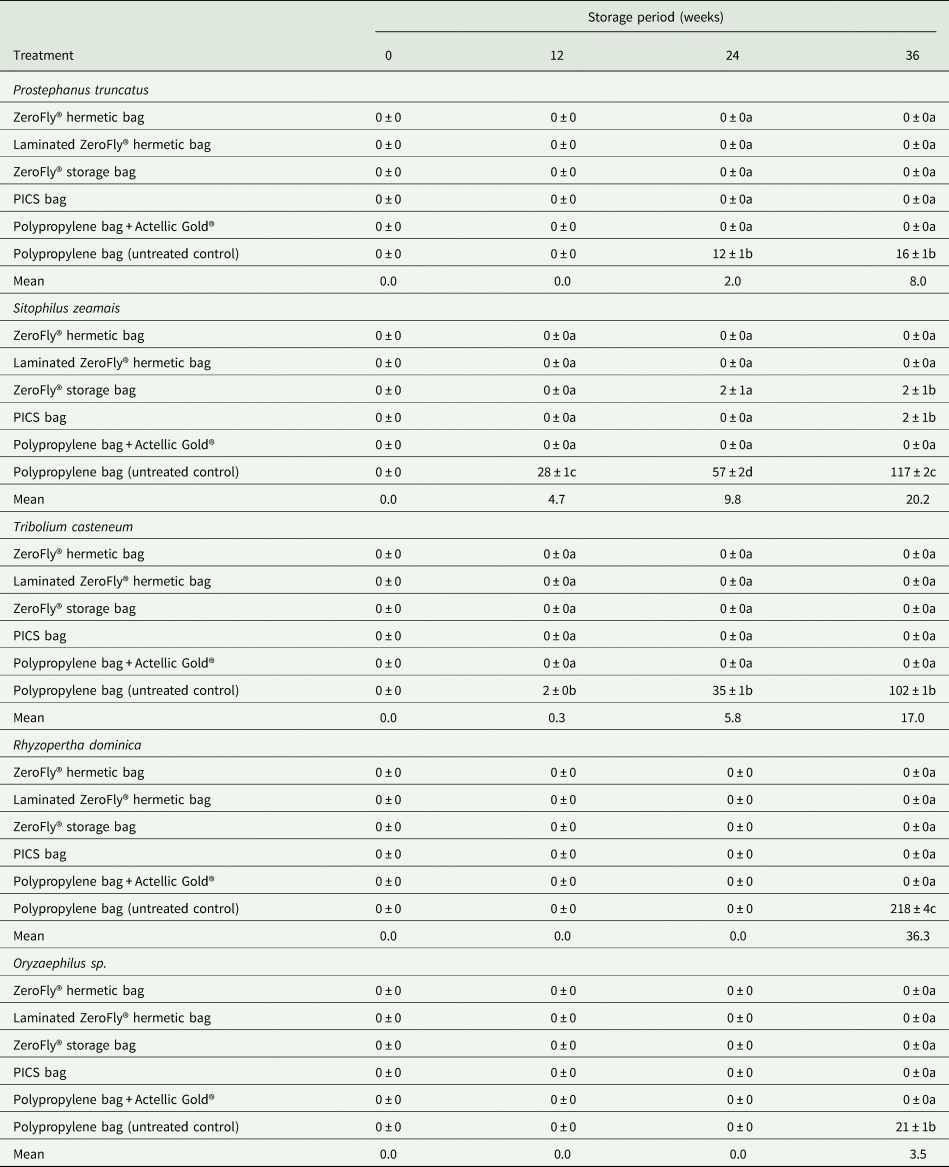
There were significant differences in the amount of dust (flour) generated by feeding activities of the insects between treatments (F = 7021.85; df = 5, 69; P < 0.001) and storage periods (F = 3271.84; df = 3, 69; P < 0.001). Treatment and storage period interaction was also significant (F = 1409.33; df = 15, 69; P < 0.001). Dust weight recorded for ZeroFly® hermetic bags remained practically the same throughout the storage duration compared to that of PP bags with untreated grain (negative control) and the non-hermetic ZeroFly® bags which contained un-infested grains. However, dust weight in non-hermetic ZeroFly® bags should not be compared since in this treatment no insects were added to the grains at set-up. From 24 to 36 weeks, dust weight recorded for PICS bags (0.35 and 0.31 g per 1 kg sample, respectively) was similar to that of PP Actellic Gold-treated bags. ZeroFly® hermetic bags recorded the least dust weight (0.23 g per 1 kg sample) while the PP bags had the highest (45.2 g per 1 kg sample) after 36 weeks of storage (table 4). Overall, there were no significant differences in the weight of dust recorded between laminated single-layer and non-laminated two-layer ZeroFly® hermetic bags and between PICS bags and PP bags with grains treated with Actellic Gold® dust throughout the entire storage period (table 4).
