Introduction
Acute tonsillopharyngitis, characterised by inflammation of the oropharyngeal cavity and surrounding lymphoid tissue, is one of the most common diseases encountered by general practitioners and otorhinolaryngologists.Reference Melio, Holmes, Rosen and Barkin1 Tonsillopharyngitis is caused by Streptococcus pyogenes, a highly virulent, group A β-haemolytic streptococcus, in 5–30 per cent of patients.Reference Shores, Tintinalli, Kelen and Stapczynski2 Accurate detection of the causative organism is useful in order to plan correct treatment (avoiding unnecessary wide-spectrum antibiotics) and to prevent complications. Although throat swab culture is the ‘gold standard’ for tonsillopharyngitis diagnosis, it takes 24–48 hours to obtain the result. Rapid antigen detection is a quick and reliable alternative which can be used for the diagnosis of group A streptococcal tonsillopharyngitis.
In cases of recurrent sore throat associated with group A β-haemolytic streptococcus, a 10-day course of antibiotics may reduce the number and frequency of attacks.3 However, treatment of a sore throat with antibiotics has only a modest beneficial effect on clinical signs, including fever.Reference Del Mar, Glasziou and Spinks4, 5 Numerous pharmaceutical preparations are available, containing disinfectants, anti-inflammatory agents and/or topical anaesthetics, which may potentially provide symptomatic relief.3
The present study aimed to investigate the effect of a mouth spray containing chlorhexidine gluconate and benzydamine hydrochloride, in comparison with placebo, on the intensity of clinical signs and the quality of life of patients with streptococcal tonsillopharyngitis, using a prospective, randomised, double-blinded, placebo-controlled trial.
Materials and methods
Study design
This study was designed as a multicentre, prospective, randomised, double-blinded, placebo-controlled, parallel-group clinical trial. The study was undertaken between May and October 2009.
The study protocol was approved by the relevant ethics committee, and a completed written consent form was obtained from each patient.
The study was conducted in the ENT clinics of three university hospitals and one tertiary hospital.
Rapid antigen detection test
The rapid antigen detection test (‘rapid strep test’) is a quick and accurate diagnostic tool used to determine the presence of streptococcal bacteria. Results can be obtained within minutes to hours. In our study, a sample of throat mucus (obtained from the surface of the palatine tonsils using a swab) was tested for group A streptococcal bacteria using the QuickVue Dipstick Test (Quidel, San Diego, California, USA).
Study protocol
We enrolled in the study patients of both genders who were admitted with clinical signs of acute tonsillopharyngitis and who had a positive streptococcal A antigen test result. The study's inclusion and exclusion criteria are shown in Table I.
Table I Inclusion and exclusion criteria
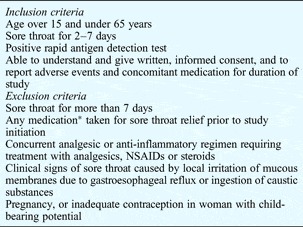
*Including herbal medicine or dietary supplements. NSAIDs = non-steroidal anti-inflammatory drugs
One hundred and seventy-one patients, encountered at four study centres, were randomised in a double-blinded manner to receive one of two parallel treatments: penicillin V plus active drug mouth spray, or penicillin V plus placebo mouth spray. The active drug mouth spray contained chlorhexidine gluconate 0.12 per cent and benzydamine hydrochloride 0.15 per cent; the placebo mouth spray contained no active ingredients. Patients used their mouth spray four times per day, and were instructed to avoid taking any other medications for the relief of sore throat.
Treatment randomisation was generated by the study coordinator (CC, the senior author), using a numbered list of patients, prior to study commencement. Numbered boxes containing either treatment or placebo were sent to the clinical investigators. Each box's number was noted on the appropriate patient's questionnaire. The clinical investigators at the four study centres were thus blinded to whether individual patients had received mouth spray containing active treatment or placebo. Two copies of the randomisation codes determining each patient's treatment were stored in two sets of sealed envelopes. One set of envelopes was kept by the study coordinator, while the other set was distributed amongst the study centres in case of emergency (i.e. in the event that knowledge of the patient's actual treatment were to become medically necessary). Revealing the randomisation code for one patient would not require other patients' codes to be revealed. In this way, all investigators and patients were blinded to treatment assignation throughout the course of the study.
