Introduction
Active fumaroles of scoria cones related to different eruptions of Tolbachik volcano are well-known for the exceptional diversity of various sulfate mineral species (Vergasova and Filatov, Reference Vergasova and Filatov2016). All of the sulfate minerals found in Tolbachik fumaroles can be divided into three groups: (1) sulfate minerals with additional O atoms (e.g. dolerophanite Cu2O(SO4), Effenberger, Reference Effenberger1985); (2) sulfates with no additional O atoms (e.g. chalcocyanite CuSO4, Siidra et al., Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018b); and (3) hydrated sulfate minerals containing OH– or/and H2O (e.g. chalcanthite CuSO4·5H2O).
Two first groups of sulfate minerals are primary and usually found in the deeper hot zones of the fumaroles. A diverse suite of sulfates with additional oxygen atoms (Krivovichev et al., Reference Krivovichev, Mentré, Siidra, Colmont and Filatov2013) is a characteristic feature for Tolbachik volcano (Siidra et al., Reference Siidra, Nazarchuk, Zaitsev, Lukina, Avdontseva, Vergasova, Vlasenko, Filatov, Turner and Karpov2017) and only a few mineral species related to this group are observed in other active fumaroles on Earth (e.g. Vesuvius, Italy). Hydrated supergene sulfates form as a result of interaction of primary sulfates with water in the surface and subsurface zones. Herein we report on the composition, structure and properties of itelmenite (Cyrillic = ительменит) an anhydrous sulfate belonging to the second group. The mineral is named for the Itelmens, an ethnic group who are the original inhabitants living in the area around Tolbachik volcano and Kamchatka Peninsula. Both the mineral and the mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2015-047). Type material is deposited at the Mineralogical Museum, St. Petersburg State University, St. Petersburg, Russia (catalogue no. 1/19637).
Occurrence and association
Itelmenite occurs as a product of fumarolic activity. It was found in the summer of 2014 by O.I.S. and E.A.L. in Saranchinaitovaya fumarole on the Naboko scoria cone (N55°46ʹ06ʺ, E160°18ʹ59ʺ, altitude 1650 m) of the Tolbachik Fissure Eruption that occurred in 2012–2013. The temperature of gases at the sampling location was ~600–620°C, nearly twice that observed in the fumaroles of the Second scoria cone of the Great Tolbachik Fissure Eruption. This means that itelmenite could be deposited directly from the gas phase as a volcanic sublimate. It is also very probable that itelmenite might form as a result of the interaction between gas and basalt scoria.
All the samples recovered were packed and isolated to avoid any contact with the atmosphere. Itelmenite forms irregularly shaped grains as well as microcrystalline masses associated closely with anhydrite (Fig. 1, Supplementary Figure S1) and form intergrowths with it. Other associated minerals are saranchinaite (Siidra et al., Reference Siidra, Lukina, Nazarchuk, Depmeier, Bubnova, Agakhanov, Avdontseva, Filatov and Kovrugin2018a), hermannjahnite (Siidra et al., Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018b), euchlorine, thénardite, aphthitalite and hematite.

Fig. 1. Itelmenite (it) grey-blue crystalline aggregates associated closely with white anhydrite (an). Green crystals are euchlorine.
General appearance and physical properties
Crystals of itelmenite are brittle with uneven fracture and lack cleavage. Itelmenite has a white streak and a vitreous lustre. Hardness and density could not be measured due to the lack of suitable material. The estimated Mohs’ hardness is 2–3. The calculated density for the empirical formula obtained by microprobe is 3.10 g/cm3. The mineral is unstable in air. Itelmenite is light grey-blue and non-pleochroic. It is biaxial (+), α = 1.535(2), β = 1.555(2), γ = 1.585(2) (589 nm) and 2V (calc.) = 79.82°. The Gladstone-Dale compatibility index (Mandarino, Reference Mandarino1981), 1 – (K p/K c) = 0.0023, is superior.
Chemical composition
Three crystals (80 × 40 to 40 × 30 µm) of itelmenite, previously checked by single-crystal X-ray, were mounted in epoxy resin and polished with an oil suspension. Ten spot analyses on the grains were obtained using a JEOL Superprobe 733 scanning electron microscope equipped with an Oxford Instruments INCA Energy Dispersive Spectrometer. The electron beam accelerating voltage was 20 kV, electron beam current measured with a Faraday cup was 2 nA and X-ray acquisition live-time was 30 s. System calibration was performed on Ni. The mineral is unstable under the focused electron beam with extensive sodium loss during analysis, therefore a defocused beam (5 µm) was used for analyses. X-ray matrix correction was carried out automatically by the Oxford Instruments INCA version of the XPP routine by Pouchou and Pichoir (Reference Pouchou, Pichoir, Heinrich and Newbury1991). No other elements with Z > 9 than those reported in Table 1 were detected. The empirical formula based on 32 O atoms per formula unit is (Na3.93K0.05)Σ3.98Mg3.12(Cu2.19Zn0.78)Σ2.97S7.97O32. The ideal end-member formula is Na2CuMg2(SO4)4 taking into account structural data discussed below. Itelmenite is soluble in H2O at room temperature and slowly transforms into a hydrate in humid air.
Table 1. Chemical composition (wt.%) of itelmenite.

