Introduction
Numerous detailed reviews, summarized below, on the biosynthesis of starch granules have been produced in the past decade (Buleon et al., Reference Buleon, Colonna, Planchot and Ball1998; Baldwin, Reference Baldwin2001, Donald et al., Reference Donald, Kato, Perry and Waigh2001; Shewry and Morell, Reference Shewry and Morell2001; Smith, Reference Smith2001; James et al., Reference James, Denyer and Myers2003 ; Tomlinson and Denyer, Reference Tomlinson and Denyer2003; Lindeboom et al., Reference Lindeboom, Chang and Tyler2004; Tester et al., Reference Tester, Karkalas and Qi2004; Svihus et al., Reference Svihus, Uhlen and Harstad2005). Cereal starch granules consist of linear and branched glucose polymers, called amylose and amylopectin, respectively, which are found in semicrystalline, water-insoluble lamellae laid down within each starch granule. The semicrystalline starch alternates with amorphous concentric growth rings which initiate at the hilum. The size and shape of the starch granules vary between and within species. There is much discussion and speculation regarding the factors responsible for starch granule differentiation, including multiple and complex genetic controls, plastid size and number, environmental conditions at seed set, complex biochemical cascades, the availability of malto-oligosaccharides or an integrated combination of all these factors.
Variation in starch granule morphology is associated with its biological origin. For example the Oryzeae and oats have distinctive compound granules. During endosperm development starch granules are formed in plastids called amyloplasts. Where multiple granules develop within a single amyloplast, the resultant granules are smaller and at maturity tend to be polyhedral in shape due to the pressure of surrounding granules. These polyhedral granules are compressed together to form compound granules which, at low magnification, have the appearance of a single granule. In contrast, the large, ‘simple’ granules typical of the Panicoideae form in individual amyloplasts. The Triticeae share a unique type of bimodal endosperm with large lenticellular A-type granules (16–26 μm diameter) in conjunction with small spherical or ovoid granules (2–10 μm diameter). This is because the A-type granules develop in the body of the amyloplasts, while the B-type granules develop within evaginations of the same cell and remain independent, without the final appearance of a compound granule (Rahman et al., Reference Rahman, Li, Batey, Cochrane, Appels and Morell2000). Recent studies on potato starch synthesis have found a direct correlation between size of the amyloplast and the size of the starch granule produced (de Pater et al., Reference de Pater, Caspers, Kottenhagen, Meima, ter Stege and de Vetten2006) which led the authors to hypothesize that endosperm morphology could be manipulated by altering the number of amyloplasts formed as well as their size. As was eloquently understated by de Pater et al. (Reference de Pater, Caspers, Kottenhagen, Meima, ter Stege and de Vetten2006), ‘It would be an advantage if starches with altered granule size distributions could be tailored in planta’.
Pores or pinholes on the surface of starch granules have been described since the 1970s (Hall and Sayre, Reference Hall and Sayre1970; Fannon and BeMiller, Reference Fannon and BeMiller1992; Sandhu et al., Reference Sandhu, Singh and Kaur2004) and have been shown to vary within and amongst individuals and species (Fannon et al., Reference Fannon, Gray, Gunawan, Huber and BeMiller2004). It has been demonstrated that sorghum, maize and millet specimens which have pores or channels on starch granules exhibit increased rates of enzymatic granule digestion (Fannon et al., Reference Fannon, Gray, Gunawan, Huber and BeMiller2004; Benmoussa et al., Reference Benmoussa, Suhendra, Aboubacar and Hamaker2006) because the channels connect the granules' outer surface to internal cavities (Huber and BeMiller, Reference Huber and BeMiller2001) and hence provide a pathway for enzymatic digestion from within the granules, starting at the hilum and moving out through the matrix (Fannon et al., Reference Fannon, Gray, Gunawan, Huber and BeMiller2004). The occurrence of these features is especially important in cereals such as sorghum which has an inherently poor digestibility which limits its end uses.
