Introduction
Onobrychis viciifolia, commonly called sainfoin in the UK, was widely cultivated in Europe, Asia and North America (Miller and Hoveland, Reference Miller and Hoveland1995; Frame et al., Reference Frame, Charlton and Laidlaw1998) in the 19th and 20th centuries. Its decline started in the middle of the last century and as the Green Revolution gathered momentum in the UK, it was gradually replaced by alfalfa (Medicago sativa) and clover (Trifolium spp.) whose higher yields and easier establishment made them more desirable to farmers. This decline in sainfoin cultivation was also seen in other parts of Europe, notably France, Italy and Spain due to the adoption of more intensive farming methods and crop choice (Hayot Carbonero, Reference Hayot Carbonero2011b; Demdoum, Reference Demdoum2012b). This decline in Europe was, in part, due to the introduction of relatively low-cost nitrogen fertilizers in the early 1970s, which helped to enable the expansion of grassland for livestock. Furthermore, supportive payments through the Common Agricultural Policy added to an increase in more intensive production methods during the 1980s (Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). The disappearance of livestock farms in hilly areas (Borreani et al., Reference Borreani, Peiretti and Tabacco2003) and the rise in animal feed imports from non-European Union (EU) countries further reduced the use and cultivation of sainfoin. This shift in cropping patterns has led to increasing dependence by Europe on imports of animal feed. It has been calculated that the EC received 2.3 Mt of nitrogen in the form of grain legumes from South America in 2004 (Galloway et al., Reference Galloway, Townsend, Erisman, Bekunda, Cai, Freney, Martinelli, Seitzinger and Sutton2008) and there are further collateral negative consequences of this change in protein sourcing. In parts of South American, for example, large areas of forests have been cleared in order to increase soya production. Europe is now concerned about its dependence on imported protein sources and negative impacts on national food security (Aigner, Reference Aigner2009; European-Commission, 2010; Weightman et al., Reference Weightman, Cottrill, Wiltshire, Kindred and Sylvester-Bradley2011; Lüscher et al., Reference Lüscher, Mueller-Harvey, Soussana, Rees and Peyraud2014). The trend within the EU is now to reduce this resource-dependence through re-establishing the use of forage legumes and improving their agronomy. Leguminous forage crops such as Sainfoin, trefoil (Lotus corniculatus) and red clover (Trifolium pratense) not only enable more efficient, locally grown supply of nitrogen, they also reduce transit of inorganic nitrogen from the soil. It has been suggested that global changes in climate and weather patterns will increase the potential for economic returns from the cultivation of both forage legumes and grasses, which can optimize the capture of heat and light, allied to nutrient sequestration (Haynes, Reference Haynes and Brady1980; Clarke et al., Reference Clarke, Davison and Fulloon2000).
Recent studies show that sainfoin has anthelmintic properties, methane-control potential from ruminants and protein-protection capability from early degradation in the rumen when used as a forage crop in the diet of ruminant animals. These properties are attributed mainly to sainfoin's foliar tannin composition (Lorenz, Reference Lorenz2011; Novobilský et al., Reference Novobilský, Mueller-Harvey and Thamsborg2011; Pellikaan et al., Reference Pellikaan, Stringano, Leenaars, Bongers, Schuppen, Plant and Mueller-Harvey2011; Theodoridou et al., Reference Theodoridou, Aufrère, Andueza, Le Morvan, Picard, Stringano, Pourrat, Mueller-Harvey and Baumont2011). Moreover, it is cited that sainfoin is a good nectar and pollen source for honey bees and many other pollinator species, including bumblebees, hoverflies and solitary bees. This characteristic, together with its drought tolerance, could play a vital role in the stability and sustainability of agro-ecosystems if sainfoin is included as a crop or as part of a pollinator mixture within the farmed environment (Kells, Reference Kells2001; Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011) (Figure 1).
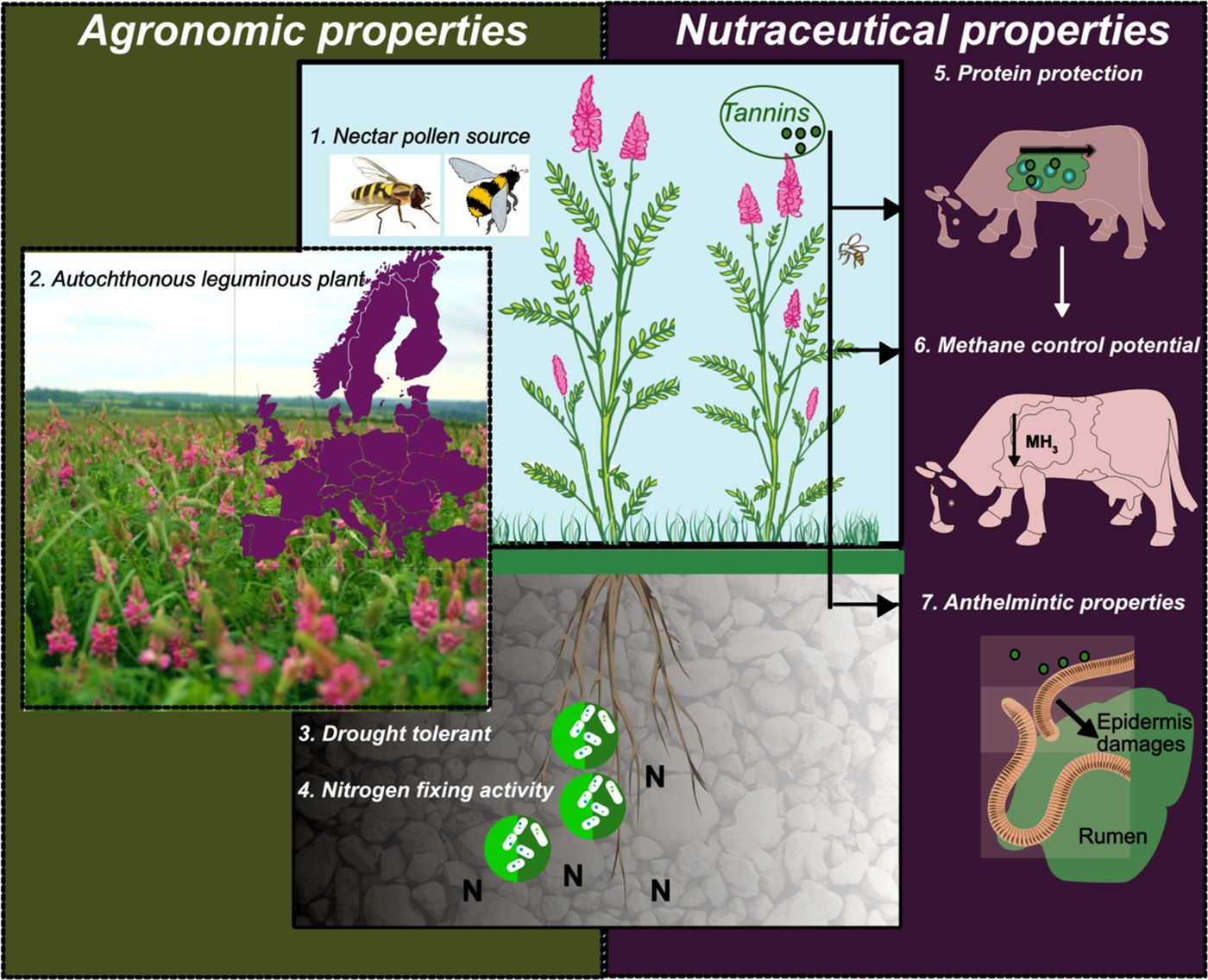
Fig. 1. (1) Sainfoin is a rich nectar and pollen source. The flowering is ‘indeterminate’ having with continuous flower development for several weeks. (2) UK Sainfoin field midway through peak flowering. Sainfoin is an autochthonous leguminous plant that could reduce the current level of nitrogen dependence from Europe. Photograph and figure donated by Cotswold Seeds Ltd. (3 and 4) Schematic representation of Sainfoin root and microbial associations. (5, 6 and 7) Schematic of impact on rumen microbiology and anthelmintic benefits.