Outcome measures
Intensity of clinical signs
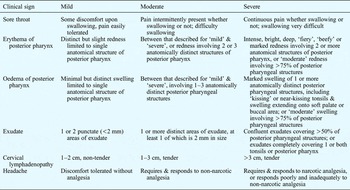
Investigators assessed the intensity of patients' clinical signs prior to treatment and on the seventh day of treatment. The assessed clinical signs comprised included sore throat, erythema and oedema of the posterior pharynx, exudate, cervical lymphadenopathy, and headache. These were graded on a four-point scale (with 0 = absent, 1 = mild, 2 = moderate and 3 = severe), and an overall clinical sign score was generated. Investigators were provided with reference guidelines to aid their classification of clinical sign intensity (see Appendix 1).
Subjective health state
Patients were asked to subjectively assess their health state using a 10 cm visual analogue scale (VAS) graded from zero (representing ‘the best imaginable health state’) to 10 (‘the worst imaginable health state’).Reference Wewers and Lowe6 Patients completed a VAS before treatment and on the third and seventh days of treatment. This information was used as a quantitative measure of patients' individual, subjective health outcome.
Quality of life
Patients completed the Short Form 36 Health Questionnaire before treatment and on the seventh day of treatment. This questionnaire (comprising a short form with 36 questions) is a well documented, self-administered, simple, standardised quality-of-life scoring system which has been widely used and validated.Reference Diaz-Buxo, Lowrie, Lew, Zhang and Lazarus7, Reference McHorney, Ware and Raczek8 The questionnaire contains eight independent scales summarised into two ‘dimensions’: the physical health dimension and the mental health dimension. Each dimension includes three specific and two overlapping scales. The questionnaire also includes a question on self-evaluated change in health during the past year (‘reported health’); this question is scored independently. Scores for the two dimensions, and the total score, are based on mathematical averaging of scale component scores.
Side effects
Patients used a customised questionnaire to assess any side effects of treatment. This questionnaire was completed on the third and seventh days of treatment. A four-point Likert scale was used to assess local side effects (e.g. taste disturbance, oral mucosal numbness, oral burning sensation, dry mouth or thirst, and tooth discolouration) and systemic side effects (e.g. nausea, vomiting, dyspepsia, vertigo and headache), with 0 = none, 1 = mild, 2 = moderate and 3 = severe.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences version 17.0 for Windows (SPSS Inc, Chicago, Illinois, USA). Shapiro–Wilk's test was used to test the normality assumption. Paired samples were analysed using Student's t-test, while repeated measures were analysed using one-way analysis of variance. Values were expressed as mean ± standard deviation. Statistical significance was accepted for p values less than 0.05.
The distribution forms for the data were determined using Shapiro–Wilk's test; all data were found to fit a normal or Gaussian distribution. In our study, the power of parametric testing was found to be greater than that of nonparametric testing. When performed with a value of α = 0.050, the testing power was found to be >0.80; this level of power was considered sufficient.
Results
We evaluated a total of 171 patients selected according to the study inclusion criteria. Sixteen patients did not complete the trial, and eight were excluded in the follow-up period. Thus, 147 patients remained enrolled in the study: 72 females (mean age 38.92 ± 14.38 years) and 75 males (mean age 36.73 ± 17.61 years). All patients used their medication as directed, and none took additional medication. Following study termination, 72 patients were found to have been assigned to the penicillin V plus treatment group, while 75 had been assigned to the penicillin V plus placebo group. The two groups had similar age and sex distributions (p = 0.413 and p = 0.367, respectively).
Intensity of clinical signs
Prior to treatment, there was no significant difference in patients' clinical sign intensity scores, comparing the treatment and control groups (p = 0.403).
At the end of the treatment period, however, there was a significant difference in clinical sign intensity scores between these two groups (p < 0.001) (Table II). Clinical sign scores significantly reduced over time in both the treatment and control groups (p < 0.001 for both). However, this reduction was significantly greater in the treatment group than in the control group (p < 0.001; Figure 1).

