INTRODUCTION
Lepeophtheirus salmonis Krøyer (1837) are copepod crustaceans that parasitize salmonid fish and other species, including the commercially farmed Atlantic salmon, Salmo salar L. The copepodid stage of the life cycle is infective and attaches to host fish before developing into chalimus, pre-adult and adult parasitic stages (Pike and Wadsworth, Reference Pike and Wadsworth2000). The ectoparasitic stages feed on skin, mucous and blood with infestation potentially leading to increased fish mortality from severe erosion and exposure of subcutaneous tissues, secondary infections, osmotic imbalances and stress (Wagner et al. Reference Wagner, Fast and Johnson2008). Dramatic recent increases in world-wide marine salmonid production have been accompanied by increased outbreaks of sea lice infection and most recent estimates put the economic cost of sea lice control across the industry at US$480 million or 4–10% of production costs (Costello, Reference Costello2009). With increasing resistance to available ectoparasiticides in sea lice and concern about the effect of sea lice on wild salmon populations, there is an urgent need for harnessing modern molecular techniques for research on the non-model organsim L. salmonis.
RNA interference (RNAi) is a gene silencing technique that is becoming an ever more powerful tool in investigating the functional role of specific genes that may be potential targets for chemotherapeutic intervention. The RNAi mechanism involves the in vivo production of small interfering RNA molecules (siRNAs) from larger introduced double-stranded RNA (dsRNA). siRNA molecules target and destroy target mRNA, silencing the target gene at the post-transcriptional stage. RNAi has been used successfully to screen and investigate genes for possible therapeutic intervention in a range of invertebrate species to date, including gut proteins in tsetse flies (Walshe et al. Reference Walshe, Lehane, Lehane and Haines2009); moulting genes in Tribolium castaneum (Minakuchi et al. Reference Minakuchi, Namiki and Shinoda2009), aquaporins in aphids (Shakesby et al. Reference Shakesby, Wallace, Isaacs, Pritchard, Roberts and Douglas2009), growth genes in white shrimps (Hui et al. Reference Hui, Tobe and Chan2008) and extensively in ticks (reviewed by de la Fuente et al. Reference de la Fuente, Kocan, Almazan and Blouin2007). In most cases administration of dsRNA to the invertebrate target is achieved by intra-haemocoelic injection, though immersion in solutions containing the dsRNA has been employed for nematodes, planarians and mosquitoe larvae.
In this study, we assessed the feasibility of gene knockdown in L. salmonis small free-living (naupilius) and infective (copepodid) stages and the larger parasitic stages (pre-adults and adults) using both immersion and intra-haemocoelic injection of dsRNA. In addition, assessment of the speed and persistence of the gene knockdown effect was determined. The chosen target gene was a putative prostaglandin E synthase 2 (PGES2) EST (Clone Id: lsal-evj-508-140) the sequence of which was in a publicly available salmon lice EST database (GiLS project, Koop B. F., Jones S. and Davidson W.; http://web.uvic.ca/grasp/gils/).
MATERIALS AND METHODS
Sea lice harvesting and maintenance
Adult and pre-adult Lepeophtheirus salmonis were collected from a commercial Atlantic salmon (Salmo salar) farm in Loch Leven on the west coast of Scotland. Some lice were kept in RNA-later (Sigma, Poole, UK) at −80°C for initial mRNA isolation. The remainder were maintained at 12°C in Loch Leven seawater in 1L beakers with constant aeration. Water was changed daily and dead lice removed. Egg strings were carefully removed from any gravid female lice and kept in similar conditions to adults. After hatching, nauplius were separated from the remaining eggs and allowed to develop into copepodids. All stages were maintained in a 15·5 h:8·5 h, light:dark regime.
RT-PCR of PGES from L. salmonis
Total RNA was prepared from whole adult female lice using Trizol (Invitrogen, Paisley, UK), according to the manufacturer's instructions. After isolation, 1 μg total RNA was DNase treated with 1 μl (1 U) of RQ1-DNase and 1 μl of RQ1 buffer and incubated at 37°C for 30 min. Then 1 μg of the DNase-treated total RNA was incubated at 70°C with 0·5 μg of oligo d(T)15 (Promega, Southampton, UK) in a total volume of 10 μl for 5 min. Material was chilled on ice for 5 min prior to the addition of 5 μl of 5× RT buffer, 1 μl of dNTPs (25 mM each), 0·5 μl of Bioscript-reverse transcriptase and DEPC water to 25 μl. The reaction was incubated at 42°C for 60 min prior to arrest by heating to 70°C for 5 min.
