Introduction
Biodiversity in South-East Asia is decreasing at an alarming rate due to habitat fragmentation, deforestation, poaching and hunting, and climate change (BirdLife International 2001, IUCN 2017a, 2017b, Tordoff et al. Reference Tordoff, Appleton, Eames, Eberhardt, Hla, Thwin and Aung2007). The exact number of avian species that have recently disappeared, and apparently have become extinct, from the region is uncertain, but the current IUCN Red List of Threatened Species has categorised an additional 39 species of birds as ‘Critically Endangered’ and on the verge of extinction (IUCN 2018). The White-bellied Heron Ardea insignis, hereafter ‘WBH’ is one species on this list (IUCN 2017a). It was listed as threatened in 1988, uplisted to ‘Endangered’ in 1994, and to ‘Critically Endangered’ since 2007 (IUCN 2017b). It is also the 94th species of the Top 100 EDGE Birds with an EDGE score of 4.9 and 7.4 ED score on the EDGE of Existence list, which highlights and protects some of the unique species on the planet which are on the verge of extinction (EDGE of Existence 2018). Although the estimated WBH population is 50–249 adults (IUCN 2017b), only 60 are confirmed to exist as per the WBH International Workshop held in Bhutan in 2015 (White-bellied Heron International Workshop Resolutions 2015).
The current WBH population is fragmented into three subpopulations; Bhutan, north-east India, and Myanmar (Tordoff et al. Reference Tordoff, Appleton, Eames, Eberhardt, Hla, Thwin and Aung2007, Maheswaran Reference Maheswaran2007, Reference Maheswaran2008, Royal Society for Protection of Nature Reference Pradhan and Frederick2011, Mondal and Maheswaran Reference Mondal and Maheswaran2014). Historically known across most of the Himalayan region, WBH is currently one of the rarest birds in the world, having disappeared from most of its historical range including Nepal and Bangladesh (Price and Goodman Reference Price and Goodman2015, IUCN 2017a). The most recent surveys in the range countries have found 29 individuals in Bhutan, 6–8 birds expected in India and fewer than 25 in northern Myanmar (Price and Goodman Reference Price and Goodman2015). The population is believed to be declining rapidly (RSPN 2009a, IUCN 2017a). Widespread loss of riverine habitats and low breeding success are presumed to be the primary threats (Pradhan et al. Reference Pradhan, Norbu and Frederick2007, RSPN 2009a, 2008, 2015, IUCN 2017a).
While there have been some infrequent sightings from the range countries, records of nesting and breeding of this species are extremely rare. Before the 1930s, only two nests presumed to be of this bird were found: one was recorded in Darjeeling, India before 1890; another in Myanmar, before 1930 (Baker et al. Reference Baker, Blanford and Oates1922, Baker Reference Baker1928, Walters Reference Walters1976, IUCN 2017a). An active nest was found in Bhutan in 2003 (RSPN 2006, Royal Society for Protection of Nature Reference Pradhan and Frederick2011) more than 70 years since the previous record, which gave new hope to conservationists. Since then, two to five active nests have been located in Bhutan from which two to eight chicks have fledged annually (Pradhan et al. Reference Pradhan, Norbu and Frederick2007, Reference Pradhan, Acharja, Tshering and Lhendup2016, Reference Pradhan, Acharja, Tshering and Lhendup2017, Pradhan Reference Pradhan2008, Royal Society for Protection of Nature Reference Pradhan and Frederick2011, RSPN 2016).
In India, one active nest was discovered in Namdapha Tiger Reserve in 2014 (Mondal and Maheswaran Reference Mondal and Maheswaran2014), but there was no evidence of breeding success. Nest monitoring throughout the breeding season was impossible due to heavy rain and flooding in the area. The research team left the site on 20 March 2014, and they did not find any remnants of the nest when they revisited the site three months later (G. Maheswaran pers. comm. 5 December 2015). There are no recent records of successful nesting and breeding in the area. There are also no records of active breeding nests in Myanmar. Therefore, Bhutan is the only country with documented nests and pairs breeding successfully every year for the last 16 years.
The Royal Society for the Protection of Nature (RSPN), a conservation NGO in Bhutan (www.rspnbhutan.org) has been working on the conservation of the WBH since the discovery of the first nest in the country in 2003. In the last 16 years, the researchers at RSPN have identified 22 nests, up to five active breeding pairs a year, and 14 active foraging localities.
