INTRODUCTION
The family Nystiellidae Clench & Turner, Reference Clench, Turner and Clench1952 includes 44 valid living species (MolluscaBase, 2016), mainly inhabiting the deep sea (Bouchet & Warén, Reference Bouchet and Warén1986). Nystiellidae was previously considered a subfamily of Epitoniidae, until Nützel (Reference Nützel1998) raised it to family rank, based on the axially ribbed protoconch.
The taxonomy of Nystiellidae is based mainly on shell morphology and is considered by some authors to be confusing, especially considering the slight knowledge of the internal anatomy of its species. Nystiellidae includes six valid genera, aside from some cases of synonymy (Bouchet, Reference Bouchet2010). The taxonomy of the group from the North-east Atlantic was revised by Bouchet & Warén (Reference Bouchet and Warén1986), whereas knowledge of the diversity of Nystiellidae from Brazil is scattered in catalogues of marine molluscs (e.g. Rios, Reference Rios1994, Reference Rios2009) or in regional faunistic surveys (e.g. Miyaji, Reference Miyaji, Amaral and Rossi-Wongtschowski2004; Absalão, Reference Absalão, Lavrado and Brasil2010), besides isolated descriptions of species (e.g. Andrade et al., Reference Andrade, Costa and Pimenta2011; Lima & Christoffersen, Reference Lima and Christoffersen2013). The current state of knowledge was summarized by Lima & Christoffersen (Reference Lima and Christoffersen2013) in a checklist of previous species records, resulting in a total of 13 names. However, these authors did not attempt a critical evaluation of species occurrences, and several dubious records remain (e.g. Narrimania cf. concinna, Eccliseogyra cf. sericea, etc.).
The present contribution reviews the occurrence of members of Nystiellidae from Brazil, adding new records and species.
MATERIALS AND METHODS
The identification of the material was based on conchological comparisons to type material and/or original descriptions and illustrations. Synonymies for the genera were mainly based on the works of Rex & Boss (Reference Rex and Boss1973) and Bouchet & Warén (Reference Bouchet and Warén1986).
Each species is illustrated by scanning electron microscope (SEM) using a JEOL JSM-6390LV at the ‘Centro de Microscopia Eletrônica’, in the ‘Departamento de Invertebrados’, Museu Nacional/UFRJ, and/or by camera Zeiss AxioCam ICc5 coupled to the stereo microscope Zeiss Discovery V20.
Geographic distribution of each species is compiled from literature records and from material of the present study. The maps of geographic distribution were built using the free online software GPS visualizer, based on the georeferenced records of the material examined and literature, in most cases, when illustrations allowed us to verify the determination. In a few cases, the literature records did not provide coordinates (e.g. Pequegnat (1983) apud Rosenberg et al. (Reference Rosenberg, Moretzsohn, García, Felder and Camp2009), for Gulf of Mexico); in such cases, the localities in the maps are approximate. Discussion about geographic and bathymetric distribution utilized the deep sea classification (UNESCO, 2009).
No live collected material was available for study. In the material examined, the number inside brackets indicates number of shells in each lot. Most of the material was obtained by dredging, in different expeditions along the Brazilian continental shelf and slope. The data from station localities that provided material for this study are summarized below; data about other collecting events are provided in the material list of each species:
• The MD 55 Cruise: French-Brazilian Expedition carried out by RV ‘Marion Dufresne’ in 1987, and samples were dredged from eastern and south-eastern Brazil (Tavares, Reference Tavares1999). All lots from MD55 were collected by P. Bouchet, J. Leal and B. Métivier. For more details, see Tavares (Reference Tavares1999): station CB76 (18°58′09″S 37°49′06″W, 637 m), 27/v/1987; station CB93 (19°36′S 38°53′03″W, 640 m), 30/v/1987;
• ‘Programa de Apoio ao Desenvolvimento Científico e Tecnológico’ – PADCT (Importance and Characterization of edge of Continental Shelf to Living and non living Resources), carried out by Instituto Oceanográfico da Universidade de São Paulo (IOUSP); Carried out between November 1997 and January 1998, collected by the Oceanographic Vessel ‘Professor W. Besnard’ PADCT station 6554 (23°53.610′S 42°45.380′W, 496 m); PADCT station 6573 (24°42.608′S 44°43.419′W, 155 m); PADCT station 6595 (26°23.55′S 46°39.49′W, 175 m); PADCT station 6601 (27°18.280′S 47°08.772′W, 228 m); PADCT station 6641 (26°159′S 45°539′W, 130 m);
• ‘Recursos Vivos da Zona Econômica Exclusiva – REVIZEE, Score Sul’ (Program of Evaluation of the Sustainable Potential of Living Resources in the Economic Exclusive Zone), carried out by ‘Ministério do Meio Ambiente, dos Recursos Naturais Renováveis e da Amazônia Legal (MMA)’. Carried out between November 1997 and January 1998, collected by the Oceanographic Vessel ‘Professor W. Besnard’: REVIZEE station 6652 (25°51.04′S 45°47.30′W, 206 m), 15/xii/1997; REVIZEE station 6660 (25°43.50′S 46°2.5′W, 155 m); REVIZEE Sul station 6672 (26°27.75′S 44°30.351′W, 165 m), 11/i/1998; REVIZEE Sul station 6676 (24°49.699′S 44°44.965′W, 153 m), 12/i/1998; REVIZEE Sul station 6679 (25°18.874′S 44°52.516′W, 808 m), 12/i/1998; REVIZEE station 6680 (25°15.064′S 44°52.865′W, 258 m), 12/i/1998; REVIZEE station 6705 (25°59.73′S 45°37.32′W, 424 m), 21/i/1998.
• –‘Caracterização Ambiental de Águas Profundas da Bacia de Campos’ (Environmental Characterization of Campos Basin Deep Waters), steps ALBACAR and Oceanprof (OP I and OP II), carried out by Petrobras SA, between 2001 and 2003, collected by Supply boat ‘Astro Garoupa’: ALBACAR station 27 (22°09′10.71″S 39°44′50.54″W, 1930 m), 08/v/2002; ALBACAR station 34 (22°33′31″S 40°12,5′W, 848 m), 18/vi/2002; ALBACAR station 40 (22°36′47.26″S 40°09′11.51″W, 1100 m), 16/v/2002; OP I station 44 (22°10′43.28″S 39°54′46.04″W, 800 m), 10/xii/2002; OP I station 49 (22°04′34.72″S 39°54′05.90″W, 750 m), 24/xi/2002; OP I station 59 (21°52′59.60″S 39°55′30.63″W, 750 m), 12/xii/2002; OP I station 64 (22°36′03.00″S 40°21′45.36″W, 750 m), 22/xi/2002; OP I station 66 (22°44′48.61″S 40°10′07.68″W, 1350 m), 22/xi/2002; OP I station 74 (22°27′31.62″S 40°09′23.19″W, 750 m), 21/xi/2002; OP I station 75 (22°31′28.28″S 40°03′50.40″W, 1050 m), 19/xi/2002; OP I station 79 (22°19′50.13″S 40°00′35.11″W, 750 m), 26/xi/2002; OP II station 44 (22°10′43.5″S 39°54′45.0″W, 750 m), 01/vii/2003; OP II station 45 (22°10′53.4″ S 39°52′18.3″W, 1039 m), 01/vii/2003; OP II station 49 (22°04′32.8″S 39°54′11.4″W, 750 m), 30/vi/2003; OP II station 50 (22°04′33.9″S 39°52′05.1″W, 1030 m), 30/vi/2003; OP II station 58 (21°57′26.8″S 39°40′34.0″W, 1942 m), 27/vi/2003; OP II station 59 (21°52′59.2″S 39°55′32.2″W, 751 m), 29/vi/2003; OP II station 74 (22°27′31.1″S 40°09′23.5″W, 749 m), 18/vi/2003; OP II station 64 (22°36′01.3″S 40°21′ 43.7″W, 750 m), 11/vi/2003; OP II station 69 (22°31′11.8″S 40°15′12.1″W, 743 m), 18/vi/2003; OP II station 75 (22°31′28.3″S 40°03′49.3″W, 1050 m), 18/vi/2003; OP II station 79 (22°20′22.4″S 40°01′24.7″W, 755 m), 21/vi/2003; OP II station 80 (22°24′30.4″S 39°57′28.6″W, 1044 m), 20/vi/2003; OP II station A2 (22°30.727′S 40°00.942′W, 1141 m), 27/viii/2003.
Other abbreviations used through the text: HMNH: Houston Museum of Natural History, Houston, USA; IBUFRJ: Instituto de Biologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MCZ: Museum of Comparative Zoology, Cambridge, USA; MNHN: Muséum National d'Histoire Naturelle, Paris, France; MNRJ: Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MZB: Museo di Zoologia, Bologna, Italy; MZSP: Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil; NHMUK: Natural History Museum, London, UK.
SYSTEMATICS
Nystiellidae Clench & Turner, Reference Clench, Turner and Clench1952
Genus Eccliseogyra Dall, Reference Dall1892
Eccliseogyra Dall, Reference Dall1892: 307. Type species (original designation): Delphinula nitida Verrill & Smith [in Verrill], Reference Verrill1885.
Solutiscala de Boury, Reference de Boury1909: 482. Type species (original designation): Scalaria dissoluta Fischer [in Locard], Reference Locard1897. – Synonymized by Rex & Boss (Reference Rex and Boss1973).
Abyssiscala de Boury, Reference de Boury1911: 212. Type species (original designation): Scalaria folini Dautzenberg & de Boury, Reference Dautzenberg and de Boury1897a – Synonymized by Bouchet & Warén (Reference Bouchet and Warén1986).
DIAGNOSIS
Shell usually turbinate-elongate and with many whorls, some species shorter, with few whorls; whorls disjunct in some species; with or without carena. Teleoconch sculpture as very low, laminar axial ribs crossed by raised spiral cords of variable number and spacing; base with or without peripheral basal cord, with or without umbilicus. Protoconch in the typical pattern of the family. Radula and operculum described by Bouchet & Warén (Reference Bouchet and Warén1986).
REMARKS
Eccliseogyra is the richest genus of Nystiellidae, with 12 deep-water species (Gofas & Bouchet, Reference Gofas and Bouchet2014; Brown & Neville, Reference Brown and Neville2015). Ten species are recorded from the Atlantic Ocean, those from Brazil, herein discussed and: E. exquisita Bouchet & Warén, Reference Bouchet and Warén1986, from the Canary Islands; E. formosissima (Jeffreys, 1884), from Gulf of Mexico, Portugal and Morocco; E. performosa (de Boury, 1917), from Gulf of Mexico; and E. sericea Bouchet & Warén, Reference Bouchet and Warén1986, from the Bay of Biscay to Mauritania – compiled from Bouchet & Warén (Reference Bouchet and Warén1986) and Rosenberg et al. (Reference Rosenberg, Moretzsohn, García, Felder and Camp2009).
The genus is characterized by an elongate shell with attached whorls in most species, but some have shorter globose shells, and others have shells with disjunct teleoconch whorls.
Two of the three species recorded from Brazil were described from the continental slope: E. maracatu, off north-eastern Brazil, and E. brasiliensis, off eastern Brazil. The third, Scalaria vermetiformis Watson, Reference Watson1886, a junior synonym of E. nitida, was described from off north-eastern Brazil.
Besides these, some doubtful records were published by Miyaji (Reference Miyaji, Amaral and Rossi-Wongtschowski2004): Eccliseogyra cf. sericea, and by Absalão (Reference Absalão, Lavrado and Brasil2010): Eccliseogyra aff. exquisita, Eccliseogyra cf. monnioti and Eccliseogyra sp. In the present paper, the material that gave rise to those records was accessed and these dubious records evaluated, some of them rejected, being determined as another species (see discussions below).
Lima & Christoffersen (Reference Lima and Christoffersen2013) recorded an undescribed morphospecies, Eccliseogyra sp., from north-eastern Brazil, based on a single, worn shell lacking a protoconch. The authors stated that it would represent a new species, but refrained from describing it until additional material should become available. In all samples studied for the present paper, no other shell that could be considered conspecific with Eccliseogyra sp. sensu Lima & Christoffersen (Reference Lima and Christoffersen2013) was found, and this record remains unclear.
Eccliseogyra brasiliensis García, Reference García2011
(Figure 1A–F)
Eccliseogyra brasiliensis García, Reference García2011: 167–169, figs 1–9: Brown & Neville (2016: 14 – not figured; annotated catalogue).
