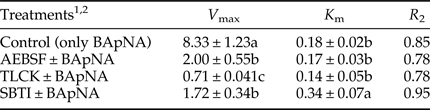Introduction
Digestive serine proteases including trypsin-like, chymotrypsin-like and elastase enzymes are crucial in digesting polypeptides in ingested food (Terra et al., Reference Terra, Ferreira, Jordao, Dillon, Lehance and Billingsley1996). The digestive trypsins are also of interest due to their prevalence amongst insect orders and being the target of synthetic or natural inhibitors. Trypsins possess three amino acids of serine, hystidine and aspartic acid in their active site as a triad, which perform covalent catalytic activity in sequential steps (Buller & Townsend, Reference Buller and Townsend2013). In the first step, an intermediate compound is created when nucleophile part attacks the carbonyl bond of substrate and the base part leads to serine (or nucleophile) activity. Then, a water molecule is displaced within broken section of polypeptide chain, the second intermediated molecule is collapsed and a C-terminal will be available in substrate (Hedstrom, Reference Hedstrom2002). Gene sequence and activity of digestive trypsins have been identified in several insect orders including Hemiptera (mainly salivary secretions), Coleoptera, Lepidoptera, Diptera and etc (Terra & Ferreira, Reference Terra, Ferriera and Gilbert2012). Complete sequence analysis revealed presence of typical components of trypsin-like enzymes as the conserved N-terminal residues IVGG, the catalytic amino acid triad of serine proteinases, three pairs of conserved cysteine residues by disulfide bonds and a residue of Asp 189 (Terra & Ferriera, Reference Terra, Ferriera and Gilbert2012).
Researches consider trypsins as a target in derangements of various physiological systems of insects because of their importance in protein digestion (de Oliveira et al., Reference de Oliveira, Luz, Paiv, Coelho, Marangoni and Macedo2011; Kanost & Clem, Reference Kanost, Clem and Gilbert2012; Terra & Ferriera, Reference Terra, Ferriera and Gilbert2012). Although synthetic inhibitors such as AEBSF.HCl (4-(2-Aminoethyl) benzenesulfonyl fluroride hydrochloride) and TLCK (N-p-Tosyl-L-lysine methyl ester hydrochloride) are commonly used to emboss the presence of trypsins in midgut secretions, many protease inhibitors mainly natural ones e.g. Cowpea Trypsin Inhibitor (CpTi) and Soybean Kunitz Trypsin Inhibitor are regarded in escalating transgenic plants (Lee et al., Reference Lee, Lee, Koo, Chaun, Lim, Mun, Song and Cho1999; Lawrence et al., Reference Lawrence, Nirmala, Jayaveeramuthu and Koundal2001). Destructive effects of synthetic insecticides on human health and so on have directed scientists to seek other approaches for pest control. Inhibitors mainly α-amylase and trypsins could provide opportunities in insect pest control due to their capability in affecting various physiological processes of insects. In fact, potential of enzyme inhibitors in genetically modified plants (resistant varieties) can pave the groundwork in develop a safe method of pest control. Such providence requires full understanding of enzymatic nature and complexity of protease–inhibitor interactions in host-pest systems (Graham & Ryan, Reference Graham and Ryan1997).
Pieris brassicae L. (Lepidoptera: Pieridae) is an economically important pest of several crop plants. We conducted a project to understand biochemical nature of digestive α-amylase and trypsin, their partial gene sequence and responses to specific inhibitors. In our previous work, a digestive α-amylase was purified, biochemically characterized and its behavior in presence of Acarbose, a specific inhibitor, was determined in vivo and in vitro (Sharifloo et al., Reference Sharifloo, Zibaee, Sendi and Talebi Jahroumi2016). In the current study, a digestive trypsin was purified from the midgut of P. brassicae larvae and its biochemical properties were determined by measuring pH, temperature and inhibition.
Materials and methods
Insect rearing
Eggs of P. brassicae were collected from radish fields, transferred to laboratory and maintained at 25 ± 2°C, 85% of relative humidity and 16L:8 h photoperiod. The hatched larvae were daily provided with fresh radish leaves until fifth larval instar (Sharifloo et al., Reference Sharifloo, Zibaee, Sendi and Talebi Jahroumi2016).
Compartmentalization of trypsin activity in the alimentary canal of P. brassicae larvae
Compartmentalization of trypsin activity in the alimentary canal of P. brassicae larvae was determined by dividing the tract into fore-gut, anterior-midgut, posterior midgut and hind-gut. Briefly, fifth instar larvae were dissected in ice cold saline solution (NaCl, 10 mM) and the mentioned sections were removed to be prepared for enzymatic assay. Each part of alimentary canal was homogenized in distilled water and centrifuged at 4°C for 20 min at 13,000 rpm, separately. Also a secretion fraction was prepared by longitudinally cutting the midgut and using the solution exited out, which we considered it as secretion fraction. The secretion was centrifuged at 4°C for 20 min at 13,000 rpm and the supernatant was used as enzyme sample. After sample preparation, trypsin activity was determined in each prepared samples using BApNA (1 mM) (Nα- benzoyl L-Arginine p-nitro aniline) as substrate. Briefly, 25 µl of substrate was mixed with 15 µl of enzyme and 50 µl of universal buffer [Containing 2-(N-Morpholino) ethane sulfonic acid, succinate and glycine 20 mM pH = 8; (Frugoni, Reference Frugoni1957; Zibaee, Reference Zibaee2012)], incubated for 10 min and the optic density was read at 405 nm. A negative control containing all biochemical mixture along with pre-boiled sample was used in the same volume to prove trypsin activity.
Sample preparation for trypsin purification
Fifth instar larvae (<24 h old) were randomly selected and dissected by scalpel in ice-cold saline solution (NaCl, 10 mM). The midguts were homogenized in distilled water and centrifuged at 4°C for 20 min at 13,000 rpm. The obtained supernatants were stored at −20°C for subsequent analyses.
Purification of enzyme
The samples of larval midguts were mixed by ammonium sulfate in concentrations of 30% and 70% prior to be centrifuged at 6000 rpm of 4°C for 20 min. Precipitated samples were dialyzed using tubes containing 12 kDa pores and exposed into a sepharyl G-100 column. The enzyme activity in each fraction (by 1.5 ml volume) was determined and the fractions containing the highest trypsin activity were selected to be loaded onto Diethylaminoethyl (DEAE)-Cellulose fast column as ion exchange chromatography. The column was eluted by different concentrations of NaCl (0.05 M, 0.1 M, 0.2 M, 0.4 M and 0.6 M) and the fractions (by 1 ml volume) with the highest trypsin activity were considered for polyacrylamide gel electrophoresis and biochemical characterization (Zibaee, Reference Zibaee2012).
Polyacrylamide gel electrophoresis
Molecular mass and purity of the trypsin from P. brassicae larvae were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli (Reference Laemmli1970) using Stacking gel of 4% (w/v) and resolving gel of 12% (w/v). Molecular mass of enzyme was estimated using beta-galactosidase (120 kDa), bovine serum albumin (90 kDa), ovalbumin (50 kDa), cardonicanhydrase (35 kDa), beta-lactoglobulin (25 kDa), lysozyme (20 kDa) (Pierce™ Prestained Protein MW Marker; #26612) as the molecular mass standards. The gel was stained by a solution of 0.2% coomassie brilliant blue R-250 in distilled water, ethanol (96%) and acetic acid (in ratio of 5:3:2) overnight. Finally, the gel was distained by distilled water, ethanol (96%) and acetic acid (in ratio of 5:2:1).