Solitary nesting behaviour is one of the distinct characteristics of WBH from its sister-species that nest colonially (Royal Society for Protection of Nature Reference Pradhan and Frederick2011). Generally, lone birds are seen feeding along the rivers, except during the courtship period when 2 to 4 birds may be found together. Juveniles and parents are seen feeding together or juveniles following the parents during the early post-fledging period, which can form colonies of up to 6, depending on brood size. The breeding season lasts nearly six months, starting in late February through July (RSPN 2009a, Reference Pradhan and Frederick2011). Observation by RSPN’s researchers found that the juveniles fledge (i.e. capable of sustained flight) after 70–74 days between June to mid-July (Royal Society for Protection of Nature Reference Pradhan and Frederick2011).
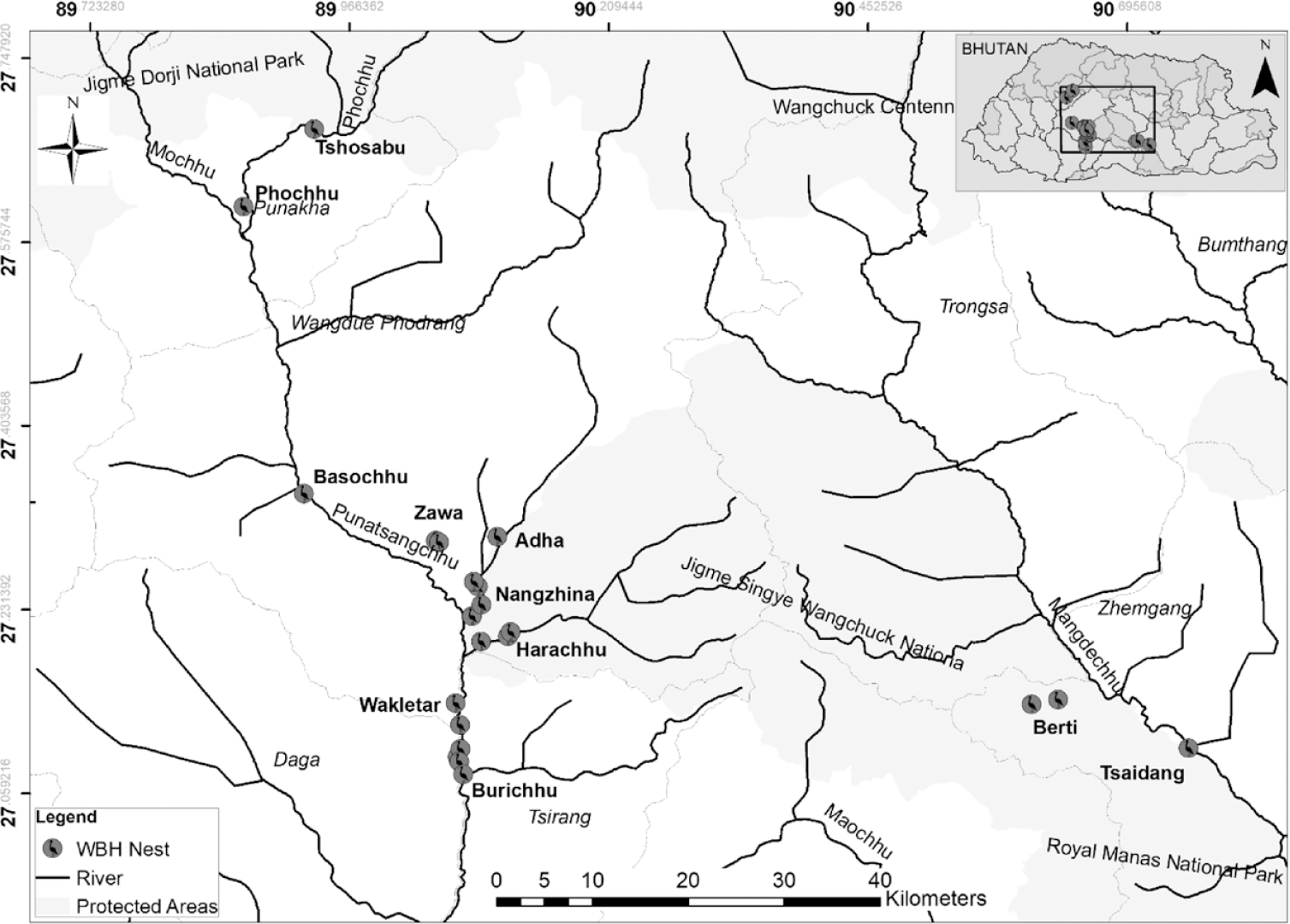
In Bhutan, WBH is distributed in temperate and mixed-broadleaf forests of two major river basins (Figure 1); Punatsangchhu and Mangdechhu, at an altitudinal range of 100–1,500 m. Prior to 2012, the altitudinal range was thought to be limited to 600–1,294 m (Dorji Reference Dorji2011). The bird is piscivorous, and it is found feeding along the shallow banks with medium to low riffle at 30–45 cm water depth (RSPN Reference Pradhan and Frederick2011). Seasonal movement and altitudinal migration of WBH are not well understood. Ali and Ripley (Reference Ali and Ripley1968) believed that it is a resident species with within-range post-breeding dispersal, while Kushlan and Hancock (Reference Kushlan and Hancock2005) suggested seasonal movement from higher breeding areas into marshy lowlands. The more recent observations by Pradhan (Reference Pradhan2008) found that WBH tends to occupy bigger rivers during winter and dry seasons and move to streams and tributaries when the rivers are turbid and the banks inundated (Dorji Reference Dorji2011, RSPN 2012). Recent records also indicate the presence of breeding birds in the same territory throughout the seasons, although they shift from one feeding ground to another, flying up to 25 km along the rivers, lakes, and streams. The literature on breeding, fledging, dispersal, philopatry, reproductive age, lifespan, and other life-history information is scarce. This knowledge is vital to preserve and revive the declining population of the bird (RSPN Reference Pradhan and Frederick2011, 2015, Price and Goodman Reference Price and Goodman2015).
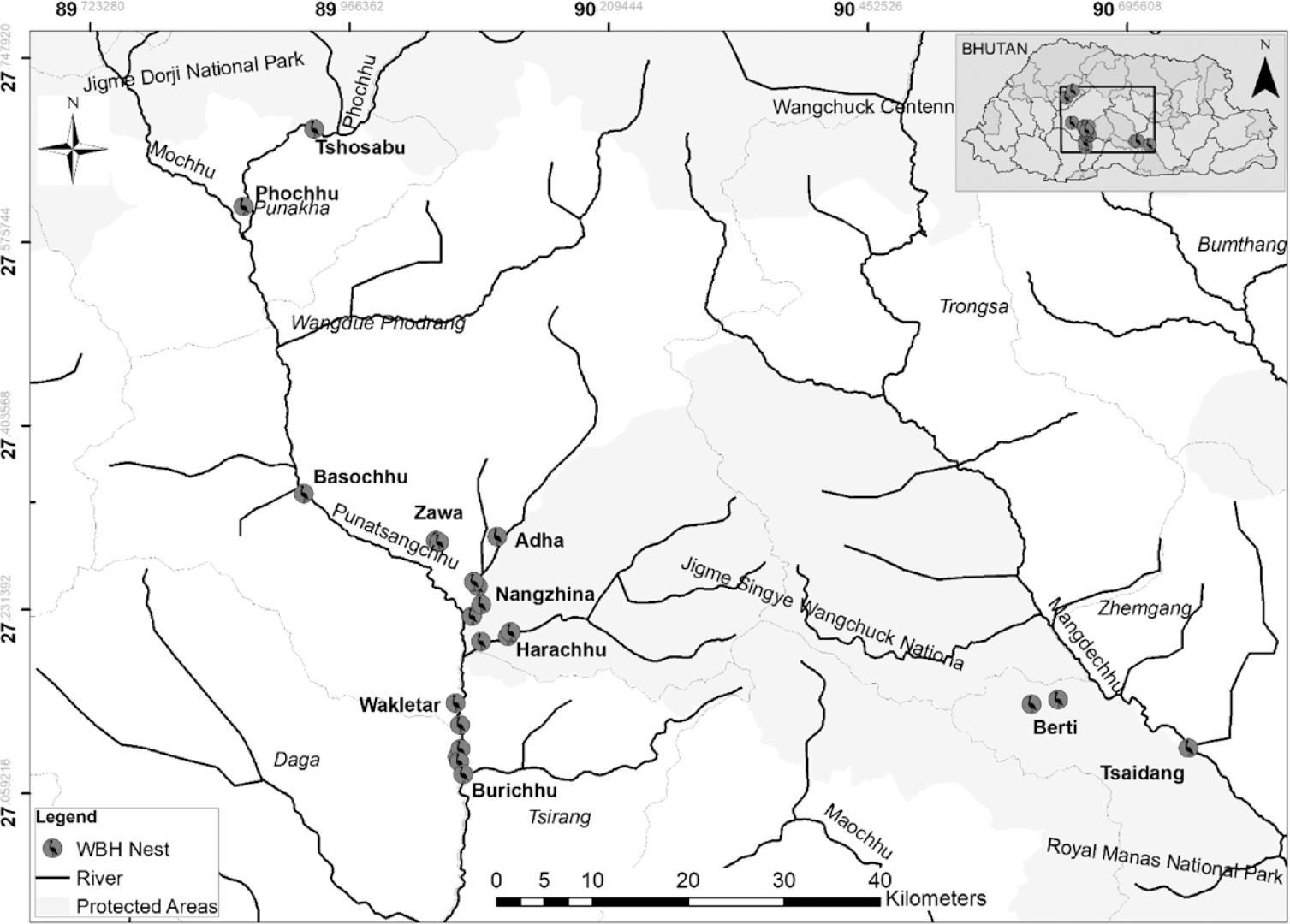
Figure 1. Distribution of White-bellied Heron nesting sites along Punatsangchhu and Mangdechhu river basins in Bhutan, 2018.
Habitat occupancy is determined by a number of proximate and ultimate factors (Block and Brennan Reference Block and Brennan1993), including habitat structures, floristic composition, biogeographical parameters, competition, food availability, potential disturbance, and predation risk (MacArthur and MacArthur Reference MacArthur and MacArthur1961, MacArthur and Levins Reference MacArthur and Levins1964, Southwood Reference Southwood1977, Noon Reference Noon1981, Muller et al. Reference Muller, Stamps, Krishnan and Willits1997). A suitable habitat could be an entire river basin, wetland, or a region which significantly varies between the taxon, genera, or even within the species. Furthermore, nest habitat selection is a complex and important determinant of reproductive success (Mainwaring et al. Reference Mainwaring, Hartley, Lambrechts and Deeming2014). It requires finding a specific site or a single tree in the vast habitat specifically for the purpose of breeding and raising offspring. According to previous studies, the selection of a suitable nest site is determined by a combination of five main factors: 1) the availability of food for both parents and offspring, 2) the risk of predation, 3) the presence and behaviour of conspecifics, 4) the availability of suitable nest material, 5) and the presence of a suitable climate for raising offspring (Collias Reference Collias1986, Hansell and Hansell Reference Hansell and Hansell2005, Mainwaring et al. Reference Mainwaring, Hartley, Lambrechts and Deeming2014). For rare and endangered birds like the WBH, it is essential to have a proper understanding of the factors defining their habitat requirements and preferences to ensure the effectiveness of conservation initiatives.