PGES transcript was isolated by PCR using a selection of primers designed from an L. salmonis EST project (http://web.uvic.ca/grasp/gils/). PCR reactions consisted of 1 μl of cDNA template, 5 μl of 10× reaction buffer, 2 μl of 50 mM MgCl2, 1 μl of dNTPs (25 mM each), 1 μl of each primer (10 mM each), 0·5 μl (1·25 U) of Taq (Bioline, London, UK) and DEPC-treated water to give a 50 μl total volume. Primer pairs used were PGES-F1 (GATATCCTCCGCATACTCGCC) and PGES-R2 (GCCCATTTCATCTTCGTAAACC) to generate a product of 1110 bp. PCR cycling conditions were as follows: 1 cycle of 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 50°C and 30 s at 72°C followed by a final extension time of 20 min at 72°C. Products were visualized on an agarose gel and specific bands excised and cloned into pCR4-TOPO TA vector (Invitrogen). Purified plasmids were submitted for sequencing to Eurofins MWG (Ebersberg, Germany) from flanking T7 and T3 promoter regions to confirm the original EST sequence data.
Bioinformatics and phylogeny
Protein sequences of related PGES were found by tBLASTx and aligned with isolated L. salmonis PGES using MEGA version 4.1 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007). Alignments were adjusted manually where necessary, and amino acid sequences used to estimate phylogeny with neighbour-joining, minimum evolution and maximum parsimony methods. Phylogenetic trees were constructed with 10 000 bootstrap replicates. All methods gave trees with similar topology and approximate bootstrap values and so only the neighbour-joining tree is presented. Percentage homology between different PGES was calculated using ClustalW (v1.83) and domains identified using the conserved domain database (Marchler-Bauer et al. Reference Marchler-Bauer, Anderson, Derbyshire, DeWeese-Scott, Gonzales, Gwadz, Hao, He, Hurwitz, Jackson, Ke, Krylov, Lanczycki, Liebert, Liu, Lu, Lu, Marchler, Mullokandov, Song, Thanki, Yamashita, Yin, Zhang and Bryant2007). Subcellular localization was predicted by PSORT (http://www.psort.org/) and TargetP (http://www.cbs.dtu.dk/services/TargetP/).
Expression of PGES transcript in developmental stages of L. salmonis
RNA from adult and pre-adult stages was extracted using the method described above. RNA from egg strings, nauplius (10 individuals) and copepodid (10 individuals) stages was isolated using a Mini RNA Isolation II Kit (Zymo Research, Orange, California, USA), as per manufacturer's instructions. After isolation of RNA, samples were reverse transcribed, as described above. The presence of the PGES in the different developmental stage cDNA was assayed by PCR. The PCR protocol and cycling conditions were carried out using PGES primers (PGES-F2 GCATCTTCCTCTTCGCCATC and PGES-R2 GCCCATTTCATCTTCGTAAACC), as described above. Gel loading was normalized to L. salmonis actin (LsActinF, GATGAAGCCCAATCCAAGAGAG and LsActinR TTGCATACGATCAGCGATACC) generating a fragment of approximately 777 bp. Additionally, the relative expression of PGES within adult female L. salmonis was determined within isolated cephalothorax, reproductive and tail samples (Fig. 1), as above.

Fig. 1. Photograph of Lepeophtheirus salmonis adult female. Arrow indicates region between dorsal carapace plates where injections were carried out. Scale bar=1 mm.
Preparation of dsRNA
PGES-dsRNA and LacZ-dsRNA, as a control, was prepared using a BLOCK-iT RNAi TOPO transcription kit (Invitrogen), according to the manufacturer's instructions. Briefly, PCR was carried out as described above using adult female L. salmonis cDNA in conjunction with PGES specific primers (PGES F2/R2), or with control LacZ-plasmid and LacZ specific primers (LacZ-F2, ACCAGAAGCGGTGCCGGAAA and LacZ-R2, CCACAGCGGTGGTTCGGAT). Products were resolved on an agarose gel, excised and purified using a Qiagen gel extraction kit (Qiagen, Crawley, UK). TOPO-T7 linker was ligated to PGES and LacZ reactions before a secondary PCR was carried out to gain sense and antisense templates. T7-RNA polymerase was used in transcription reactions with target templates to generate sense and antisense RNA. Finally, RNA strands were annealed and the resultant dsRNA purified and quantified in a micro-spectrophotometer (Nanodrop Technology Ltd). dsRNA was diluted with DEPC-treated water to a working concentration of 1 μg/l μl and stored at −80°C.