Protein assay
Protein concentration was determined by the method of Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951) using bovine serum albumin as standard.
Effect of different range of pH and temperature on purified trypsin
Briefly, 15 µl of the purified trypsin was added into a mixture containing 25 µl of substrate and 50 µl of universal buffer (20 mM, range of 5–12). The mixture incubated at laboratory temperature for 10 min before the OD was read at 405 nm. For optimal temperature, the purified enzyme was added into 25 µl of substrate and 50 µl of universal buffer (20 mM, pH 8 as optimal value) and incubated in different range of temperature including 20, 25, 30, 35, 40, 45, 50 and 55°C. After 10 min of incubation, the trypsin activity was read at 405 nm.
Effect of specific inhibitors on the purified trypsin in vitro
Different concentrations (0.5, 1 and 2 mM) of AEBSF.HCl (Thermo Scientific, USA, 78431) (Walsmann et al., Reference Walsmann, Richter and Markwardt1972), TLCK (Sigma-Aldrich, T5012) (Shaw et al., Reference Shaw, Mares-Guia and Cohen1965) and SBTI (Soybean Trypsin Inhibitor, Sigma-Aldrich, T6522) were selected to find changes in activity of the purified trypsin from P. brassicae larvae. The reaction mixture contained 10 µl of purified enzyme, 50 µl of universal buffer (pH 8), 25 µl of substrate and 10 µl of each inhibitor in the given concentrations, separately. The mixtures were incubated for 10 min at laboratory temperature and the OD was read at 405 nm.
Kinetic parameters of the purified α-amylase
Kinetic parameters of the purified trypsin were determined using different concentrations (0.1, 0.3, 0.5, 0.7, 1 mM) of BApNA as substrate. The enzymatic assay was done as described earlier and maximum velocity (V max) and constant of Michealis–Menten (K m) were estimated by Sigma plot software, version 6. Moreover, different concentrations of BApNA were incubated by 1 mM of each substrate (AEBSF.HCl, TLCK and SBTI) to find type of inhibition.
In vivo assay of specific inhibitors
Radish leaves were treated by 1 mM concentration of SBTI, TLCK, AEBSF.HCl prepared in Triton X-100 solution (0.02%) while control leaves were only treated by Triton X-100 solution (0.02%). Leaves were separately immersed in each solution for 20 seconds then put on filter paper (Whatman No. 1) for 30 min to be dried (Sharifloo et al., Reference Sharifloo, Zibaee, Sendi and Talebi Jahroumi2016). The fourth instar larvae were weighted and fed on control and treated leaves (already weighted) separately for 72 h. During this period, several items such as fresh leaves, larvae, feces and leaves remnants were weight in intervals of 24 h (dry weight). Then, nutritional indices were measured according to Scriber & Slansky (Reference Scriber and Slansky1981); Approximate digestibility (AD) = (dry weight of food consumed-dry weight of feces produced/dry weight of food consumed)× 100, efficiency of conversion of ingested food (ECI) = (dry weight gained o insects/dry weight of food consumed) × 100, efficiency of conversion of digested food (ECD) = dry weight gain of the insect/(dry weight of food consumed-dry weight of feces produced) × 100, consumption index (CI) = dry weight of food consumed/mean of insect dry weight over unit time, relative consumption rate (RCR) = dry weight of food consumed/(initial weight of larvae × duration of feeding period), relative growth rate(RGR) = dry weight of gain of the insect/(initial weight of larvae × duration of feeding period) and metabolic cost (MC) = 100-ECD. For each treatment, 16 larvae were selected in four replicates. At the end of experiment, the live larvae were dissected and used for trypsin assay.
Statistical analysis
All data were compared by one-way analysis of variance (ANOVA) followed by Tukey test using SAS software (Version 9.3, SAS Inc.). Differences between samples were considered statistically significant at P < 0.05 and marked in figures and tables by letters.
Results
Compartmentalization of trypsin activity in the alimentary canal of P. brassicae larvae
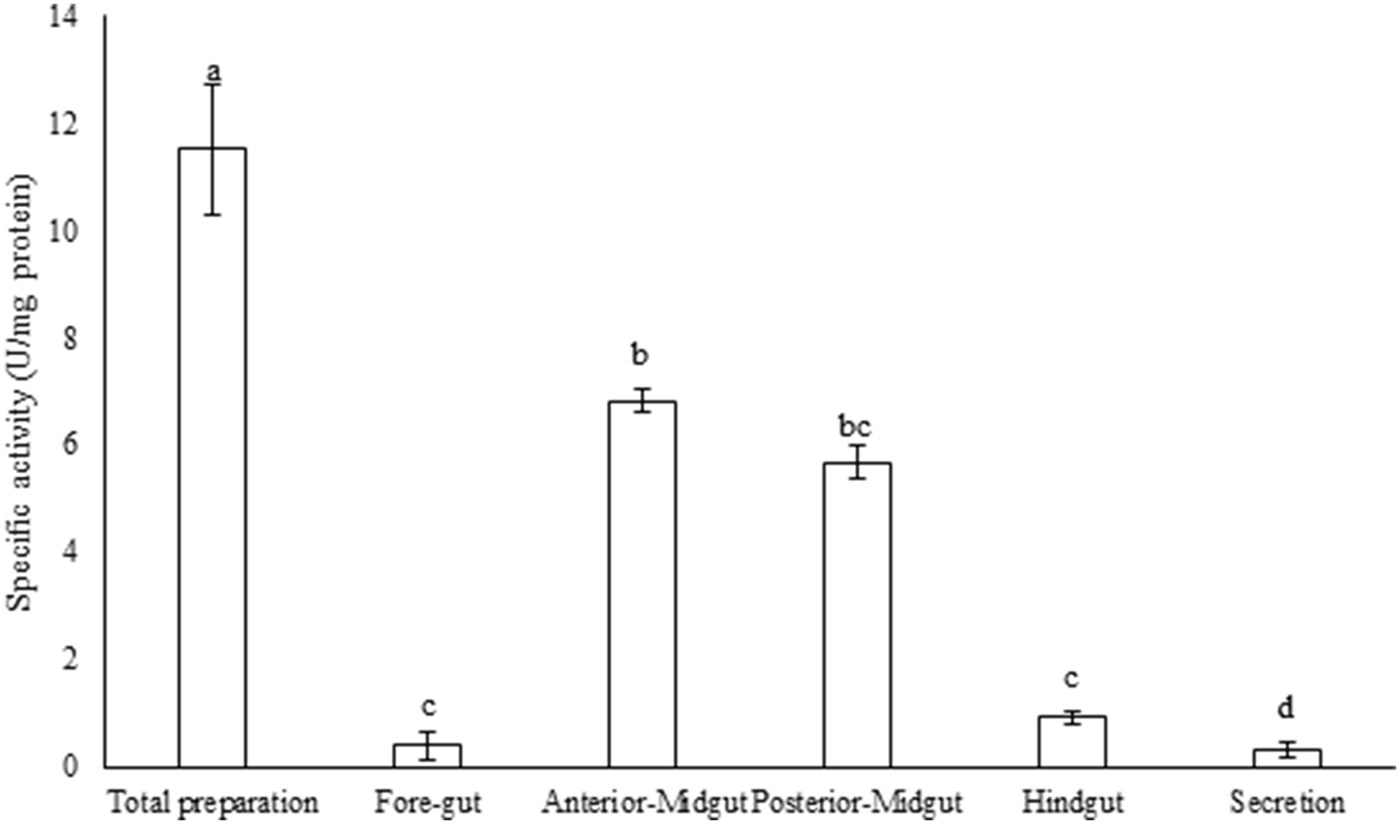
Compartmentalization in the alimentary canal of P. brassicae larvae showed the highest trypsin activity in the larval midgut whereas only trace activities were observed in fore and hindgut although the lowest trypsin activity was obtained in the secretion preparation of the midgut (fig. 1; F = 4.654, df = 10, P < 0.000483). In midgut preparations, anterior-midgut showed statistically higher trypsin activity than posterior-midgut (fig. 1). Moreover, the cumulative activity of trypsin has been obtained in the total preparation as 11.08 U mg−1 protein (fig. 1).