Table 4. The effect of the treatment on dust weight (mean ± SEM) in grams, due to feeding activities of insects

Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Effect of treatment on live adult insect count and bag perforation
Means of total live adult insect counts in the different treatments during the 36-week trial are presented in table 5. The insects recorded during the storage period were P. truncatus, S. zeamais, Rhyzopertha dominica, Tribolium castaneum and Oryzaephilus sp. (table 6). At the onset of the study, the maize did not have any emergent infestation. There were significant differences between treatments (F = 885.17; df = 5, 69; P < 0.001) and storage periods (F = 281.48; df = 3, 69; P < 0.001). The interaction effect between treatment and storage period was significant (F = 118.95; df = 15, 69; P < 0.001). On all sampling occasions, no live adult insects were recorded in the grains stored in ZeroFly® hermetic bags and in grains treated with Actellic Gold® dust (positive control). For 12 weeks storage duration, no insects were detected except in the untreated grain stored in the PP bags (negative control) in which proliferation of insects continued. Significant numbers of insects became evident starting from 12th week of storage and a drastic increase in the mean number of insects per kg grain sample occurred in the PP bags between the 24th and 36th week of storage, reaching a mean of 814 per kg of grain at 36 weeks storage (table 5).
Table 5. The effect of the treatment on the mean (±SEM) number of live adult insects per 1 kg grain sample

Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Table 6. Details of total live adult insect counts per kilogram of sampled grain

At the end of the study, the PICS hermetic bags recorded a higher mean number of holes (20 ± 3) per inner liner bag perforated by P. truncatus compared to both the laminated single-layer and non-laminated two-layer ZeroFly® hermetic bags which had no holes. Two replications of non-hermetic ZeroFly® bags were damaged by rats. Dead insects (mostly P. truncatus) were observed on the non-hermetic ZeroFly® bag fabric.
Effect of treatment on grain moisture content
There were significant differences in grain moisture levels between treatment (F = 62.16; df = 5, 69; P < 0.001) and storage periods (F = 1431.99; df = 3, 69; P < 0.001). Treatment and storage period interaction was also significant (F = 22.55; df = 15, 69; P < 0.001). Although significant differences were observed, the moisture content remained below the FAO recommended limit of 13.5% (Walker and Farrell, Reference Walker and Farrell2003) for safe storage of maize grain (table 7). Unexpectedly, moisture content in all treatments dropped between 0 and 12 weeks of storage by at least 1.2% from the initial average of 13.2%. From 12 to 26 weeks of storage, the moisture content of grains stored in hermetic bags remained practically the same (12%).
Table 7. The effect of the treatment on changes in grain moisture content (mean ± SEM)

Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Effect of treatment on grain germination
Grain germination capacity for the treatments is presented in table 8. There were significant differences in grain germination between treatments (F = 1228.12; df = 5, 87; P < 0.001) and storage period (F = 3296.56; df = 4, 87; P < 0.001). Treatment and storage period interaction was also significant (F = 472.83; df = 20, 87; P < 0.001). At the start of the evaluation, the mean germination rate was 90.5%. The germination rate had dropped substantially in hermetic PICS and ZeroFly® bags and Actellic Gold dust-treated PP bags by 30% after 36 weeks of storage duration. The highest drop in germination capacity was recorded for untreated grains stored in PP bags (86%). No significant differences were detected in germination capacity for grains stored in hermetic PICS and ZeroFly® bags and PP bags treated with Actellic Gold® dust.