Unfortunately, the reintroduction of sainfoin remains a challenge and it is necessary to significantly improve our understanding of the crop and to improve both its agronomy and crop genetic resources. Of particular interest are improvements in weed control, establishment, seed dormancy and genetic characterization to facilitate targeted pre-breeding and breeding programmes in the future.
History of the crop, introduction to Europe from Asia and posterior expansion to America
The centre of origin of sainfoin is South Central Asia, where it was common as a component of mixed swards in Asia Minor, particularly on the Anatolian Plateau of Turkey and the districts of the Caucasus and the Caspian fringes, it was originally cultivated by Arabian cultures through whom it was introduced to Greece and Italy, although it was neither formally cultivated by the ancient Greeks as a crop nor by their descendants (de Candolle, Reference de Candolle1883; Stebler et al., Reference Stebler, Schroeter and Volkart1894), although a related wild species Onobrychis caputgalli was documented at this time (Stebler et al., Reference Stebler, Schroeter and Volkart1894). Most authors agree that it was introduced into southern continental Europe in the late 14th century not reaching northern Europe and the UK until at least a century later. It was introduced into North America later (Burton and Curley, Reference Burton and Curley1968; Frame et al., Reference Frame, Charlton and Laidlaw1998) probably early in the 16th century, the exact date is difficult to establish due to inconsistencies and contradictions in the literature.
Different documents testify to the historic cultivation of sainfoin in the UK; mostly in England. Cultivation in England has been documented in the south and south-east, south of Wales; north to the Humber and west to the river Severn since 18th century; in the Vale of Glamorgan in the 19th and early 20th century (Davies, Reference Davies1815; Rees, Reference Rees1928) and in East Anglia (Bland, Reference Bland1971). This distribution is linked to its preference for light free-draining, neutral to alkaline soil. In ‘The English Improver Improved’ (Blith, Reference Blith1652) and ‘Horse Hoeing Husbandry’ there is evidence that many thousands of acres in England were used for sainfoin production due to its importance in animal nutrition and soil quality preservation. The cultivation methods are described in ‘General View of the Agriculture of Oxforshire’ (Tull, Reference Tull1733; Young, Reference Young1913) it is traditionally sown mixed with a companion species, usually a non-invasive grass such as Festuca pratensis or Phleum pratense. This strategy enabled farmers to suppress weed invasion, which is a significant challenge in establishment and cultivation.
The decline of sainfoin in Britain started in the 1920s, and increased significantly as long-term leys were ploughed up during the 1939–1945 war period (Bland, Reference Bland1971). This loss of popularity was partly associated with a decrease in cultivation of long-term pasture for sheep production in favour of the cultivation of higher yielding ryegrasses (Edmunds, personal communication cited in Hayot Carbonero (Reference Hayot Carbonero2011b)). The decline was also attributable to the replacement of horses by machinery (Newman, Reference Newman, Lane and Wilkinson1997). It was recorded that about 150 tonnes of sainfoin seed were sold every year in the late 1950s, this was sufficient for circa 2400 hectares (Hill, Reference Hill, Lane and Wilkinson1997). Seed sales dropped to an amount sufficient for 150 hectares in the 1970s (Sheehy and Popple, Reference Sheehy and Popple1981) and just 50 hectares in the 1980s (Aldrich, Reference Aldrich1984), but UK cultivation has increased in the past decade to more than 1970s levels and is still rising. Sainfoin is still an important crop in parts of Asia, Turkey and Iran. Currently, sainfoin cultivation also persists in parts of North America, Italy and Spain (Koivisto, Reference Koivisto2001).
Etymology and common denomination of sainfoin
The name Onobrychis viciifolia is probably derived from the Greek, ónos (ὄνος, ‘donkey’) and brýkein (‘to eat avariciously’) (Carniol, Reference Carniol1771). However, Jaques (1894–1897) and (Demdoum, Reference Demdoum2012b) suggest that the real origin of –brychis is brýcho (‘bray’) is due to the happy sound that is made by donkeys when they eat it.
The crop has many common names, the most popular is probably sainfoin, but it has been known as St. Foin, which comes from the old French sain foin (‘healthy hay’). It has also been called Medicinal Plant and Luzerne. Other names and derivations originate from the French and Spanish name esparceto. Some examples of this include esparceta or pipirigallo in Spanish, esparsette in Danish, esparcette in Dutch, sparceta in Polish, or Эспарцет (espartset) in Russian. It has been called ‘holy grass’ due to its beneficial properties, ‘French grass’ or ‘Cock's head’ in English, or ‘crête de coq’ in French (Zolla, Reference Zolla1904). The Spanish pipirigallo, also originates from this source meaning ‘cockscomb’, all of which latter names refer to the morphology of the spiny husk of the seed (Moliner, Reference Moliner1982). Foin de Bourgogne, Fenasse, Bourgogne or Herbe éternelle (Stebler et al., Reference Stebler, Schroeter and Volkart1894) are other names found in the literature, referring to its use and perennial growth.
Taxonomy of sainfoin
The crop known as ‘sainfoin’ has been located in many different genera during its history, for example, Hedysarum and Sartoria (Badoux, Reference Badoux1965). Old drawings were published by Johann Georg Sturm in 1796, illustrating an example of Onobrychis viciifolia called Esparsette, but classified as H. onobrychis. Some authors have named it Onobrychis foliis viciae due to its similarity to those species in the genus Vicia and due to the resemblance between foliage morphology of Onobrychis sativa (Stebler et al., Reference Stebler, Schroeter and Volkart1894). Sainfoin has also been called Dendrobrychis and Xanthobrychis in the past (Table 1).
Table 1. Onobrychis viciifolia, a summary of the classification (Širjaev, Reference Širjaev1925)

Onobrychis viciifoila is now the accepted name for the crop known as sainfoin and most authors now accept that this genus has 126 species closely–following the classification originally developed by (Širjaev, Reference Širjaev1925). This inlcudes a large representation from Turkey, where 57 species are described of which, 27 are endemic (Aktokly, Reference Aktokly1995). Onobrychis is in the Fabaceae family, previously called Leguminoseae. In this widely accepted classification (Table 1) the genus is organized into two sub-genuses, Eunobrychis and Sisyrosemae, with four sections each.