Protocol of dsRNA injections of adults and pre-adults
Adult and pre-adult L. salmonis were removed from aerated seawater, placed on sterile Petri dishes and the dorsal region dabbed dry with filter paper. Injections were carried out using either pulled glass capillary needles in conjunction with a Harvard micro-injector system, or with 2·5 μl Hamilton micro-syringes fitted with 33 gauge needles. Lice were injected with 0·5 μl (1 μg/μl) of either PGES-dsRNA or LacZ-dsRNA in the dorsal carapace between the plates, as indicated in Fig. 1. If penetration into the soft interplate region was difficult, then the louse was held in position with fine forceps and the body squeezed gently to allow separation of the plates. It was noted that it was vital to avoid injecting air bubbles into the lice and to angle needles away from the central region where the vital organs are found. Injected lice were left for 1–2 min to allow the injection site to ‘seal’. Lice were returned to vigorously aerated fresh seawater and dead or unhealthy looking lice were removed after 1 h (<10%). Mortality was monitored over 6 days in LacZ-dsRNA, PGES-dsRNA and non-injected lice.
To validate RNAi in the adults and pre-adult and to investigate persistence of its effect, total RNA was extracted from injected lice after 24, 48, 72, 96 and 120 h using Trizol (Invitrogen) prior to DNase-treatment and reverse transcription, as described previously. PCR was carried out using either PGES-dsRNA or LacZ-dsRNA treated sample cDNA in conjunction with primers specific for actin or PGES using primers and cycling conditions, as described above. Products were visualized on an agarose gel normalized to actin loading. ImageJ software was used to carry out semi-quantititative densitometric analysis on gel images.
Protocol of dsRNA soaking of nauplius and copepodids
As nauplius and copepodid stages are too small and fragile for microinjection we attempted to soak these stages in dsRNA to achieve gene silencing. Newly hatched nauplius (10 individuals) or fresh copepodids (10 individuals) were removed from aerated beakers and placed in 1 ml microfuge tubes in 45 μl of sterile sea water along with 5 μl (1 μg/μl) of either PGES-dsRNA or LacZ-dsRNA and left at 4°C for 7 h. After 7 h, lice were removed from dsRNA-sea water and placed into 30 ml of aerated seawater in universal tubes and kept at 12°C. To validate gene silencing and assay the persistence of the treatment, lice were collected at 7, 24 and 48 h. Mortality was determined at each time-point.
Total RNA was extracted from soaked lice after 7, 24 and 48 h using a Mini RNA Isolation II Kit (Zymo Research) prior to DNase-treatment and reverse transcription, as described previously. PCR was carried out using either PGES-dsRNA or LacZ-dsRNA treated sample cDNA in conjunction with primers specific for actin or PGES using primers and cycling conditions, as described above. Products were visualized on an agarose gel normalized to actin loading.
RESULTS
Bioinformatics and phylogeny
Partial sequences of a putative microsomal prostaglandin E synthase type-2 (mPGES2), were isolated from adult female L. salmonis cDNA. The cloned region was 1110 bp in length and shared 97% nucleotide and amino acid identity with a contig from an L. salmonis EST project, from which original primers were designed. The consensus open reading frame of these sequences encodes a protein of 391 amino acids. Conserved domain analysis revealed that LsPGES2 has 2 highly conserved regional motifs. A GST-N domain, similar to microsomal fold is found the N-terminus region at position C118–L186, and includes a redox-active CXXC site at positions C118–C121. A GST-C domain is present in the C-terminus at position K236–V382. The subcellular localization of LsPGES2 was predicted to be mitochondrial or microsomal with a high degree of certainty by both PSORT and TargetP programmes.
BLAST analysis using LsPGES2 nucleotide and protein sequences resulted in multiple hits; with arthropod PGES2s yielding the highest identity scores. A putative prostaglandin-E-synthase type-2 from the sea lice, Calagus rogercresseyi (BT077234.1), shares 76% amino acid conservation, followed by 66% with Triboleum castaneum (XM_968559.1), and 58% with Aedes aegyptii (XM_001659433.1). Phylogenetic analysis of LsPGES2 with sequences from the PGES family placed it in a clade containing members of the PGES2 family (Fig. 2). Within this clade, LsPGES2 is in a group with other arthropod PGES2s and on a branch containing the closely related sea lice C. rogercresseyi.