Knowledge of habitat selection and preferences allows inferences both of specific habitat management measures as well as the opportunity to develop models to estimate the distribution, potential habitats, or population sizes (Guisan and Zimmermann Reference Guisan and Zimmermann2000). Such models can facilitate establishing priorities and conservation planning. Moreover, an in-depth understanding of nesting and breeding habitat selection and preferences of threatened species is necessary to maintain protected habitats and enhance breeding success. Therefore, considering the current dearth of information on characteristics of nesting habitat, habitat selection, and preferences, this study was conducted to explicitly evaluate the nesting habitat and site preferences of the critically endangered WBH in Bhutan.
Methods
Study site
This study was conducted in two river basins: Punatsangchhu (27.1232°N, 90.0704°E) and Mangdechhu (27.1633°N, 90.6637°E), both in Bhutan (Figure 1). The climate of the study area is classified as humid subtropical (Cwa) by the Köppen-Geiger system (Climate-data.org 2018). The average annual precipitation range is 3,016–3,719 mm, and the average temperature range is 18.2–19.7°C (Climate-data.org, 2018).
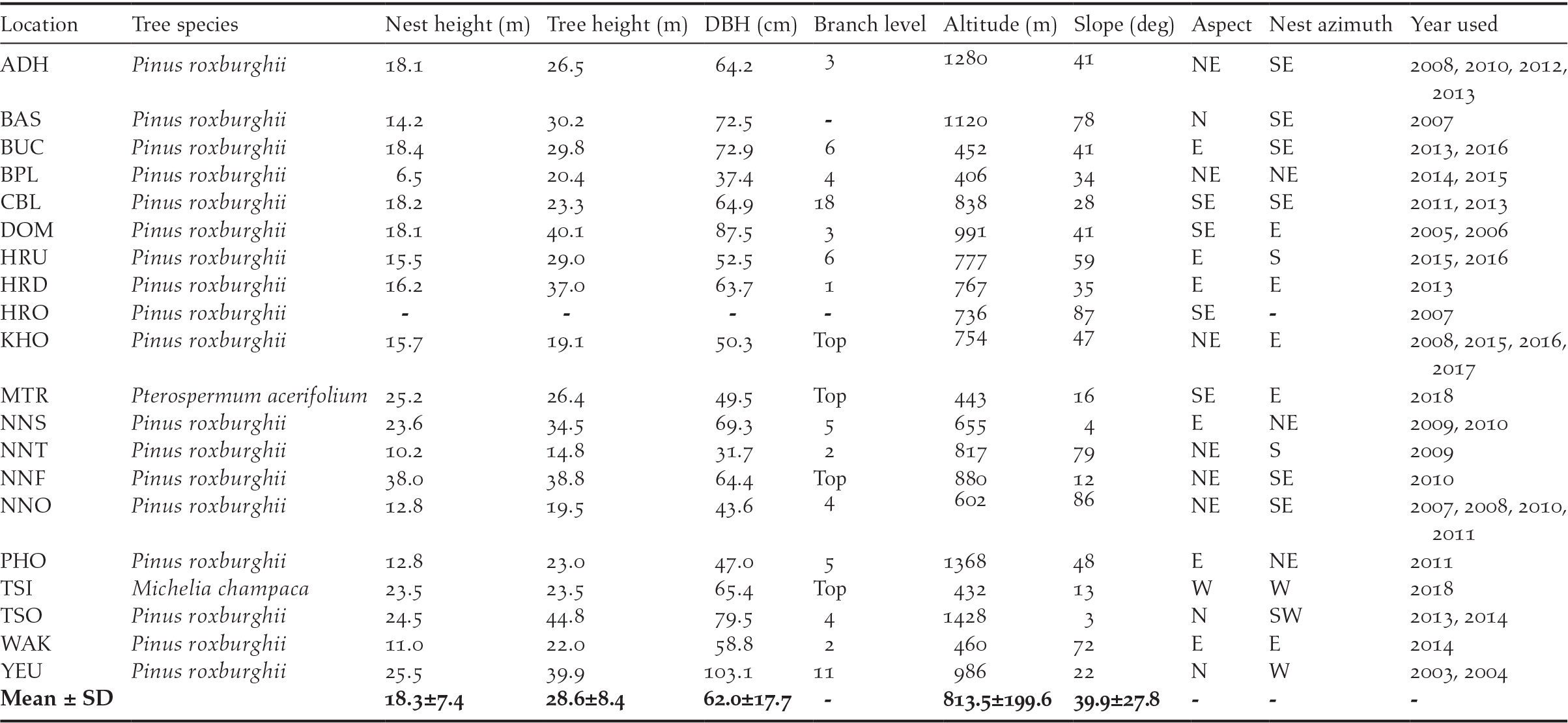
The area is mostly dominated by chir pine Pinus roxburghii, temperate forest at 400–1,450 m altitude. The common understorey shrub species are Woodfordia fruticose, Eupatorium odoratum, Murraya koenigii, Eriolaena spectabilis, Phyllanthus emblica, Holarrhena pubescens, Lagerstroemia parviflora, Bridelia retusa, Glochidion velutinum and, Phoenix loureirii, and the dominant grass species are Cymbopogon khasianum, Curcuma aromatica, and Cymbopogon jwarancusa. Although the WBH is distributed to the mixed-temperate and sub-tropical forest, down to an altitude of 105 m, nests have only been documented at altitudes between 400 m and 1,450 m in Bhutan. All nests found before 2017 were on Pinus roxburghii while the few recent ones are on broad-leaved species in the mixed broad-leaved forest along the base of river valleys, where the dominant vegetation in the landscape remains Pinus roxburghii.
Within these two basins, a total of 22 known nests, including those in which the breeding pair failed to incubate or produce fledglings, were evaluated for this nesting habitat and site preferences research. The nests were located as close as 300 m to up to 100 km apart in central and south-central Bhutan. Of the 22 nests, three were located in the Mangdechhu river basin and 19 in Punatsangchhu river basin. Two nests within Mangdechhu and eight within Punatsangchhu basin were within the jurisdiction of Jigme Singye Wangchuck National Park (JSWNP), one nest within Mangdechhu was inside the Royal Manas National Park (RMNP), five nests were within the Lobesa Territorial Division and six within the Tsirang Territorial Division. The nests were as close as 250 m to up to 7 km from the nearest settlement or vehicular roads. All the nests were solitary, in an elevated position having a clear view of the surrounding areas and 10–172 m from the nearest river(s).
Field methods
GPS locations of nesting trees from 2003 to 2017 were collected from RSPN’s database. Identification of nest tree and information on the position of the nest, year of use, azimuth, and branch level of the nest were obtained with the help of the senior ecologist and field assistant at RSPN and Local Conservation Support Group (LCSG) member who monitored the particular nest when it was active. LCSG members are individuals from the local community appointed and paid by RSPN to monitor, collect data, and safeguard the WBH in their locality (RSPN 2010). Additional background information on habitat, distribution, feeding and breeding ecology and community engagement in the conservation of the WBH were obtained from Rangzhin, a quarterly-published newsletter of RSPN since 1994.
To determine the specific characteristics of each nest, nest height, tree height, species, diameter at breast height, branch level, crown diameter, slope, aspect, nest azimuth, altitude, year in which the nest was used, distance to nearest river, distance to settlement, and distance to road/walking trail from all the nests, were recorded.
To determine nest habitat and site characteristics, a circular plot of 20 m diameter, with the nesting tree as a centre, was used to assess the tree and shrub composition and structure of the nesting habitat. Species, height, DBH, canopy cover, distance to the nest tree and, abundance of all trees and shrubs in the plot were recorded. Similarly, herb abundance, percentage herb cover, percentage litter cover, percentage rock cover, and percentage barren ground were measured in four 1 m × 1 m plots, 10 m from the nest tree, nested within the 20 m circular plot, situated perpendicularly from the base of nesting tree. These data were of interest in order to analyse the ground cover and debris which are essential nest-building materials.