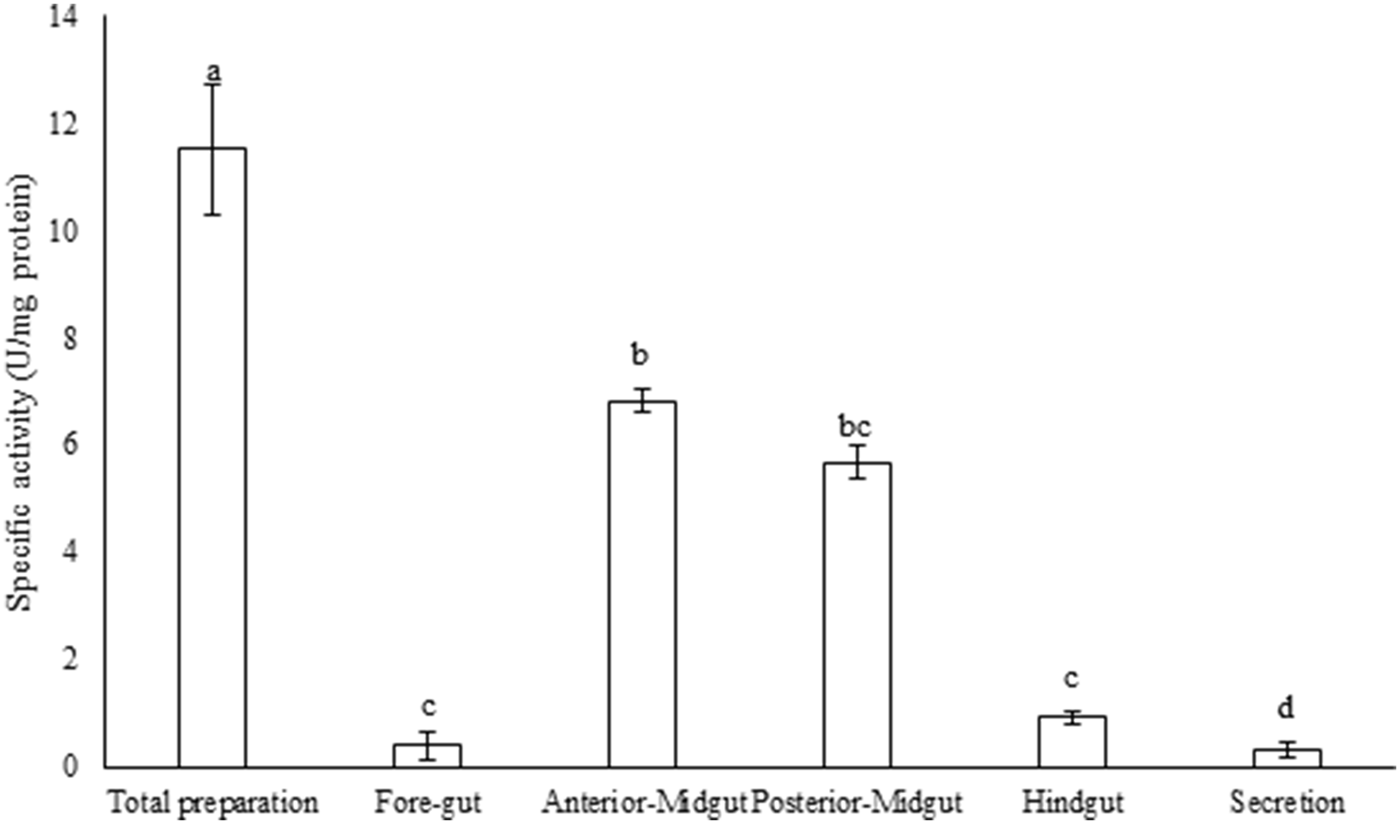
Fig. 1. Compartmentalization of trypsin activity in the alimentary canal of the fifth instar larvae of Pieris brassicae. Statistical differences have been marked by different letters (P ≤ 0.05, Tukey test).
Purification of digestive trypsin
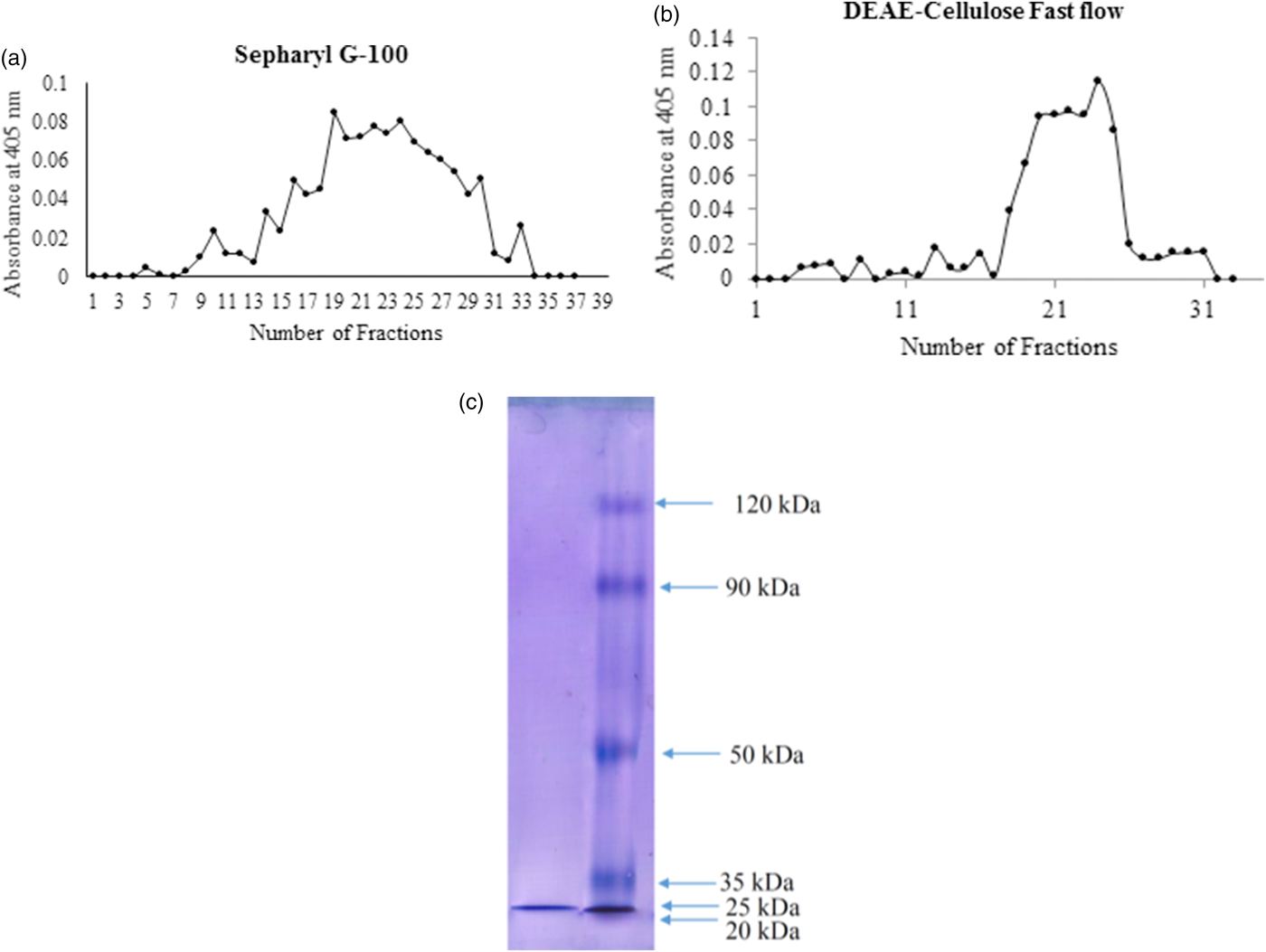
After final ammonium sulfate precipitation (70%), the enzyme had a specific activity of 0.92 U mg−1 protein and a purification fold of 1.22 (table 1). These samples were loaded onto Sepharyle G-100 column to get 37 fractions by 1.5 ml of volume. The fractions 19–25 with the highest trypsin activity were pooled to be exposed on ion exchange chromatography (fig. 2a). In DEAE-cellulose fast flow column, fractions 20–25 demonstrated the highest trypsin activity so they were pooled and considered to determine purity and molecular weight (fig. 2b). The samples obtained by the final chromatography step had the specific activity of 21 U mg−1 protein, recovery of 22.8% and purification fold of 28-fold (table 1). Moreover, a single band was observed in SDS-PAGE and molecular weight of the purified trypsin found to be 25 kDa (fig. 2c).