Fig. 2. Phylogeny of the MAPEG PGES family. Phylogenetic tree constructed using amino acid sequences with neighbour-joining method using MEGA (4.0). Scale bar represents an estimate of the number of amino acid substitutions per site. Numbers are bootstrap values. Accession and database sequence identifiers are as follows: mPGES1 (microsomal PGES1); human NP_004869, Gallus gallus XP_415475, Tetradon nigroviridis CAF97117, Drosophila mojavensis XP_002010102 Culex quinquefasciatus XP_001863047. mPGES2 (microsomal PGES2); Human NP_079348, Gallus gallus XP_415498, Caligus rogercresseyi ACO11658, Triboleum castaneum XP_973652, Aedes aegypti XP_001659483. cPGES3 (cytosolic PGES3); Human EAW96954, Gallus gallus B56211, Oncorhynchus mykiss ACO08219.
Developmental and tissue distribution of LsPGES
RT-PCR analysis of life stages revealed that LsPGES2 mRNA was present in whole tissue extracts from white and black egg strings, nauplius, copepodid, chalimus, pre-adult and adult male and female lice (Fig. 3). LsPGES2 transcripts were also detected with approximately equal abundance in isolated cephalothorax, reproductive and tail samples of adult female lice (data not shown). Semi-quantitative RT-PCR on total RNA from all stages and tissue samples revealed that LsPGES2 is a relatively abundant transcript compared to the housekeeping gene actin.

Fig. 3. Relative life-stage expression of LsPGES2 transcript determined by RT-PCR in different stadia of Lepeophtheirus salmonis using LsPGES2-specific primers and gel loading normalized to actin. Wh, white (immature) egg string; Blk, black (mature) egg string; Na, nauplius; Co, copepodid; Ch, chalamus; Pa, pre-adult; AF, adult female; AM, adult male. Results are representative of ⩾3 replicates, except for Ch and AM which were performed in duplicate.
Mortality and validation of knockdown in microinjected adult lice
Lice were monitored for 120 h post-injection to determine if microinjection with LacZ or PGES-dsRNA caused significant mortality. No difference was seen between injected and control (non-injected) mortality levels in adult sea lice (Fig. 4). To validate that injected PGES-dsRNA suppresses LsPGES2 levels and to establish the persistence of this effect, transcript levels were determined from samples of adult female L. salmonis 24–120 h post-injection. LsPGES2 mRNA was detectable in extracts of whole body female L. salmonis 24 h post-treatment in both LacZ and PGES-dsRNA injected lice, albeit at reduced levels in the latter (Fig. 5). Densitometry, standardized by actin gel-loading, indicated that gene knockdown of LsPGES2 was evident from 48 to 72 h post-injection with levels of transcript significantly reduced to as low as 5% of control levels in some individuals. Transcript levels of LsPGES2 recovered towards control levels after 96 and 120 h. Simliar knockdown of LsPGES2 was also observed in adult male and pre-adult L. salmonis (data not shown).

Fig. 4. Mortality of injected and non-injected Lepeophtheirus salmonis over the 120 h experimental period. Sea lice were injected with either LacZ or PGES-dsRNA or left uninjected. Closed circles represent non-injected lice; open circles represent LacZ-dsRNA injected lice and triangles represent PGES-dsRNA injected lice. Data are percentage daily cumulative mortality for each treatment.

Fig. 5. Persistence of dsRNA-LsPGES2 gene knockdown in adult Lepeophtheirus salmonis. (A) LsPGES2 transcipt levels were determined by RT-PCR up to 120 h post-dsRNA treatment and gel loading normalized to actin. (B) Semi-quantitative LsPGES2 RT-PCR band intensities were determined by densitometry and the relative abundance to the within-sample actin band calculated. The level of PGES2: actin knockdown is presented as a percentage of the PGES2: actin in control lice injected with LacZ-dsRNA at each given time-point. Circles represent individual lice performed in duplicate at each time-point and triangles represent the mean.