Fig. 1 Clinical sign intensity scores for treatment and control groups, pre- and post-treatment.
Table II Clinical sign intensity scores pre- and post-treatment

Data represent means ± standard deviations.
Subjective health state
Before treatment, there was no significant difference in patients' VAS scores for subjective health state, comparing the treatment and control groups (p = 0.928).
However, following treatment initiation, a significant difference in VAS scores was seen between the treatment and controls groups, on both the third and seventh days of treatment (p < 0.001 and p < 0.001, respectively) (Table III, Figure 2).

Fig. 2 VAS scores for subjective health state, for treatment and control groups, before and during treatment.
Table III Vas scores for subjective health state pre- and post-treatment

Data represent means ± standard deviations. VAS = visual analogue scale
Quality of life
Analysis of Short Form 36 Health Questionnaire quality-of-life scores on the seventh day of treatment revealed no statistically significant difference between treatment and control groups (p > 0.05; Table IV, Figure 3). A significant improvement in quality-of-life scores was seen over time in both the treatment and control groups (p < 0.001 for both).

Fig. 3 Short Form 36 Health Questionnaire (SF36) scores for quality of life, for treatment and control groups, pre- and post-treatment.
Table IV SF36 Qol scores pre- and post-treatment

Data represent means ± standard deviations. SF36 = Short Form 36 Health Questionnaire; QoL = quality of life
Side effects
Analysis of side effects in the treatment and control groups revealed a statistically significant difference on the third day of treatment (p = 0.004) but no significant difference on the seventh day of treatment (p = 0.937) (Figure 4). Mild taste disturbance and mild to moderate oral mucosal numbness were the most frequent side effects, being reported by 28 of the 72 patients in the treatment group.