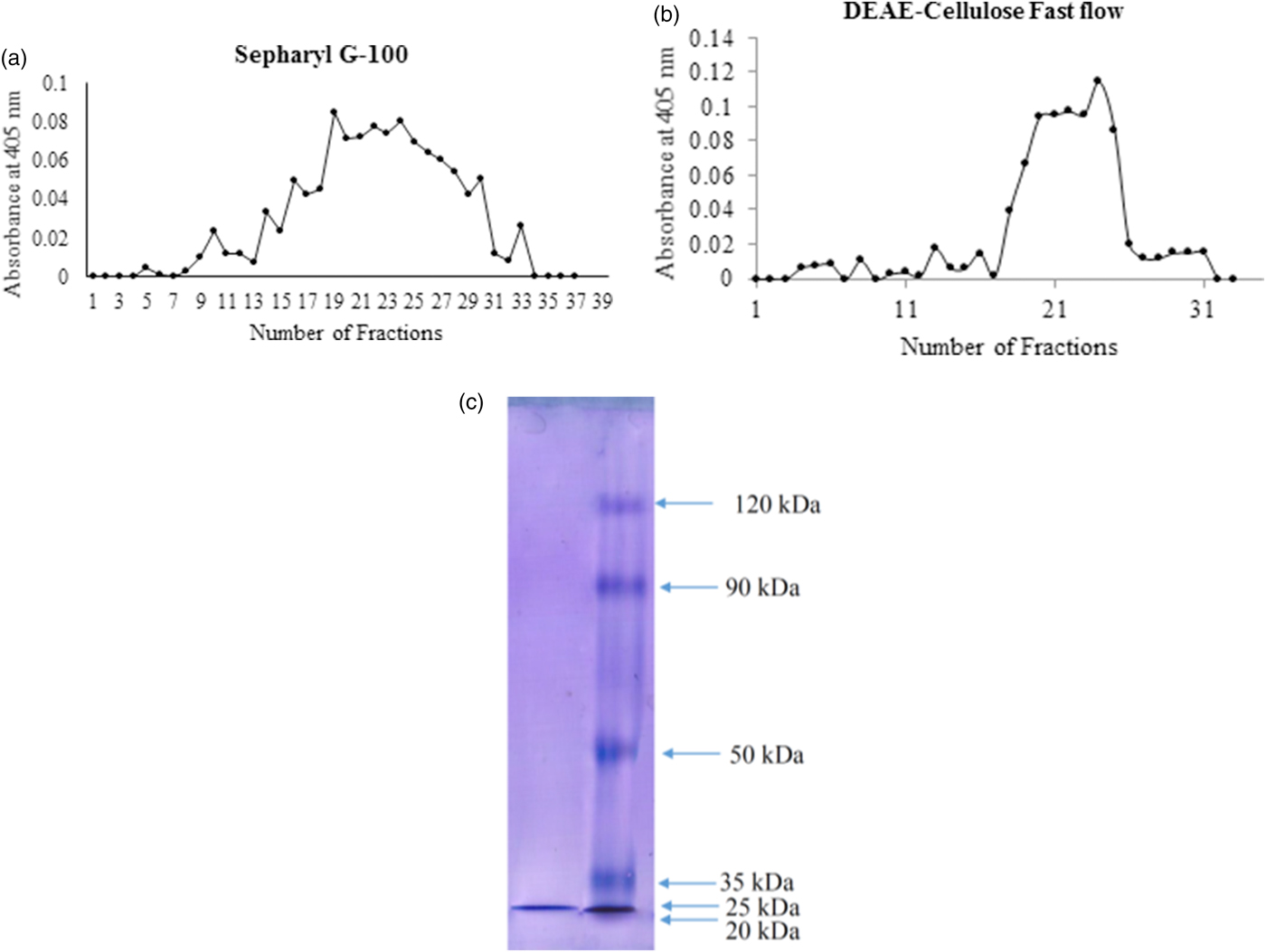
Fig. 2. Sepharyl G-100 (a), DEAE chromatographies (b) and molecular mass (c) determination of the digestive trypsin in the fifth instar larvae of Pieris brassicae.
Table 1. Purification of digestive trypsin in the fifth instar larvae of Pieris brassicae.