To assess nesting habitat preferences, a random tree, having similar DBH, height, and crown cover to the nest tree, was identified at a random direction within 100–120 m from the nest tree and the same site variables were measured.
Data were collected from 14 nests and 14 random sites (pseudo-nests) from 1 June to 27 July 2018. Data could not be collected from four nests at Nangzhina because of the monsoon and lack of accessibility. The nest tree at Basachhu was burned, and the tree had fallen off, and a landslide eroded the nest tree at Harachhu. However, I used the nest-specific information collected in February 2017 during pre-survey observations of four nests at Nangzhina and the data available at RSPN about the fallen nests in this research. Because the juveniles were still in the two 2018 active nests, I could only collect nest-specific information remotely, to avoid disturbance to the breeding pair and the chicks.
To analyse nest architecture, nest dimension, quantity, quality, and the size of nesting materials were measured from an abandoned but intact nest at Burichhu. An incubating heron had abandoned the nest after the eggs were presumably predated by some small mammalian predator. Measurements were taken a week after the nest was abandoned.
Further details of nest and habitat variables are provided in Appendix S1 in the online supplementary materials.
Statistical analyses
I broadly characterised nests by calculating a mean and standard deviation of nest specific data from the 22 nests. I used paired sample t-tests (nest and random sites) to compare each of the habitat variables (Table 3). Vegetation composition and species diversity were calculated using the R package vegan 2.5-2 (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O’Hara, Simpson, Solymos, Henry, Stevens, Szoecs and Wagner2018). I performed a simple 1-sample proportions test with continuity correction to test if the WBH has a preference for taller trees and particular aspect among other potential trees available within 20 m diameter in each nest site. A simple linear regression model was fitted to predict the change in the height of the surrounding trees with a relative change in distance to the nest tree to determine the preference to lone tree and visibility. The data were analysed using the R statistical computing environment, version 3.5.1 (R Core Team 2018).
Results
Nest sites
In the past 16 years (2003–2018), the WBH has successfully bred 38 times in Bhutan. While it is difficult to identify the number of breeding pairs precisely, there are 20 successful nest sites distributed across Punatsangchhu and Mangdechhu basins. Successful nests here are those from which at least one juvenile fledged, based on the records maintained by RSPN. I collected data from 22 nests, of which 20 were successful (Table 1). One nest was predated at the incubation stage while the other failed at the nest-building stage.
Table 1. Measurements of variables from 14 successful White-bellied Heron nests from 2003 to 2018 in Bhutan (Locations: ADH = Adha, BAS = Basachhu, BUC = Burichhu Confluence, BPL = Burichhu Plantation, CBL = Changbaling Berti, DOM = Domthang, HRU = Harachhu upstream, HRO = Harachhu old, HRD = Harachhu downstream, KHO = Khoti Berti, MTR = Mithuntar, NNS = Nangzhina 2, NNT = Nangzhina 3, NNF = Nangzhina 4, NNO = Nangzhina1, PHO = Phochhu, TSI = Tsaidang, TSO = Tshosabu, WAK = Wakletar, YUE = Yeutama).
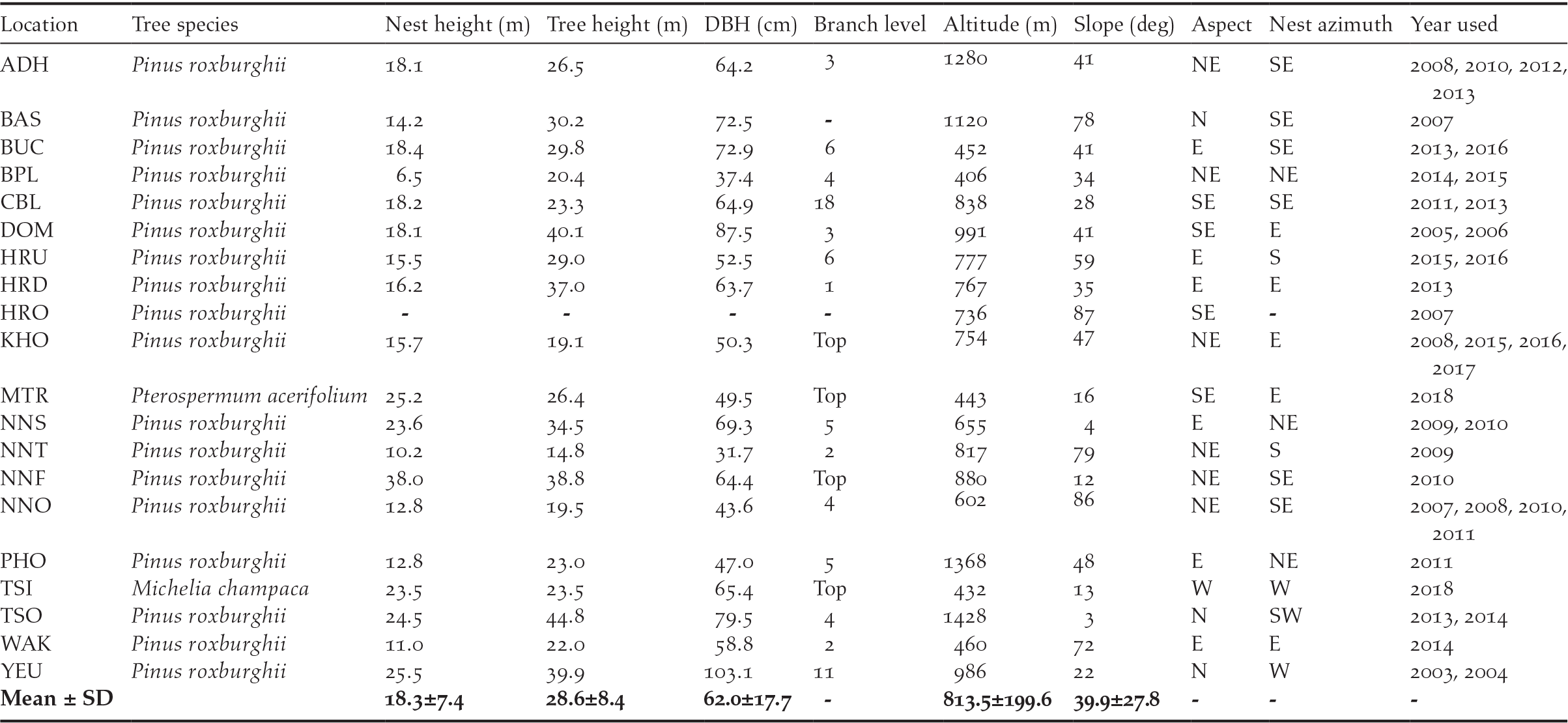
Before 2018, all the nests were in conifer species (Pinus roxburghii), while both the successful 2018 nests were in broad-leaved trees (Michelia champaca and Pterospermum acerifolium). All nests were found in the temperate forest between the altitudes of 400 and 1,430 m asl. The average height of nests was 18.3 ± 7.4 m. The mean height of the nesting tree was 28.6 ± 8.4 m with a mean diameter at breast height of 62.0 ± 17.7 cm, and mean crown diameter of 15.7 ± 6.9 m. Of the 20 nests, 80% were on branches within or under the canopy, and 20% were on the canopy top. Nine nests (45%) had been used once, 8 (40%) had been used twice, and 3 (15%) had been used more than twice by the herons.
Nests were well exposed with a large open space, giving enough space for parents with ∼2 m wingspans to fly around without hindrance. All the nests were found within a mean distance of 74 m from the nearest water body on a slope of 40°, mostly facing either north-east, east, or south-east (Table 1). Only four nests were found on north- or west-facing slopes. Similarly, 90% of the nests were built on a branch facing south-east, east, or south, while the remaining 10% were on a north-east or west-facing branch. Looking at the spatial distribution of nests from settlement and proximity to human activities, the farthest nest was 2.6 km from the nearest village, while the closest was 68 m; 75% of the nests were closer than 1 km to a human settlement, and 80% of the nests were closer than 200 m to the nearest walking trails.