Classification by other authors has used various contradictory systems based on different components of morphology (Emre et al., Reference Emre, Turgut-balik, Sahin and Kursat2007). ILDIS included between 140 and 150 species (ILDIS, 2005) in contrast, only 23 were included in the encyclopaedia of European Flora (Wallace, Reference Wallace1969). Based mainly on the seed morphology, 170 species were estimated by Yildiz (Reference Yildiz, Ciplak and Aktoklu1999) but this was shown to be flawed due to the high variability observed in the seeds depending upon environmental conditions (Yildiz et al., Reference Yildiz, Ciplak and Aktoklu1999; Hayot Carbonero, Reference Hayot Carbonero2011b). Guner et al. (Reference Guner, Ozhatay, Ekim and Baser2000) building on earlier work (Badoux, Reference Badoux1965) estimated that the 54 species can be divided into five sections.
Of the species within the genus Onobrychis, only five have been shown to have useful agricultural attributes: Onobrychis sativa Lam. (O. Viciifolia Scop.), Onobrychis sativa var. Persica (Širjaev pro var.), Onobrychis arenaria (Kit.) Ser., Onobrychis transcaucasica Gross. and Onobrychis montana D.C. (Badoux, Reference Badoux1965).
Badoux (Reference Badoux1965) named these five species as O. viciifolia Scop. sensu lato, and O. viciifolia Scop. sensu stricto O. sativa, in its three forms and varities: f. communis, f. bifera and var. persica (cited in Demdoum (Reference Demdoum2012b) and Lewke Bandara et al. (Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013)). Hayot Carbonero et al. (Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011) re-defined the classification of divisions according to flowering date and morphological traits, indicating that there are two coherent clusters dividing O. viciifolia originally from Western Europe and Eastern Europe, Asia and USA (Hayot Carbonero, Reference Hayot Carbonero2011b). The taxonomic classification of O. viciifolia still remains a matter of debate (Lewke Bandara et al., Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013).
Polyploidy and karyotype of Onobrychis viciifolia
Sainfoin has been characterized in terms of the chromosome number and morphology. An analysis of the ploidy of world seed collections of sainfoin yielded three possible ploidy levels (2x, 4x and 8x) and 2 basic chromosome numbers (x = 7 and x = 8) (Abou-El-Enain, Reference Abou-El-Enain2002). Sainfoin can be diploid (2n = 2x = 14) or tetraploid (2n = 4x = 28) (Figure 2), the basic number of chromosomes being 7. Whereas diploid accessions of sainfoin are infrequent and not very well described in the literature, tetraploid makes up the majority of sainfoin accessions held in most collections (Sacristan, Reference Sacristan1966; Frame et al., Reference Frame, Charlton and Laidlaw1998; Tamas, Reference Tamas2006; Hayot Carbonero et al., Reference Hayot Carbonero, Carbonero, Smith and Brown2013). Polyploidy level has been linked with domestication and improvement of the crop, such that productive tetraploid plants have been selected (Hayot Carbonero, Reference Hayot Carbonero2011b). These tetraploid accessions can be either autopolyploids or allopolyploids, and it is uncertain whether the inheritance is tetrasomic or disomic (Corti, Reference Corti1930; Sacristan, Reference Sacristan1966; Vicente and Arús, Reference Vicente and Arús1996; Abou-El-Enain, Reference Abou-El-Enain2002).

Fig. 2. Meristematic root tips of O. viciifolia stained with feulgen solution showing (a) diploid nucleus with 2n = 2x = 14 chromosomes (accession 1257), and (b) tetraploid nuclei with 2n = 4x = 28 chromosomes (accession 1292). Scale bars are 5 µm (reproduced by permission of (Hayot Carbonero, Reference Hayot Carbonero2011b)).
Most of the cytological research on Onobrychis has been focused on ploidy characterization (Karshibaev, Reference Karshibaev1992; Slavicvk et al., Reference Slavicvk, Jarolivmovav and Chrtek1993), while karyological studies are less common (Surayya and Syed Irtifaq, Reference Surayya and Syed Irtifaq1991; Karshibaev, Reference Karshibaev1992; Mesicek and Sojak, Reference Mesicek and Sojak1992). The karyotype analyses of O. viciifolia show an average size of 3.39 µm (Tamas, Reference Tamas2006) and more recent analysis indicates that arm ratios range from 1.41 to 2.22 µm (Somay Akcelik et al., Reference Somay Akcelik, Avci, Uzun and Sancak2012). The analysis of the karyotype was also used to define the taxonomy of different species within the Onobrychis genus and to determine its evolution. Through the karyotype analysis of different Onobrychis species, it has been suggested that the two populations of Onobrychis transcaucasica should be placed in one group, while Onobrychis altissima and O. viciifolia should be placed in a second group (Massoud et al., Reference Massoud, Karamian and Hadadi2010). During one of the last karyotype analyses developed in sainfoin, the 2C value was determined to be approximately 2.5 pg (Hayot Carbonero et al., Reference Hayot Carbonero, Carbonero, Smith and Brown2013). This cytological data is potentially useful alongside genetic profile to support crop genetic improvement in the future.
Genetic characterization and phylogenetic analysis
The understanding of sainfoin molecular genetics is limited to a few recent studies. These studies focused on DNA extraction methods, marker discovery, phylogenetic analysis of sainfoin accessions, gene discovery (focused on polyphenol metabolism) and most recently, de-novo transcriptome investigation for gene identification and putative marker discovery (Hayot Carbonero, Reference Hayot Carbonero2011b; Demdoum et al., Reference Demdoum, Munoz, Delgado, Valderrabano and Wuensch2012; Hayot-Carbonero et al., Reference Hayot-Carbonero, Carbonero, Smith and Brown2012; Thill et al., Reference Thill, Regos, Farag, Ahmad, Kusek, Castro, Schlangen, Carbonero, Gadjev and Smith2012; Lewke Bandara et al., Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013; Kempf et al., Reference Kempf, Mora-Ortiz, Smith, Kolliker and Skot2016; Mora-Ortiz et al., Reference Mora-Ortiz, Swain, Vickers, Hegarty, Kelly, Smith and Skøt2016).
Polyphenols and high levels of many types of high molecular weight tannins are present in sainfoin foliage. These interfere with successful DNA and RNA extraction, producing problems in isolating high-quality samples. Very low yield, contamination by (i) phenols, (ii) proteins and (iii) RNA, or (iv) degradation of the samples, are some of the most common issues described in the literature (Hayot Carbonero, Reference Hayot Carbonero2011b; Mora-Ortiz, Reference Mora-Ortiz2015). Several extraction methods have been tested in order to extract high-quality DNA. These methods included standard procedures such as those described by (Hormaza, Reference Hormaza1999) and (Fulton et al., Reference Fulton, Chunwongse and Tanksley1995). Other commercial options such as the kits from Qiagen (DNeasy Plant Mini Kit) and GE Healthcare (Nucleon PhytoPure Genomic DNA) have also been tried. The most successful method reported was Nucleon PhytoPure and the modified (Doyle and Doyle, Reference Doyle and Doyle1987) ‘DNA extraction protocol’ described by Hormaza (Reference Hormaza1999) (Hayot Carbonero, Reference Hayot Carbonero2011b, Demdoum et al., Reference Demdoum, Munoz, Delgado, Valderrabano and Wuensch2012, Hayot-Carbonero et al., Reference Hayot-Carbonero, Carbonero, Smith and Brown2012). Similar methodological difficulties were encountered when attempting to obtain high-quality RNA extracts. Many methods have been evaluated including (i) TRIzol® (Invitrogen, USA) RNA isolation method, (ii) RNeasy® (Qiagen, Germany) and (iii) CTAB Total RNA Extraction Protocol. The Plant/Fungi Total RNA Purification Kit from NORGEN from Biotek Corporation was identified as a suitable method to extract high-quality RNA for Next Generation Sequencing (Mora-Ortiz, Reference Mora-Ortiz2015; Mora-Ortiz et al., Reference Mora-Ortiz, Swain, Vickers, Hegarty, Kelly, Smith and Skøt2016).