Granule morphology has been shown to have an important impact on the starch physiochemical properties (Da Silva et al., Reference Da Silva, Oliveira and Rao1997; Lindeboom et al., Reference Lindeboom, Chang and Tyler2004) and most of the potential food and industrial applications of starch are mediated by the starch granule size ( Ji et al., Reference Ji, Oomen, Vincken, Bolam, Gilbert, Suurs and Visser2004). Studies of different wheat granule types indicated that large granules have an increased enthalpy of gelatinization, increased retrogradation, lower gelatinization temperatures (Peng et al., Reference Peng, Gao, Abdel-Aal, Huel and Chibbar1999; Singh and Kaur, Reference Singh and Kaur2004) and softer textured flours than smaller granules (Gaines et al., Reference Gaines, Raeker, Tilley, Finney, Wilson, Betchel, Martin, Seib, Lookhart and Donelson2000). Varieties of maize with small starch granules also had the lowest swelling power, solubility, amylose content and retrogradation (Sandhu et al., Reference Sandhu, Singh and Kaur2004). A study comparing waxy sorghum, waxy millet and amaranth, a very low amylose species, suggested that the smaller starch granule size of amaranth was associated with its slower retrogradation (Choi et al., Reference Choi, Kim and Shin2004). Small granule size, low amylose and continuity of shape of the starch granules made amaranth starch ideal for applications such as soups and thickeners, and also identified it as having excellent potential as a fat replacer due to its creamy texture. Outside of the food industry, granule morphology affects starches' utility for applications such as cosmetics, binders in pharmaceuticals, paper, photographics and plastics (Lindeboom et al., Reference Lindeboom, Chang and Tyler2004).
In order to determine the extent to which starch granule morphology and/or protein distribution differed from that observed in cultivated cereals, this study utilized scanning electron microscopy to examine the endosperm of 19 cereal wild relatives, including three taxonomically diverse members of the Poaceae. Five of the species exhibited rice-like endosperm morphology, while the sorghum and wheat wild relatives were observed to have simple and bimodal granules, respectively. In general, the wild species retained a greater proportion of the endosperm cell wall at maturity than cultivated cereals. The wild sorghum relatives exhibited an abundance of endosperm variations due to variation in starch granule size, shape and surface morphology, and the distribution of protein bodies.
Methods and materials
Plant material
Grains from 19 Australian native grasses (Table 1) were examined. Criteria for selection included adaptation to a broad range of environments, an ability to produce high grain yield, large grain size and close taxonomic relationship to cultivated cereal species. Native Sorghum and Oryza grain was accessed through the Australian Tropical Crops and Forages Collection, Queensland Department of Primary Industries and Fisheries, Biloela, Australia (www.dpi.gov.au/auspgris/). Elymus and Austrostipa species were sourced from Native Seeds Pty Ltd Australia (http://www.nativeseeds.com.au). Astrebla and Microlaena species were acquired through field collection in northern NSW.
Table 1 Table of accession details for specimen identification and collection records of 19 Australian wild crop relatives

Plant collection records prefixed with AC04 are lodged with the Australian Plant DNA Bank, http://www.dnabank.com.au and records prefixed with AusTRCF are lodged with the Australian Plant Genetic Resource Information Service, www.dpi.qld.gov.au/auspgris/.n/a, Not applicable.
Microscopy
Observations were made on 2–7 specimens from each species. Grains were manually dehusked prior to being fixed overnight in 10% formalin. Each sample was then washed with distilled water for 15 min followed by a standard dehydration process of 25%, 50%, 75%, 95% ethanol, each for 20 min. The samples were then washed three times in 100% ethanol for 30 min and immediately dried using a Polaron E3100 critical-point drier.
Once dried, grains were scored along one side with a shallow scalpel line and snapped in half transversely between two pairs of forceps. Specimens were mounted on aluminium stubs. Specimens were then gold coated for 30 s at 35 ma. Images were recorded on a Leo440Stereoscan scanning electron microscope with the probe current of 100 pA, accelerated voltage (EHT) at 10 kV and a working distance of 15 mm.
Results and discussion
There were no clear observable differences between the two wild rices in this study (Fig. 1) and domesticated rice, as described by multiple studies (Hoover et al., Reference Hoover, Sailaja and Sosulski1996; Dang and Copeland, Reference Dang and Copeland2004; Wang and Wang, Reference Wang and Wang2004). Starch granules were rigidly polyhedral, evenly sized at between 1.5 and 3 μm for Oryza australiensis and 2 and 5 μm for Oryza rufipogon, which is similar to the 3–5 μm reported for O. sativa. Starch granules were organized in compound granules with few protein bodies and little protein matrix between them. A similar comparison of an American wild rice to long-grain cultivated O. sativa found no observable differences in the starch granule morphology, and further chemical composition analysis of the endosperm indicated that both had a very low protein and lipid content (Hoover et al., Reference Hoover, Sailaja and Sosulski1996). Hoover did report significantly lower gelatinization temperatures and faster initial enzymatic digestion for the wild rices relative to domesticated species, emphasizing that although the endosperm of these wild rices is not morphologically different, it does not rule out nutritional or functional differences.