Mortality and validation of knockdown in nauplius and copepodid stages
As the relatively small size of nauplius and copepodid stages made microinjection technically impractical, gene-silencing was achieved by ‘soaking’ the free-living L. salmonis stages in small volumes of sea water and target dsRNA. Nauplius were monitored for 48 h post-treatment to determine whether soaking with LacZ or PGES-dsRNA caused significant mortality. No difference was observed between treated and background (non-injected) mortality levels, with ~80–90% of nauplius being dead at 72 h, regardless of treatment (Fig. 6). Interestingly, the remainder of the surviving lice from all treatments had undergone moult to the copepodid stage, indicating that dsRNA per se does not interrupt this process.

Fig. 6. Mortality of soaked Lepeophtheirus salmonis nauplius over the 72 h experimental period. Nauplius were soaked in either LacZ-dsRNA or PGES-dsRNA or left untreated. Black circles represent non-treated nauplius; black squares represent LacZ-dsRNA soaked nauplius and open triangles represent PGES-dsRNA soaked nauplius. Data are percentage daily cumulative mortality from each treatment.
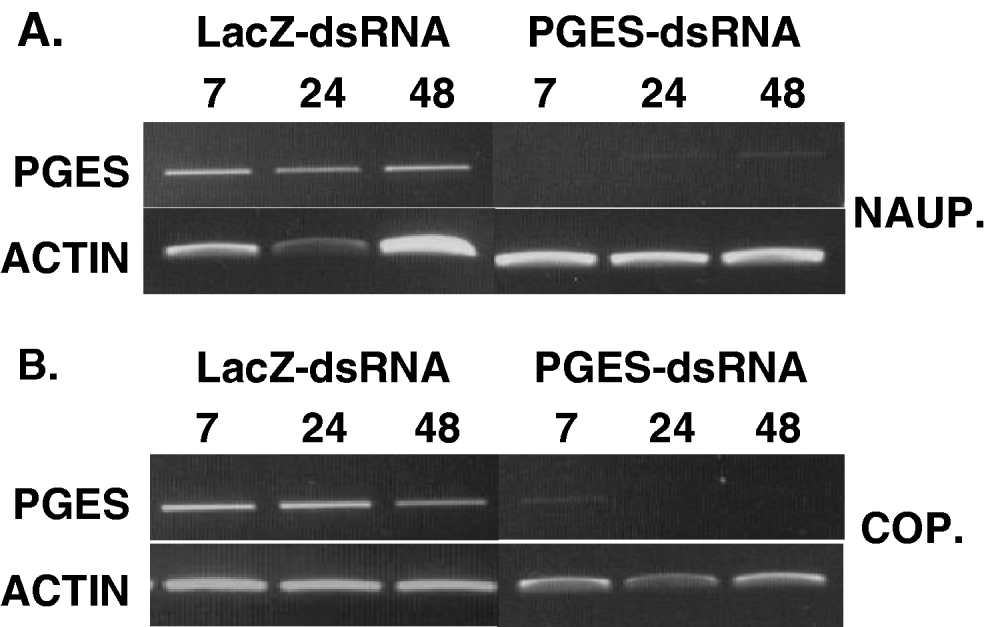
To validate that nauplius and copepodids soaked in PGES-dsRNA have lower levels of LsPGES2 mRNA, and to establish the persistence of this effect, transcript abundance was determined from samples of 10 pooled individuals at 7, 24 and 48 h post-treatment. LsPGES mRNA was undetectable in samples of nauplius stages 7 h post-treatment, whereas dsLacZ-RNA-soaked lice had abundant LsPGES2 transcript (Fig. 7A). Samples from 24 and 48 h PGES-dsRNA post-treated nauplius had very low, but detectable, levels of LsPGES2 transcript, whereas dsLacZ-RNA-soaked lice had LsPGES2 transcript at far higher levels. Copepodids treated with PGES-dsRNA had greatly reduced, but detectable, transcript levels after 7 h (Fig. 7B). After 24 and 48 h LsPGES2 mRNA was undetectable, whereas LacZ-dsRNA-soaked copepodids had abundant LsPGES2 transcript at all sampling time-points.