In view of the lack of markers available for sainfoin, initial marker development studies were based on the transferability of genetic markers from related species like Medicago truncatula and Glycine max (L.), nuclear internal transcribed spacer region and the trnH-psbA, trnT-trnL intergenic spacers of the chloroplast genome, and nuclear (ITS) and chloroplast (matK) markers (Hayot Carbonero, Reference Hayot Carbonero2011b; Demdoum et al., Reference Demdoum, Munoz, Delgado, Valderrabano and Wuensch2012; Lewke Bandara et al., Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013). EST-SSR from Medicago truncatula were found to be 81% amplifiable in sainfoin, and 52% were polymorphic. The amplification size found in sainfoin was 79–865 bp, and between 79 and 240 bp in Medicago truncatula (Demdoum et al., Reference Demdoum, Munoz, Delgado, Valderrabano and Wuensch2012). In a recent study, de-novo transcriptome interrogation was completed, which allowed the identification of 3786 potential SSRs and 77,000 putative SNPs markers (Mora-Ortiz et al., Reference Mora-Ortiz, Swain, Vickers, Hegarty, Kelly, Smith and Skøt2016).
Phylogenetic studies have indicated that there is a significant division between western European varieties of sainfoin versus eastern European and Asiatic. This division is similar to that identified using morphological and agronomic traits (Hayot Carbonero, Reference Hayot Carbonero2011b; Hayot-Carbonero et al., Reference Hayot-Carbonero, Carbonero, Smith and Brown2012). Furthermore, in a phylogenetic study (Lewke Bandara et al., Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013) using nuclear (ITS) and chloroplast (matK) markers, Onobrychis species were resolved as paraphyletic, with species of the genera Eversmannia Bunge and Hedysarum L. nested within it. In this study, uncertainty in defining species delimitation in the Onobrychis genus has been attributed to recent speciation, hybridization, and introgression events, particularly between cultivated species and their wild relatives (Lewke Bandara et al., Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013) (Figure 3). More recent phylogenetic studies interrogating sainfoin transcriptome have shown that O. viciifolia is more closely linked to red clover and Medicago truncatula, than other legumes like Lotus japonicas, bean and soybean, which are more distant relatives (Mora-Ortiz et al., Reference Mora-Ortiz, Swain, Vickers, Hegarty, Kelly, Smith and Skøt2016).

Fig. 3. Phylogenetic study developed using nuclear (its) and chloroplast (matk) markers. The section in green represents Onobrychis subgenus Onobrychis section Onobrychis. The lower section from accession 1319 to 1328 represents O. subgenus Lophobrychis. The bottom section from 1302 to 1315 represents, O. subgenus Sisyrosema section Hymenobrychis and from 1301 to 1360, O. subgenus Sisyrosema section Heliobrychis. Robustness of the analysis is explained above branches: the first number corresponds to the Bayesian support, the second to the bootstrap (maximum parsimony) support, and the third to the decay values (Lewke Bandara et al., Reference Lewke Bandara, Papini, Mosti, Brown and Smith2013). Reproduced with permission from the authors.
Similar findings were observed using SSRs markers in different subsequent studies. When the polymorphism information content (PIC)Footnote 1 (Botstein et al., Reference Botstein, White, Skolnick and Davis1980) was studied for 25 different accessions from Onobrychis species in Demdoum (Reference Demdoum2012b), it was shown that PIC ranged from 0.45 to 0.85. Onobrychis viciifolia lines clustered with a high genetic similarity. This was much lower compared with O. argentea and O. pyrenaica. This closely clustered with a PIC of 0.95, which was unexpected by the authors because, in terms of morphology, O. argenta is more similar to O. viciifolia than to O. pyrenaica (Demdoum et al., Reference Demdoum, Munoz, Delgado, Valderrabano and Wuensch2012). This investigation also showed that British accessions are phylogenetically associated with Western European accessions such as UK varieties known as ‘Cotswold-Common’ and ‘Sombourne’, which are phylogenetically more distant to those from the cluster formed by Eastern and West continental European accessions (Demdoum et al., Reference Demdoum, Munoz, Delgado, Valderrabano and Wuensch2012). This study was, however, based on EST-SSR markers obtained from Medicago truncatula. A later study based on O. viciifolia putative SSR markers showed similar results; confirming clusters according to geographical origin and separating species into two major groups from Southern and Eastern Europe, and Switzerland and UK, respectively. In this case, PIC reached lower values, ranked between 0.14 and 0.36 (Kempf et al., Reference Kempf, Mora-Ortiz, Smith, Kolliker and Skot2016) (Figure 4). These results agree with the phylogenetic study based on the morphology developed by Hayot Carbonero (Reference Hayot Carbonero2011b); Hayot Carbonero et al. (Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011).

Fig. 4. Cluster dendrogram of individuals based on the modified Rogers’ distance. Values at branches are AU P-values (blue). Different colours of genotype labels give the affiliation to the two groups determined by k-means partitioning. The accessions that were tested separated into two main clusters. One was associated with varieties from southern & eastern Europe and a second one included accessions from the UK and Switzerland. Figure is adapted from Kempf et al. (Reference Kempf, Mora-Ortiz, Smith, Kolliker and Skot2016).
These latest advances in molecular genetics, have provided a large number of molecular markers for further breeding programmes and a better understanding of sainfoin phylogeny. Breeding and pre-breeding programmes now have tools for focussed development in the future. The selection of new highly productive varieties is demanded by farmers and the seed industry (Mora-Ortiz and Smith, Reference Mora-Ortiz and Smith2016). Breeding programmes will need to include recently identified attributes in selection processes to ensure that multi-beneficial properties attributed to its condensed tannins content are maximized. There are 12 cDNAs encoding genes implicated in sainfoin flavonoid biosynthesis pathway, which have been cloned and sequenced (Thill et al., Reference Thill, Regos, Farag, Ahmad, Kusek, Castro, Schlangen, Carbonero, Gadjev and Smith2012). These sequences, together with a collection of genes encoding enzymes in this pathway from KEGG, allowed the identification of 63 transcripts involved in the tannin biosynthesis pathway and their associated transcriptional levels (Mora-Ortiz et al., Reference Mora-Ortiz, Swain, Vickers, Hegarty, Kelly, Smith and Skøt2016). During this study, the gene ontology (GO) analysis of the transcriptome also enabled the annotation of 18,000 transcripts with at least one GO term (Mora-Ortiz et al., Reference Mora-Ortiz, Swain, Vickers, Hegarty, Kelly, Smith and Skøt2016).