S.D. – standard deviation.
X-ray crystallography
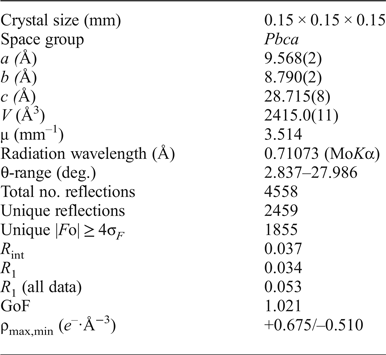
Powder X-ray diffraction data were collected using a Stoe IPDS II diffractometer (MoKα radiation) at the Department of Crystallography, St. Petersburg State University, Russia after crushing the crystal fragments used for the single-crystal analysis. Data (in Å) are given in Table 2. Unit-cell parameters refined from the powder data are as follows: monoclinic, space group Pbca, a = 9.575(5) Å, b = 8.786(4) Å, c = 28.78(1) Å, V = 2416(1) Å3 and Z = 4.
Table 2. Powder X-ray diffraction data for itelmenite.

The strongest lines are given in bold.
A transparent equant crystal fragment of itelmenite was mounted on a thin glass fibre for X-ray diffraction analysis using Bruker APEX II DUO X-ray diffractometer with a micro-focus X-ray tube operated with MoKα radiation at 50 kV and 40 mA. The data were integrated and corrected for absorption using a multi scan type model implemented in the Bruker programs APEX and SADABS (Bruker-AXS, 2014). More than a hemisphere of X-ray diffraction data was collected. The structure was solved by direct methods and successfully refined using SHELX (Sheldrick, Reference Sheldrick2015). The refinement in the Pbca space group converged to R 1 = 0.034 (Table 3). The final model included anisotropic displacement parameters for all atoms. The final atomic coordinates and anisotropic displacement parameters are given in Tables 4 and 5 and selected interatomic distances in Table 6. Lists of observed and calculated structure factors have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Crystallographic data and refinement parameters for itelmenite.
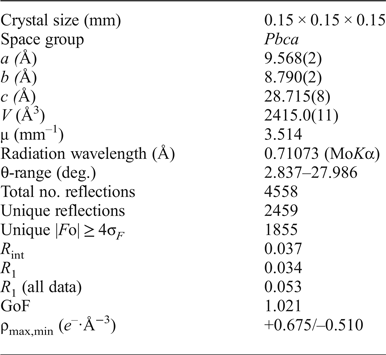
Table 4. Coordinates and isotropic displacement parameters (Å2) of atoms in itelmenite.

*Bond-valence sums, calculated using the parameters for Cu–O, Mg–O, Na–O and S–O bonds from Brown and Altermatt (Reference Brown and Altermatt1985).
Table 5. Anisotropic displacement parameters (Å2) of atoms in itelmenite.

Table 6. Selected interatomic distances (in Å) in itelmenite.

There are four S sites with site-scattering values compatible with occupancy by S6+ and tetrahedrally coordinated by O anions (Table 6). The <S–O> distances demonstrate very similar values of ~1.47 Å, which is in a good agreement with the <S–O> distance of 1.473 Å reported for sulfate minerals by Hawthorne et al. (Reference Hawthorne, Krivovichev, Burns, Alpers, Jambor and Nordstrom2000).
Two symmetrically independent Na sites have different coordination environments (Fig. 2). The Na1 atom forms ten Na–O bonds <3.5 Å thus forming NaO10 polyhedron with the average <Na1–O> distance of 2.742 Å. Coordination environments of the Na2 atom are different with formation of a NaO8 irregular polyhedron and <Na2–O> = 2.561 Å. The Na1 site is partly substituted by K+ in agreement with microprobe data.