Table 8. The effect of the treatment on the percentage of grain germination (mean ± SEM)

Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Effect of treatment on aflatoxin levels
There was significant effect on aflatoxin levels by treatment (F = 113.77; df = 5, 69; P < 0.001) and storage period (F = 392.48; df = 3, 69; P < 0.001). The interaction between treatment and storage period was also significant (F = 21.42; df = 15, 69; P < 0.001). Doubling of aflatoxin levels occurred within 8 weeks in PICS bags and PP bags with or without Actellic Gold® dust, and laminated ZeroFly® hermetic bags (table 9). However, no clear trend in changes of aflatoxin levels was observed. Aflatoxin levels in all treatments remained below the maximum allowable level of 10 ppb throughout the 36-week storage period.
Table 9. The effect of the treatment on the aflatoxin levels (mean ± SEM)

Analysis was performed up to 24 weeks of storage only.
Means within the same column followed by the same letter are not significantly different at P = 0.05 level (Tukey test). Data are means of four replications.
Discussion
Smallholder farmers store their maize grain to assure their food supply between the harvests. However, factors such as use of improved varieties more susceptible to storage insect damage and the spread of exotic storage insect pests such as P. truncatus negatively impact effective storage practices. Although farmers apply insecticides and various traditional protective measures, few achieve adequate control of the insect pests. Grain damage due to insect infestation is a serious concern that threatens food security and livelihoods of smallholder farmers.
Hermetic PICS and ZeroFly® bags are airtight and depletion of oxygen entrapped in them during filling occurs through the natural respiration of the maize grains and insects, effectively suppressing insect development and survival and consequently preventing grain damage. Low oxygen (6–10%) and enhanced carbon dioxide (3–12%) in the inter-granular atmosphere led to suppression of the insect infestation and mortality thus preventing grain damage during storage; which has been documented in maize studies in West Africa (Baoua et al., Reference Baoua, Amadou, Ousmane, Baributsa and Murdock2014) and Zimbabwe (Mlambo et al., Reference Mlambo, Mvumi, Stathers, Mubayiwa and Nyaboko2017). Oxygen depletion and carbon dioxide evolution was faster after 8 weeks of storage in non-laminated two-layer ZeroFly® hermetic bags compared to laminated ZeroFly® and PICS bags. However, the oxygen level in non-laminated two-layer ZeroFly® hermetic bags increased gradually after 16 weeks of storage while that in laminated ZeroFly® hermetic single-layer bags increased after 24 weeks storage. This probably suggests lamination, which is used to prevent oxygen ingress, did not help limit oxygen from entering the liner. The liner was not perforated and we are unable to explain why oxygen started to increase. Since the maize grains were dried to 13% moisture content before loading and the same number of live insects were introduced in the bags, the observed differences in the oxygen depletion and carbon dioxide evolution rates in the present study could probably be due to the differences in gas permeability rates between ZeroFly® hermetic and PICS bags, which are reported by the manufacturers to be ≤50 and 50–150 cc m−2 day−1, respectively.
For the 36 weeks storage duration, PICS, ZeroFly® hermetic, non-hermetic ZeroFly® and Actellic Gold® dust-treated grain in PP bags kept grain damage and weight loss below 4 and 3%, respectively, compared to untreated PP bags (negative control). Since insects had been added to the grains in all treatments except for non-hermetic ZeroFly® storage bags, the damage and weight loss it recorded are not comparable. Non-hermetic ZeroFly® storage bag technology was invented to prevent insects outside the sacks from penetrating the sacks and attacking grain after it has been loaded insect-free into the bag. Field studies on its efficacy in Zimbabwe (Mlambo et al., Reference Mlambo, Mvumi, Stathers, Mubayiwa and Nyaboko2017), Tanzania (Abass et al., Reference Abass, Fischer, Schneider, Daudi, Gasper, Rust, Kabula, Ndunguru, Madulu and Msola2018) and Malawi (Singano et al., Reference Singano, Mvumi and Stathers2019) show the occurrence of live insects and high damage to grain stored within it, although no initial artificial seeding of insects was done in the trials. It is likely if insects had been added to non-hermetic ZeroFly® storage bags, high grain damage and weight loss would have occurred as happened in the untreated grain in PP bags. As expected, grain damage and weight loss were high in untreated PP bags and the grains were of low quality that is indicative of losses farmers would incur if maize was stored unprotected for 36 weeks. Our study shows the use of hermetic storage bags or Actellic Gold dust-treated PP bags which cost US$2.5 and US$1.7 for a bag of 90 kg grain capacity, respectively, offer farmers the opportunity to save storage losses of US$10.5 (assuming a 30% weight loss after 6 months of storage) per 90 kg bag of maize, as maize in Kenya in 2019/2020 was marketed at US$35 per 90 kg bag 6 months after harvest.