Botanical description of sainfoin
Plant morphology
Sainfoin has epigeal germination, which means that during germination, the hypocotyl extends and the cotyledons emerge from the soil during early growth, as opposed to hypogeal germination. In hypogeal germination the epicotyl extends and the cotyledons stay below the soil surface until germination whereby the cotyledons are pushed above ground after germination. Crop plant vigour during this early stage depends on the stored substrate in the seed during the first 7 d of growth. Following initial germination, photosynthesis in the cotyledon leaves then plays an important role in the development of normal first-leaves and their expansion (Cooper and Fransen, Reference Cooper and Fransen1974).
The plant habit is mainly erect or sub-erect; however, some accessions have a more prostrate or rosette habit and most accessions will die back to a short prostrate plant over the winter months. The morphological plasticity of sainfoin adds to difficulties in characterizing varieties, malleability contributes to its ability to cold stress during winter and early spring (Frame et al., Reference Frame, Charlton and Laidlaw1998; Seker et al., Reference Seker, Rowe and Brink2003; Drobná, Reference Drobná2010; Hayot Carbonero, Reference Hayot Carbonero2011b).
In spring, many hollow stems grow from basal buds and form a branched crown. They are defined as sub-woody until the height of about 70 cm and can be hairless to slightly hairy, and hardly ramified. Height varies between 100 and 20 cm and normally has between 16 and 18 stems per plant, with a variable thickness of 3–9 mm (Frame et al., Reference Frame, Charlton and Laidlaw1998; Valdes, Reference Valdes2000; Hayot Carbonero, Reference Hayot Carbonero2011b). Foliage is green, rarely with some red pigmentation. Significant variability has been observed in the green colour of the different accessions and occasionally the leaves have hairs in the middle nerve (Canals et al., Reference Canals, Peralta and Zubiri2009; Hayot Carbonero, Reference Hayot Carbonero2011b). Stems have pinnate leaves in a variable number between 6 and 14 and the leaves are compound normally with between 10 and 28 leaflets per leaf. The individual leaflets are oblong, oblong-elliptic and elliptic on average 10.3 mm long and 6 mm wide. The leaves are classified as impapirinnante, pinaticomposed and oppositepinnate (Allaby, Reference Allaby1987; Font Quer, Reference Font Quer1987; Lancha and Sempere, Reference Lancha and Sempere1988; Polunin, Reference Polunin1991; Valdes, Reference Valdes2000; Canals et al., Reference Canals, Peralta and Zubiri2009; Hayot Carbonero, Reference Hayot Carbonero2011b).
Root morphology and development
Sainfoin has over a 2-meter-long taproot in mature plants, partly responsible for its drought tolerance. The root is quite branched, especially at the bottom and multiples of thin lateral roots constitute the bulk of the root system (Hayot Carbonero, Reference Hayot Carbonero2011b; Kempf, Reference Kempf2016; Mora-Ortiz and Smith, Reference Mora-Ortiz and Smith2016). Roots have often been measured at more than 2 m and in dry conditions over 3 m. The Sainfoin root systems rival Lucerne for its ability to access water in the lower soil horizons (personal communication, Beat Boller, ETH, 2011)
Flower development and morphology
Inflorescences, developed on auxiliary tillers have broad finely pointed stipules. The inflorescences are dense with 10–80 flowers and peduncle between 12 and 20 cm. The calyx is loosely tomentose or sub-glabrous. The corolla of the flowers is pinkish. This base colour has high diversity from white to purple with darker linear patterns of greater intensity than the primary colour. The corolla is 1.5–2 times larger than the calyx. The keel is curved in an obtuse angle and 1.5 times longer than the calyx. The flowers have bracteoles 0.6–1.5 mm (Valdes, Reference Valdes2000; Canals et al., Reference Canals, Peralta and Zubiri2009; Hayot Carbonero, Reference Hayot Carbonero2011b).
Sainfoin is an indeterminate species and generally flowers for more than 5 weeks, from green flower buds to set seed in UK field conditions. During this period, nine phonological stages have been defined: (1) green bud, (2) red bud, (3) keel out, (4) open flower, (5) wilted flower, (6) calyx, (7) swollen calyx, (8) green seed and (9) dry seed. Fruit set depends upon the variety, but it can be correlated with the suitability and adaptability of its environment. The flowers open gradually over 24-h especially between night and sunrise (McGregor, Reference McGregor1976; Demdoum, Reference Demdoum2012b). Authors disagree on the time at which the flower becomes receptive to fertilization. This may occur before the stage of keel out or not until the flower is fully open (Galloni et al., Reference Galloni, Podda, Vivarelli and Cristofolini2007; Demdoum, Reference Demdoum2012b). This may be a strategy to avoid self-pollination (Knuth, Reference Knuth1906). The pollen is more viable at the stage of keel out (Pavlova and Manova, Reference Pavlova and Manova2000; Demdoum, Reference Demdoum2012b). Sainfoin was thought to be an obligate allogamous species due to its flower morphology; however, recent studies (Demdoum, Reference Demdoum2012b) suggest that although the species requires pollination by insects, it can tolerate a low level of selfing. The lack of success in selfing could be due to a combination of the morphology of the flower and the level of activity of pollinating insects to cover the asynchronous maturation of the stigmas (Demdoum, Reference Demdoum2012b). It has also been observed that a physical jolt is required by visiting pollinators in order to break the stigma cuticle, which improves pollination by 4.1% (Thomson, Reference Thomson1938).
The seeds are kidney-shaped with a brown pod whose size varies between 2.5 and 7 mm long, 2–3.5 wide and 1.5–2 mm thick (Valdes, Reference Valdes2000; Hayot Carbonero, Reference Hayot Carbonero2011b; Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). The pod-spinyness is variable and has been used as a taxonomic character (Thomson, Reference Thomson1951). The hull is downy due to the presence of short hairs. The contour of the seed is described as orbicular and from 0.1 to 0.3 mm in length. The embryo is large and rich in starch reserves, protein and lipid. The maturity of the fruit at the time of harvesting is the major factor that determines the final colour of the seed. Seed are either sold with their hull intact (those that have not been processed and retain the pod coat, are known as ‘un-milled seeds’ among farmers) or as de-hulled seeds (which have been processed to remove the pod coat, also known as ‘milled seeds’ among farmers). Seed weight is between 24 g/1000 seed for the former and 15 g/1000 for the latter. The dispersion of the fruit is by animals, which is improved by the presence of the spines on the seed hull (Valdes, Reference Valdes2000; Hayot Carbonero, Reference Hayot Carbonero2011b; Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). Under certain conditions, dehulling or ‘milling’ the seed assists in early synchronous germination (Hayot Carbonero, Reference Hayot Carbonero2011b).