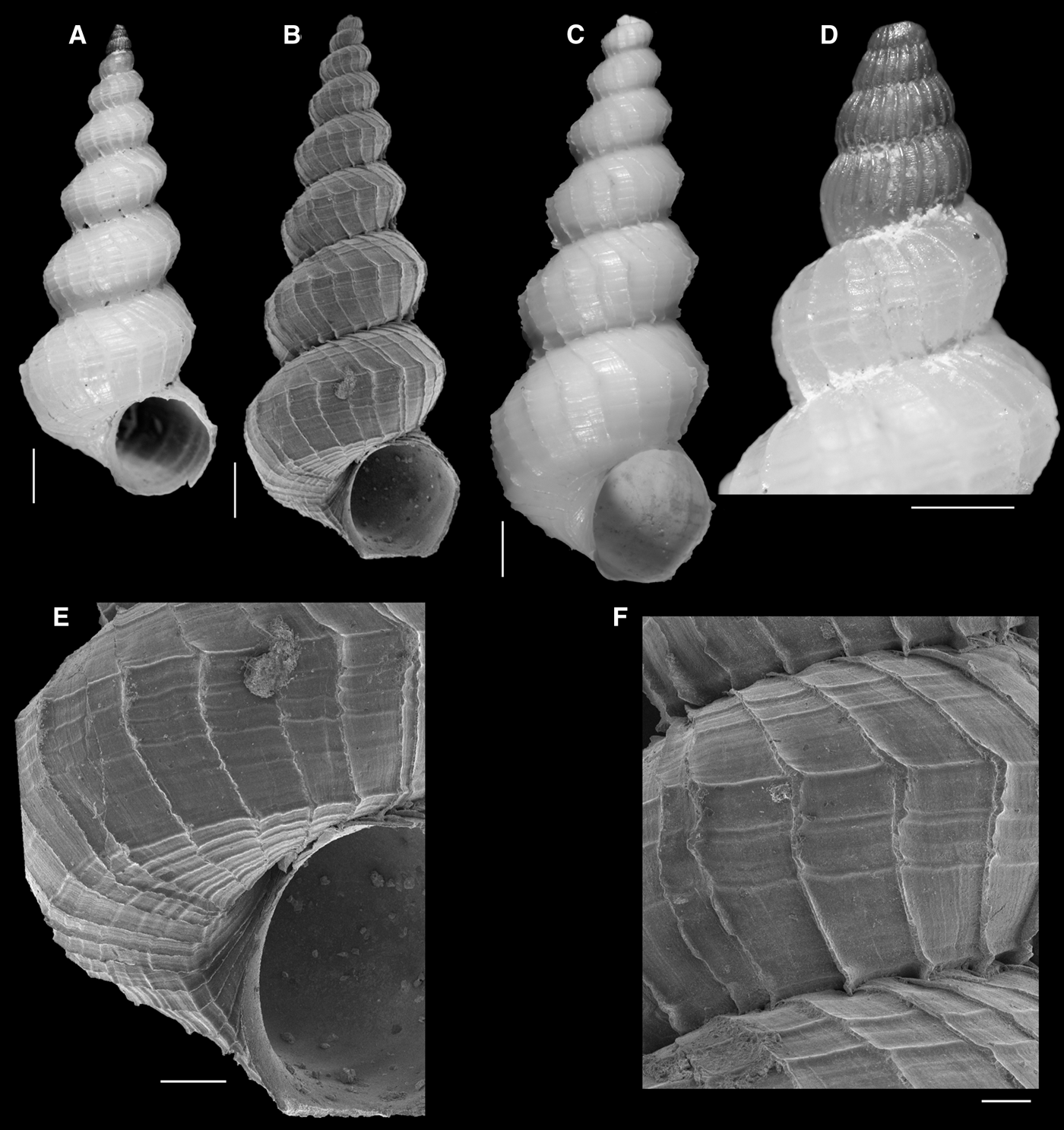
Fig. 1. Eccliseogyra brasiliensis García, Reference García2011: (A, D) holotype; (B, E, F) paratype MZSP 100523; (C) MNRJ 28181. (A–C) whole shells; (D) protoconch; (E) last whorl; (F) detail of sculpture on fifth whorl. Scale bars: A–C, 1 mm; D, E, 200 µm; F, 500 µm.
TYPE MATERIAL
Holotype (Figure 1A, D): MNHN 24428; paratypes, as cited in García (Reference García2011).
TYPE LOCALITY
South-western Atlantic: east of Cabo de São Tomé (21°35′S 40°31′W, 900 m), eastern Brazil.
MATERIAL EXAMINED
Paratype MZSP 100523 (type locality) (Figure 1B, E, F) and: – Brazil: continental slope off Arraial do Cabo, Rio de Janeiro state, MBT-150 (23°56′S 41°54′W, 710 m): MNRJ 28181 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
South-western Atlantic: eastern Brazil; 640–1550 m (García, Reference García2011; this study).
REMARKS
Eccliseogyra brasiliensis (Figure 1) is restricted to eastern Brazil, and was previously known only from the type material (besides the additional shells also cited in the original description). A new record of this species, based on a single shell from a southern locality, on the continental slope off Arraial do Cabo, Rio de Janeiro state, is presented herein (Figure 1C). The holotype is illustrated again (Figure 1A, D) as is a larger paratype, with additional details of the sculpture on the teleoconch whorls (Figure 1B, E, F).
Eccliseogyra folini (Dautzenberg & de Boury, Reference Dautzenberg and de Boury1897a)
(Figure 2A–D)
Scalaria folini Dautzenberg & de Boury, Reference Dautzenberg and de Boury1897a: 31, Reference Dautzenberg and de Bouryb: 65, figs. 1, 2.

Fig. 2. Eccliseogyra folini (Dautzenberg & de Boury, Reference Dautzenberg and de Boury1897a): (A) MNRJ 28180; (B, C) IBUFRJ 16749; (D) MNRJ 17754. (A, B) whole shells; (C) last teleoconch whorl; (D) detail of protoconch sculpture. Scale bars: A–C, 1 mm; D, 100 µm.
Eccliseogyra folini: Bouchet & Warén (Reference Bouchet and Warén1986: 485, figs 1131, 1143–1144 – lectotype designation); Segres et al. (2009: 106, pl. 15, fig. 11); Ardovini & Cossignani (Reference Ardovini and Cossignani2004: 138, fig); Brown & Neville (2016: 15 – not figured; annotated catalogue).
TYPE MATERIAL
Lectotype designated by Bouchet & Warén (Reference Bouchet and Warén1986: 485): the figured specimen in Musée Océanographique, Monaco (fide Bouchet & Warén, Reference Bouchet and Warén1986); Ardovini & Cossignani (Reference Ardovini and Cossignani2004: 138) illustrated the same shell, but referred as lectotype in MNHN.
TYPE LOCALITY
North-eastern Atlantic, off Azores Archipelago (39°21′N 31°06′W, 1360 m).
MATERIAL EXAMINED
– Brazil: continental slope of Campos Basin, off Espírito Santo state: (21°14′31.9″S 39°08′02″W, 2400 m): MNRJ 17754 [1 shell]; continental slope of Campos Basin, off Rio de Janeiro state: OP II station A2: IBUFRJ 16749 [1 shell]; continental slope off Arraial do Cabo, Rio de Janeiro state MBT-150 (23°56′S 41°54′W, 710 m): MNRJ 28180 [1 shell]; – Rio Grande Rise: ERG station 014 (30.6073°S 36.1003°W, 1500 m): MNRJ 29149 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-eastern Atlantic: off Portugal, Madeira, Azores, 450–2005 m (Bouchet & Warén, Reference Bouchet and Warén1986). South-western Atlantic: eastern Brazil, 710–2400 m; Rio Grande Rise, 1500 m (this study).
REMARKS
The only three shells of Eccliseogyra folini from Brazil are somewhat damaged (Figure 2A, B), lacking a protoconch or with a broken last whorl and aperture. In spite of this, the overall shape of the teleoconch whorls (Figure 2A, B), sculpture (Figure 2C) and narrow umbilicus (Figure 2C) can be compared to the illustration of this taxon by Bouchet & Warén (Reference Bouchet and Warén1986: 487, figs 1143–1144). These shells represent the first record of this species from the South Atlantic.
Eccliseogyra maracatu Lima & Christoffersen, Reference Lima and Christoffersen2013
(Figure 3A–F)
Eccliseogyra cf. sericea: Miyaji (Reference Miyaji, Amaral and Rossi-Wongtschowski2004: 79).

Fig. 3. Eccliseogyra maracatu Lima & Christoffersen, Reference Lima and Christoffersen2013: (A, D–F) MNHN; (B, C) MNRJ 17744; (A, B) whole shell; (C) detail of sculpture on last whorl of teleoconch; (D) detail of sculpture on penultimate whorl of teleoconch; (E) protoconch; (F) detail of last whorl of protoconch. Scale bars: A–C, 1 mm; D, 200 µm; E, F, 100 µm.
Eccliseogyra maracatu Lima & Christoffersen, Reference Lima and Christoffersen2013: 290–292, figs. 1A–E.
Eccliseogyra maracuta [misspelled]: Brown & Neville (2016: 17 – not figured; annotated catalogue).
TYPE MATERIAL
Holotype (not located): IBUFRJ 18825; paratype: IBUFRJ 19231 [1 shell], type locality.
TYPE LOCALITY
South-western Atlantic: off Alagoas state (10°06′35″S 35°46′41″W, 720 m), north-eastern Brazil.
MATERIAL EXAMINED
The paratype and: – Brazil: continental slope of Abrolhos, off Bahia/Espírito Santo states: MD 55 station CB76: MNHN [1 shell]; – east of Rio Doce fall, off Espírito Santo state: MD 55 station CB93: MNHN [15 shells]; continental slope of Campos Basin, off Rio de Janeiro state: (22°32′50.8″S 40°04′02″W, 1067 m), 12.xi.2001: MNRJ 17739 [1 shell]; MNRJ 17741 [1 shell]; (22°22′S 40°06′W, 650 m), 22/viii/2009: MNRJ 35864 [1 shell]; OP I sta44: IBUFRJ 21115 [1 shell]; OP II #64: IBUFRJ 16111 [1 shell]; continental slope off Arraial do Cabo, Rio de Janeiro state: MBT-150 (23°56′S 41°54′W, 710 m): MZSP 131834 [2 shells]; continental slope of Santos Basin, off São Paulo state: (24°11′58.21″S 43°15′23.97″W, 610 m), 26.xi.2002: MNRJ 17742 [1 shell]; (24°11′57.91″S 43°15′26.23″W, 600 m), 06.x.2002: MNRJ 17738 [1 shell]; (24°11′55.96″S 43°15′23.52″W, 625 m), 06.x.2002: MNRJ 31145 [1 shell]; (24°11′55.82″S 43°15′26.05″W, 618 m), 27.xi.2002: MNRJ 17744 [1 shell]; (24°11′53.10″S 43°15′28.35″W, 550 m), 28.xi.2002: MNRJ 17755 [1 shell]; (24°11′44.37″S 43°15′35.89″W, 560 m), 28.xi.2002: MNRJ 17740 [1 shell]; MNRJ 17743 [1 shell]; continental slope off São Paulo state: REVIZEE Sul station 6679: MNRJ 28179 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
South-western Atlantic: north-eastern, eastern and south-eastern Brazil, 550–1067 m (Lima & Christoffersen, Reference Lima and Christoffersen2013; this study).
REMARKS
Eccliseogyra maracatu (Figure 3) was described based on two shells from north-eastern Brazil. The holotype could not be found in IBUFRJ and is herein considered ‘not located’.
The additional material of E. maracatu recorded herein shows that this species is moderately common in deep-sea samples, and extends its geographic range southward to the Santos Basin (~24°S). The bathymetric range reaches 1067 m in the Campos Basin. Some conchological features are more visible in these new specimens: the umbilicus (Figure 3A–C), described as deep and rather large, is indeed one of the widest in the genus, comparable to those in Eccliseogyra formosissima (Jeffreys, 1884), not including the open-coiled species. The overall shell shape is also distinct, with inflated whorls of a rather convex outline (Figure 3A, B). The sculpture lacks the lamellar ribs of the other species, and the spiral cords are regularly spaced (Figure 3A–D), being most similar to those in E. monnioti (Figure 4A, B) and E. nitida (Figure 5E). The maximum shell length recorded by Lima & Christoffersen (Reference Lima and Christoffersen2013) was 4.2 mm in 3.5 teleoconch whorls; the longest shell examined herein (MNHN) measures 11.4 mm, with six teleoconch whorls (lacking a protoconch).

Fig. 4. Eccliseogyra monnioti: (A, D, E) MNRJ 35727; (B, C, F) MNRJ 29150; (A, B, D) whole shells; (E) detail of apical view; (C) protoconch; (F) detail of protoconch sculpture. Scale bars: A, B, D, 1 mm; C, F, 100 µm; E, 200 µm.

Fig. 5. Eccliseogyra nitida (Verrill & Smith, Reference Verrill1885): (A) holotype of Scalaria dissoluta MNHN-IM-2000-3963; (B) MNRJ 17746; (C, E–G) MNRJ 35524; (D) MNRJ 27216; (A–D) whole shell; (E) detail of sculpture on second teleoconch whorl; (F) protoconch; (G) detail of last protoconch whorl. Scale bars: A–D, 1 mm; E–G, 100 µm.