Effect of temperature and pH on purified trypsin activity
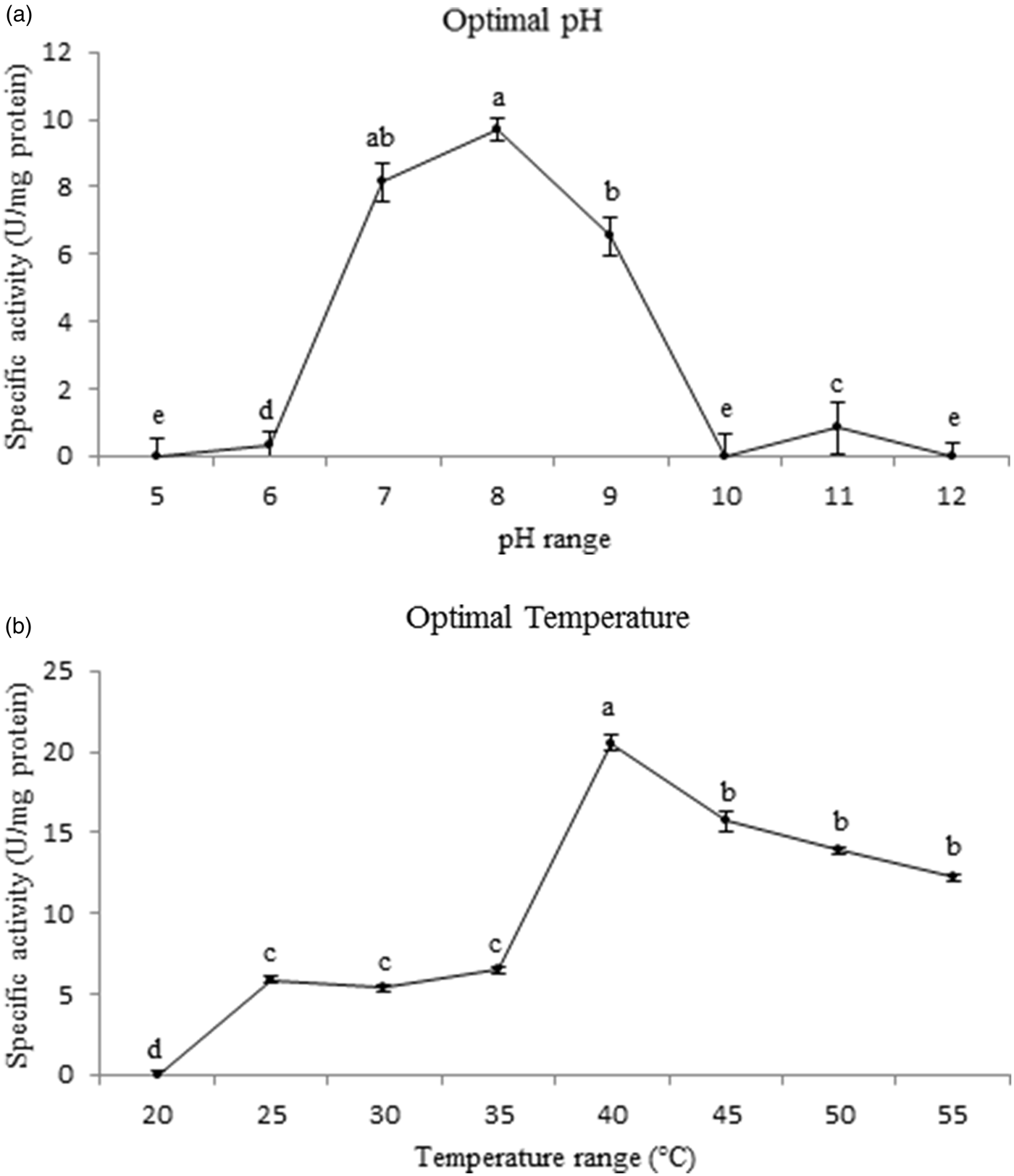
The purified trypsin of P. brassicae larvae showed the highest activity at pH 8 although a slight significant difference was found in comparison with pH 7 (fig. 3; F = 4.8962, df = 16, P < 0.001393). The enzyme also had showed significantly highest activity at 40°C among all temperature regimes (20–55°C) (fig. 3; F = 4.8966, df = 16, P < 0.000478).
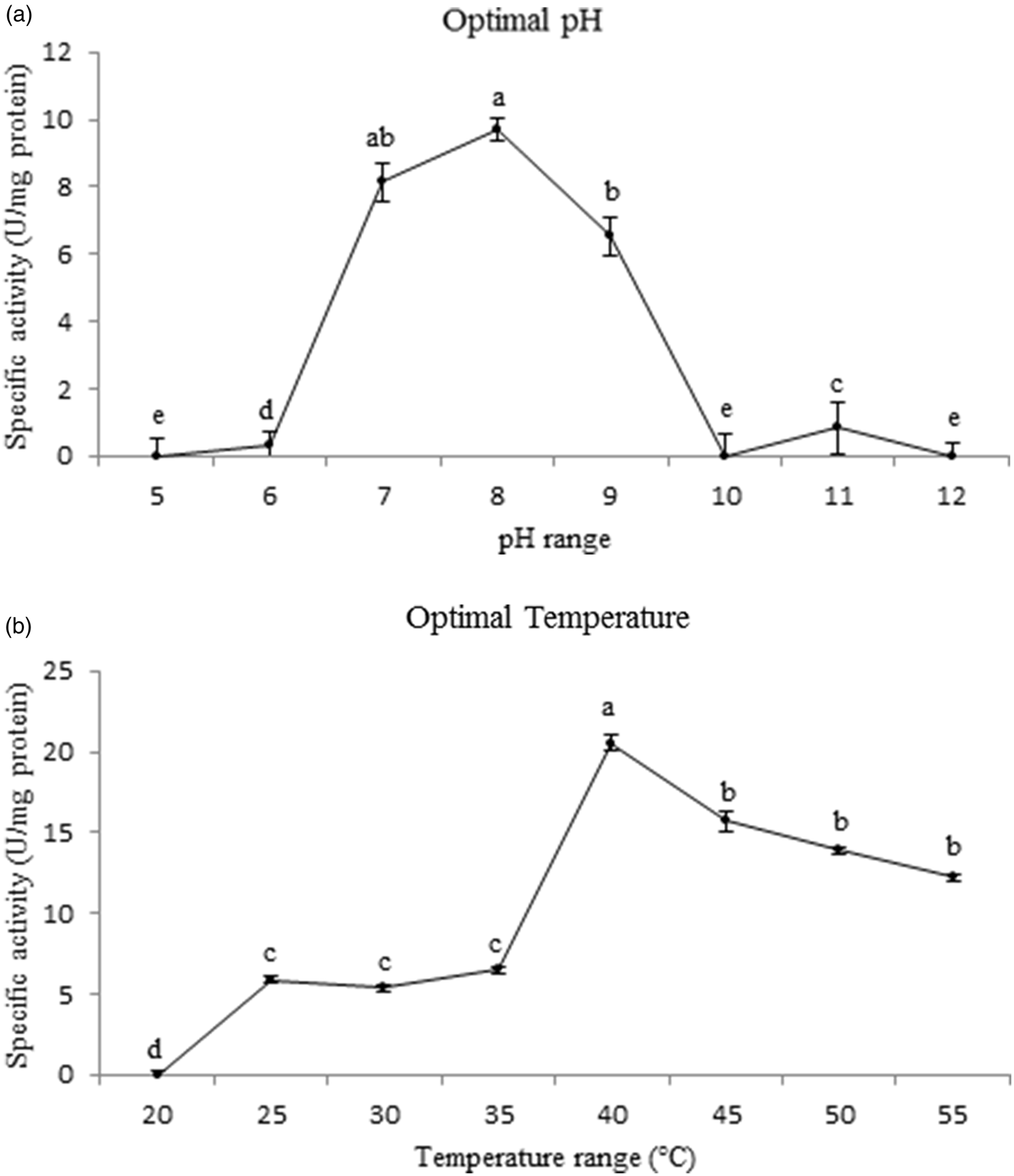
Fig. 3. Effect of pH and temperature on the purified trypsin activity in the fifth instar larvae of Pieris brassicae. Statistical differences have been marked by different letters (P ≤ 0.05, Tukey test).