Agronomic characterization
Varieties
Sainfoin has been traditionally divided into two types, ‘giant or two-cuts sainfoin’ and ‘common or single-cut sainfoin’. They have different characteristics and morphology (Table 2). In general, the common type has more stems per plant, while the giant type has longer stems, more internodes per stem and more leaflets per leaf. The giant type is normally recommended for fertile lands, while the common type is more suited to a higher altitude (Badoux, Reference Badoux1965; Michelena, Reference Michelena1983; Prosperi et al., Reference Prosperi, Demarquet, Angevain and Mansat1994; Delgado et al., Reference Delgado, Muñoz, Demdoum and Buil2008; Demdoum, Reference Demdoum2012b).
Table 2. Morphological and agronomic differences between giant and common sainfoin also called ‘two-cuts’ and ‘single-cut’, respectively

Most of the varieties that are now commercialized were developed in the 1970s and are an intermediate type between the giant and common sainfoin. The most popular varieties available in Europe are Ambra, Vala and Zeus from Italy; Perly from Switzerland, Emyr from Hungary; Fakir from France; Višňovský from Czech Republic; and Cotswold-Common and Cholderton-Hampshire-Common from the UK. In Canada, varieties such as Melrose and Nova are popular; Eski, Remont, Remunex and Shoshone are from USA and G35 in New Zealand (Hayot Carbonero, Reference Hayot Carbonero2011b; Demdoum, Reference Demdoum2012b).
Climate, habitat and soil
Sainfoin is adapted to a range of climatic and abiotic conditions existing in Asia, Europe, North America, New Zealand and Australia. (García Salmerón et al., Reference García Salmerón, Montserrat, Buendía, Ruiz-del-Castillo and Allue1966). It prefers the Mediterranean sub-humid climates with some Central-European trend, being compatible with Mediterranean semi-arid climates, moderately warm and dry, and with the climates of High Mountain. In the Mediterranean basin, it prefers altitudes above 600 m but is cultivated in a range between 100 and 2500 m (García Salmerón et al., Reference García Salmerón, Montserrat, Buendía, Ruiz-del-Castillo and Allue1966; Demdoum, Reference Demdoum2012b). In a survey of 40 Spanish farmers producing sainfoin seeds, 90% of their farms were located in altitudes between 600 and 1474 m, where the climate was semi-arid and the soil was limestone (Delgado et al., Reference Delgado, Andrés, Sin and Ochoa2002; Demdoum, Reference Demdoum2012b).
Sainfoin grows well in very slightly acid, neutral and alkaline soils with a pH above 6.5; sainfoin is intolerant to acid soil, especially of subject to high rainfall, and can grow in both, dry-lands and irrigated areas (Bland, Reference Bland1971; Frame et al., Reference Frame, Charlton and Laidlaw1998). In the absence of irrigation, annual rainfall should be at least 330 mm (Miller and Hoveland, Reference Miller and Hoveland1995). Sainfoin is not tolerant to waterlogging and prefers well-drained areas (Sheldrick et al., Reference Sheldrick, Thomson and Newman1987). In the UK, sainfoin has generally been linked to calcareous chalky or limestone soils (Frame et al., Reference Frame, Charlton and Laidlaw1998). The poor establishment was obtained on clay soil at pH 6 with failures on the alluvial sand at/or below 5 (Bland, Reference Bland1971; Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). In Spain, it is traditionally linked to neutral or slightly alkaline brown-earth soils. It is incompatible with poor draining soils such as podzols, greysolic acid brown, grey forest and oxisols and combinations of any of the latter (García Salmerón et al., Reference García Salmerón, Montserrat, Buendía, Ruiz-del-Castillo and Allue1966; Demdoum, Reference Demdoum2012b). Sainfoin does not need fertile soil to thrive as long as the requirement for lime and humidity are satisfied. Sainfoin can thrive in less fertile soils than alfalfa and clovers, but can also grow well in more fertile soils. Alfalfa and clover will, however, produce better yields in fertile and irrigated lands, but sainfoin provides better outcomes when the soil is of low fertility compared with alfalfa (Benaiges, Reference Benaiges1971; Demdoum, Reference Demdoum2012b).
Long periods of hot temperature can negatively affect sainfoin and therefore, reduce yields and this is particularly important following defoliation when the ability of the plant to cope with high metabolic rates is decreased (Kallenbanch et al., Reference Kallenbanch, Matches and Mahan1996). Although sainfoin is considered to be intolerant to high temperatures, there is some evidence to show it can grow at temperatures above 32°C in Spain and Greece when irrigation is well managed (Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). There are few studies on its ability to tolerate frost, and this relates to variety choice. Most accessions can withstand winter frost, but not frost in combination with prolonged snow cover. Young sainfoin seedlings were found to be better able to withstand such conditions than seedlings from other legumes, such as M. sativa and several Trifolium species, with the exception of Trifolium hybridum (Benaiges, Reference Benaiges1971; Meyer and Badaruddin, Reference Meyer and Badaruddin2001).
Sowing
In the warm Mediterranean basin, sainfoin is normally drilled either in early autumn or at the beginning of spring. Conversely, in colder areas like the UK, it is recommended to drill sainfoin between April and July after the soil temperature has become warm and humid enough to facilitate a quick germination and subsequent growth (Jensen and Sharp, Reference Jensen and Sharp1968; Goplen et al., Reference Goplen, Richards and Moyer1991). Sainfoin has an extended optimum temperature range for germination, but it is normally advised to drill it between 10 and 20°C and never below 5°C (Jensen and Sharp, Reference Jensen and Sharp1968; Smoliak et al., Reference Smoliak, Jonston and Hanna1972). Early sowing can improve the development of the plants thanks to the early development of the vegetative plant and roots, and yield in the first year.
The seeds can be drilled either de-hulled or hulled (Thomson, Reference Thomson1951). There is a controversy among authors as to the preferred option (Wiesner et al., Reference Wiesner, Carleton and Cooper1968; Chen Reference Chen1992). Use of de-hulled seed could provide staggered germination and thus cushion potential weather disturbances (Wiesner et al., Reference Wiesner, Carleton and Cooper1968; Chen, Reference Chen1992; Demdoum, Reference Demdoum2012b). The use of large fully mature seeds increases establishment success giving stronger plants, with more nodules and higher rates of nitrogen fixation (Cash and Ditterline, Reference Cash and Ditterline1996).
In order to establish a population of 70–150 plants/m2 in the first year, authors recommend seed rates of 40–50 kg/ha of de-hulled seed (or 80–120 kg/ha hulled) (Sheldrick et al., Reference Sheldrick, Newman and Roberts1995; Frame et al., Reference Frame, Charlton and Laidlaw1998) at a depth of 1 and 2 cm in Canada (Hill, Reference Hill, Lane and Wilkinson1997). Conversely, in China, a depth of 4–5 cm was recommended (Chen, Reference Chen1992). These variations in planting depth are attributed to differences in the soil texture and moisture at the different sites (Hayot Carbonero, Reference Hayot Carbonero2011b). The recommended row spacing is between 50 and 60 cm (Goplen et al., Reference Goplen, Richards and Moyer1991; Stevovic et al., Reference Stevovic, Stanisavljevic, Djukic and Djurovic2010).