Nest architecture
Unlike most of the other large herons, which breed in colonies (Jenni Reference Jenni1969, Kushlan and Hancock Reference Kushlan and Hancock2005, Reynaud and Kushlan Reference Reynaud and Kushlan2004, Stier et al. Reference Stier, Ricardou, Uriot, Pracontal and Kushlan2017), WBH is a solitary breeder. No two active nests have been found closer together than 1 km in Bhutan. The WBH nest can be best categorised as a simple platform based on the nest description guidelines by Simon and Pacheco (Reference Simon and Pacheco2013). The abandoned nest (Figure 2), from which the measurements were taken had open access and was fully exposed without any canopy cover above. A similar pattern of exposure was also observed in all other nests. The nest was a new construction, although old nests are commonly reused (RSPN 2006).

Figure 2. The White-bellied Heron nest a week after it was predated and abandoned at Burichhu in Bhutan, 2018.
The diameter of the nest was 106 cm in the frontal intersection and 87 cm across the sides, excluding the extended edges. These measurements are close to the 120 cm observed in earlier nests (Royal Society for Protection of Nature Reference Pradhan and Frederick2011). The nest materials were mostly dried twigs and small branches of nearby available plant species without any foliage. Interestingly, most of the larger materials in the nest were remains of cattle fodder chopped by herders near the nest site. Like other heron species, the architecture of the nest was not very complex, but there was a noticeable pattern in the arrangement of materials depending on length, diameter, shape, and rigidity. Bigger and longer twigs were placed at the bottom and along the edges, and smaller and more pliable materials were on top and towards the inner side to establish the desired shape and stable platform for incubation, and to