Fig. 1 Representative images of wild grain endosperm exhibiting rice-starch starch granule structure. (a) Oryza australiensis, (b) Oryza rufipogon, (c) Microlaena stipoides, (d) Microlaena stipoides endosperm cell wall at high magnification with visible pores, (e) Astrebla lappacea, (f) Austrostipa aristiglumis. S, Starch granule; C, channels; E, endosperm cell wall; B, protein bodies; M, protein matrix; P, pores; X, exudate; D, dimples.
Some endosperm variation was exhibited by these wild rice species. The starch granules of O. australiensis (Fig. 1a) were consistently slightly smaller than those of O. sativa. Similar to O. sativa, dimples can be seen on the starch granule surfaces, which are presumably indentations left by protein bodies which have been removed during the fracturing process. The protein matrix in the O. rufipogon (Fig. 1b) endosperm was thicker than that reported for O. sativa and consistently appeared to have small protrusions from its surface, reminiscent of exudate. Very thin remnants of the endosperm cell walls were also observed.
Microlaena stipoides, a member of the same sub-family as rice (Ehartoideae) and two species from sub-families other than the Ehartoideae, Austrostipa aristiglumis (Pooideae) and Astrebla lappacea (Chlorodoideae), all exhibited compound granules and endosperm morphology similar to rice (Fig. 1). These wild species may therefore provide an alternative starch source from plants far better adapted to the world's drying climate than cultivated rice. Similar to rice, starch granules were rigidly polygonal and were organized in compound granules. The size of the individual starch granules varied slightly, with M. stipoides having diameters of 1-4 μm; A. aristiglumis, 1–2 μm; and A. lappacea, 2–5 μm, compared to 3–5 μm for cultivated rice. The protein matrix was generally thin or not visible and the protein bodies were scattered between the compound granules. M. stipoides retained entire endosperm cell wall structure at maturity, as opposed to the majority of cereals which only have remnants at maturity. Some of the cell walls were ruptured during the fracturing process, allowing investigation of the cell contents (Fig. 1c). Pores within the cell wall were visible even at low magnification and were consistent across the entire endosperm for the samples examined. Compound granules within the endosperm cells were so tightly packed that the entire cell appeared to be one single granule; however, it was confirmed that each cell was made of many compound granules. A. lappacea exhibited ‘dimpling’ of the starch granules and conforms most closely to the appearance of cultivated rice, while A. aristiglumis is structurally similar though with slightly smaller granules.
Since granule morphology has been associated with biological source, it was expected that M. stipoides endosperm morphology may be similar to rice, given their relatively close taxonomic relationship. However, the same logic would suggest the endosperm of A. aristiglumis would be similar to the Triticeae and the A. lappacea should more closely reflect the Sorghum species, as these are their closest taxonomic relationships. Contrary to this, prior descriptions of variation in the starch granule morphology across the Poaceae noted that compound granule formation is the most common morphology amongst grass endosperms, with exceptions being the majority of species in the Panicoideae, which exhibit simple granules, and the Triticeae tribe, which have bimodal granules (Tateoka, Reference Tateoka1962). The Triticeae is part of the sub-family Pooideae. Partial phylogenetic relationships within this sub-family are simplified in Fig. 2, with the types of granules observed in multiple specimens across the species annotated (Tateoka, Reference Tateoka1962). Granule descriptions have been supported by the current study and recent literature (Tomlinson and Denyer, Reference Tomlinson and Denyer2003). Explanation of these tribal variations as the evolution of compound granules to more complex bimodal granules is confounded by the relationship of Aveneae and Poeae to the other species, which following this explanation have reverted to the ancestral morphology.

Fig. 2 Partial schematic of the phylogenetic relationships within the Pooideae. Adapted from Kellogg (2002).
Recent studies also observed compound granules within Eragrostis tef, Chloridoideae, (Bultosa et al., 2002) and Setaria italica Beauvos, Panicoideae, (Choi et al., Reference Choi, Kim and Shin2004). Sorghum species, also Panicoideae though in a separate tribe, have large, simple granules described with a range of shapes from spherical/ovoid through to regular polyhedrons, and do not have compound granules such as the endosperm exhibited in S. italica.