Inoculation and nitrogen fixation indications in sainfoin
Sainfoin, along with other leguminous species, will establish symbiotic relationships with gram-negative bacteria from the family Rhizobiaceae and with arbuscular mycorrhizal fungi. The symbiosis with Rhizobiaceae is sited in specialized root nodules, which in sainfoin can exhibit a range of morphologies; spherical, to branched and coralloid are formed (Figure 5). In these nodules, differentiated bacteria use nitrogenase enzyme complex to reduce atmospheric nitrogen to ammonia. Sainfoin benefits from this ammonia to synthesize amino acids and proteins (Baimiev et al., Reference Baimiev, Baimiev, Gubaidullin, Kulikova and Chemeris2007). Both, mycorrhizal fungi and the Rhizobia, associated with sainfoin plants benefit from food in the form of carbohydrates produced from photosynthesis in the host plant. The inoculation of sainfoin with Rhizobium sp. can be developed using strains isolated from related legumes such as Hedysarum, Coronilla or Dalea or from healthy nodules on sainfoin (Burton and Curley, Reference Burton and Curley1968) Isolation of Rhizobia from more cold tolerant legumes such as Astragalus alpinus, Oxytropis madelliana and Oxytropis arctobia, led to an improvement in nitrogen fixation during cold conditions (Prevost et al., Reference Prevost, Bordeleau and Antoun1987). Several authors have noted that the level of nitrogen fixation in sainfoin nodules is inadequate in some situations and nitrogen deficiency symptoms can be seen, even when the crop has been inoculated (Burton and Curley, Reference Burton and Curley1968; Sims et al., Reference Sims, Muir and Carleton1968; Sheehy and Popple, Reference Sheehy and Popple1981). The higher requirement of energy from sainfoin has been attributed to the smaller leaf area index compared with alfalfa. This would reduce the use of the light energy and carbon fixation, which is indirectly related to the level of nitrogen fixation. That would explain the high nodular activity of sainfoin and weight of their nodules compared with other legumes (Sheehy and Popple, Reference Sheehy and Popple1981; Hayot Carbonero, Reference Hayot Carbonero2011b; Demdoum, Reference Demdoum2012b) (Figure 5).

Fig. 5. (a) Nodules found in sainfoin. The size of sainfoin nodules is unusually large compared with other legumes. Research on these symbioses is limited. (b) Products of photosynthesis, are transferred to both rhizobium and arbuscular mycorrhizal partners. (c) Rhizobium fixes nitrogen that sainfoin will use for protein synthesis and (d) Mycorrhizas enhance phosphate uptake.
The nitrogen fixation rate of sainfoin has been compared with that of alfalfa. In sainfoin, it was estimated at between 130 and 160 kg N/ha and for alfalfa between 140 and 160 kg N/ha. This resulted in a yield improvement of 17 and 25%, respectively (Provorov and Tikhonovich, Reference Provorov and Tikhonovich2003). In an experiment with the nitrogen-free growing medium, the sainfoin variety Melrose was tested against 47 rhizobia strains. The results ranked from 8 to 140 mg total nitrogen/pot showing that efficient symbiosis depends on finding an efficient rhizobial symbiont (Prevost et al., Reference Prevost, Bordeleau and Antoun1987). A combined application of phosphorus, nitrogen and Rhizobium inoculum was found by some authors to produce the best improvement in sainfoin yields over untreated controls (Tufenkci et al., Reference Tufenkci, Erman and Sonmez2006).
Arbuscular mycorrhizal (AM) symbiosis
AM symbiosis is a close association between plant roots and fungi; at least 80% of the vascular flowering plants worldwide are able to form this type of symbiosis, and it is one of the most widespread symbioses found in plants. The fungus partner(s) supply sainfoin with phosphate and other nutrients from the soil, improves water use efficiency and promotes plant resistance to pathological infections. The plant provides the fungus with carbon compounds (Harrison, Reference Harrison1998). Pilot studies of mycorrhizal inoculation have indicated that sainfoin can benefit from improved access to symbionts under field conditions, especially in the presence of Rhizobia inoculants (NIAB, L. M. J. Smith, unpublished data).
Fertilization
Fertilization requirements in sainfoin can be highly variable. It is traditionally believed that sainfoin does not require fertilizers. Studies have, however, shown that use of low levels of inorganic N fertilizer applications stimulated nitrogen fixation in sainfoin (Sims et al., Reference Sims, Muir and Carleton1968). But high dosages inhibited nodulation and fixation rates (Badoux, Reference Badoux1965; Koter, Reference Koter1965; Meyer, Reference Meyer1975; Sheehy and McNeill, Reference Sheehy and McNeill1988; Hartwig and Nösberger, Reference Hartwig, Nösberger and Younie1996). These contradictory responses to nitrogen could be partly attributed to differences in the original nitrogen content of the soil, which is not always defined (Hayot Carbonero, Reference Hayot Carbonero2011b; Demdoum, Reference Demdoum2012b).
In a comparative study total N, P and K extracted from the soil by Medicago sativa and sainfoin were evaluated and expressed as fertilizer equivalents. It was concluded that sainfoin needs more P2O5 and NO3 than alfalfa, and that alfalfa needs more K2O and CaCO3 than sainfoin (Sheehy et al., Reference Sheehy, Minchin and McNeill1984). Meyer (Reference Meyer1975) observed only small effects from applications of P2O5 and K2O fertilizer while (Sheldrick et al., Reference Sheldrick, Newman and Roberts1995) noted a better response.
Seed production
Every sainfoin flower has the biological capacity to produce a seed, but on average only 55% of these will succeed and produce a viable seed (Goplen et al., Reference Goplen, Richards and Moyer1991). Sainfoin can produce between 5 and 40 tillers, each of which has between 3 and 5 inflorescences. Sainfoin inflorescences are composed of 5 and 80 flowers. Both the variety and the environment will have an impact on the final seed production (Carleton and Wiesner, Reference Carleton and Wiesner1968). Honey bees (Apis mellifera) and leafcutting bees (Megachile rotundata) are the recommended pollinators to assist in sainfoin seed production. Bee-assisted pollination in sainfoin is considered to be more successful than in alfalfa due to the longer morphology of the flower (Wallace, Reference Wallace1968).
Seed yield can be improved by the presence of at least two to three colonies of honey bees per hectare. Alternatively, it is possible to use leafcutting bees; in this case, it would be necessary to have at least 20,000 per hectare (Goplen et al., Reference Goplen, Richards and Moyer1991). To optimize seed yields, seeds are swathed after they have dried to a maximum of 40% water content and then allowed to dry further in the windrow before threshing. In this way, yields vary between 500 and 900 kg of clean seeds per hectare. Following these protocols, a maximum yield of 1100 kg/ha has been obtained in Canada (Thomson, Reference Thomson1951; Goplen et al., Reference Goplen, Richards and Moyer1991; Prosperi et al., Reference Prosperi, Demarquet, Angevain and Mansat1994). Some authors consider that it is better to leave the seeds with hulls intact if they are going to be stored, to maintain maximum viability for longer (Thomson, Reference Thomson1951).
There are several factors involved in seed production that can have a secondary impact on the final yields. The best yields have been obtained when the flowers are cross-pollinated. Seed size increases as the number of seeds per plant head decreases. Plant density also impacts on seed production; seed production per plant decreased when there was competition between densely planted individuals. In dry areas, such as Italy, seed production improves with irrigation (Carleton and Wiesner, Reference Carleton and Wiesner1968; Martinello and Ciola, Reference Martinello and Ciola1994; Demdoum, Reference Demdoum2012b).