The record of Eccliseogyra cf. sericea from south-eastern Brazil (Miyaji, Reference Miyaji, Amaral and Rossi-Wongtschowski2004) actually refers to a shell of E. maracatu, after examination of the material (MNRJ 28179).
Eccliseogyra monnioti Bouchet & Warén, Reference Bouchet and Warén1986
(Figure 4A–F)
Eccliseogyra monnioti Bouchet & Warén, Reference Bouchet and Warén1986 (482, figs 1140–1141): Weil et al. (Reference Weil, Brown and Neville1999: 36, fig. 87); Segres et al. (2009: 106, pl. 15, fig. 12); Brown & Neville (2016: 17 – not figured; annotated catalogue).
Eccliseogyra cf. monnioti: Absalão (Reference Absalão, Lavrado and Brasil2010: 91).
TYPE MATERIAL
Holotype: MNHN-IM-2000-4325.
TYPE LOCALITY
North-eastern Atlantic, Madeira abyssal plain (34°54′N 21°26′W, 5160 m).
MATERIAL EXAMINED
– Brazil: continental slope of Campos Basin, off Rio de Janeiro state: OP II station 45: IBUFRJ 16598 [2 shells], MNRJ 35727 [2 shells]; uncertain precise locality: MNRJ 17732 [1 shell]; – Rio Grande Rise: ERG station 51 (31.1657°S 34.7999°W, 870 m): MNRJ 29150 [2 shells].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-eastern Atlantic: south of 35°N (off northern Africa, including records in the middle Atlantic), 3850–5867 m (Bouchet & Warén, Reference Bouchet and Warén1986). South-western Atlantic (this study): eastern Brazil, 1037 m; Rio Grande Rise, 870 m.
REMARKS
Eccliseogyra monnioti (Figure 4) differs from its congeners in the overall shell shape, with few teleoconch whorls in a somewhat globular shape. This was the main diagnostic feature used by Bouchet & Warén (Reference Bouchet and Warén1986) to differentiate this species from the usually turriform shells of the other species of Eccliseogyra, aside from the open-coiled E. nitida.
Bouchet & Warén (Reference Bouchet and Warén1986) indicated that the early whorls are only loosely coiled, while the coiling of the body whorl is completely open. In the material from the South Atlantic examined herein, this feature is also present; some shells with fewer teleoconch whorls lack the open coiling (Figure 4B), and the largest specimen has open coiling on the last whorl (Figure 4A).
As in the material of the original description (holotype illustrated by Bouchet & Warén, Reference Bouchet and Warén1986 and Weil et al., Reference Weil, Brown and Neville1999, and the image available at MolluscaBase, 2016), most of the shells herein examined lack a protoconch (Figure 4A, D, E). Bouchet & Warén (Reference Bouchet and Warén1986) referred to the corroded apex and justified the inclusion of this species in Nystiellidae by the same type of axial sculpture on the teleoconch as in other species of Eccliseogyra. In a single shell from eastern Brazil, as well as in a shell from the Rio Grande Rise (Figure 4B, C), the protoconch is intact and therefore the typical morphology of Nystiellidae is confirmed. In the remaining shells, the protoconch seems not to be corroded, but decollated, since a kind of internal septum sealing the shell can be seen in all shells lacking the protoconch (Figure 4E); anterior to this area, the axial cords are easily visible, and the spiral cords begin to appear on the second teleoconch whorl. In some shells, laminar expansions, resembling a varix (Figure 4B, D), are present along the teleoconch whorls.
Eccliseogyra nitida (Verrill & Smith, Reference Verrill1885)
(Figure 5A–G)
Delphinula nitida Verrill & Smith [in Verrill], Reference Verrill1885: 424–425, pl. 44, fig. 11.
Scalaria vermetiformis Watson, Reference Watson1886: 142, pl. 9, fig. 6. – Synonymized by Rex & Boss (Reference Rex and Boss1973).
Liotia (Laxispira) nitida: Dall, Reference Dall1889: 166.
Liotia (Eccliseogyra) nitida: Dall (Reference Dall1892: 307).
Scalaria dissoluta Fischer [in Locard], Reference Locard1897: 407, pl. 19, figs 1–3. – Synonymized by Rex & Boss (Reference Rex and Boss1973).
Opaliopsis (Eccliseogyra) nitida: Rios (Reference Rios1994: 101, fig. 412).
Nystiella (Eccliseogyra) nitida: Rios (Reference Rios2009: 186, fig. 455).
Epitonium (Eccliseogyra) nitidum: Rex & Boss (Reference Rex and Boss1973: 93–98).
Eccliseogyra nitida: Bouchet & Warén (Reference Bouchet and Warén1986: 482, fig. 1139); Weil et al. (Reference Weil, Brown and Neville1999: 14, fig. 15); Nakayama (Reference Nakayama2003: 37, pl. 4, figs 1–2); Absalão (Reference Absalão, Lavrado and Brasil2010: 91); Lima & Christoffersen (Reference Lima and Christoffersen2013: 290, tab. 1); Brown & Neville (2016: 17 – not figured; annotated catalogue).
Solutiscala (Solutiscala) vermetiformis: Clench & Turner (Reference Clench, Turner and Clench1952: 347, pl. 170, figs 1–2).
Epitonium (Solutiscala) vermetiforme: Bayer (Reference Bayer1971: 133, fig. 11D).
TYPE MATERIAL
Holotype: USNM 44648 (destroyed, E. Strong, personal communication, 8 July 2016); holotype of Scalaria dissoluta (Figure 5A): MNHN-IM-2000-3963, Sardegna (39°48′N 09°51′W, 3307 m); holotype of Scalaria vermetiformis: NHMUK (09°05′S 34°50′W, 645 m).
TYPE LOCALITY
North-western Atlantic, off Chesapeake Bay, USA (37°39′N 73°16′W, 2600 m).
MATERIAL EXAMINED
– Brazil: continental slope of Campos Basin, off Rio de Janeiro state: OP II station 59: IBUFRJ 19790 [2 shells]; (21°53′47″S 39°50′1″W, 1170 m, 24.viii.2001): MNRJ 13805 [1 shell]; OP II station 58: IBUFRJ 17713 [1 shell]; OP II station 50: MNRJ 35527 [1 shell]; ALBACAR station 27: IBUFRJ 19789 [2 shells]; OP II station 45: MNRJ 35526 [1 shell], IBUFRJ 16957 [1 shell]; OP I station 79: IBUFRJ 16878 [1 shell]; OP II station 69: IBUFRJ 15511 [1 shell]; OP I station 75: MNRJ 35524 [8]; (22°32′50″S 40°04′02″W, 1067 m, 17.xi.2002): MNRJ 17746 [1 shell]; ALBACAR station 34: MNRJ 35528 [1 shell]; ALBACAR station 40: MNRJ 35525 [1 shell]; OP I station 66: IBUFRJ 16177 [1 shell]; OP II station 79: IBUFRJ 16799 [1 shell]; OP II station 80: IBUFRJ 19786 [2 shells]; OP II station 74: IBUFRJ 17253 [3 shells]; OP II station 75: IBUFRJ 19787 [1 shell]; continental slope of Santos Basin, off São Paulo state: (24°12′09.63″S 43°15′13.93″W, 600 m), 05.x.2002: MNRJ 13806 [2 shells]; – Rio Grande Rise: ERG 27 (31.5656° S 34.2850° W, 2200 m): MNRJ 27216 [2 shells].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-western Atlantic: northern USA, Gulf of Mexico, Straits of Florida; North-eastern Atlantic: off Portugal, Cape Verde, 2022–4693 m (Bayer, Reference Bayer1971; Rex & Boss, Reference Rex and Boss1973; Bouchet & Warén, Reference Bouchet and Warén1986; Pequegnat (1983) apud Rosenberg et al., Reference Rosenberg, Moretzsohn, García, Felder and Camp2009). South-western Atlantic (this study): north-eastern, eastern and south-eastern Brazil, 600–1942 m; Rio Grande Rise, 2200 m. Pacific Ocean: Japan: Kyushu, 993–1103 m (Nakayama, Reference Nakayama2003).
REMARKS
Eccliseogyra nitida (Figure 5) is a moderately common species in deep-sea localities in the North Atlantic (Rex & Boss, Reference Rex and Boss1973; Bouchet & Warén, Reference Bouchet and Warén1986). Although it was regarded as rare by Weil et al. (Reference Weil, Brown and Neville1999), E. nitida is one of the most abundant nystiellids in the slope samples off Brazil. It is readily distinguished from the other species in Nystiellidae by its open-coiling adult shell (Figure 5A–D), which is present since the first teleoconch whorl, resulting in an exposed protoconch base (Figure 5F, G).
Eccliseogyra pyrrhias (Watson, Reference Watson1886)
(Figure 6A–G)
Scalaria (Acirsa) pyrrhias Watson, Reference Watson1886: 145, pl. 9, fig. 7.
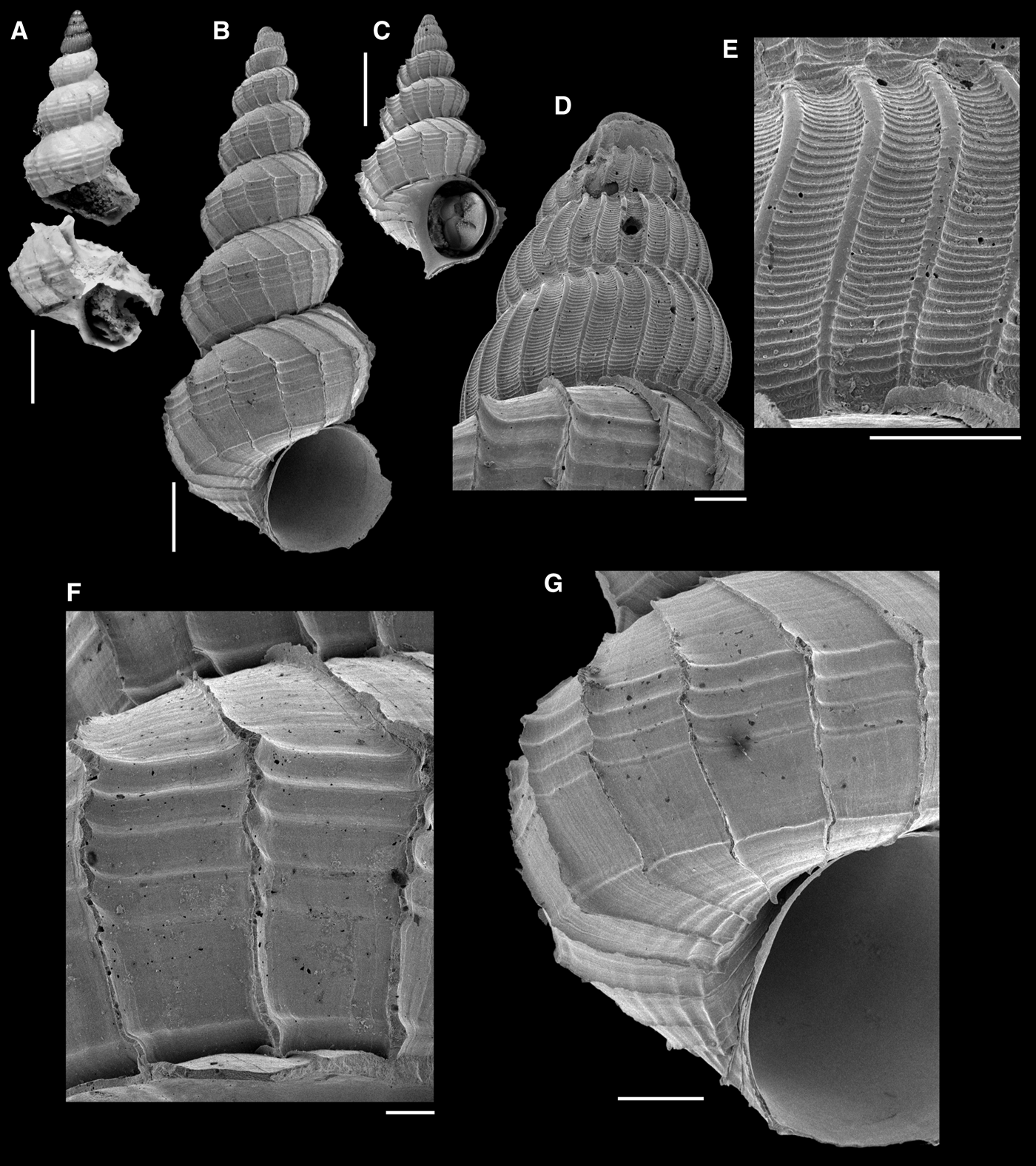
Fig. 6. Eccliseogyra pyrrhias: (A) holotype; (B, G; C–F) MNRJ 35726; (A–C) whole shell; (D) protoconch; (E) detail of last protoconch whorl; (F) detail of third teleoconch whorl; (G) detail of sixth teleoconch whorl. Scale bars: A–C, 1 mm; D–F, 100 µm; G, 200 µm.