Fig. 4 Side effect scores for treatment and control groups on the third and seventh days of treatment.
Sixteen patients did not complete the trial, and eight patients were excluded from the study in the follow-up period. A telephone survey of the 16 patients not completing the trial revealed various reasons for noncompletion: 14 stated that they could not get time off work to attend their follow-up appointment, while two stated that they were too ill to continue with the trial. Eight patients were excluded from the study in the follow-up period due to other reasons.
Discussion
The aetiology of exudative pharyngitis is usually infectious: 40–60 per cent of cases are of viral origin and 5–40 per cent of bacterial origin.3, Reference Wei, Kasperbauer, Weaver and Boggust9 However, the combination of symptoms and clinical signs is usually inadequate to differentiate with certainty between viral and bacterial aetiology. For this reason, several different authorities (including the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the American Heart Association, the American Academy of Pediatrics, and the Infectious Diseases Society of America) have recommended that a diagnosis of streptococcal sore throat, in patients suspected for clinical and epidemiological reasons, should be confirmed by microbiological testing.Reference Bisno, Gerber, Gwaltney, Kaplan and Schwartz10–Reference Spadetto, Camara, Ingles, Escuriet, Barcelo and Sanchez14
Although throat swab culture is the ‘gold standard’ for tonsillopharyngitis diagnosis, results are not available for 24–48 hours. An alternative is supplied by the rapid antigen detection test, a quick and reliable test used in the diagnosis of group A streptococcal tonsillopharyngitis. Spadetto et al. assessed the reliability and validity of a rapid test for the identification of S pyogenes in pharyngeal exudate (a test similar to that used in the present study), in 430 patients presenting with tonsillopharyngitis; the test sensitivity was 91.2 per cent (negative predictive value = 96.5 per cent) and the test specificity 96.2 per cent (positive predictive value = 90.4 per cent).Reference Spadetto, Camara, Ingles, Escuriet, Barcelo and Sanchez14 In our study, the rapid antigen detection test was used to diagnose patients with group A β-haemolytic streptococcal pharyngitis.
In cases of tonsillopharyngitis associated with group A β-haemolytic streptococcus, the limited evidence available suggests that a 10-day antibiotic course may reduce the number and frequency of attacks.3 Once group A streptococcal tonsillopharyngitis is diagnosed, either by throat culture or rapid testing, penicillin V is the first choice of treatment. The efficacy of this antibiotic has been demonstrated by many clinical studies. A 10-day penicillin course (given twice daily) is as efficacious as more frequent dosing regimens in the treatment of streptococcal tonsillopharyngitis.Reference Efstratiou15 Hence, our study used penicillin V to treat patients with group A β-haemolytic streptococcal pharyngitis. However, while penicillin V is generally effective in the eradication of group A β-haemolytic streptococcus, it is not effective in reducing the associated pain. Thus, the main objectives of supportive treatment are to reduce tonsillopharyngeal inflammation and thus reduce pain, while also improving oral intake to avoid dehydration (and possible hospitalisation).Reference Wei, Kasperbauer, Weaver and Boggust9
At present, numerous pharmaceutical preparations (containing disinfectants, anti-inflammatory agents and/or topical anaesthetics) have been approved for the local treatment of acute pharyngitis.3 Our study assessed the effect of a mouth spray containing chlorhexidine gluconate and benzydamine hydrochloride.Reference Matthijs and Adriaens16 Benzydamine hydrochloride is an effective anti-inflammatory agent, while chlorhexidine gluconate is an antimicrobial agent frequently used for topical antiseptic effects. The effectiveness of chlorhexidine against gingivitis and recurrent oral mucosa ulceration has been shown in many studies.Reference Killoy17, Reference Burgess, Johnson and Sommers18 Chlorhexidine is effective against a wide variety of bacteria, including Gram-positives, Gram-negatives, aerobes and anaerobes.Reference Emilson19 Chlorhexidine is effective against bacteria commonly found in the oral cavity, and against organisms associated with oral cavity disease.Reference Hennessey20–Reference Hesselgren, Dahl and Larje22 In addition to its in vitro antibacterial activity, studies have shown that chlorhexidine mouth rinse reduces the number of oral bacteria.Reference Briner, Grossman, Buckner, Rebitski, Sox and Setser23, Reference Ferretti, Ash, Brown, Largent, Kaplan and Lillich24
The present prospective, randomised, double-blinded, placebo-controlled study investigated the effect of chlorhexidine gluconate and benzydamine hydrochloride mouth spray, compared with placebo. The active treatment mouth spray was found to significantly reduce the intensity of clinical signs, without any evident side effects. The active treatment mouth spray was also associated with improved quality of life over time, compared with placebo, although this improvement was not statistically significant.
Chlorhexidine gluconate is reported to have infrequent side effects, including tooth discolouration, taste disturbance and oral mucosa desquamation.Reference Field and Allan25 In our treatment group, those patients who reported side effects noted some taste disturbance or oral mucosa numbness for a few days following treatment. In our control group, no statistically significant side effects were observed on either the third or seventh day. The chlorhexidine–benzydamine mouth spray was generally very well tolerated, at the dosage administered, and no serious adverse events were observed.
• Chlorhexidine gluconate and benzydamine hydrochloride mouth spray, added to standard antibiotic treatment, significantly alleviates the intensity of clinical signs in patients with streptococcal pharyngitis, compared with placebo
• Further research is necessary to fully assess the efficacy of this treatment in combating group A streptococcal tonsillopharyngitis
It is possible that chlorhexidine–benzydamine mouth spray may be appropriate for patients who are unable to tolerate nonsteroidal anti-inflammatory drugs or steroids. Although this question was not addressed by the present study, chlorhexidine–benzydamine mouth spray may represent a safer and less costly form of analgesia for such patients, compared with current treatment options.
The present study had certain limitations. The power of the study was limited by its short duration (six months) and modest number of participants. Further research may be necessary to fully assess the efficacy of chlorhexidine–benzydamine mouth spray in treating group A streptococcal tonsillopharyngitis, using larger sample sizes or alternative control groups.
Conclusion
Chlorhexidine gluconate and benzydamine hydrochloride mouth spray, used in conjunction with standard antibiotic treatment, significantly decreases the intensity of clinical signs in patients with streptococcal pharyngitis.
Appendix I Guidelines for assessing the intensity of clinical signs of group a β-haemolytic streptococcal tonsillopharyngitis