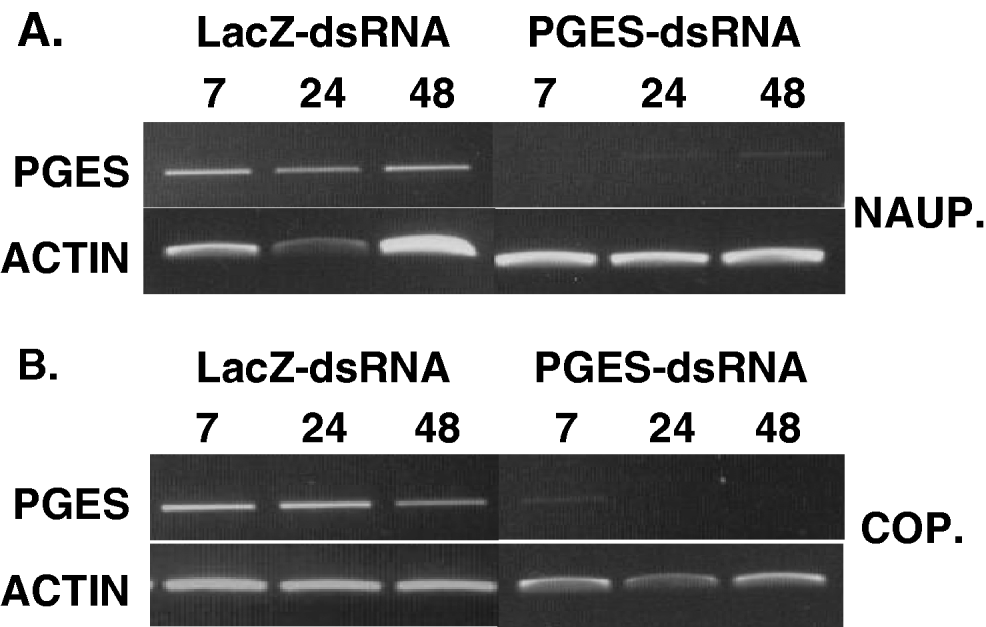
Fig. 7. Persistence of dsRNA-LsPGES2 gene knockdown in (A) nauplius and (B) copepodid stages. Sea lice (n=10) were assayed by RT-PCR up to 48 h post-treatment with dsRNA and gel loading normalized to actin. Data are representative of multiple assays.
DISCUSSION
Previously, adult L. salmonis were reported to secrete prostaglandin E2 (PGE2) in response to the neurotransmitter dopamine (Fast et al. Reference Fast, Ross, Craft, Locke, MacKinnon and Johnson2004), a secretion suggested to play an anti-inflammatory and immunosuppressive role in L. salmonis feeding on the host (Fast et al. Reference Fast, Johnson, Eddy, Pinto and Ross2007; Wagner et al. Reference Wagner, Fast and Johnson2008). In the present study, we cloned and sequenced a putative prostaglandin E synthase-2 from L. salmonis collected from farmed salmon on the west coast of Scotland. This PGES2 had 97% amino acid identity to a full-length EST from Pacific L. salmonis (GenBank AC012313.1). The PGES2 gene family contained within the superfamily MAPEG (Membrane Associated Proteins in Eicosanoid and Glutathione Metabolism) catalyses the isomerization of PGH2 into the bioactive PGE2 (Jakobsson et al. Reference Jakobsson, Thoren, Morgenstern and Samuelsson1999; Samuelsson et al. Reference Samuelsson, Morgenstern and Jakobsson2007). However, some members of MAPEG play no role in eicosanoid synthesis, but, rather, exhibit glutathione S-transferase (GST) or peroxidise activity (Jakobsson et al. Reference Jakobsson, Thoren, Morgenstern and Samuelsson1999; Hayes et al. Reference Hayes, Flanagan and Jowsey2005).
Although BLAST homology analysis indicates the putative LsPGES2 is likely a PGES2, and the L. salmonis EST project has assigned this gene as a PGES2, there are several caveats in accepting this assignation. LsPGES2 contains the characteristic N-terminus region of the PGES2 family, but this occurs about mid-way in the LsPGES2. Indeed, the LsPGES2, along with numerous other PGES2 genes from invertebrates, contains an extra 110 amino acid stretch at the N-terminus. LsPGES2 was present in far higher abundance than would be expected for a gene involved in PGE2 synthesis. Additionally, LsPGES2 was present in every L. salmonis stadia investigated including the non-parasitic, free-living stages and, most notably, in the early developing embryos (white egg strings) and the more developed embryos (black egg strings). LsPGES2 was present in equal abundance in the cephalothorax, reproductive and tail sections of adult female lice. Taken together, the temporal and tissue distribution of LsPGES2 indicates that it is a ubiquitous and constitutively expressed gene, more like a detoxifying GST than a gene involved in PGE2 synthesis for immunosuppressing the host.