hold chicks at a later stage. The mean diameter of the twigs was 0.7 ± 0.02 cm (n = 247), and the mean length was 25.8 ± 1.04 cm (n = 247). The largest twig in the nest was 1.6 cm diameter, and the longest was 112 cm. Similar twig size of 0.5–2.5 cm, and nest diameter of 105 cm and 121 cm were found in other two successful nests build in broad-leaved trees (Khandu et al. submitted). However, much larger twigs have been observed in other nests in the past (Royal Society for Protection of Nature Reference Pradhan and Frederick2011). The thickness of the nest bedding was 17 cm at the centre and gradually decreased to just a few sticks spread at the edges. Similarly, the centre of the nest was 12 cm deeper than the extended edges. The nest was placed on the canopy of a broad-leaf tree, fully exposed, facing towards the river with a clear overview of the surrounding and easily accessible from the feeding sites.
Vegetation composition and structure
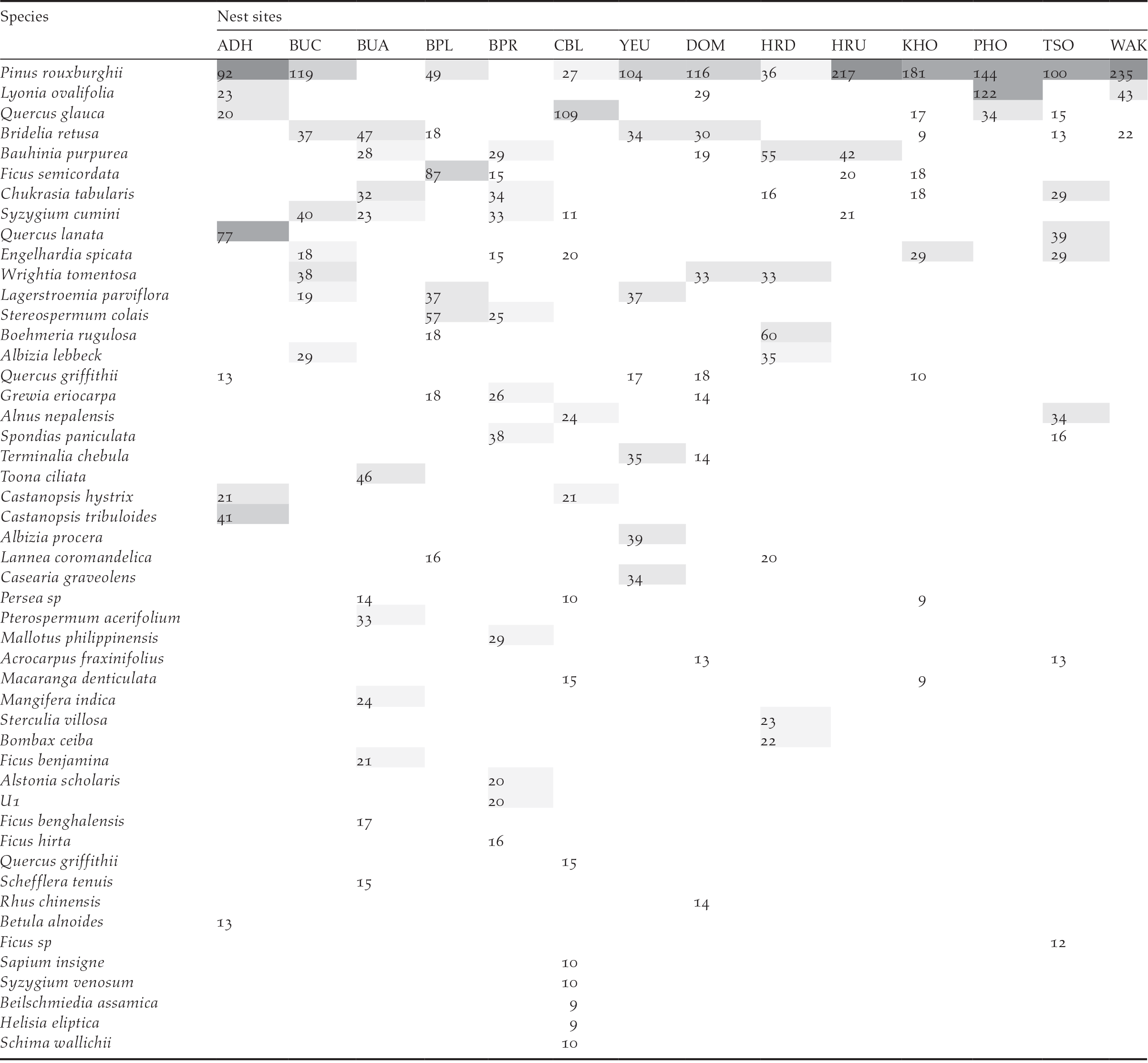
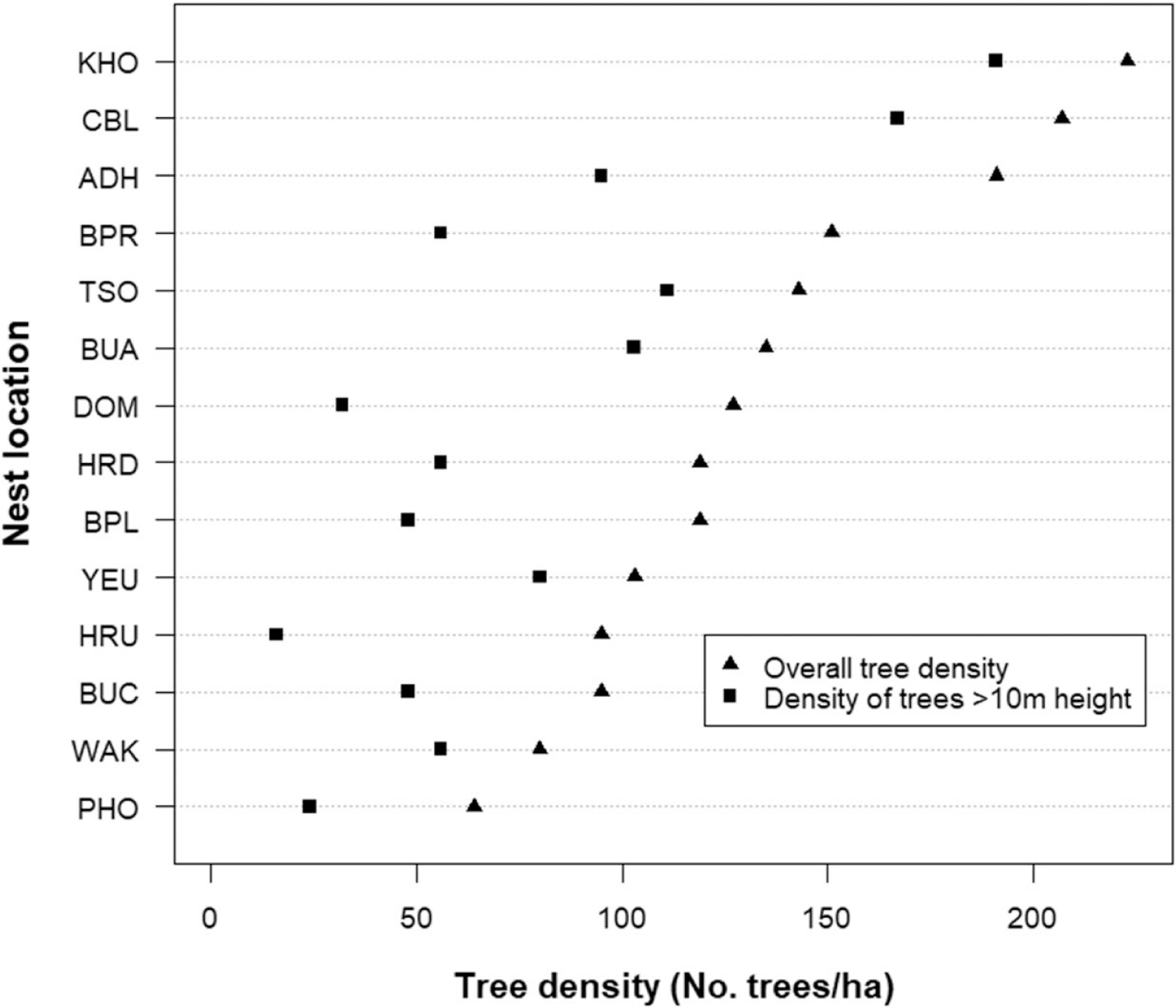
Vegetation composition and structure were analysed based on the data collected from 14 nests and 14 random sites. A total of 49 species of tree and 30 species of shrub belonging to 40 families were recorded from nest plots. However, 82% of the nests (18) were on Pinus roxburghii and only 18% (4) were on broad-leaved tree species. Of the four nests on broad-leaved trees, only two were successful while one was abandoned half-built and the other was predated during incubation. The five most abundant tree species in the nesting habitat were Pinus rouxburghii, Bridelia retusa, Quercus glauca, Lagerstroemia parviflora, and Bauhinia purpurea. Pinus roxburghii was also found to have highest importance value in nine of the 14 sites (Table 2), while Quercus glauca, Bridelia retusa, Ficus semicordata, Boehmeria rugulosa, and Spondias paniculate had