Solutiscala (Foratiscala) pyrrhias: Clench & Turner (Reference Clench, Turner and Clench1952: 346–347, pl. 170, figs 3–4).
Eccliseogyra pyrrhias: Bouchet & Warén (Reference Bouchet and Warén1986: 488, fig. 1149); Weil et al. (Reference Weil, Brown and Neville1999: 16, fig. 17); Brown & Neville (2016: 17 – not figured; annotated catalogue).
Eccliseogyra aff. exquisita auct. non Bouchet & Warén, Reference Bouchet and Warén1986: Absalão (Reference Absalão, Lavrado and Brasil2010: 50–51, 91, fig.).
TYPE MATERIAL
Holotype (Figure 6A): NHMUK 1887.2.9.506.
TYPE LOCALITY
North-western Atlantic, Challenger station 24: Culebra Island, West Indies (18°38′30″N 65°05′30″W, 713 m).
MATERIAL EXAMINED
– Brazil: continental slope of Campos Basin, off Rio de Janeiro state: OP I station 59: IBUFRJ 15191 [1 shell]; OP I station 74: IBUFRJ 15411 [1 shell]; OP II station 74: IBUFRJ 17772 [1 shell]; IBUFRJ 17254 [1 shell]; OP II station 69: IBUFRJ 15510 [2 shells]; MNRJ 35726 [1 shell]; OP I station 64: IBUFRJ 16110 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-western Atlantic: Gulf of Mexico; Puerto Rico: Culebra Island, 713 m (Watson, Reference Watson1886; Pequegnat (1983) apud Rosenberg et al., Reference Rosenberg, Moretzsohn, García, Felder and Camp2009); Gay-Bermuda Transect, (39°49′N 50°41′W, 1102 m (Bouchet & Warén, Reference Bouchet and Warén1986). South-western Atlantic: eastern Brazil, 735–750 m (this study).
REMARKS
Prior to the present new records, Eccliseogyra pyrrhias (Figure 6) was known only from its type locality in Puerto Rico, besides a single shell from the Gay-Bermuda Transect in the North Atlantic, illustrated by Bouchet & Warén (Reference Bouchet and Warén1986); this shell, however, has slightly more axial ribs per whorl. Based on the original description and on the holotype, in which the features are visible in spite of extensive damage (Figure 6A), we consider that the shells from eastern Brazil (Figure 6B–G) belong to this species. As indicated by Bouchet & Warén (Reference Bouchet and Warén1986) and also by García (Reference García2011), this species is characterized by few spiral cords (three to four), restricted to the area anterior to the peripheral angulation of the teleoconch whorls. In the shells from Brazil, this feature is very clear, although some weak spiral growth lines are seen below the suture (Figure 6F, G).
The shells from Brazil have a spiral cord at the periphery of the base (Figure 6G). The base is sculptured with axial cords, continuing from the axial lamellae of the last whorl, and thin spiral cords, which are stronger on the portion close to the narrow umbilicus (Figure 6G). This sculpture is visible in the photograph of the holotype provided by Weil et al. (Reference Weil, Brown and Neville1999), although the spiral cords are indistinct.
Re-examination of the material recorded as Ecclisiogyra aff. exquisita by Absalão (Reference Absalão, Lavrado and Brasil2010), from the Campos Basin off eastern Brazil, revealed that it is Eccliseogyra pyrrhias. Eccliseogyra exquisita Bouchet & Warén, Reference Bouchet and Warén1986 is distinguished by more numerous spiral cords over the entire length of the teleoconch whorls, including the region posterior to the peripheral angulation.
Genus Iphitus Jeffreys, Reference Jeffreys1883
Iphitus Jeffreys, Reference Jeffreys1883: 113. Type species by monotypy: Iphitus tuberatus Jeffreys, Reference Jeffreys1883.
Iphitella Thiele, Reference Thiele1925: 59 (according to Bouchet & Warén, Reference Bouchet and Warén1986, an unnecessary new name for Iphitus Jeffreys, Reference Jeffreys1883 non Rafinesque).
Sculptifer Beu & Climo, Reference Beu and Climo1974: 323. Type species (original designation): Stilifer neozelanicus Dell, 1956. – Synonymized by Beu (Reference Beu1978).
DIAGNOSIS
Shell trochiform-turriculate, teleoconch with few whorls of rounded to angulated outline. Teleoconch sculpture of usually equally strong axial and spiral cords, sometimes with dominant spiral cords; some species with development of peripheral spiral cords, with or without umbilicus. Protoconch in the typical pattern of the family, with its axis strongly deviating from the teleoconch axis. Operculum described by Bouchet & Warén (Reference Bouchet and Warén1986).
Iphitus cancellatus Dautzenberg & H. Fischer, 1896
(Figure 7A–E)
Iphitus cancellatus Dautzenberg & H. Fischer, 1896: 450, pl. 19, fig. 1; Bouchet & Warén (Reference Bouchet and Warén1986: 492, figs 1138, 1156–1157); Brown & Neville (2016: 17 – not figured; annotated catalogue).
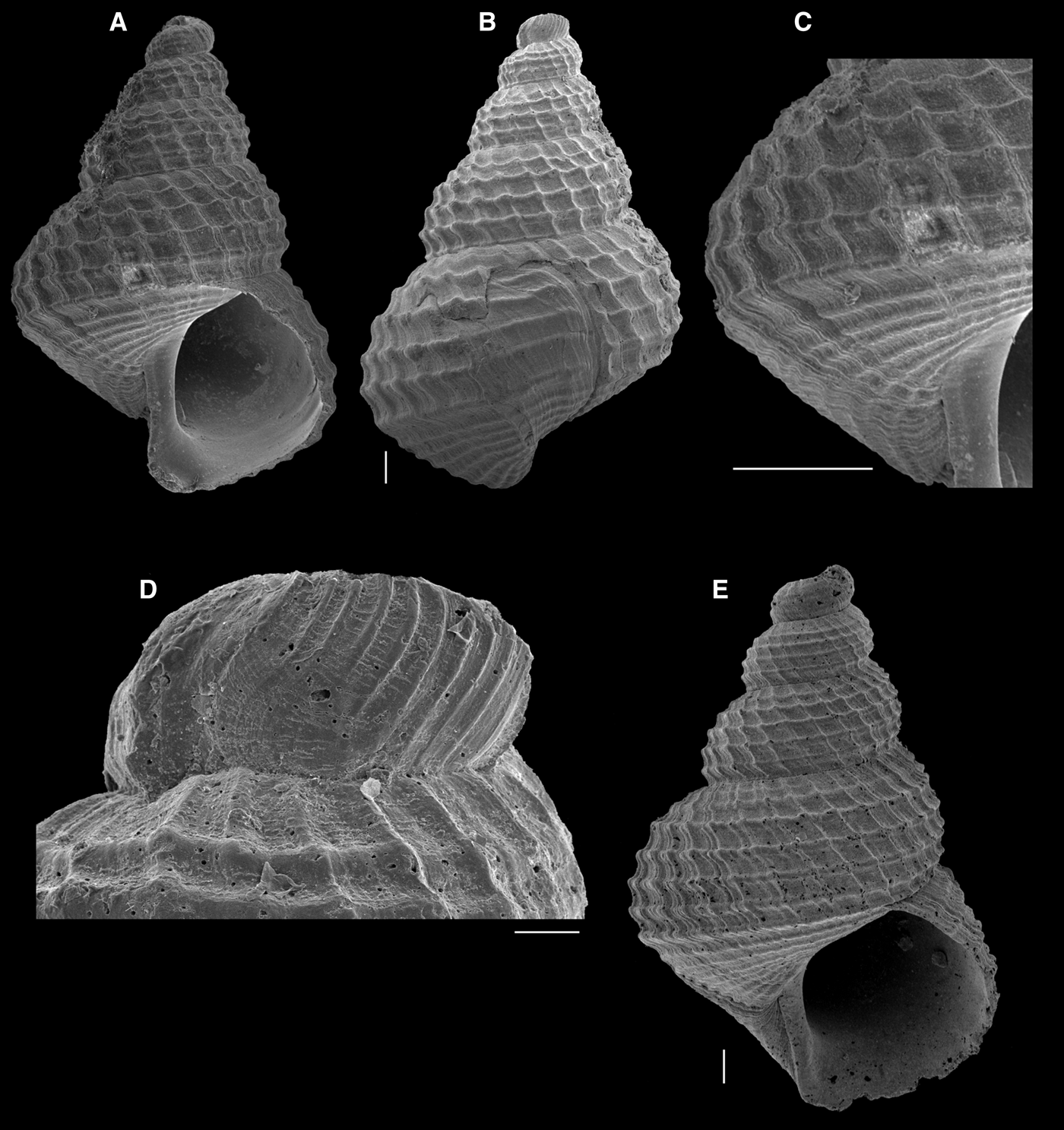
Fig. 7. Iphitus cancellatus: (A–D) MNHN; (E) MNRJ 29152. (A, B, E) whole shells, broken protoconch; (C) detail of last whorl; (D) detail of last protoconch whorl. Scale bars: A–C, E, 1 mm; D, 50 µm.
Iphitus reticulatus Dall, Reference Dall1927: 116. – Synonymized by Bouchet & Warén (Reference Bouchet and Warén1986).
TYPE MATERIAL
Holotype in Musée Océanographique, Monaco (fide Bouchet & Warén, Reference Bouchet and Warén1986).
TYPE LOCALITY
North-eastern Atlantic, Azores (1300 m).
MATERIAL EXAMINED
– Brazil: continental slope of Abrolhos: MD 55 station CB76 (18°58′09″S 37°49′06″W, 637 m): MNHN [1 shell]; continental slope of Campos Basin, off Rio de Janeiro state: OP I station 74: IBUFRJ 15406 [1 shell]; – Rio Grande Rise: ERG 005 (30.0094°S 36.1437°W, 1380 m): MNRJ 29152 [2 shells].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-eastern Atlantic: Morocco and Azores, 785–1300 m (Bouchet & Warén, Reference Bouchet and Warén1986). Western Atlantic: Georgia, USA (Bouchet & Warén, Reference Bouchet and Warén1986); eastern Brazil, 750 m (this study); Rio Grande Rise, 1380 m (this study).
Iphitus robertsii Sabelli & Taviani, Reference Sabelli and Taviani1997
(Figure 8A–F)
Iphitus robertsi Sabelli & Taviani, Reference Sabelli and Taviani1997: 94–96, figs 1–4: Hernández et al. (Reference Hernández, Swinnen, Gómez and Pérez2011: 110, figs 33F–H); Brown & Neville (2016: 18 – not figured; annotated catalogue).

Fig. 8. Iphitus robertsi: MNRJ 26212. (A–C) whole shells; (D) protoconch; (E) detail of last protoconch whorl; (F) detail of umbilical region on the third whorl. Scale bars: A–C, 200 µm; D, F, 100 µm; E, 20 µm.
TYPE MATERIAL
Holotype: USNM 880185; paratypes: USNM 880186 [1 shell]; MZB 11621 [3 shells]; MZB 11622 [1 shell]; MNHN-IM-2012-40996 [1 shell]; HMNH 42544 [1 shell]. All from type locality.
TYPE LOCALITY
North-western Atlantic, Green Canyon Block 189, Gulf of Mexico (27°46′48″N 93°17′74″W, 184 m).
MATERIAL EXAMINED
The paratypes deposited in MZB and: – Brazil: off Amapá state: 04°27′54″N 49°58′05″W, 160 m, ‘Saltro 25′ coll., 13.x.2000: MNRJ 26212 [5 shells]; off São Paulo state: REVIZEE Sul station 6672: MNRJ 27819 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
Eastern Atlantic: Canary Islands, 90 m (Hernández et al., Reference Hernández, Swinnen, Gómez and Pérez2011); Western Atlantic: Gulf of Mexico, 184 m (Sabelli & Taviani, Reference Sabelli and Taviani1997); northern and south-eastern Brazil, 160–184 m (this study).
TYPE MATERIAL
Holotype: MNRJ 29151.

Fig. 9. Iphitus notios sp. nov.: holotype. (A) whole shell; (B) protoconch. Scale bars: 200 µm.
TYPE LOCALITY
South-western Atlantic: Rio Grande Rise, ERG station 005 (30.0094°S 36.1437°W, 1380 m).
ETYMOLOGY
G. notios = southern. This species is named after its occurrence in the South Atlantic.
DIAGNOSIS
Axial and spiral sculpture of equivalent expression on early whorls; axial sculpture becoming fainter each subsequent whorl, while the spiral becomes slightly stronger in each whorl.