In vitro effect of specific inhibitors on purified trypsin
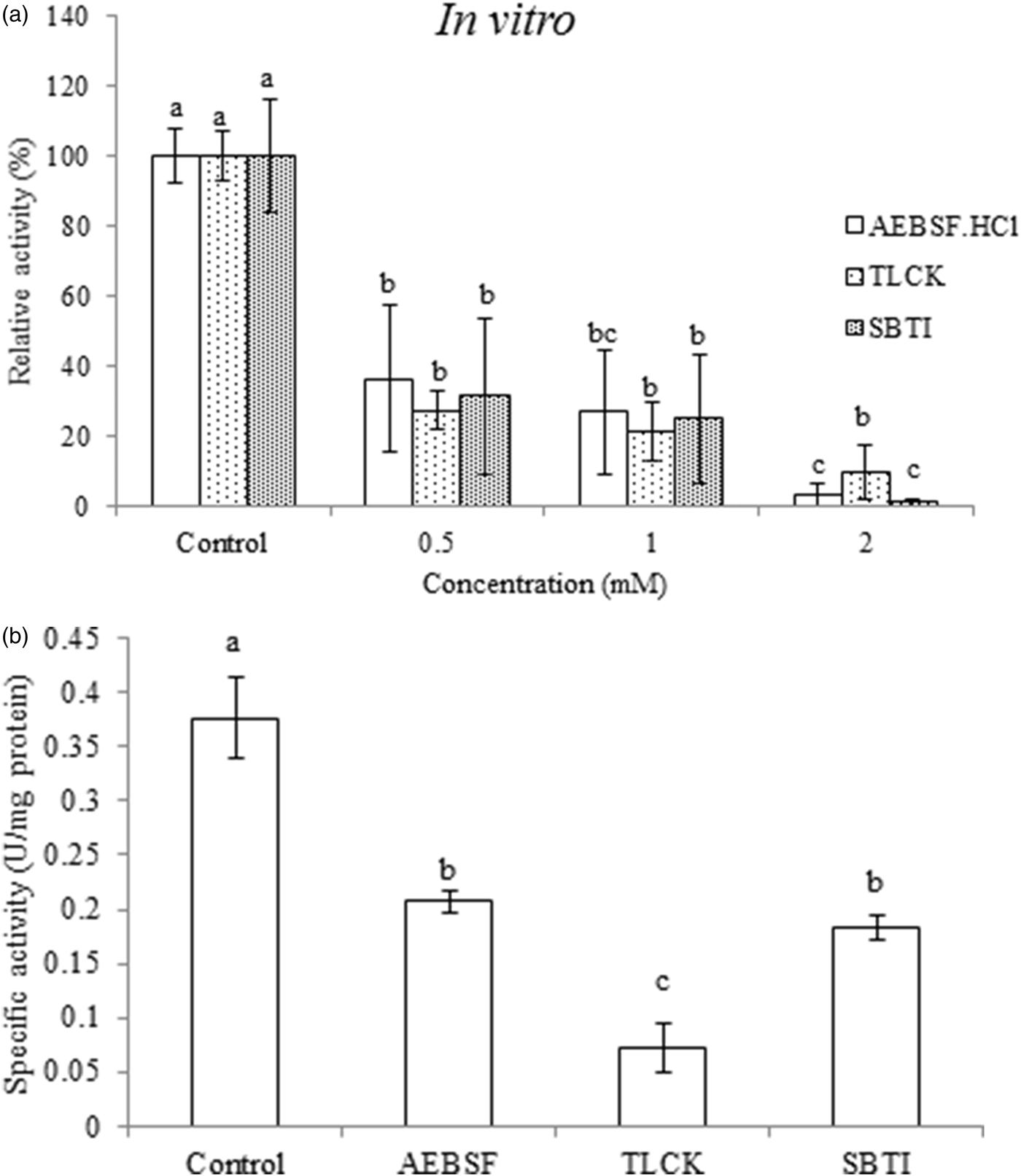
Three specific inhibitors including AEBSF.HCl (Inhibitor of serine proteases), TLCK and SBTI (Inhibitor of trypsin proteinases) were prepared in different concentrations (0.5, 1 and 2 mM) and were incubated with purified trypsin of P. brassicae larvae in vitro. All inhibitors significantly lowered the activity of enzyme from 36 to 1% in a dose-dependent manner (fig. 4a; AEBSF.HCl; F = 4.528, df = 8, P < 0.000112; TLCK F = 4.528, df = 8, P < 0.000079; SBTI F = 4.582, df = 8, P < 0.000011). Moreover, kinetic analysis by Linweaver–Burk plots indicated a lower V max value in presence of AEBSF.HCl, TLCK and SBTI in which the lowest value was found in case of TLCK (table 2; F = 7.544, df = 8, P < 0.000039). The highest value of K m was found in the purified trypsin incubated with SBTI while no statistically significant differences were found among AEBSF.HCl, TLCK and the control (table 2; F = 11.614, df = 8, P < 0.000379).
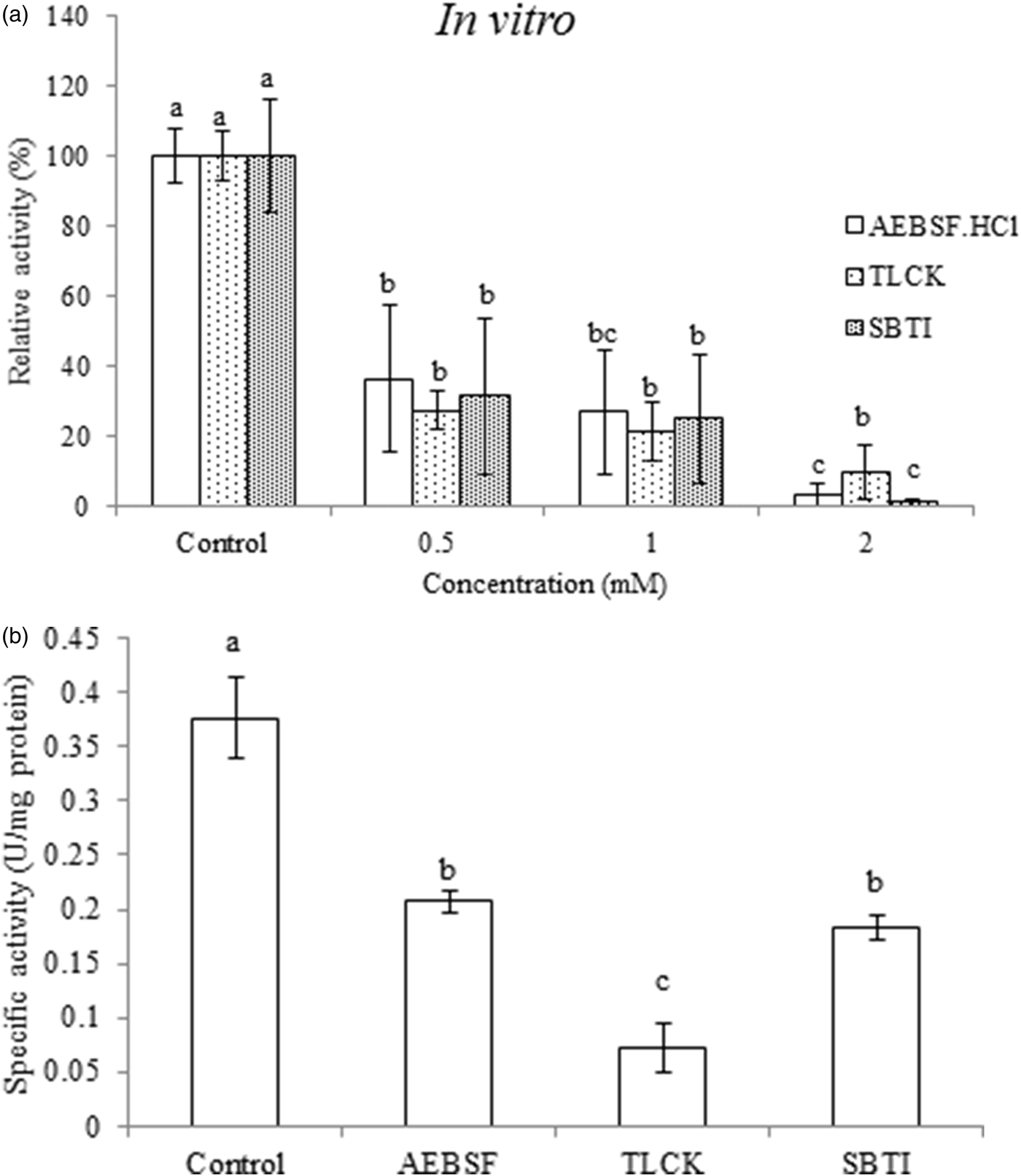
Fig. 4. (a) Effect of different concentrations of specific inhibitors on the purified trypsin in the fifth instar larvae of Pieris brassicae. (b) In vivo effects of specific inhibitors on the trypsin activity in the fourth instar larvae of Pieris brassicae. Statistical differences have been marked by different letters (P ≤ 0.05, Tukey test).