Insect infestation was high in untreated PP bags for 36 weeks of storage. Although PP bags are simple to use and available in different sizes, they allow easy access to the stored grain by insect pests. The finding is in agreement with other work that reported that untreated grains kept in PP bags during storage were of low quality and incurred high losses (Manandhar et al., Reference Manandhar, Milindi and Shah2018; Kitinoja et al., Reference Kitinoja, Dandago and Abdullahi2019). In contrast, multiplication of insect pests was drastically reduced in PICS, ZeroFly® hermetic and treated PP bags. The grain damage levels observed in the storage technologies were mainly as a result of P. truncatus, S. zeamais and R. dominica insect infestation. Whereas infestation was observed in non-hermetic ZeroFly® storage bags; it performed better than untreated PP bags in suppressing insect population build-up. However, it is not known if the same level of grain damage as occurred in the non-hermetic ZeroFly® storage bags would be obtained if artificial insect seeding was not done to untreated grain in PP bags.
The hermetic and Actellic Gold dust-treated grain in PP bags kept the weight of dust generated by insect feeding activities to below 0.4 g per 1 kg sample compared to the untreated grain PP bags for 36 weeks of storage. The bostrichids P. truncatus and R. dominica are known for generating extensive dust during feeding and boring of grains, which negatively reduces grain quality and germination capacity.
Unexpectedly, grain moisture content dropped by 1.2% from the initial 13.2% between 0 and 12 weeks of storage. The hermetic liners are bags with good gas-tight and water barrier properties. Interaction of grains stored in the liners with the environment leading to moisture content changes in response to ambient relative humidity therefore is not expected (Williams et al., Reference Williams, Murdock and Baributsa2017; Baoua et al., Reference Baoua, Bakoye, Amadou, Murdock and Baributsa2018). The moisture content of maize stored in SuperGrain bag™ bags for 7 months in Benin dropped by 0.6% from the initial 13.4% while that in PICS bags remained unchanged (Baributsa et al., Reference Baributsa, Bakoye, Baoua and Murdock2020). Earlier field study reported a drop in moisture content in grains kept in PP bags in response to ambient relative humidity and temperature (Mlambo et al., Reference Mlambo, Mvumi, Stathers, Mubayiwa and Nyaboko2017). Since the same moisture meter was used throughout the trial, it is speculated that grain moisture drop observed in the hermetic bags could have been to a smaller extent affected by prevailing ambient conditions. Studies done elsewhere show that moisture content of grains stored in hermetic technologies is to a lesser degree affected by prevailing ambient conditions (Williams et al., Reference Williams, Murdock and Baributsa2017; Baoua et al., Reference Baoua, Bakoye, Amadou, Murdock and Baributsa2018).
Smallholder farmers who recycle own saved hybrid seed over time experience poor germination potential. The present work demonstrates that germination rates for all treatments dropped significantly during the 36 weeks of storage. The lowest germination rate (4.7%) was observed in untreated highly infested grain kept in PP bags. Better germination rates (60.0%) were recorded for PICS, ZeroFly® hermetic and Actellic Gold® dust-treated PP bags after 36 weeks of storage, although this was still a 30% drop from the initial levels. Since a random selection of grain from each treatment was taken for the sample, it is likely that heavily damaged grains would not germinate. Insect damage among other factors may have affected the germination. A 50–80% germination drop in maize stored in uncontrolled warehouse after 8 months (about 32 weeks) has been documented in the USA (Tekrony et al., Reference Tekrony, Shade, Rucker and Egli2005). Further, the rate of grain germination has been documented to differ with storage method used over time (Tefera et al., Reference Tefera, Teshome and Singano2018). However, the finding that the germination rate dropped for grain held in hermetic bags contrasts with other studies that showed seeds of all types stored in ultra-hermetic storage devices maintained high germination capacity (86.1%) (Villers, Reference Villers2017). The observed difference in germination potential in the present study could be attributed to microflora that infect all grains, insect damage of the grains and warmer ambient temperatures. The maximum temperature experienced at Kiboko slightly exceeded 30 °C. For certified seeds, a germination rate of 85% is required after at least 1 year of storage (Villers, Reference Villers2017; Fufa et al., Reference Fufa, Abera and Demissie2020). Since many smallholder farmers recycle seed for planting, the finding suggests that reasonable germination can be achieved when maize grain is stored in PICS, ZeroFly® hermetic and treated PP bags. Further study is required to better understand the factors responsible for the decline in germination capacity.