DESCRIPTION
Shell turriculated, up to 3.71 mm in length, 2.04 mm in width, conic profile. Protoconch amber, teleoconch white coloured. Protoconch conic-subcylindrical, deviated from axis of teleoconch, with four whorls; readily distinct of teleoconch, transition marked by protoconch sculpture becoming faint; embryonic whorl dome-shaped, smooth; remaining whorls with sinuous profile, more convex abapically, sculpture by ~25 axial ribs crossing over suture, where small nodules are formed and 10 fine spiral cords regularly spaced, crossing axial ribs near sutural area only. Teleoconch with up to 4.5 whorls of rounded, convex profile; axial sculpture of numerous low ribs of regular width and spacing, becoming faint and more spaced on subsequent whorl, reaching suture; ~40 ribs on each whorl up to 4th whorl; interspaces as wide as ribs on first whorl, becoming wider on each subsequent whorl; three to four spiral cords of same strength as axial ribs on first whorl, becoming slightly stronger on each subsequent whorl, forming small nodules on intersections with axial sculpture; varices lacking. Suture impressed. Last whorl with basal cord of same strength as spiral cords on same whorl; basal disc with spiral cords and axial ribs that evanesce into umbilical area. Aperture elliptical; inner lip smooth. Umbilicus closed but forming a weak chink created by inner lip.
Dimensions: holotype with 4.5 teleoconch whorls; total length: 3.71 mm; protoconch: 560 µm height, 470 µm width.
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
Known from type locality, only.
REMARKS ON IPHITUS
Beu (Reference Beu1978) and Bouchet & Warén (Reference Bouchet and Warén1986) provided historical reviews of the taxonomy and biology of Iphitus. The genus is characterized by a somewhat trochiform shell with a wide body whorl and strong spiral cords crossed by thin to equally strong axial ribs.
Only seven species of Iphitus are known, four of them from the North Atlantic (Bouchet & Warén, Reference Bouchet and Warén1986; Hernández et al., Reference Hernández, Swinnen, Gómez and Pérez2011): Iphitus cancellatus (Dautzenberg & H. Fisher, 1896), I. robertsi (Sabelli & Taviani, Reference Sabelli and Taviani1997), I. tenuisculptus (Seguenza, 1876), and I. tuberatus Jeffreys, Reference Jeffreys1883. The single species from the western Atlantic, I. robertsi, was described from the Gulf of Mexico and subsequently recorded from the Canary Islands (Hernández et al., Reference Hernández, Swinnen, Gómez and Pérez2011).
The present study records the genus from the South Atlantic, based on the records of I. cancellatus (Figure 7) and I. robertsi (Figure 8) from Brazil, besides the record of I. cancellatus and Iphitus notios sp. nov. (Figure 9), from the Rio Grande Rise.
While the shells of I. cancellatus from the South Atlantic (Figure 7) are very similar to those from the north-eastern Atlantic illustrated by Bouchet & Warén (Reference Bouchet and Warén1986), with insignificant variation, those of I. robertsi (Figure 8) show considerable variation in ornamentation in comparison to the type series from the Gulf of Mexico. In the shells from the type series of I. robertsi, the spiral cords are more distinct, with the two peripheral cords stronger than the median ones; this is also evident in the shell from the Canary Islands (Hernández et al., Reference Hernández, Swinnen, Gómez and Pérez2011: fig. 33F–H); but in the shells from Brazil, the two peripheral spiral cords and the median ones are approximately of the same strength (Figure 8A–C). This less-pronounced peripheral cord in the shells from Brazil gives rise to a more convex, less-angulated outline, especially apparent in the larger shell (Figure 8A). However, Figure 8B and C show shells that are more similar to the holotype of I. robertsi, although the peripheral spiral cords are still not as strong. This variation is herein considered as intraspecific.
The larger shell of I. robertsi from northern Brazil (Figure 8A) exhibits three spiral cords between the two median ones on the third teleoconch whorl, while the paratype with three whorls has only two cords. The remaining features of the shell, such as the general shape and proportions, umbilical sculpture, and protoconch agree with the type series. The sculpture in the umbilical region is important since it is variable in the type series: the holotype has a very strong basal cord and spiral cords in the basal disc, while in the paratypes these cords are less pronounced.
Iphitus notios sp. nov., from the Rio Grande Rise, South Atlantic, is somewhat similar to I. robertsi in the pattern of sculpture of the initial teleoconch whorls, but in this new species, the expression of the overall sculpture varies conspicuously on its single shell. The initial whorls (Figure 9A) bear a reticulate pattern of equal proportions between the axial and spiral sculptures. This pattern of sculpture changes on latter whorl, as the axial sculpture becomes fainter, while the spiral sculpture grows slightly stronger (Figure 9A). The first teleoconch whorl of I. notius bears three spiral cords of approximate equal strength; on the second whorl, a new cord arises subsuturally, although it is nearly obsolete by the fourth whorl. In comparison, I. robertsi has two strong, peripheral spiral cords on all teleoconch whorls (the adapical one, close to the suture) and secondary, weaker cords: one posterior to the adapical peripheral cord and one to two median ones. The umbilicus of I. notios (Figure 9A) is closed, different from that of I. robertsi and like that of I. cancellatus.
All species of Iphitus studied here have the axis of the protoconch strongly deviating from the teleoconch axis, a feature also present in Iphitus tuberatus (Jeffreys, Reference Jeffreys1883).
Genus Narrimania Taviani, Reference Taviani1984
Narrimania Taviani, Reference Taviani1984: 240–241. Type species (original designation): Cerithiopsis concinna Sykes, Reference Sykes1925.
DIAGNOSIS
Shell turbinate-turriculate, teleoconch with several whorls of somewhat rounded outline. Teleoconch sculpture of usually equally strong axial and spiral cords, forming weak to strong nodules on intersections; with microscopic granules in the squared interspaces of the teleoconch whorls, as pits or irregular, spirally oriented striae; with development of peripheral basal cord, without umbilicus; basal area with weak to nearly obsolete sculpture. Protoconch in the typical pattern of the family, with nodulous abapical end of the axial ribs.
Narrimania azelotes (Dall, Reference Dall1927)
(Figure 10)
Epitonium azelotes Dall, Reference Dall1927: 61.

Fig. 10. Narrimania azelotes (Dall, Reference Dall1927): (A) Holotype; (B, E) MNRJ 28176; (C, D, F) MNRJ 28177. (A–C) whole shells; (D) protoconch; (E) last teleoconch whorl; (F) detail of sculpture on fifth teleoconch whorl. Scale bars: A–C, 1 mm; D 55, 100 µm.
Nystiella azelotes: Clench & Turner, 1954: 342, pl. 167; Miyaji (Reference Miyaji, Amaral and Rossi-Wongtschowski2004: 79 – not figured); Brown & Neville (2016: 14 – not figured; annotated catalogue).
TYPE MATERIAL
Holotype USNM 108370 (Figure 10A).
TYPE LOCALITY
North-western Atlantic, off Fernandina, Florida, USA, Albatross station 2415 (30°44′N 79°26′W, 805 m).
MATERIAL EXAMINED
– Brasil: off Paraná/Santa Catarina states: REVIZEE Sul station 6680: MZSP 131835 [1 shell]; REVIZEE Sul station 6660: MNRJ 28177 [1 shell]; PADCT station 6641: MNRJ 28178 [1 shell]; PADCT station 6595: MNRJ 28176 [2 shells]; – Rio Grande Rise: ERG station 019 L2 (29.954°S 36.4969°W, 1280 m): MNRJ 29147 [1 shell]; ERG station 068 (31.1667°S 35.6565°W, 895 m): MNRJ 29148 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-western Atlantic: Carolinian region, 805 m (Clench & Turner, 1954); South-western Atlantic: south-eastern Brazil, 130–258 m (this study); Rio Grande Rise, 895–1280 m (this study).
Narrimania concinna (Sykes, Reference Sykes1925)
(Figure 11)
Cerithiopsis concinna Sykes, Reference Sykes1925: 184, pl. 9, fig. 1.

Fig. 11. Narrimania concinna: (A, 60–61) MNRJ 28176; (B–D) MNRJ 18104. (A, B) whole shells; (C) protoconch; (D) detail of protoconch; (E) last teleoconch whorl; (F) detail of sculpture on fifth teleoconch whorl. Scale bars: A, B, 1 mm; C, D, F, 100 µm; E, 200 µm.
Narrimania concinna: Taviani (Reference Taviani1984: pl. 1); Bouchet & Warén (Reference Bouchet and Warén1986: 490, figs 1135, 1153–1154); Ardovini & Cossignani (Reference Ardovini and Cossignani2004: 139, fig); Brown & Neville (2016: 14 – not figured; annotated catalogue).
Narrimania cf. concinna: Miyaji (Reference Miyaji, Amaral and Rossi-Wongtschowski2004: 79 – not figured).
TYPE MATERIAL
Syntypes: NHMUK 1985005.
TYPE LOCALITY
Mediterranean, Adventure Bank, between Sicily and Tunisia, 170 m.
MATERIAL EXAMINED
Brazil: Campos Basin, off Rio de Janeiro state: OP II station 79: MNRJ 18104 [1 shell]; off Rio de Janeiro state: PADCT station 6554: MZSP 131836 [1 shell]; off São Paulo state: REVIZEE Sul station 6652: MNRJ 28175 [1 shell]; REVIZEE Sul station 6705: MNRJ 28174 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
Western Mediterranean, 130–695 m (Sykes, Reference Sykes1925; Bouchet & Warén, Reference Bouchet and Warén1986); South-western Atlantic: south-eastern Brazil, 206–755 m (this study).
Narrimania raquelae new species
(Figure 12)
Narrimania sp.: Absalão (Reference Absalão, Lavrado and Brasil2010: 92 – not figured).
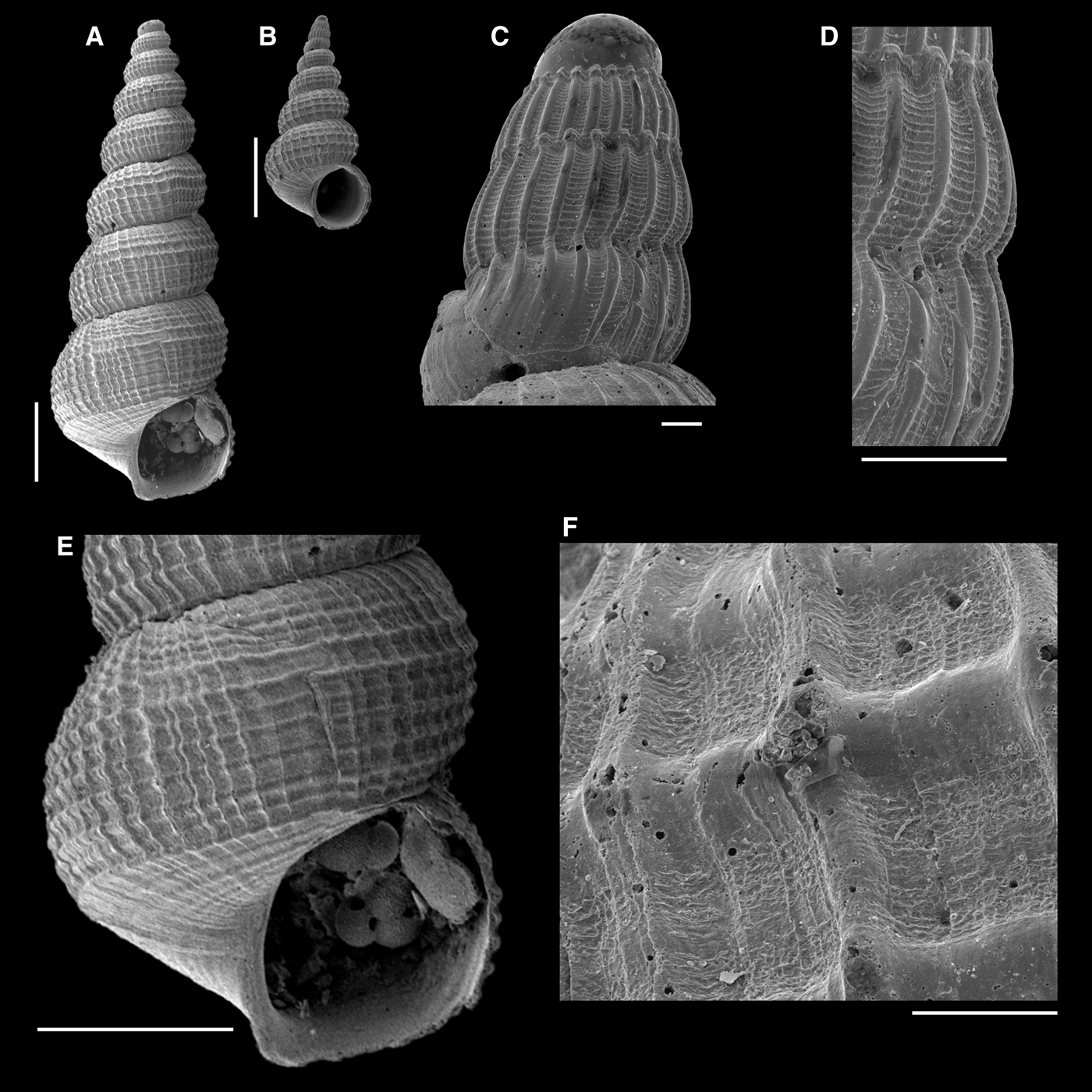
Fig. 12. Narrimania raquelae new species: (A, E) holotype; (B–D, F) paratype MNRJ 17217; (A, B) whole shells; (C) protoconch; (D) detail of penultimate and last protoconch whorls; (E) last teleoconch whorl; (F) detail of microsculpture on fourth teleoconch whorl. Scale bars: A, B, E, 1 mm; C, D, F, 100 µm.