the highest importance value in the remaining five plots. The mean overall tree density in the area was 132 trees/ha. The Khoti nest was found to have the highest overall tree density of 223 trees/ha, followed by Changbaling (207 trees/ha) and Adha (191 trees/ha). The Phochhu site had the lowest density of 64 trees/ha. Similarly, the mean density of trees with a height greater than 10 m (i.e. potential nesting trees) was 77 trees/ha (n = 14). Again, the Khoti and Changbaling nest had the highest density of potential nesting trees (191 and 167 trees/ha, respectively) (Figure 3). See also Table S1 for further details.
Table 2. List of tree species and their importance value (IVI) of 14 White-bellied Heron nesting sites in Bhutan 2018.
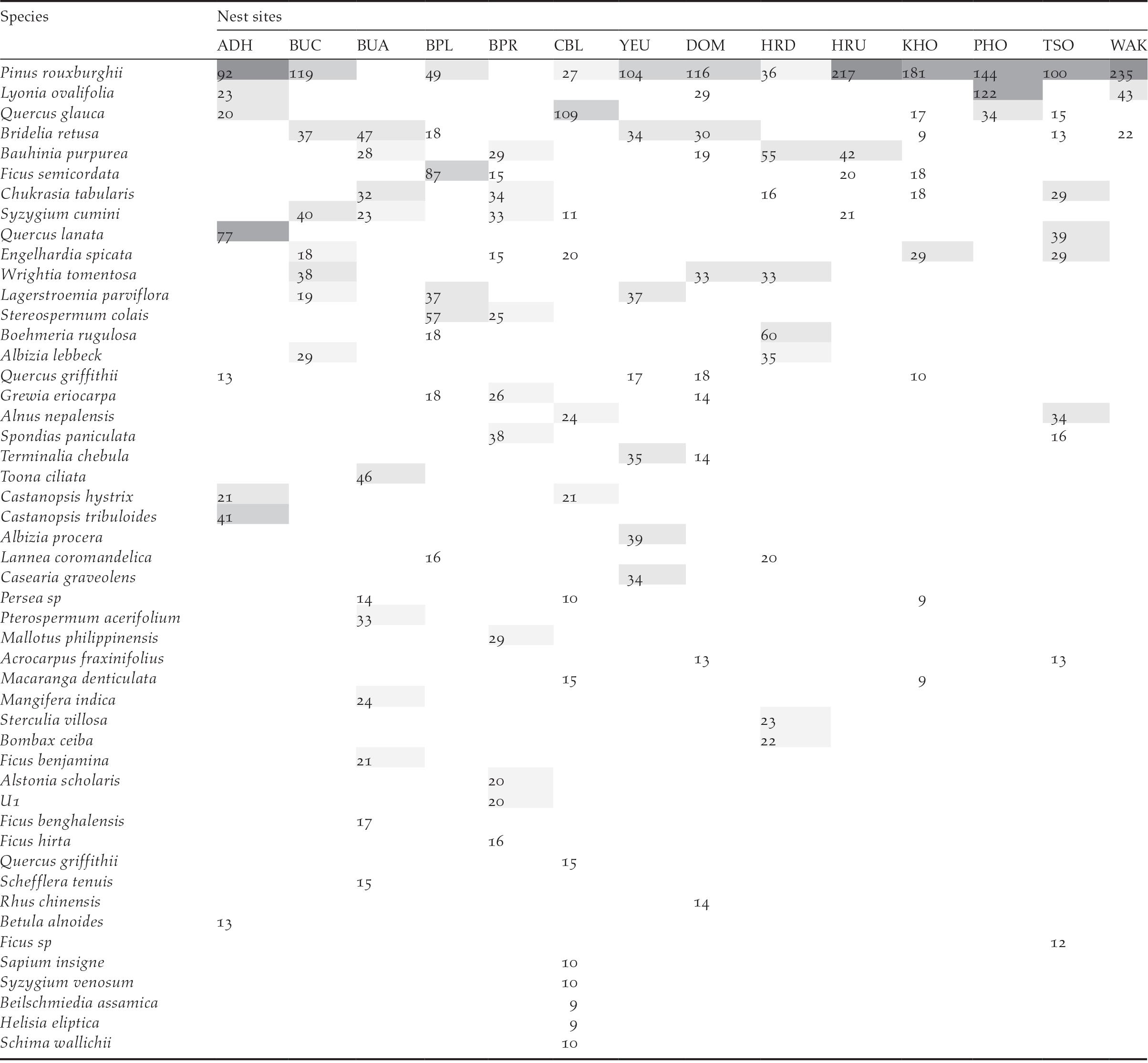
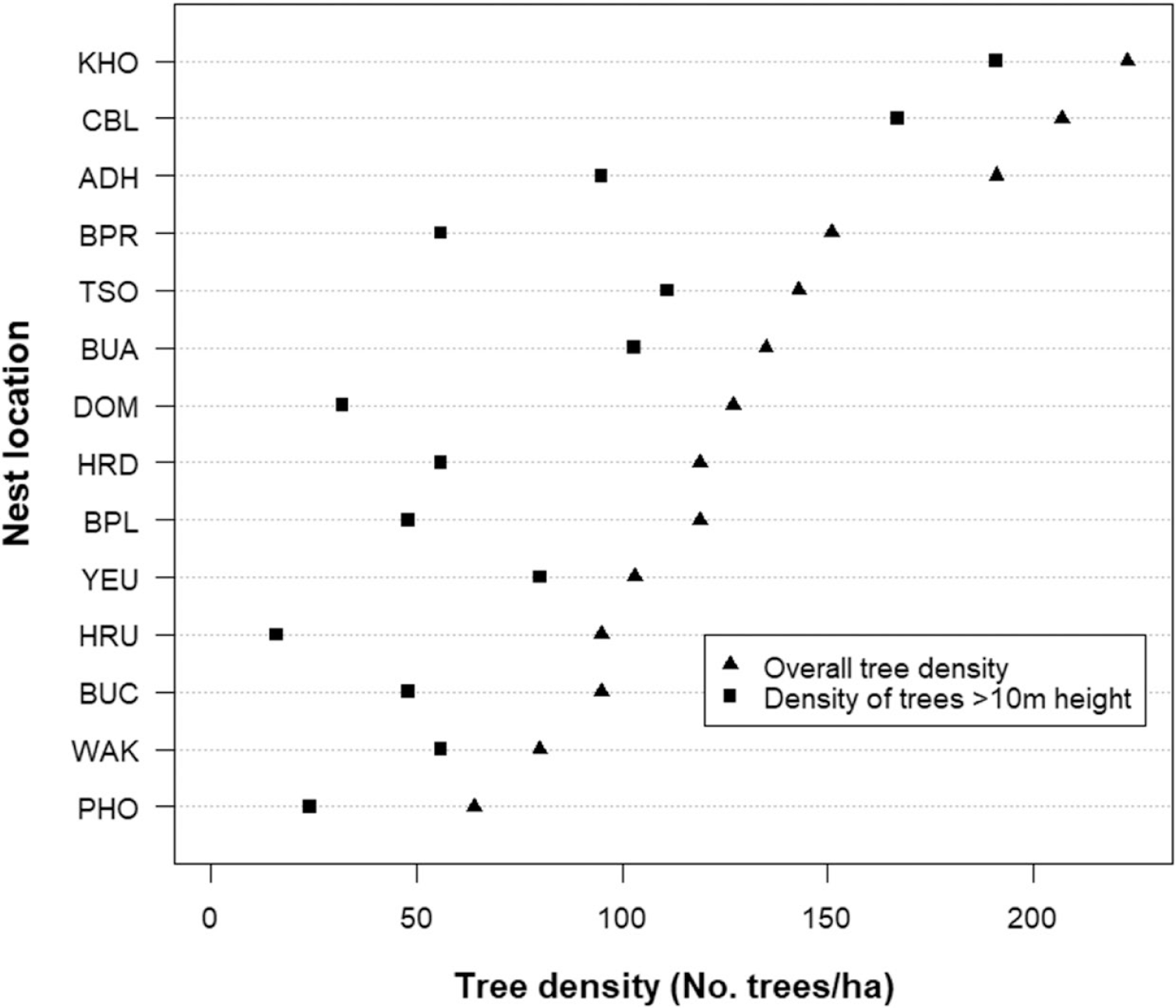
Figure 3. The mean overall tree density and mean density of trees ≥ 10 m height of White-bellied Heron nest sites in Bhutan, 2018.
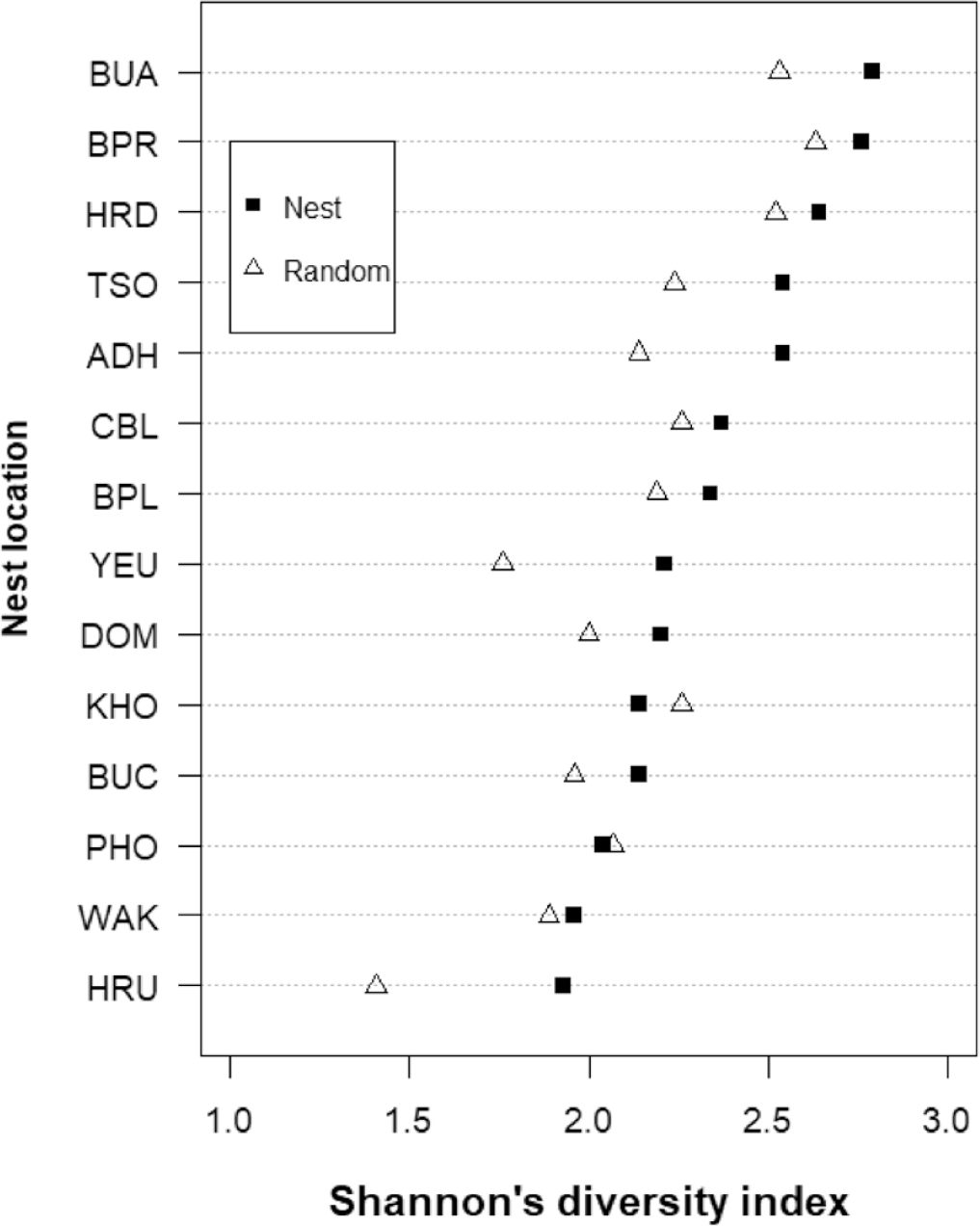
The mean Shannon diversity index (H) for tree species in the nesting habitat was 2.2 ± 0.2 (n = 14) (Figure 4). The greatest diversity was in the nests located in broad-leaved forest closest to the river, and the lowest was in the Pinus roxburghii-dominated sites on the drier and steeper slopes (Table S1).

Figure 4. The Shannon diversity indices of tree species in White-bellied Heron nest and random sites in Bhutan, 2018.
The mean shrub abundance was 3.7 ± 4.6 (n = 14), mean shrub height was 3.0 ± 2.6 m (n = 14) and mean shrub richness was 9.8 ± 1.7 (n = 14) across all nest sites. The most abundant shrubs across all sites were Eupatorium odoratum, Woodfordia fruticose, Murraya koenigii, Desmodium oojeinense and Holarrhena pubescens. Phoenix rupicola, a common palm species of family Arecaceae and Pueraria sp., a climber, were also frequent in the four broad-leaved nest sites.