Upon termination, inspection of the plastic liners showed no physical damage (perforation) for ZeroFly® hermetic bags. These bags are made of multi-layered recyclable PE, with good gas and water barrier properties. Therefore, grain volatiles would not be released to the outside to elicit movement of insects into the bags while searching for food and the insects inside the bags at set-up died due to hypoxia. In comparison, PICS bags were perforated. Although the holes were evident to the naked eye, their examination by use of hand-held magnifying glass showed that the scratch and tear were less marked around the holes on the side from which the insects perforated the liner, an indication of exit holes (Riudavets et al., Reference Riudavets, Salas and Pons2007). The holes might have been made by P. truncatus, while attempting to escape the bags when exposed to an oxygen-depleted environment. Prostephanus truncatus is known to bore through harder plastic materials such as 35 mm-thick plastic (Li, Reference Li1988) than any other maize storage insect pests. The holes were made near the bottom of the bags. The holed bags therefore were unable to attain air-tight conditions resulting in inadequate control of the storage pests. The observation is in agreement with other researchers’ findings that mostly the inner liners were perforated when maize was stored in PICS bags for 150 days (Ognakossan et al., Reference Ognakossan, Tounou, Lamboni and Hell2013) and 180 days in SuperGrain™ bags (De Groote et al., Reference De Groote, Kimenju, Likhayo, Kanampiu, Tefera and Hellin2013). The cowpea bruchid, Callosobruchus maculatus F. (Coleoptera: Bruchidae), was found to bore PICS bags during storage in Niger (Baoua et al., Reference Baoua, Amadou and Murdock2012) but the hermetic conditions were not completely lost because of the unperforated second liner. In the present evaluation, both inner (20 holes) and outer liner (six holes) of the four PICS bags were perforated. The integrity of the holed liners was lost and consequently could not be re-used under the trial conditions.
Maize is among the crops that can exhibit high levels of aflatoxin in hot and humid climatic conditions (Villers, Reference Villers2017). In the present study, aflatoxin was heterogeneously distributed and present at very low levels in the maize samples. Substantial increases in the level of aflatoxin were recorded after 8 weeks in PICS and PP bags. However, a reduction in the levels to almost those recorded at the onset of the study was observed in all treatments at 24 weeks of storage. The observed difference in the aflatoxin levels between 16 and 24 weeks could be attributed to the challenges inherent in sampling heterogeneously distributed low concentrations of mycotoxins in maize grain samples. The international maximum standard for acceptable aflatoxin levels in food is 10–20 ppb (Villers, Reference Villers2017). In 2010, 10% of the Kenya's maize crop was destroyed for having aflatoxin levels exceeding 20 ppb, which caused deaths in some areas after consumption (FAO, 2011). No detection of aflatoxin concentration above Kenya's tolerance specification of 10 ppb in maize grain was recorded in any of the treatments in the current trial. Very low differences in aflatoxin levels were detected among the treatments and between storage periods to make meaningful inference.
Conclusion and recommendation
Based on the results, the PICS, both ZeroFly® hermetic bags and the Actellic Gold® dust-treated grain stored in PP bags were very effective in protecting stored maize grain from insect infestation during a 36 weeks storage period. Grain damage, weight loss and insect populations remained very low in these storage technologies. The study was done in one on-station site and for one season. A multi-season and multi-site on-farm participatory study is required to evaluate the efficacy of these hermetic storage bag brands under smallholder farmers’ use in locations with different pest pressure and temperatures. The findings of the study will help farmers, development agencies and government to make informed decisions on adoption and promotion of grain storage technologies.
Acknowledgements
The authors thank Teresa Warigia, Mark Limo and Eliud Njoroge for their technical support. This work was financed by Vestergaard S.A., Switzerland. The authors thank drivers Michael Kimunya Njuguna and Jeremiah Kioko who were always punctual and made sure that the car was well maintained before and after every trip. This report presents results of research only. Mention of trade names or commercial products in this report is solely for the purpose of providing specific information and does not imply recommendation or endorsement by KALRO or Vestergaard.