Clearly, there are specific genetic controls for granule formation that do vary between biological sources; however, the taxonomic relationships between sources is not an accurate predictor of granule morphology. This may mean that wild relatives of commercial species, which can be hybridized in traditional breeding programmes, may in fact have novel and beneficial starch granule characteristics which would allow the manipulation of the starch in planta. For example, the low digestibility of sorghum and poor functional performance of the flour may be alleviated by breeding a more rice-like starch granule within the grain while concurrently maintaining the agronomic production advantages of the sorghum plant.
Elymus scaber is within the same tribe as wheat and barley, the Triticeae. The endosperm of these grasses is characterized by their bimodal starch distribution (Fig. 3). As expected E. scaber grains had both large, lenticellular, A-type starch granules of 10–18 μm, and smaller, spherical B-type granules of 2–3 μm diameter. The barley A- and B-type granules examined in this study ranged from 10–15 μm and 3–5 μm, respectively, and the wheat, 10–20 μm and 3–5 μm, which is in line with previous reports. The A-type granules of E. scaber did exhibit the equatorial groove common to the Triticeae, although it is difficult to observe in this image (Fig. 3e). While the starch granule was very similar, some subtle endosperm variations were observed. Similar to other wild species, E. scaber retained its endosperm cell wall at maturity and appeared to have a greater ratio of A-type to B-type granules. The interaction between the endosperm protein matrix, cell walls and starch granules affects the malting quality of barley, a feature of importance to the brewing industry. In addition, B-type granule residuals can form haze and other difficulties during the brewing process, making a higher A-type granule ratio preferable (Chmelik et al., Reference Chmelik, Krumlova, Budinska, Kruml, Psota, Bohacenko, Mazal and Vydrova2001). The protein matrix of E. scaber appears to be slightly denser than that of barley, though similar to that of wheat, and has a similar protein body distribution to both. Some dimpling can be seen on the starch granules, though there was no evidence of either pores or channels in this species.

Fig. 3 Representative images of the Triticeae endosperm morphology examined. (a) and (b) Wheat, Triticum aestivum cv. Chinese Spring; (c) and (d) barley, Hordeum vulgare cv. Chebec; (e) and (f) Elymus scaber. Sa, A-type starch granule; Sb, B-type starch granule; E, endosperm cell wall; M, protein matrix; P, pores; X, exudate; G, equatorial groove.
The remainder of the species examined were wild relatives of Sorghum bicolor found in Australia, mainly across North Queensland and the Northern Territory. S. bicolor endosperm presents as two regions, the outer region known as the ‘vitreous’ layer and the more central ‘floury’ endosperm. Vitreous endosperm has closely packed starch granules surrounded by a continuous protein matrix embedded with individual protein bodies (Serna-Saldivar and Rooney, Reference Serna-Saldivar, Rooney and Dendy1995). The floury endosperm is typically more loosely packed and the polygonal starch granules soften to almost spherical shapes, with the protein bodies tending to exist without the matrix (Duodo et al., Reference Duodo, Nunes, Delgadillo, Parker, Mills, Belton and Taylor2002, Reference Duodo, Taylor, Belton and Hamaker2003). The proportion of vitreous and floury endosperm has been cited as determining the hardness of the grain, with increases of floury endosperm being associated with softer grains (Tesso et al., Reference Tesso, Ejeta, Chandrashekar, Huang, Tandjung, Lewamy, Axtell and Hamaker2006). Soft endosperms are associated with higher nutrient values, for example high lysine content; however, hard grains are generally preferred due to better functional characteristics and disease resistance (Chandrashekar and Mazhar, Reference Chandrashekar and Mazhar1999; Tesso et al., Reference Tesso, Ejeta, Chandrashekar, Huang, Tandjung, Lewamy, Axtell and Hamaker2006).
Due to the presence of distinct vitreous and floury endosperm in the sorghums, the endosperm was examined on a transect line from the centre of the seed to a point just below the sub-aleurone layer. Along this line three images were captured; central, middle and outer endosperm zones. S. bicolor has a distinct sub-aleurone layer, approximately 15–30 μm thick between the aleurone layer and the vitreous endosperm, in which the starch granules are smaller and the protein matrix appears to be thicker than in the remainder of the endosperm. The sub-aleurone starch granules range from 1–4 μm in diameter, are densely packed, polyhedral and many of the wild Sorghums also exhibit compound granules within the sub-aleurone layer, as did one of the S. bicolor specimens. In addition, a putatively novel sub-aleurone layer was also identified and has been described (Shapter et al., unpublished).