TYPE MATERIAL
Holotype: IBUFRJ 17249.
Paratypes: Campos Basin, Rio de Janeiro state: type locality: IBUFRJ 17073 [1 shell]; MNRJ 17171 [1 shell]; (22°04′32.8″S 39°54′11.4″W, 722 m), 30.vi.2003: IBUFRJ 17564 [2 shells]; OP I station 44: IBUFRJ 17217; OP II station 79: IBUFRJ 16802 [1 shell].
TYPE LOCALITY
South-western Atlantic, Campos Basin, off Rio de Janeiro state, OP I station 59 (21°52′59.60″S 39°55′30.63″W, 750 m).
ETYMOLOGY
Named after Raquel Absalão, daughter of Ricardo Absalão.
DIAGNOSIS
Numerous axial and spiral sculpture elements, forming small nodules at intersection; interspaces with irregular short elongate granules.
DESCRIPTION
Shell turriculate, up to 8.47 mm in length, 1.97 mm in width (larger shell with broken apex), conical-rectilinear profile. Protoconch amber; teleoconch white. Protoconch conical-subcylindrical, with four whorls, readily distinct of teleoconch, transition marked by protoconch sculpture becoming faint; embryonic whorl dome-shaped, smooth, remaining whorls with sinuous profile, more convex abapically, sculptured with ~20 axial ribs projecting over the crenulated suture, where small nodules are formed adapically; spiral sculpture of 18 fine cords regularly spaced, crossing ribs in sutural region. Teleoconch with up to 8 whorls of rounded, convex profile; axial sculpture formed by numerous low axial ribs of irregular width and spacing, reaching suture, ~50 ribs on eight whorl; interspaces twice as wide as ribs; spiral sculpture of 11 cords of same strength as axial ribs, forming small nodules in intersections, Varices absent. Suture impressed. Last whorl with very weak peripheral cord; basal disc with spiral cords and axial growth lines. Aperture subovate, inner lip smooth. Dimensions: holotype with eight teleoconch whorls (lacking protoconch); total length: 6.4 mm; protoconch: 580 µm height, 360 µm width.
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
Eastern Brazil, 722–800 m.
REMARKS ON NARRIMANIA
Narrimania was described to include only two species, both from the North Atlantic: N. concinna (Sykes, Reference Sykes1925), from the Mediterranean, and N. azelotes (Dall, Reference Dall1927), from Florida. The distinguishing features of the genus, as indicated by Taviani (Reference Taviani1984), are the equally strong axial and spiral sculpture, giving rise to very deep square excavations.
The precise taxonomic distinction between the two species has remained unclear, since both Taviani (Reference Taviani1984) and Bouchet & Warén (Reference Bouchet and Warén1986) expressed doubt as to whether the subtle difference in the number of spiral cords represents intraspecific or interspecific variation: Narrimania concinna has four spiral cords on the sixth teleoconch whorl, while N. azelotes has only three. Both Taviani (Reference Taviani1984) and Bouchet & Warén (Reference Bouchet and Warén1986) stated that additional material from the western Atlantic was necessary to evaluate the possibility of intraspecific variation or distinction between the two species.
Examination of the additional material from Brazil showed that N. azelotes (Figure 10) and N. concinna (Figure 11) are indeed distinct, and confirmed the difference in the number of spiral cords. A large shell of N. azelotes (Figure 10B) has only three spiral cords on its eighth teleoconch whorl, while in N. concinna a fourth whorl is present on the sixth teleoconch whorl (slightly visible on the fifth whorl) (Figure 11A). Moreover, the overall sculpture is coarser in N. azelotes, with a deeper suture (Figure 10A–C, E). Taviani (Reference Taviani1984) and Bouchet & Warén (Reference Bouchet and Warén1986) also stated that the eroded type of N. azelotes (Figure 10A) precluded the recognition of spiral cords on the base of the shell; inspection of the material for the present study confirmed that this sculpture is indeed present (Figure 10E).
Taviani (Reference Taviani1984) recognized the presence in N. concinna of a punctate microsculpture arranged in parallel spiral lines. This sculpture pattern is visible in the material of the present study in both species (Figure 10F and 11F); in N. azelotes, in spite of the somewhat eroded squared interspaces, this microscopic sculpture is visible in some areas of the shell (Figure 12F).
Narrimania raquelae sp. nov. (Figure 12) agrees with the general diagnosis of the genus, being a turriculated shell with equally strong axial and spiral sculpture, although this sculpture is weaker and has more numerous axial and spiral elements than in N. azelotes and N. concinna.
The axial and spiral sculpture of the teleoconch of Narrimania raquelae are of similar strength, also forming squared interspaces (Figure 12E), but they are much thinner and more numerous than those of N. azelotes and N. concinna, with up to 10 spiral cords on the seventh teleoconch whorl, and almost 50 axial ribs in the new species (Figure 12E). The nodules formed by the crossing of the axial and spiral sculpture are much thinner, giving rise to a rather convex whorl outline, in contrast to the crenulate outline of the other two species. The basal disc of N. raquelae (Figure 12E) has the same pattern as that of N. azelotes and N. concinna, with spiral cords. The microscopic sculpture in the squared interspaces is also present, although in a somewhat different pattern, not as punctuations, but as short, irregular spirally oriented lines (Figure 12F).
Two other features can be added to the Narrimania characterization: the nodulous abapical end of the axial ribs on the protoconch (Figures 10D, 11D & 12D), which is shared with Iphitus; and the microscopic granules in the squared interspaces of the teleoconch whorls, which are formed by pits (Figures 10F & 11F) or irregular, spirally oriented striae (Figure 12F). Both features are visible in the illustrations of N. concinna by Taviani (Reference Taviani1984: fig. 1c, 1b, 2b) and by Bouchet & Warén (1986: figs 1135, 1153, 1154), as well as in N. raquelae, new species (Figure 12D, F).
Genus Opaliopsis Thiele, Reference Thiele1928
Opaliopsis Thiele, Reference Thiele1928: 92. Type species (original designation): Scala elata Thiele, Reference Thiele1925.
Nystiella Clench & Turner, Reference Clench, Turner and Clench1952: 337. Type species (original designation): Epitonium opalinum Dall, Reference Dall1927. – Synonymized by Kilburn (Reference Kilburn1985).
DIAGNOSIS
Shell turbinate-turriculate, teleoconch with several whorls of somewhat rounded outline. Teleoconch sculpture of broad axial costae, from low to high height, and spiral sculpture weak to nearly obsolete, with or without nodules on intersections; with or without a well-marked peripheral spiral cord, without umbilicus; basal area with weak to nearly obsolete sculpture, or with pattern similar as the palatal area. Protoconch in the typical pattern of the family.
Opaliopsis cearensis Andrade, Costa & Pimenta, Reference Andrade, Costa and Pimenta2011
(Figure 13A, B)
Opaliopsis cearense Andrade, Costa & Pimenta, Reference Andrade, Costa and Pimenta2011: 1564, figs 10–16; Brown & Neville (2016: 13 – not figured; annotated catalogue).

Fig. 13. Opaliopsis from Brazil: (A, B) Opaliopsis cearensis Andrade et al., Reference Andrade, Costa and Pimenta2011: MNRJ 13964; (C) Opaliopsis atlantis (Clench & Turner, Reference Clench, Turner and Clench1952); MNRJ 13515; (D) Opaliopsis opalina (Dall, Reference Dall1927): MNRJ 13347; (B) detail of sculpture on seventh teleoconch whorl. Scale bars, A, C, D, 1 mm; B, 100 µm.
TYPE MATERIAL
Holotype: MNRJ 15500; and paratypes, as listed by Andrade et al. (Reference Andrade, Costa and Pimenta2011).
TYPE LOCALITY
South-western Atlantic, Canopus Bank, 96 miles off Ceará State, Brazil (02°14′25″S 38°22′50″W, 240–260 m).
MATERIAL EXAMINED
The types.
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-western Atlantic: north-eastern Brazil, 240–260 m (Andrade et al., Reference Andrade, Costa and Pimenta2011).
Opaliopsis atlantis (Clench & Turner, Reference Clench, Turner and Clench1952)
(Figure 13C)
Nystiella atlantis Clench & Turner, Reference Clench, Turner and Clench1952: 343, pl. 168; Rios (2009: 186, figure 454, holotype photograph reproduced).
Opaliopsis atlantis: Bouchet & Warén (Reference Bouchet and Warén1986: 489, figs 1133, 1151–1152); Weil et al. (Reference Weil, Brown and Neville1999: 26, figure 67); Giannuzzi-Savelli et al. (1999: 88, fig. 230); Ardovini & Cossignani (Reference Ardovini and Cossignani2004: 140, fig.); Peñas et al. (Reference Peñas, Rolán, Luque, Templado, Moreno, Rubio, Salas, Sierra and Gofas2006: 94, figs 148–149); Segers et al. (Reference Segers, Swinnen and de Prins2009: 106, pl. 15, fig. 13); Luque (Reference Luque, Gofas, Salas and Moreno2010: 223, fig.); Andrade et al. (Reference Andrade, Costa and Pimenta2011: 1562, figs 1–3); Hernández et al. (Reference Hernández, Swinnen, Gómez and Pérez2011: 110, figs 33I–K); Brown & Neville (2016: 13 – not figured; annotated catalogue).
Opaliopsis cf. atlantis: Miyaji (2002: 79 – not figured).
TYPE MATERIAL
Holotype: MCZ 187988; Paratypes: T. McGuinty collection and USNM 417386 (fide Weil et al., Reference Weil, Brown and Neville1999).
TYPE LOCALITY
North-western Atlantic, off Bahía de Cochinos, Cuba (22°09′N 81°10′W, 420–484 m).
MATERIAL EXAMINED
Those cited in Andrade et al. (Reference Andrade, Costa and Pimenta2011) and: – Brazil: off Ilha Bela, São Paulo state: REVIZEE Sul station 6676: MZSP 131837 [1 shell]; off Santa Catarina state: PADCT station 6601 : MNRJ 28185 [1 shell].
GEOOGRAPHIC AND BATHYMETRIC DISTRIBUTIONS
Eastern Atlantic: Azores, Strait of Gibraltar, Andalucia, Canary Islands, Madeira, 228–825 m (Bouchet & Warén, Reference Bouchet and Warén1986; Segres et al., 2009; Gofas et al., 2010; Hernández et al., Reference Hernández, Swinnen, Gómez and Pérez2011); Western Atlantic: southern Florida, Gulf of Mexico and Caribbean, 115–485 m (Clench & Turner, Reference Clench, Turner and Clench1952; Tunnell et al., Reference Tunnell, Woods, Kindinger and Kindinger1978); north-eastern, eastern and south-eastern Brazil, 140–260 m (Andrade et al., Reference Andrade, Costa and Pimenta2011).
REMARKS
Giannuzzi-Savelli (1999) recorded O. atlantis from Meridional Mediterrannean, without precise location.
Opaliopsis opalina (Dall, Reference Dall1927)
(Figure 13D)
Epitonium opalinum Dall, Reference Dall1927: 61.
Epitonium lavaratum Dall, Reference Dall1927: 62. – Synonymized by Clench & Turner (Reference Clench, Turner and Clench1952).
Opalia ? dromio Dall, Reference Dall1927: 63. – Synonymized by Clench & Turner (Reference Clench, Turner and Clench1952).
Nystiella opalina: Clench & Turner (Reference Clench, Turner and Clench1952: 337, pls 163–164).
Opaliopsis opalina: Andrade et al. (Reference Andrade, Costa and Pimenta2011: 1562: figs 4–9).
Opaliopsis opalinum: Brown & Neville (2016: 18 – not figured; annotated catalogue).
TYPE MATERIAL
Holotype USNM 108368 (Clench & Turner, Reference Clench, Turner and Clench1952).
TYPE LOCALITY
North-western Atlantic, off Fernandina, Florida, USA, Alatross station 2415 (30°44′N 79°26′W, 805 m).