Weed control
Weed invasion in recently drilled sainfoin fields in the UK often leads to the poor competitive establishment, especially with broad leaf weeds such as Galium aparine, Senecio vulgaris, Chenopodium album, Lamium purpurum and Stellaria media (Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). Sainfoin has 50% less leaf surface area than alfalfa and a diffuse canopy structure during the first 4 months of growth (Sheehy and Popple, Reference Sheehy and Popple1981; Frame et al., Reference Frame, Charlton and Laidlaw1998). The main strategies used to control weed invasion in sainfoin fields are herbicide treatments and use companion crops. Different herbicide approaches have been tested, but research in this area is limited. Previous studies have shown that control of dandelion (Taraxacum officinale) in sainfoin using metribuzin [4-amino-6-tert-butyl-3-methylsulfanyl-1,2,4-triazin-5-one] improved sainfoin yields by up to 28% (Moyer et al., Reference Moyer, Hironaka, Kozub and Bergen1990). It has also been noted that, in the absence of herbicide treatments, weeds can represent up to 98% of the final yields in the first cut (Moyer, Reference Moyer1985). A range of herbicides aimed at pre and post-crop emergence was tested in a field scale screen including; bentazone [3-Isopropyl-1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide] and imazethapyr [5-ethyl-2-[(RS)-4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl] nicotinic acid] and weed control were improved by the inclusion of an adjuvant (tween 80 or ammonium sulphate). Imazethapyr performed best, which, unlike bentazone, did not reduce sainfoin biomass (Amiri et al., Reference Amiri, Karimmojeni, Majidi and Boromand2013). The use of pre-emergence herbicides like pendimethalin, metazachlor and prosulfocarb significantly controlled weed numbers and increased yields by 8 and 30%, respectively in the UK. These findings highlight the importance of good weed control strategies during early establishment of the crop (Mora-Ortiz et al., Reference Mora-Ortiz, Wood, Philpott, Skøt, Smith and De Ron2015a, Reference Mora-Ortiz, Wood, Skøt, Smith and De Ronb). The use of carbetamide [(R)-1-(ethylcarbamoyl) ethylcarbanilate] was tested for maintenance of a clean crop during the winter; and MCPA [a.i. 4-(4-Chloro-2-methyl-phenoxy) acetic acid] and MCPB [a.i. 4-(4-Chloro-2-methyl-phenoxy) butyric acid] for the control of broad leaf weeds during the spring (Sheldrick and Thomson, Reference Sheldrick and Thomson1982; Frame et al., Reference Frame, Charlton and Laidlaw1998). In one study, yields were increased by 20% using hexazione [3-Cyclohexyl-6-dimethylamino-1-methyl-1,3,5-triazine-2,4-dion] and terbacil (3-tert-butyl-5-chloro-6-methyluracil) treatments (Malik and Waddington, Reference Malik and Waddington1988). In the USA, sainfoin natural tolerance to Glyphosate was the basis for weed control using post-emergence multiple applications of low dosage Glyphosate (N-(phosphonomethyl) glycine) (Lauriault et al., Reference Lauriault, Contreras, VanLeeuwen and Kirksey2009).
Weed control can also be addressed through the use of companion crops. Depending upon local environmental conditions, farmers have favoured the use of mixtures containing Phleum pratense, Festuca pratensis or have under-sowed with spring barley (Hordeum vulgare), tetraploid perennial ryegrass (Lolium perenne), Russian wild rye (Psathyrostachys juncea) or crested wheatgrass (Agropyron desertorum). Co-cultivation with a second leguminous species, Lotus corniculatus, has also been considered (Dubbs, Reference Dubbs1968; Bland, Reference Bland1971; Cooper, Reference Cooper1972; Goplen et al., Reference Goplen, Richards and Moyer1991; Frame et al., Reference Frame, Charlton and Laidlaw1998; Liu et al., Reference Liu, Baines, Lane and Davies2009; Hayot Carbonero et al., Reference Hayot Carbonero, Mueller-Harvey, Brown and Smith2011). Chicory and oat have also been considered as potential alternatives due to their antiparasitic properties and nutritional profile respectively. Recent studies have shown that they can grow together with sainfoin during short periods, for example, in rotation systems, but further studies are necessary (Mora-Ortiz, Reference Mora-Ortiz2015). The use of companion crops has been shown to reduce the proportion of weeds to crop by 65%, to increase the symbiotic N2 fixation rate by up to 158 kg/ha and increase total yields by 31% compared with monocultures (Malisch et al., Reference Malisch, Suter, Studer and Luscher2017). This approach can be combined with reduced herbicide use.
Finally, sainfoin crops have not suffered significant impact from most common pest and disease problems in Northern Europe compared with other legumes (Goplen et al., Reference Goplen, Richards and Moyer1991; Frame et al., Reference Frame, Charlton and Laidlaw1998). This has been attributed to the presence of a range of complex secondary metabolites within the foliage, including high molecular weight condensed tannins and polyphenols. Some minor damage through insect and nematode predation has been noted such as Sitona scissifrons, a weevil from the family Curculionidae (Morrill et al., Reference Morrill, Ditterline and Cash1998) and other members from this genus including S. lineata, S. calloso and S. crinite have been reported to damage sainfoin (Wallace, Reference Wallace1968). Similarly, sainfoin is rarely damaged by diseases, only certain Fusarium spp have been found to have an economic impact on the crop affecting survival over winter (Mathre, Reference Mathre1968). Farmers have occasionally noted the presence of other minor pathogens including, Stemphyllium sp. where infection led to black stems and characteristic pepper spots in leaves (Mathre, Reference Mathre1968).
General conclusions and perspectives
Sainfoin has significant potential for benefits to the farmer when included in a rotation, due to its environmental and nutraceutical attributes; however, its low productivity and difficulty relating to reliable establishment prevent many farmers from considering this crop a viable alternative to other forage legumes. Recently, advances in high-throughput sequencing have yielded markers that will enable further potential advances in targeted breeding programmes. Marker-Assisted Selection (MAS) programmes could ideally focus on improving the major disadvantages in comparison with other leguminous forage crops, such as slow establishment, poor competition with weeds and low yields; especially during the first establishment year. Phenotypic approaches like ‘phenomics’ or metabolomics strategies using NMR interrogation, could represent a significant step forward in the characterization of current varieties, and the combination of these techniques with MAS could promote the selection of new and more competitive varieties of sainfoin, that retain the many positive attributes it possesses. Weed control is another area where more research is necessary, the current knowledge of herbicide options and companion species choice have only been tested in a few geographical locations and some results are contradictory in the literature. In summary, advances in recent years have provided more opportunities for sainfoin to be considered as an alternative choice for farmers, particularly those interested in producing locally sourced protein and lower input, sustainable agricultural practices.
Acknowledgements
The authors thank the European Commission for funding this study (Marie Curie Initial Training Network, ‘LegumePlus’, PITN-GA-2011-289377). The authors wish to thank Javier Mora-Ortiz for support with graphics software and the authors who allowed them to replicate some of their pictures. Finally, thanks to Cotswold-Seeds LTD for the sainfoin drawing and picture.