Variation within and between the wild sorghum endosperm is clearly visible. Representative images of the morphological differences observed have been collated (Figs 4–6) for each of the outer, middle and central zones of the endosperm, respectively. Each of the species has different combinations of the various zone morphologies represented by the images. Each image is representative of the typical endosperm morphological features of that zone and the cells of Table 2 list the wild species in which these variations occur.

Fig. 4 Representative images of the variation in endosperm morphology observed in Sorghum species by scanning electron microscopy. (a) S. bicolor, (b) S. timorense, (c) S. extans, (d) S. leiocladum. S, Starch granule; R, rice-like starch granules; B, protein bodies; M, protein matrix; D, dimpling; P, pores; C, channels; Cp, compound granules.

Fig. 5 Representative images of the variation in mid-endosperm morphology in Sorghum species. (a) S. bicolor, (b) S. laxiflorum, (c) S. brachypodum, (d) S. stipoideum, (e) S. leiocladum, (f) S. angustum. S, Starch granule; R, rice-like granules; B, protein bodies; M, protein matrix; P, pores; C, channels; D, dimpling; X, exudate.

Fig. 6 Representative images of the variation in central endosperm morphology observed in Sorghum species. (a) S. bicolor, (b) S. laxiflorum, (c) S. timorense, (d) S. nitidum, (e) S. brachypodum, (f) S. leiocladum. S, Starch granule, R, rice-like granules; B, protein bodies; M, protein matrix; P, pores; C, channels; X, exudate.
Table 2 Species distribution table of the representative endosperm types across the three regions of Sorghum endosperm
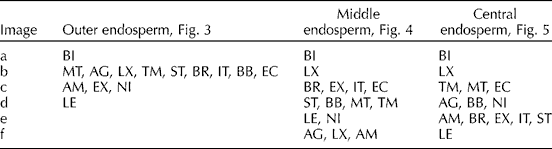
S. bicolor (BI), S. amplum (AM), S. angustrum (AG), S. brachypodum (BR), S. bulbosum (BB), S. ecarinatum (EC), S. extans (EX), S. intrans (IN), S. laxiflorum (LX), S. leicladum (LE), S. matarankense (MT), S. nitidum (NI), S. stipoideum (ST), S. timorense (TM).
Variations in the outer, vitreous endosperm are summarized in Fig. 4. Nine of the wild Sorghum species maintained the starch granule morphology and protein body distribution characteristic of S. bicolor (Fig. 4b). The starch granules of three of the species, S. amplum, S. nitidum and S. extans, were more spherical, had evidence of both pores and channels, and a lack of protein body dimpling on the granule surface and a lower proportion of protein bodies in the matrix than S. bicolor. The decrease in protein and increase in channelling and pores on the granule surface suggests that this part of the endosperm in these species has the potential to be more digestible than S. bicolor. The final wild species, S. leiocladum, exhibits sections of the outer endosperm with rice-like granules formed into compound granules, a feature previously unreported in the Sorghum species. Recent studies of barley mutants found that a deletion in the isa1 gene caused the initiation of multiple granules per amyloplast, which resulted in rice-like starch granule formation (Burton et al., Reference Burton, Jenner, Carrangis, Fahy, Fincher, Hylton, Laurie, Parker, Waite, van Wegen, Verhoeven and Denyer2002) similar to those seen in S. leiocladum (Figs 4d, 5e, 6f).
The middle and central regions of the endosperm exhibited more variation between the species than the outer zone. All of the wild species varied to some degree from the reference S. bicolor endosperm morphology (Figs 4a, 6a). Again, starch granule channels were more evident in particular species, with S. laxiflorum exhibiting this feature in both zones (Figs 5b and 6b). S. laxiflorum was also of particular note as its endosperm did not appear to have distinct middle and central layers, but rather was consistent throughout and had relatively few protein bodies, a discontinuous protein matrix and uniform granule morphology. Over the same zones, S. angustum remains consistent with very spherical granules and a discontinuous protein matrix. S. matarankense and S. timorense also appeared to have a consistent endosperm; however, in these species there was little variation across the entire grain and the endosperm was characterized by having a comparatively large volume of protein bodies distributed across the entire surface of the uniformly shaped starch granules, reminiscent of the vitreous layer. An increase in vitreous layer has been reported to increase the grain hardness and thereby improve the functionality of the grain (Tesso et al., Reference Tesso, Ejeta, Chandrashekar, Huang, Tandjung, Lewamy, Axtell and Hamaker2006). Concurrently the increases in protein body concentration in the endosperm in these species may cause a decrease of their starch digestibility, due to the protein interactions detailed below, which would also lower the glycaemic index of any grain end products.