Fig. 2. Coordination of atoms in itelmenite.
Three M sites are characterized by the different cationic substitutions in the structure of itelmenite. The M1 and M2 sites form similar MO5 distorted tetragonal pyramids, whereas M3 is roughly symmetrically coordinated by six oxygen atoms thus forming MO6 octahedron (Fig. 2). As the X-ray scattering power of Cu and Zn atoms cannot be distinguished, Cu and Zn were regarded as one group with one scattering factor for the refinement of the M1 and M2 site occupancies. Copper and zinc atoms were summed to form Cu* in atomic fractions. The occupancy of the M3 atom position was refined using scattering factors for Mg and Zn. All M-site occupancies were subsequently fixed at the values given in Table 4 so as to be in agreement with the results of chemical analysis (see above). Refined site scattering and calculated site scattering are in good agreement with each other for all M sites in itelmenite. The [5]-coordinated polyhedron as observed for M1- and M2-centred distorted tetragonal pyramids is rather typical for both Cu2+ and Mg2+ cations.
A projection of the structure of itelmenite along the b axis is shown in Fig. 3. Sulfate tetrahedra are packed into pseudolayered arrangements perpendicular to the a axis (Fig. 4). Na1O10 and Na2O8 polyhedra are also arranged in pseudolayers, but perpendicular to the c axis. The structure topology of itelmenite is rather simple. Each MO5 or MO6 polyhedron shares all common corners with sulfate tetrahedra thus forming heteropolyhedral framework with voids filled by Na+ cations. The framework can be split into A and B layers as specified in Fig. 3. The A layers are formed by M2O5 polyhedra and sulfate tetrahedra, whereas B layers consist of M3O6 octahedra and sulfate tetrahedra. Chains based on M1O5 polyhedra occur in the channels between the layers.

Fig. 3. General projection of the crystal structure of itelmenite along the b axis (above). The heteropolyhedral framework structure of itelmenite consists of A and B corrugated layers formed by M2O5 polyhedra (designated as A) and M3O6 octahedra (designated as B) and SO4 tetrahedra with M1O5-based chains inserted in between.

Fig. 4. Sulfate pseudolayers (above) (SO4 = yellow) and arrangement of Na-centred polyhedra (below) (Na1O10 = purple, Na2O8 = orange) in the structure of itelmenite.
Discussion
The [A 2+3(SO4)4]2– sulfate framework in itelmenite is unique and has not been described before. Itelmenite is the second sulfate mineral after dravertite CuMg(SO4)2 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2017) with Mg and Cu as essential elements. However itelmenite from the Saranchinoitovaya fumarole formed at significantly higher temperature (~600°C) compared to dravertite (~350°C) from the Arsenatnaya fumarole, Second scoria cone of the Great Tolbachik Fissure Eruption. The temperature of gases in the Saranchinaitovaya fumarole had dropped to ~180°C in the summer of 2016, which resulted in the extinction of a rich Cu-sulfate mineralization.
We have obtained by the solid-state method a synthetic analogue of itelmenite in the CuSO4–MgSO4–Na2SO4 anhydrous system. The mixed cationic character of the M1 and M2 sites is very similar to that observed in the natural sample. The symmetrical M3 octahedral site is occupied exclusively by Mg, which is also similar to the structure of itelmenite with a minor admixture of zinc reported above. In a symmetrical coordination environment it is not preferable for Cu2+ to replace Mg2+, however partial substitution is possible for Zn2+ (Siidra et al., Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018b). The following structural formula for synthetic itelmenite was obtained from refinement: Na2(Cu0.81Mg0.19)(Mg0.78Cu0.22)Mg(SO4)4. The synthetic analogue of itelmenite was obtained from a mixture of anhydrous Na2SO4, MgSO4 and CuSO4 by solid-state synthesis in a platinum crucible at 600°C. The temperature of the successful synthesis is in a good agreement with the temperature of gases observed in Saranchinaitovaya fumarole.
It is very probable that a Zn-dominant analogue of itelmenite exists as a mineral species in fumaroles of the Tolbachik Fissure Eruption and the Great Tolbachik Fissure Eruption. Synthetic analogues of Cu exhalative minerals may be of interest from the viewpoint of materials science. Physical properties of the synthetic analogue of itelmenite will be reported in a separate publication in the near future.
Acknowledgements
We are grateful to Nikita Chukanov, Henrik Friis and Peter Leverett for valuable review comments. This work was financially supported by the Russian Science Foundation through the grant 16-17-10085. Technical support by the SPbSU X-ray Diffraction Resource Centre is gratefully acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.089