MATERIAL EXAMINED
Those cited in Andrade et al. (Reference Andrade, Costa and Pimenta2011).
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTIONS
Western Atlantic: Carolinian region, USA, eastern Caribbean, 538–805 m (Clench & Turner, Reference Clench, Turner and Clench1952); north-eastern Brazil, 240–610 m (Andrade et al., Reference Andrade, Costa and Pimenta2011; this study).
Opaliopsis arnaldoi new species
(Figure 14)
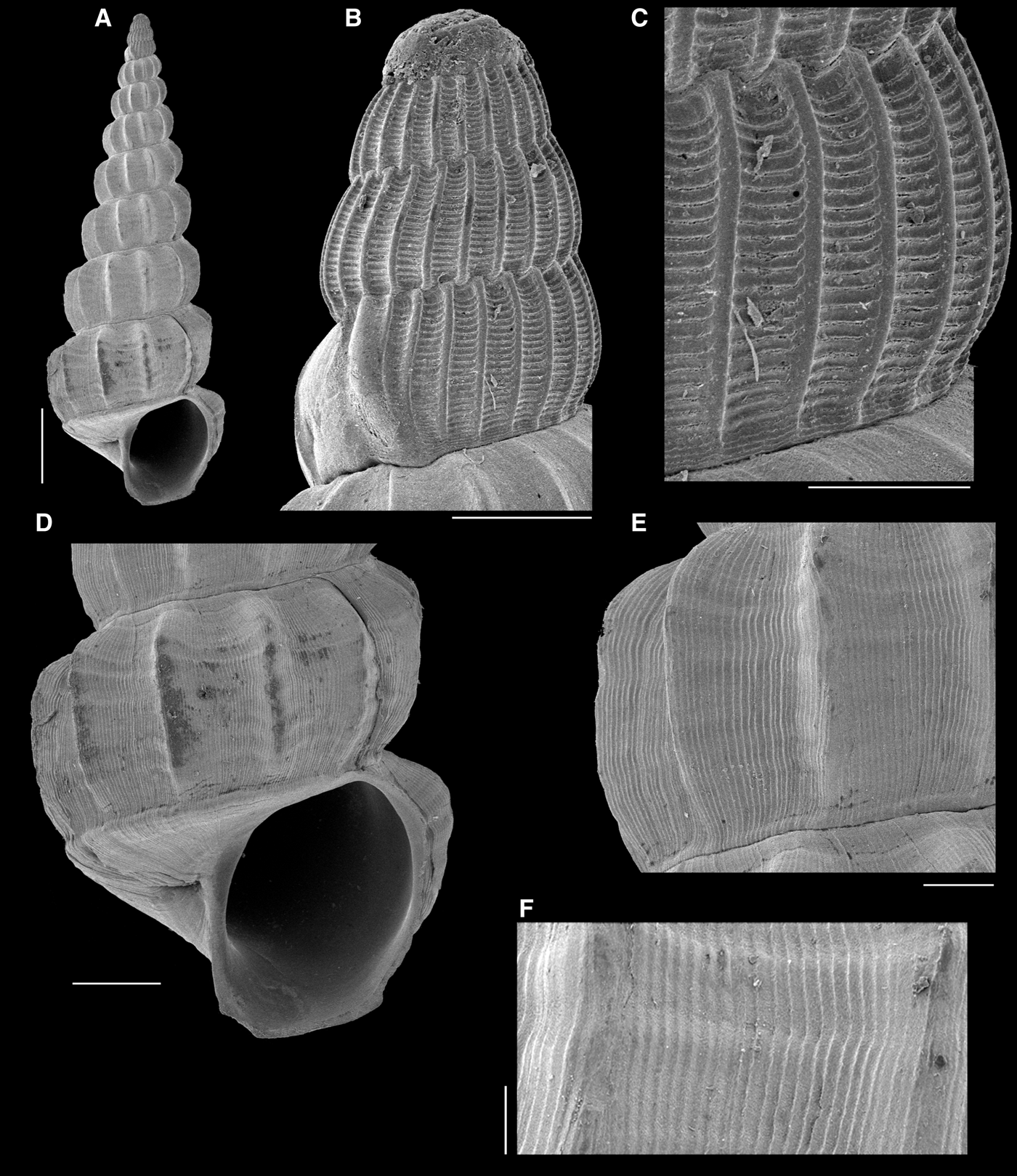
Fig. 14. Opaliopsis arnaldoi new species: Holotype. (A) whole shell; (B) protoconch; (C) detail of last whorl of protoconch; (D) last teleoconch whorl; (E, F) detail of sculpture on sixth teleoconch whorl. Scale bars: A, 1 mm; B, E, 200 µm; C, F, 100 µm; D, 500 µm.
Opaliopsis sp.: Miyaji (2002: 79).
TYPE MATERIAL
Holotype: MNRJ 18103; Paratypes: – Brazil: Campos Basin, off Rio de Janeiro state: (21°41′7″S 40°05′2″W, 701 m): MNRJ 17199 [3 shells]; OP I station 49: IBUFRJ 15096 [2 shells]; IBUFRJ 15074 [3 shells]; (22°22′37″S 41°06′28″W, 650 m): MNRJ 35871 [1 shell]; MNRJ 33071 [2 shells]; off São Paulo state: PADCT station 6573: MZSP 131838 [1 shell]; MNRJ 28193 [1 shell].
TYPE LOCALITY
South-western Atlantic, continental slope of Campos Basin, off Rio de Janeiro state, eastern Brazil, OP I station 59 (21°52′59.60″S 39°55′30.63″W, 750 m).
ADDITIONAL MATERIAL EXAMINED
– Brazil: Campos Basin, off Rio de Janeiro state: OP II station 49: MNRJ 33074 [1 shell]; IBUFRJ 17562 [7]; OP II station 45: IBUFRJ 10959 [1 shell]; OP I station 44: IBUFRJ 17224 [5 shells]; OP II station 74: IBUFRJ 17268 [2 shells]; OP I station 59: IBUFRJ 17086 [2 shells]; OP II station 44: IBUFRJ 20149 [3 shells]; off Rio de Janeiro city: PADCT station 6554 : MNRJ 28194 [1 shell].
ETYMOLOGY
Named after the late Professor Arnaldo Campos dos Santos Coelho, former curator of the Molluscan Collection and Director of Museu Nacional.
DIAGNOSIS
Shell with strong, shouldered axial ribs and numerous microscopic axial striae; spiral sculpture almost indistinct.
DESCRIPTION
Shell turriculate, up to 8.3 mm in length (protoconch missing), 3.05 mm in width, conical profile; protoconch amber in colouration, teleoconch white. Protoconch conical-subcylindrical, with four whorls, readily distinct of teleoconch, transition marked by a strong varix; embryonic whorl dome-shaped, smooth, remaining whorls with convex profile; axial sculpture with ~24 axial ribs projecting over suture; spiral sculpture of 24 fine, regularly spaced cords not covering ribs, except for a short region above suture. Teleoconch with up to eight strongly shouldered whorls; axial sculpture formed by ~12 regularly spaced ribs not touching suture; interspaces wide, ~2× rib width, sculptured by 6–7 very weak, almost imperceptible spiral cords; numerous microscopic, cover interspaces, also crossing over axial ribs. Varices absent. Suture impressed, bordered adapically and abapically by very thin spiral cord. Last whorl with rounded, smooth basal cord. Basal disc conspicuous, sculpture by growth lines. Aperture sub-ovate; inner lip smooth. Umbilicus absent.
Dimensions: holotype with eight teleoconch whorls; length: 6.4 mm; protoconch: 580 µm height, 360 µm width.
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
South-western Atlantic: eastern and south-eastern Brazil, 650–750 m.
REMARKS ON OPALIOPSIS
Opaliopsis includes nine species worldwide (Bouchet & Gofas, Reference Bouchet and Gofas2011), most from the Western Atlantic Ocean: O. canium (Dall, Reference Dall1927), O. cearensis Andrade, Costa & Pimenta, Reference Andrade, Costa and Pimenta2011, O. concava (Dall, Reference Dall1889), O. rabalaisi Garcia, 2005, O. opalina (Dall, Reference Dall1927) and O. atlantis (Clench & Turner, Reference Clench, Turner and Clench1952); this last one, being the only one also recorded from Eastern Atlantic (Bouchet & Warén, Reference Bouchet and Warén1986).
The species of Opaliopsis from Brazil were revised by Andrade et al. (Reference Andrade, Costa and Pimenta2011), who recognized three species (Figure 13A–D). A fourth species is described herein, from the Campos Basin, eastern Brazil.
Opaliopsis arnaldoi sp. nov. (Figure 14) is very similar to Opaliopsis cearensis (Figure 13A, B) in general shell shape, dimensions and overall ornamentation, so that the diagnosis presented for that species by Andrade et al. (Reference Andrade, Costa and Pimenta2011) would fit Opaliopsis arnaldoi as well. However, some subtle differences are present in the axial and spiral sculpture. In O. cearensis, the axial ribs are stronger and more regularly convex in outline (Figure 13A), and a varix is formed, while in O. arnaldoi, there is no varix formation and the whorls are strongly shouldered (Figure 14A, D). In O. arnaldoi, the spiral cords are weaker and less numerous, so that the microscopic parallel axial striae have some predominance (Figure 13E); compare the details of this feature in approximately the same shell region and scale in Figures 13B & 14F. In addition, the basal disc is smooth in O. arnaldoi (Figure 14D), similar to that of O. opalina, while in O. cearensis, a set of spiral furrows are present.
Genus Papuliscala de Boury, Reference de Boury1911
Papuliscala de Boury, Reference de Boury1911: 220. Type species (original designation): Acirsa praelonga Jeffreys, Reference Jeffreys1877.
Pustuliscala de Boury in Cossmann & Peyrot, Reference Cossmann and Peyrot1921: 124. Type species (original designation): Acirsa praelonga Jeffreys, Reference Jeffreys1877. – synonymized by Bouchet & Warén (Reference Bouchet and Warén1986).
DIAGNOSIS
Shell turriculate, teleoconch with several whorls of somewhat rounded outline; with or without carena. Teleoconch sculpture of usually equally strong axial and spiral cords, forming weak to strong nodules on intersections; with development of peripheral basal spiral cord, without umbilicus; basal area with weak to nearly obsolete sculpture. Protoconch paucispiral, with whorls somewhat globose, sculptured by few axial axial striae restricted to its terminal portion. Radula described by Bouchet & Warén (Reference Bouchet and Warén1986).
Papuliscala elongata (Watson, Reference Watson1881)
(Figure 15)
Fenella elongata Watson, Reference Watson1881: 249; Reference Watson1886: 621, pl. 34, fig. 4.
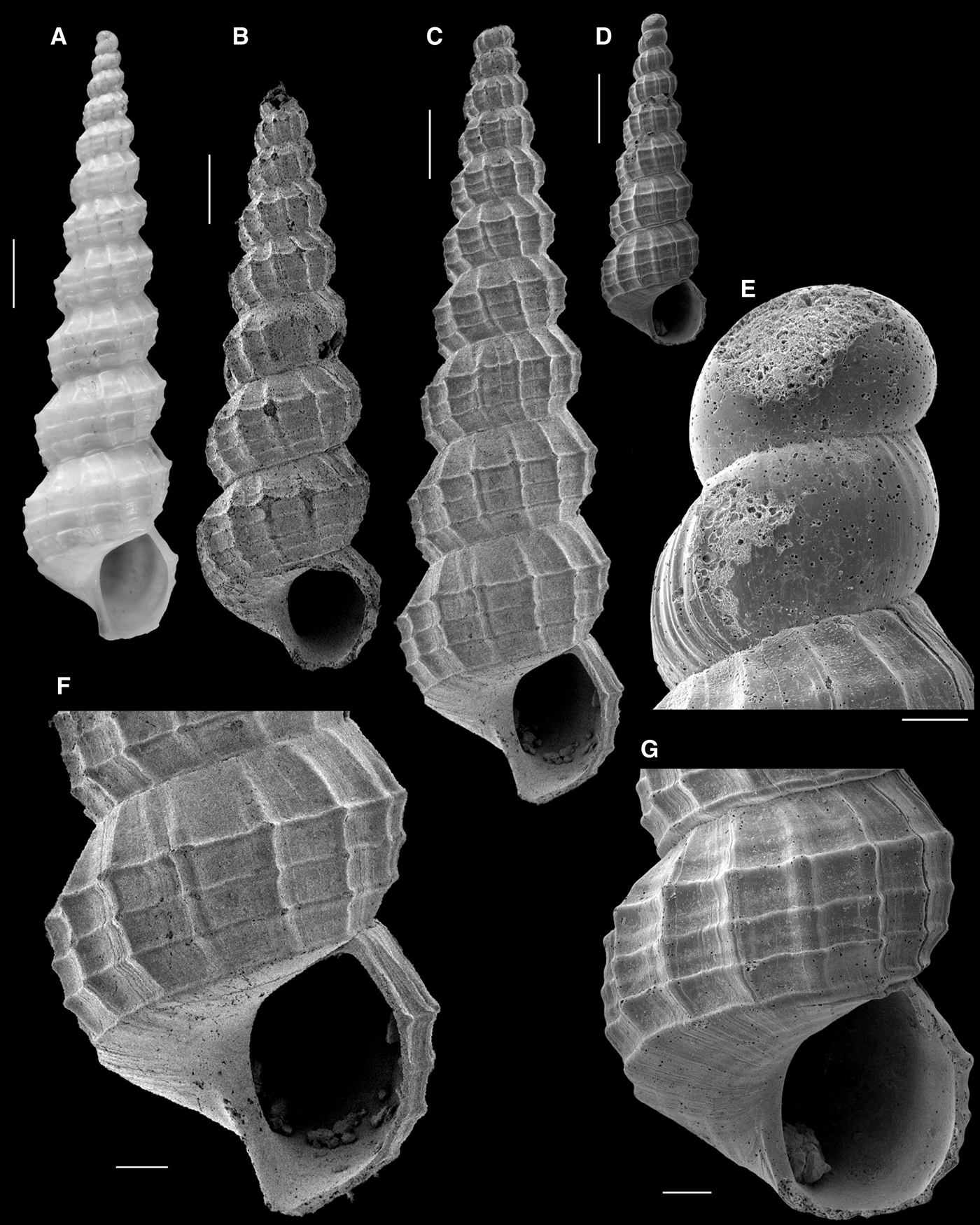
Fig. 15. Papuliscala elongata: (A) Lectotype; (B) Holotype of Papuliscala nordestina Lima & Christoffersen, Reference Lima and Christoffersen2013; (C, 82) MNRJ 35833; (D, E, G) IBUFRJ 15099. (A–D) whole shells (apex partially broken in B and C); (E) protoconch; (F, G) last whorl. Scale bars: A–D, 1 mm; E, 100 µm; F, G, 500 µm.