The distribution of the matrix and protein bodies varied greatly amongst the Sorghum species. Arrangement of protein bodies around the starch granules has been citied as a barrier to amylase digestion and therefore a cause of reduction in sorghum starch digestibility (Shull et al., Reference Shull, Chandrashekar, Kirleis and Ejeta1990). This hypothesis was supported by the finding that treatment of S. bicolor flour with pepsin, which degraded the native protein prior to cooking, resulted in an increase in starch digestibility (Zhang and Hamaker, Reference Zhang and Hamaker1998). SEM imaging of protein bodies before and after in vitro digestibility trials using reducing agents during cooking (Rom et al., Reference Rom, Shull, Chandrashekar and Kirleis1992) concur with reports that sorghum mutants with increased digestibility have deeply pitted protein bodies (Taylor et al., Reference Taylor, Novellie and Liebenberg1985; Duodo et al., Reference Duodo, Taylor, Belton and Hamaker2003). It is hypothesized that these invaginations allow for increased enzyme penetration to the digestible α-kafirin centres of the protein bodies allowing enzymes to circumvent the indigestible γ-kafirins on the surface of the granule (Oria et al., Reference Oria, Hamaker, Axtell and Huang2000). Since the protein bodies of the wild sorghum appeared to have no such invaginations and they are presumed to act as a barrier to digestion, their concentration and distribution within the endosperm may be an indicator of the digestibility of the wild starches.
The occurrence of pores and channels on the surface of the starch granules varied between species. Since the surface of starch granules is also the major storage area for lipids in the endosperm, it suggests that lipid/starch complexes may provide a chemical barrier which also reduces starch digestion (Svihus et al., Reference Svihus, Uhlen and Harstad2005). Studies have shown the lipid content of smaller granules to be higher than that of larger granules and this affects digestibility (Raeker et al., Reference Raeker, Gaines, Finney and Donelson1998). Pores or channels may provide a means for enzymes to penetrate this lipid barrier. Singularly, or in combination, it is expected that variations in protein body distribution and the occurrence of pores and/or channels on the starch granule surface will affect the digestibility of the starch, and hence wild species may provide new genetic diversity for sorghum breeding programmes and germplasm for characterization of the genes controlling these traits.
General observations of cereal grains have led to the hypothesis that starch granules only maintain a sharp-edged morphology when they are tightly packed within the endosperm (Earp et al., Reference Earp, McDonough and Rooney2003). The softening of the morphology of sorghum starch granules from the outer vitreous endosperm, which typically has consistently polyhedral granules, to almost smooth oval or spherical starch granules in the centre of the floury endosperm, appears to coincide with the density of starch granules in these zones. In S. bicolor these changes from the outer to inner endosperm are very distinct and clearly described by the sub-aleurone, vitreous and floury endosperm morphologies. In conjunction with this, starch granules initiate in the centre of the endosperm earlier in seed development than the outer endosperm. Therefore, the largest granules are located in the central endosperm at maturity. These features are clearly evident in S. bicolor (Figs 4a, 6a), while in the wild sorghum species little size differentiation occurred across the different zones of the endosperm.
The wild Sorghum species diverge from S. bicolor starch granule morphology and protein body arrangement throughout the endosperm (Figs 4–6). There are two groups of starch granule shape observed across the images, spherical and polyhedral. S. amplum, S. extans and S. nitidum have spherical granules throughout the endosperm while S. angustum, S. bulbosum, S. intrans, S. stipoideum, and S. brachypodum have these spherical granules confined to the central and middle endosperm. None of the wild species exhibited granules as large as those that are common in S. bicolor, nor the more ovoid shapes common to the floury endosperm of S. bicolor. Smaller granule size should provide a greater surface area to volume ratio for the starch granules, which may present an opportunity for increased digestibility.