Table 2. Kinetic parameters of the purified trypsin from the midgut of Pieris brassicae larvae.

1 IC50 concentration of AEBSF.HCL, TLCK and SBTI were used for kinetic experiment.
2 Statistical differences have been marked by different letters (Tukey test, P ≤ 0.05).
In vivo assay of specific inhibitors on trypsin activity and larval nutritional indices
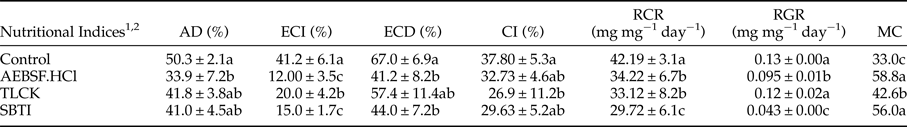
Larval treatments by 1 mM concentration of AEBSF.HCl, TLCK and SBTI caused significantly alleviated activity of digestive trypsin so that larvae fed on leaves treated with TLCK showed the lowest enzymatic activity (fig. 4b; F = 4.52887, df = 8, P < 0.00154). All the three inhibitors significantly affected nutritional indices in comparison with the control (table 3). The larvae fed on AEBSF.HCl treated leaves showed the lowest values of AD (table 3; F = 5.236, df = 8, P < 0.00712) while the lowest values of ECI and ECD were found in the larvae fed on both AEBSF.HCl and SBTI treated leaves. However, the control larvae showed the highest values in all parameters (table 3; ECI: F = 4.251, df = 8, P < 0.000173; ECD: F = 4.5842, df = 8, P < 0.00159). The larvae fed on TLCK treated leaves demonstrated the lowest value of CI (F = 5.729, df = 8, P < 0.00284) but the lowest values of RCR and RGR values were obtained in the leaves treated by used inhibitors (table 3; RCR: F = 6.581, df = 8, P < 0.00145; RGR: F = 7.925, df = 8, P < 0.000432). Finally, larvae feeding on both AEBSF.HCl and SBTI were imposed to have the highest value of MC in comparison with control larvae (table 3; F = 3.883, df = 8, P < 0.00563).
Table 3. Nutritional Indices in Pieris brassicae larvae that treated with AEBSF.HCl, TLCK and SBTI.
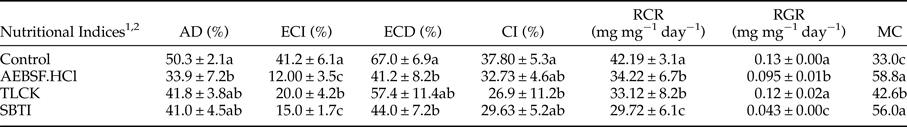
1 Approximate digestibility (AD), efficiency of conversion of ingested food (ECI), efficiency of conversion of digested food (ECD), consumption index (CI), relative consumption rate (RCR), relative growth rate (RGR), metabolic cost (MC).
2 Statistical differences have been marked by different letters (Tukey test, P ≤ 0.05).
Discussion
Trypsins are among the most widespread enzymes in insects that play several important roles in insects including, molting, tissue remodeling, innate immunity, diapause, fertilization, activation of enzymatic precursors and virulence of entomopathogens (Lazarevic & Jankovic-Tomanic, Reference Lazarevic and Jankovic-Tomanic2015). As a digestive enzyme, trypsin can be found in almost all insect orders by different feeding habits and engaged in both digestion of dietary proteins and some xenobiotic compounds (de Oliveira et al., Reference de Oliveira, Luz, Paiv, Coelho, Marangoni and Macedo2011). Although majority of researches have shown its activity as a lumen free enzyme, its distribution varied across digestive tract mainly midgut. In our study, compartmentalization of trypsin activity showed the highest activity in the midgut of P. brassicae larvae although anterior-midgut contained a higher trypsin activity than posterior-midgut. Varied distribution of trypsins has been reported in different parts of insect midguts such as midgut caeca of Orthoptera, anterior midgut of Phthiraptera, Phasmida, Hymenoptera and Lepidoptera, whole midgut of Coleoptera and Diptera as well as posterior midgut of Dictyoptera (Lazarevic & Jankovic-Tomanic, Reference Lazarevic and Jankovic-Tomanic2015). These differences may reflect feeding habit, pH midgut and involvement of different proteases in digestion of proteins in an insect. A previous study reported presence of all serines (Trypsin, Chymotrypsin and Elastase), amino- and carboxypeptidases in the midgut of P. brassicae larvae so, there may be an arrangement in engagements of proteases in digestive process (Zibaee, Reference Zibaee2012). Since dietary proteins are initially digested by endopeptidase e.g like serines and cysteines so, the higher activity of trypsin in anterior-midgut of P. brassicae larvae may point out its role as a pioneer enzyme to digest proteins.
Researches on purification and characterization of insect digestive trypsins show a molecular mass of 18–38 kDa, alkali pH of ≥8 and temperatures of 20–60°C (See Below). Our results showed an enzyme by molecular mass of 25 kDa, optimal pH of 8 and optimal temperature of 40°C. In lepidopterans, the purified trypsins have shown molecular mass of 24.6 kDa from Ostrinia nubilalis Hubner (Lepidoptera: Crambidae), to 24 kDa in Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), then 28.7 in Diatraea saccharalis Fabricius (Lepidoptera: Crambidae), then 24–27 kDa in Sesamia nonagrioides Lefebvre (Lepidoptera: Noctuidae), Helicoverpa armigera Hubner (Lepidoptera: Noctuidae), further 35.8 kDa in Ectomyelois ceratoniae Walker (Lepidoptera: Pyralidae) and finally 18.8 kDa in H. armigera (Bernardi et al., Reference Bernardi, Tedeschi, Ronchi and Palmieri1996; Marchetti et al., Reference Marchetti, Chiaba, Chiesa, Bandiera and Pitotti1998; Novillo et al., Reference Novillo, Castanera and Ortego1999; Telang et al., Reference Telang, Giri, Sainani and Gupta2005; Lopes et al., Reference Lopes, Juliano, Marana, Juliano and Terra2006; Ranjbar et al., Reference Ranjbar, Zibaee and Sendi2014; Grover et al., Reference Grover, Kaur, Gupta, Kumar and Kaur2016). Although two exceptions have been reported in the molecular mass of two trypsins from Anticarsia gemmatalis Hubner (Lepidoptera: Noctuidae) and Naranga aenescens Moore (Lepidoptera: Noctuidae) as 66–91 and 88.5 kDa (Oliveira et al., Reference Oliveira, Simone, Xavier and Geudes2005; Zibaee et al., Reference Zibaee, Bandani, Fazeli-Dinan, Zibaee, Sendi and Maleki2011). The similar range of trypsin molecular mass in Lepidoptera may reflect the genetic basis of their secretion or phylogenetic distance of species. Moreover, the exceptional high molecular mass observed in some species may be attributed to oligomerization during protein digestion, which interferes with binding of proteinaceous inhibitors within their food (Lazarevic & Jankovic-Tomanic, Reference Lazarevic and Jankovic-Tomanic2015).