LsPGES2, used as a test gene, was successfully knocked down in the free-living nauplius and the infective, host-seeking copepodid stages by immersing the lice in a small volume of sea water containing the dsRNA for 7 h. The knockdown was apparent at the end of the 7-h treatment and maintained for 48 h without any effect on mortality. Thus, this technique would allow investigators to observe the effect of the knockdown of their target gene immediately after the 7-h soaking period and for the next 48 h. In the case of the nauplius, this 48 h period would enable studies on the genes involved on development and moulting through to the copepodid stage. Indeed, in our present studies several dsRNA-treated nauplius moulted through to copepodids, but the quantity of material remaining was too small to assay for persistence of gene-silencing through the moulting process. Knockdown of certain genes in the copepodid stage employing the methods described in this paper would allow investigators to assess many aspects of host seeking behaviour, settling and initial changes from a free-living to a parasitic mode of life.
Gene knockdown of LsPGES2 was readily achieved within 24 h of intra-haemocoelic injection of dsRNA in adult and pre-adult L. salmonis and the knockdown effect was still apparent after 5 days. Injection between the dorsal carapace plates was relatively straightforward and, after a little practice, 20–30 lice could be injected per hour. Mortality was higher using the micro-syringes than using the more complex micro-injector system, but any mortality was typically observed within the first couple of hours of the injection trauma and such lice can be removed from the experimental groups. However, since the micro-syringe method is easier and more rapid, investigators without access to a micro-injector system can simply inject more lice and figure the higher mortality levels into their experimental design.
Variation in knock-down responses between individuals was apparent at the beginning and toward the end of the effective period (Fig. 5B). Such variation may be due to different size and ages of the lice, differences in metabolic rates or slight differences in the RT-PCR procedures, but the most likely cause of variation is differences in the injection site and volumes. Researchers wishing to use our approach for trials on their gene of interest would be advised to optimize the amount of dsRNA injected for their particular gene. It should be noted that each gene will have slightly different requirements for optimal and persistent knockdown depending on its initial level of abundance, its rate of turnover and its location. However, our methods described above are a good starting point for an abundant and seemingly ubiquitous gene and the proof of concept has been established.
Employing dsRNAi in adult and pre-adult L. salmonis would allow investigators to determine the effect of knockdown of certain genes on mate finding, fertilization and oogenesis. Likewise, specific genes thought to be involved in host inflammation could be knocked down and the response of the host salmon investigated at the feeding site by histopathology or at a global gene scale by microarray analysis. With the enormous cost of ectoparasiticide development estimated at US$57–100 million (Graf et al. Reference Graf, Gogolewski, Leach-Bing, Sabatini, Molento, Bordin and Arantes2004; Woods and Williams, Reference Woods and Williams2007), new drug targets within L. salmonis can be genetically validated by gene knockdown before embarking on large-scale lead compound development (Lees and Bowman, Reference Lees and Bowman2007). Additionally, dsRNAi can be used to investigate the role of certain genes. For example, there is doubt whether LsPGES-2 is indeed involved in PGE2 synthesis or performs some other function. This uncertainty could be investigated by determining the amounts of PGE2 secreted by PGES2-dsRNA injected L. salmonis compared to control L. salmonis, as described by Fast et al. (Reference Fast, Ross, Craft, Locke, MacKinnon and Johnson2004).
In conclusion, we have established dsRNA knockdown in the economically important L. salmonis, using the PGES2 as a test candidate gene, to our knowledge, the first report to perform dsRNAi in any copepod. The marine environment of L. salmonis is not a major impediment to applying gene knockdown approaches to this parasite. Methods suitable both to the small free-living stages and the larger parasitic stages were established. The degree of knockdown was essentially complete and the effect persisted for several days. The dsRNAi approach will allow researchers to make full use of all the L. salmonis gene sequences becoming available and to tackle some very interesting questions in this important parasite.
We are indebted to Professor Ben Koop, University of Victoria British Columbia for advice about the L. salmonis GiLS EST database. We are also grateful to Dr Alan Pike and Professor Jenny Mordue, University of Aberdeen for advice on maintenance of L. salmonis.
FINANCIAL SUPPORT
This work was supported by a pump-priming grant from the College of Life Sciences and Medicine, University of Aberdeen and part-supported by the Biotechnology and Biological Sciences Research Council (ASB, grant number BBC5178331).