Compound granules are not characteristic of sorghum endosperm; however, a recent study of the pericarp development of domesticated sorghums identified small (1–2 μm) polyhedral starch granules arranged in compound granules in the developing pericarp. By maturity these had been broken into singular granules in the thick pericarp varieties and were not visible in the thin pericarp varieties (Earp et al., Reference Earp, McDonough and Rooney2003). In S. leiocladum these small rice-like polyhedral and compound starch granules were observed in the starchy endosperm (Figs 4d and 6f). In one specimen, channels of the rice-like endosperm extended deep into the vitreous endosperm. The potential for manipulating sorghum varieties so they produce a more rice-like starch has implications for global nutrition, especially in developing countries where sorghum is the staple diet due to its ability to grow in arid conditions. Malnutrition is common in these countries, due in part to the poor digestibility of the starch and protein in sorghum after it has been cooked (Duodo et al., Reference Duodo, Taylor, Belton and Hamaker2003).
The endosperm of some specimens had the appearance of having been sheared (Fig. 7). These sheared areas were observed across several of the species around the sub-aleurone layer but in S. brachypodum and S. bulbosum some of these areas extended throughout the endosperm. Higher magnification of this sheared surface revealed circular patterns which had the appearance of starch granules or protein bodies sitting in a matrix that had been sliced through the centre, rather than fracturing around the granule surface. In sorghum, it has been observed that the fracture pattern is strongly associated with grain hardness, with the ‘softer’ genotypes tending to fracture along the cell lines while the ‘harder’ varieties tend to break through the cells (McDonough et al., Reference McDonough, Floyd, Waniska and Rooney2004). After harvesting, ageing and high temperatures can cause an increase in the starch–protein interaction and an associated ‘hardening’ of the endosperm (McDonough et al., Reference McDonough, Floyd, Waniska and Rooney2004). It is therefore possible that the sheared appearance may be an artefact of the preparation process or the conditions of seed storage prior to the experiment. However, in wheat a large proportion of the starch granule associated proteins are stored close to the surface of the granules and this affects the binding between the starch granules in the endosperm, and hence whether the endosperm fractures between or through the starch granules (Svihus et al., Reference Svihus, Uhlen and Harstad2005). In sorghum, the positive association between grain hardness and proportion of vitreous endosperm is well established and images similar to those of Fig. 7 have been recorded previously in a hard variety (Parker et al., Reference Parker, Ng and Waldron2005). The species exhibiting the sheared endosperm tended to be those which had increased vitreous endosperm and it is therefore more likely that the sheared appearance is due to increased grain hardness in these varieties.

Fig. 7 Representative images of the sheared endosperm morphology observed in some Sorghum species. (a) S. stipoideum, (b) S. bulbosum. S, Starch granule; B, protein bodies; M, protein matrix; C, channels; D, dimpling.
Globally, sorghum is the fifth highest consumed cereal species. Unlike all other cereals, the digestibility of sorghum is reduced by cooking (Duodo et al., Reference Duodo, Nunes, Delgadillo, Parker, Mills, Belton and Taylor2002). It is particularly important in developing countries, despite it having lower nutrient availability than other cereals, because it is better adapted to the low water and low-nutrient soils which are prevalent in many subsistence communities (Duodo et al., Reference Duodo, Nunes, Delgadillo, Parker, Mills, Belton and Taylor2002; Tesso et al., Reference Tesso, Ejeta, Chandrashekar, Huang, Tandjung, Lewamy, Axtell and Hamaker2006). Wild sorghums have already been successfully targeted as a novel source of germplasm for breeding programmes (Dillon et al., Reference Dillon, Lawrence, Henry, Ross, Price and Johnston2004). The observations reported here suggest that there are useful variations in the endosperm morphology of wild grass species and these provide a valuable genetic resource for enhancing the nutritional and functional value of cereals into the future.
Acknowledgements
The authors wish to acknowledge the tutelage of Maxine Dawes and the contribution of the Scanning Electron Microscopy Department, School of Environmental Science and Management, Southern Cross University. Seed for this study was supplied by Australian Tropical Crops and Forages Collection, Queensland Department of Primary Industries and Fisheries (http://www.dpi.qld.gov.au/auspgris/) and Native Seeds Pty Ltd Australia (http://www.nativeseeds.com.au). Funding was provided by the Grain Foods Cooperative Research Centre.