The alkali pH and temperature of 40°C have been observed as the optimal value for activity of the purified trypsin in P. brassicae larvae. The alkali pH has been reported for almost all purified lepidopteran trypsins including O. nubilalis (9.5), S. littoralis (9–11), S. saccharalis (105.), S. nonagrioides (10.5), A. gemmatalis (8), H. armigera (10–11), N. aenescens (10), E. ceratoniae (9) (Bernardi et al., Reference Bernardi, Tedeschi, Ronchi and Palmieri1996; Marchetti et al., Reference Marchetti, Chiaba, Chiesa, Bandiera and Pitotti1998; Novillo et al., Reference Novillo, Castanera and Ortego1999; Oliveira et al., Reference Oliveira, Simone, Xavier and Geudes2005; Telang et al., Reference Telang, Giri, Sainani and Gupta2005; Lopes et al., Reference Lopes, Juliano, Marana, Juliano and Terra2006; Zibaee et al., Reference Zibaee, Bandani, Fazeli-Dinan, Zibaee, Sendi and Maleki2011; Ranjbar et al., Reference Ranjbar, Zibaee and Sendi2014). The alkali pH in the midgut of lepidopterous larvae indicates two main phenomena in their digestion. First, it increases efficacy of food processing by increasing solubility of digested peptides to be further hydrolyzed by other proteases or absorption via midgut epithelial membrane. Second, it provides adaptability when the larvae fed on the diets rich in plant allelochemicals like tanins, plyphenols, terpenes, glucosinolates and etc. The latter phenomenon solubilizes hemicelluloses and other constituents of plant fibers to improve extractability of proteins and to elevate autoxidative cleaving plant allelochemicals and lignin-carbohydrate complexes (Lazarevic & Jankovic-Tomanic, Reference Lazarevic and Jankovic-Tomanic2015). Since larvae of P. brassicae utilize leaves of Brassicaceae plants rich in allelochemicals, alkali pH of their midgut (which also has been already reported for digestive lipase and α-amylase of P. brassicae) is a critical factor to increase digestibility of dietary proteins from host leaves (Zibaee, Reference Zibaee2012; Sharifloo et al., Reference Sharifloo, Zibaee, Sendi and Talebi Jahroumi2016).
Temperature is one of the biochemical characteristics of enzyme, which somehow is related to the environmental conditions of insect evolution (Lazarevic & Jankovic-Tomanic, Reference Lazarevic and Jankovic-Tomanic2015). Also, structural nature of an enzyme may affect the optimal and extreme temperatures of its activity or denaturation. The optimal temperature of 40°C was found for the activity of purified trypsin in P. brassicae larvae, which was the highest value in comparison with optimal temperatures of 30°C and 35°C for the purified lipase and α-amylase in this insect (Zibaee, Reference Zibaee2012; Sharifloo et al., Reference Sharifloo, Zibaee, Sendi and Talebi Jahroumi2016). Since the source of larval collection from field and rearing condition were the same for both previous and current researches, differences in optimal temperature values may reflect structural nature of the purified lipase, α-amylase and trypsin of P. brassicae larvae. However, 30°C was the optimal temperature values for trypsins of lepidopterous larvae except for O. nubilalis (53°C), Spodoptera litura Fabricius (Lepidoptera: Noctuidae), (50–60°C), Spilosoma oblique Walker (Lepidoptera: Erebidae) (50–60°C) and E. ceratoniae (20°C) (Ahmad et al., Reference Ahmad, Saleemuddin and Siddiqui1980; Bernardi et al., Reference Bernardi, Tedeschi, Ronchi and Palmieri1996; Anwar & Saleemudin, Reference Anwar and Saleemudin2002).
Digestive trypsins of insects are inhibited by specific trypsin and serine inhibitors of TLCK and AEBSF.HCL. Although some of the purified trypsins were susceptible to a plant origin one called SBTI (Terra & Ferriera, Reference Terra, Ferriera and Gilbert2012). In our study, all three mentioned inhibitors significantly decreased trypsin activity both in vitro using purified enzyme and in vivo when the larvae fed on leaves treated separately with AEBSF.HCl, TLCK and SBTI. Moreover, these inhibitors significantly decreased nutritional indices of the treated larvae in comparison with control. Kintetic analysis of the purified trypsin using Linweaver–Burk plots revealed biochemically the inhibitory mechanisms of each inhibitor. Since V max in the purified enzyme incubated with AEBSF.HCl and TLCK showed statistically lower values but K m showed no significant differences in comparison with control. It can be concluded that these inhibitors suppressed enzymatic activity via non-competitive mechanisms. While SBTI pointed out a mixed inhibition mechanism, since the value of V max in the treated enzyme was significantly decreased but K m showed a higher value in comparison with the control. In non-competitive inhibition, the inhibitor usually binds to enzymes regardless of substrate binding and reduces the activity of target enzyme (Stryer, Reference Stryer2000). On the other hand, the created complex of enzyme-substrate-inhibitor has no ability to get product and can only be converted back to the enzyme-substrate or the enzyme-inhibitor complexes. This type of inhibition is similar to mixed inhibition but in the current one, the inhibitor has an equal affinity as substrate to be bound with the enzyme (Stryer, Reference Stryer2000). Moreover, mixed inhibition imposes an allosteric effect, which leads to conformation changes (i.e. tertiary structure or three dimensional shape) and alleviating affinity of the substrate to the active site of enzyme (Zibaee et al., Reference Zibaee, Bandani, Fazeli-Dinan, Zibaee, Sendi and Maleki2011).
The current study reported several biochemical characteristics of a digestive trypsin in P. brassicae larvae. The characterized trypsin here showed higher activity in anterior midgut, alkali pH, sensitivity to the common inhibitors and somehow similar nucleotide sequence to other butterflies from Papilionidae to Saturnidae. Trypsin is one of the enzymes, which plays crucial roles not only in digestive processes of lepidopterans but also in severally other physiological processes. So, full understanding of its structure, reactions to synthetic or natural compounds and distribution in digestive tract or other tissues would elucidate its importance in an insect. Similarly, important are understanding the potential of physiological techniques such as natural trypsin inhibitors and RNA-interfering to suppress biological fitness of insect pests. Moreover, serine proteases mainly serines, which are stable against high temperatures and some synthetic compounds have shown interesting potential to be used in industrial processing as additives in stain removers, detergents, and other bio-formulations which is the case in the current study that has highlighted other applications of insect proteases.
Acknowledgements
This research was supported by a grant of University of Guilan. We appreciate Dr Ali Aalami for his assistances in gene expression facilities. The primers to find trypsin sequence of P. brassicae were kindly sent by Prof. Sudeshna Mazumdar–Leighton from Delhi university whom the authors appreciate her attitude and help during the experiments.