Papuliscala elongata: Bouchet & Warén (Reference Bouchet and Warén1986: 494, figs 1160–1161); Weil et al. (Reference Weil, Brown and Neville1999: 46 – not figured); Segres et al. (2009: 107, pl. 15, fig. 14); Brown & Neville (2016: 15 – not figured; annotated catalogue).
Papuliscala nordestina Lima & Christoffersen, Reference Lima and Christoffersen2013: 292, fig. 3A–C; Brown & Neville (2016: 17 – not figured; annotated catalogue). – New synonym.
TYPE MATERIAL
Lectotype selected by Bouchet & Warén (Reference Bouchet and Warén1986) (Figure 15A): NHMUK 1887.2.9.2057; paralectotype: NHMUK 1887.2.9.2058, type locality; NHMUK 1887.2.9.2056, Isla Culebra; Challenger #624 (38°59′N 028°18′W, 713 m), Puerto Rico.
Holotype of Papuliscala nordestina (Figure 15B): IBUFRJ 18827, Sergipe-Alagoas Basin (10°06′35″S 35°46′41″W, 720 m), north-eastern Brazil.
TYPE LOCALITY
North-eastern Atlantic: São Miguel, Azores; Challenger #78 (37°26′N 25°13′W, 1830 m).
MATERIAL EXAMINED
Holotype of Papuliscala nordestina and: – Brazil: Rio Grande do Norte state: (04°34′50″S 36°49′77″W, 460 m): MNRJ 35833 [1 shell]; Espírito Santo: off Rio Docés fall, MD 55 station CB93, MNHN [1 shell]; Rio de Janeiro, Campos Basin: OP I station 49: IBUFRJ 15099 [1 shell].
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
North-eastern Atlantic: south-western Europe, Madeira and Azores, 1689–2120 m (Bouchet & Warén, Reference Bouchet and Warén1986; Segers et al., Reference Segers, Swinnen and de Prins2009); South-western Atlantic: north-eastern and eastern Brazil, 460–750 m (Lima & Christoffersen, Reference Lima and Christoffersen2013; this study).
REMARKS
Papuliscala comprises, alongside with Murdochella Finaly, 1926, the two genera of Nystiellidae that have lost the planktotrophic larval stage (Bouchet & Warén, Reference Bouchet and Warén1986), and thus the protoconch lacks the typical strong sculpturing characteristic of the family.
Papuliscala includes eight deep-water species (Bouchet & Gofas, Reference Bouchet and Gofas2010). Besides P. nordestina Lima & Christoffersen, Reference Lima and Christoffersen2013, discussed in detail below, the genus includes five species from the Atlantic Ocean: P. cerithielloides Bouchet & Warén, Reference Bouchet and Warén1986, from Portugal; P. diminuta Castellanos, Rolán & Bartolotta, 1987, from Argentina; P. elongata (Watson, Reference Watson1881), from Madeira and Azores; P. praelonga (Jeffreys, Reference Jeffreys1877), from north-west Atlantic; P. taviani Bouchet & Warén, Reference Bouchet and Warén1986, from Canary Islands.
The first record of Papuliscala from Brazil was published by Lima & Christoffersen (Reference Lima and Christoffersen2013) based on a new species, P. nordestina, which was described based on a single worn shell with a broken apex (Figure 15B), with no sign of a protoconch and with a somewhat eroded teleoconch sculpture.
The additional shells from Brazil recorded in the present study, from both the north-east and eastern coasts of Brazil (Figure 15C, D), allowed a better interpretation of the intraspecific variation in the teleoconch sculpture, which led to the conclusion that P. nordestina is a junior synonym of P. elongata.
Lima & Christoffersen (Reference Lima and Christoffersen2013) recognized that P. nordestina was ‘strongly correlated with P. elongata’. However, the differences on which they relied to distinguish the two species are interpreted herein as artifacts of the eroded state of the holotype of P. nordestina, or of intraspecific variation. The shape of the aperture cannot be evaluated since it is considerably broken in the holotype of P. nordestina; the non-spiny periphery of the whorls is clearly due to the much-eroded sculpture; and the more widely flattened subsutural region is a feature which cannot be evaluated objectively. Examination of a new record from the north-eastern coast (Figure 15C) revealed a better-preserved shell, in which the above features agree with those of P. elongata.
The only discernible difference was the number of spiral cords per whorl (four to five in P. nordestina vs three in P. elongata). The new records from Brazil revealed that some variation is present in this feature, since the shells in Figure 15C and D have the typical three strong spiral cords, but also some intercalated weak cords. Thus, we consider both the number of spiral cords and their strength are variable features.
The protoconch is paucispiral (Figure 15E), as in the holotype of P. elongata (Figure 15A), and the shell has the characters of the genus Papuliscala as defined by Bouchet & Warén (Reference Bouchet and Warén1986).
Although the type series of P. elongata includes specimens from the Azores (Challenger Station 78) and Puerto Rico (Challenger Station 24), Bouchet & Warén (Reference Bouchet and Warén1986) showed that the type from Puerto Rico (NHMUK 1887.2.9.2056) is a shell of Papuliscala praelonga, so they restricted the distribution of P. elongata to the eastern Atlantic. The recognition herein of the synonymy between P. elongata and P. nordestina confirms the bathyal amphi-Atlantic distribution of P. elongata.
DISCUSSION
Of the 46 valid species of Nystiellidae worldwide (Bouchet, Reference Bouchet2010, and including the present findings), 30 species occur in the Atlantic Ocean, some of them with Mediterranean records. The Lower Bathyal Province of the North Atlantic includes 23 species, while the South Atlantic Province includes 18, with 11 species widely distributed in both provinces. The remaining species occur in the New Zealand–Kermadec Province (five species), West Pacific (five species), and Indian Ocean (five species), and only one in the Antarctic Province. Despite recent advances in molluscan surveys in the Indo-Pacific (Bouchet et al., Reference Bouchet, Bary, Héros, Marani, Héros, Strong and Bouchet2016), which led to the discovery of several new species from that region in different families, the Nystiellidae have not yet been acknowledged.
So far, the Atlantic Ocean is the richest area for Nystiellidae in the world (pending better surveys off the coast of Africa) and the fauna from the south-western Atlantic (mainly Brazil) shows a close affinity to that from the North Atlantic (Figures 16–18). Six species from the south-western Atlantic are also present in the Caribbean/Gulf of Mexico region, and eight species co-occur in the south-western Atlantic and the north-eastern Atlantic/Mediterranean region. While only four species (N. raquelae, O. cearensis, O. arnaldoi and E. maracatu) are endemic to the Brazilian continental slope, E. nitida, I. cancellatus, I. robertsi and O. atlantis are widely distributed, being present in the North Atlantic, from Europe to the Gulf of Mexico, and off southern Brazil, whereas E. nitida has been recorded from Japan as well.

Fig. 16. Records of the studied species of Eccliseogyra in the Atlantic Ocean based on the material examined and literature records. White symbol indicates type locality of each species. Record of E. nitida from Pacific not shown.

Fig. 17. Records of Iphitus and of the studied species of Opaliopsis in the Atlantic Ocean based on the material examined and literature records. White symbol indicates type locality of each species.

Fig. 18. Records of Narrimania and of the studied species of Papuliscala in the Atlantic Ocean based on the material examined and literature records. White symbol indicates type locality of each species.
Prior to the present study, Lima & Christoffersen (Reference Lima and Christoffersen2013) listed 14 nystiellid taxa from the South Atlantic, some with dubious or imprecise taxonomic determinations (cf. or sp.). Lima & Christoffersen (Reference Lima and Christoffersen2013) stated that ‘the scarcity of records of nystiellids from the deep-sea of the South Atlantic is most probably due to the small sampling effort in the region’. In the present study, several samples from recent (last 20–30 years) oceanographic campaigns in different localities along the Brazilian continental slope were sorted, searching for nystiellids. This effort resulted in the recognition of 16 species from Brazil. In addition to these, Iphitus notios sp. nov. and Papuliscala diminuta are so far known only from their type localities, the Rio Grande Rise and the Gulf of San José (Argentina), respectively.
The present account confirms the primary depth range of Nystiellidae from the deep sea. While Bouchet & Warén (Reference Bouchet and Warén1986) recorded Nystiellidae from the north-eastern Atlantic at depths from 130 m to 5867 m, in the south-western Atlantic the specimens ranged from around 155 m to 2200 m, although collections from the abyssal plain are still sparse. In general, the depth range of each species from the South Atlantic agrees with (or is within the range of) those recorded from the North Atlantic. For example, the species recorded from the shallowest depths in the North Atlantic, Iphitus robertsi (184 m in the Gulf of Mexico and 90 m in the Canary Islands), is also a shelf species in the two localities where it was collected in Brazil (160 m off the northern coast; 165 m off the south-eastern coast). A striking similarity in depth range is also present in Narrimania concinna, from the outer shelf to the upper bathyal (130 m to 695 m in the Mediterranean, 206 m to 755 m in Brazil). The exceptions, in which the occurrences in the South Atlantic are in shallower depths than in the North Atlantic, are E. monnioti, E. nitida and P. elongata.
In Eccliseogyra, which seems to inhabit a wide depth range in the north-east Atlantic, from the lower bathyal (~450 m) to truly abyssal depths (~5800 m), the deepest record from the South Atlantic is around 2400 m, probably owing to the scarcity of sampling at greater (i.e. abyssal depths).
ACKNOWLEDGEMENTS
We are grateful to P. Bouchet and P. Maestrati (MNHN), L.R.L. Simone (MZSP), E.C. Rios (in memoriam) and P.S. Oliveira (MORG), J.C.N. Barros (UFRPE) and P.M.S. Costa (FIPERJ; MNRJ), C. Miyaji (Unimonte), for loan or donation of material; C. Walter, E. Strong, J. Harasewych (USNM), V. Héros (MNHN), A. Bonfitto (MZB, University of Bologna) and A. Salvador (NHMUK) facilitated loans of photos of types and helped with other specimen related matters; Dr S.F.B. Lima (UFCG/CFP/UACEN), for discussions about Nystiellidae taxonomy and critically reviewing the manuscript. C. Simões and B. Cordeiro, for operating the SEM at the Departamento de Invertebrados (MNRJ); Mr A. Schneider, for allowing the use of the maps build with GPS Visualizer; Dr J. Reid revised the English text; the two anonymous referees, for their important criticism and suggestions that improved the text; Petrobras SA, for making possible the collection and study of material from the Oceanprof Project; Serviço Geológico do Brasil (CPRM), Grupo Operacional do Programa da Prospecção e Exploração de Recursos da Área Internacional do Atlântico Sul e Equatorial (GO - PROAREA), Grupo de Trabalho de Biologia Marinha, for making possible the collection and study of the material from Rio Grande Rise.
FINANCIAL SUPPORT
FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) provided financial support through project E-26/110.325/2014 and project E-26/110.068/2014.




