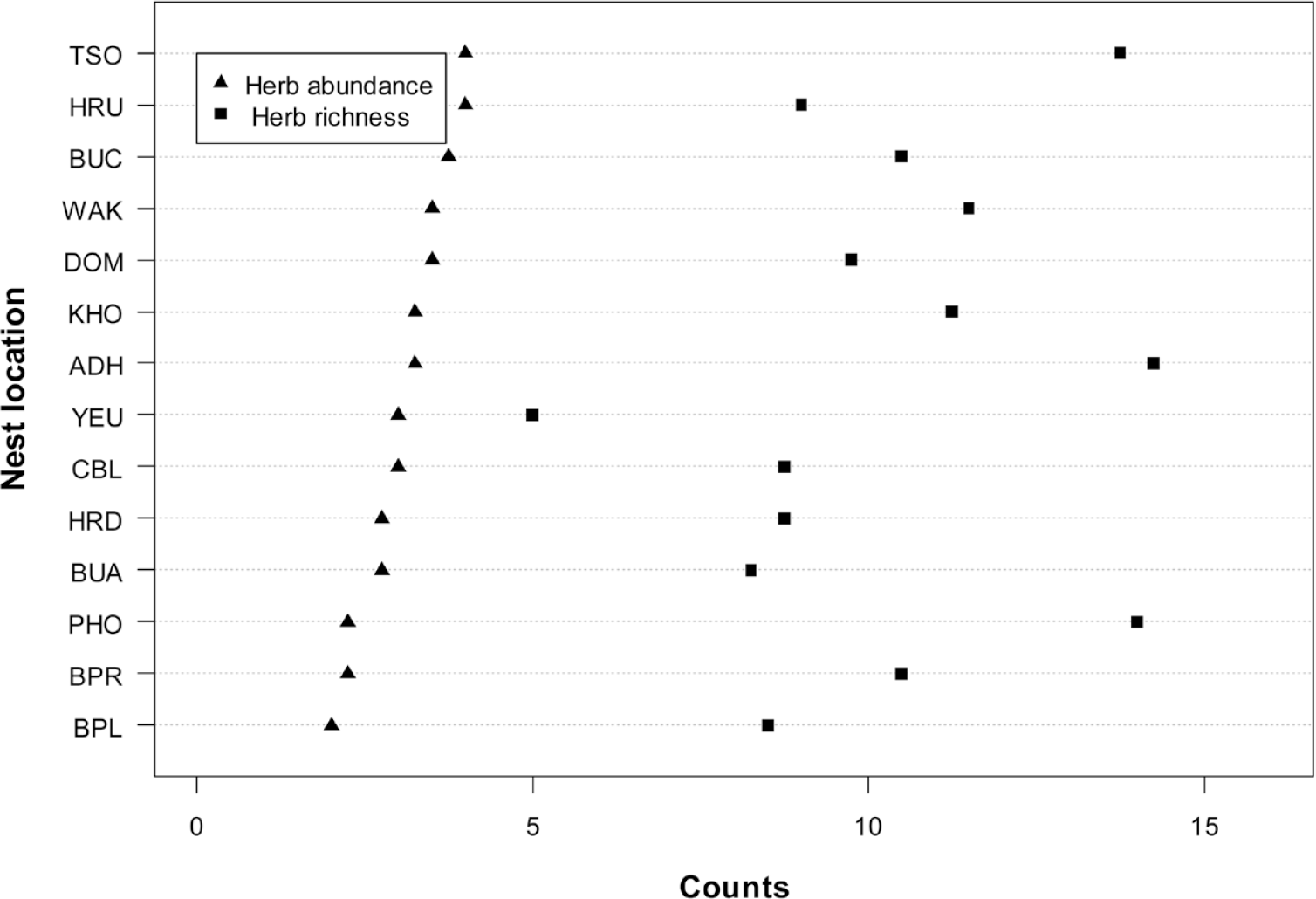
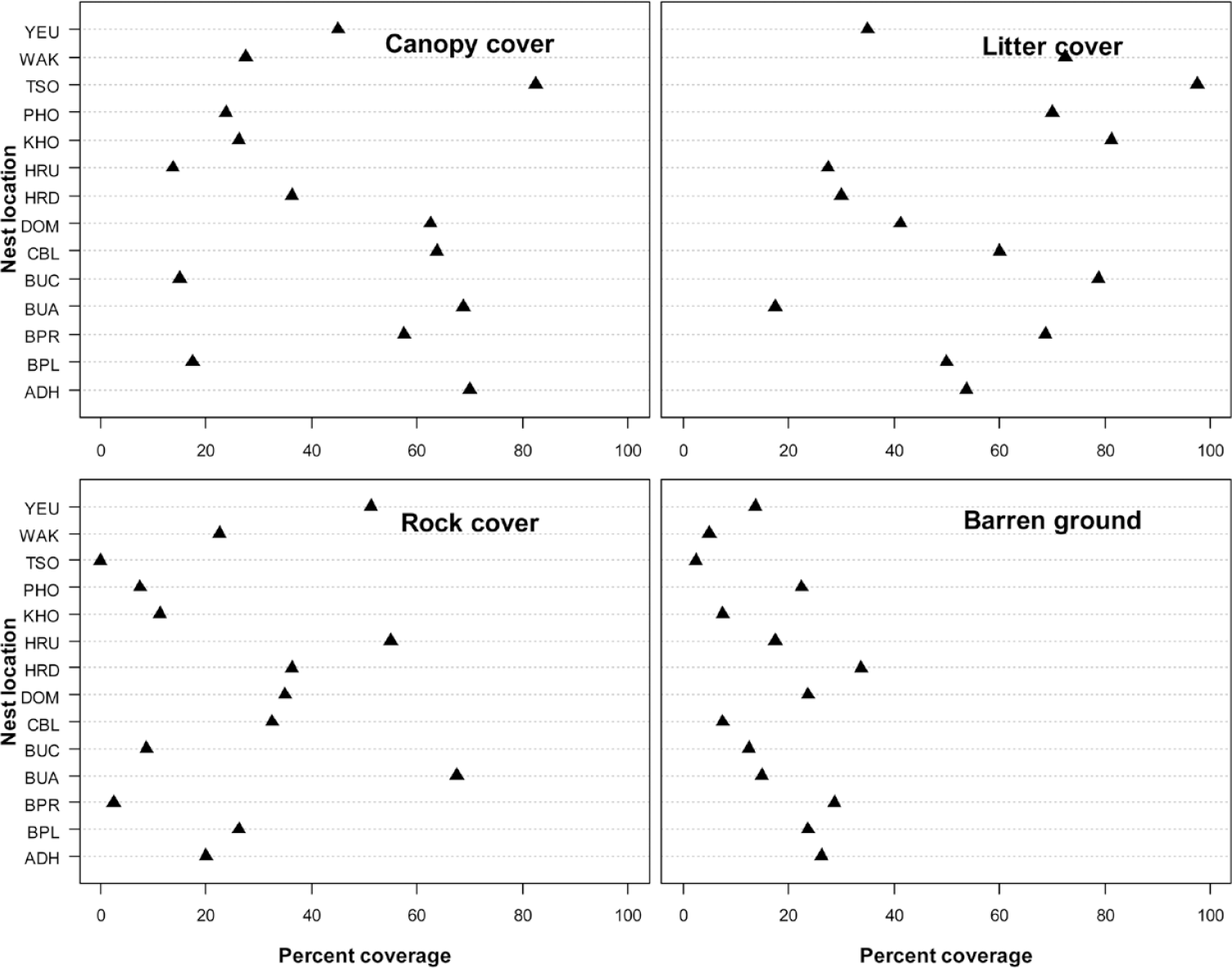
The mean herb richness across all sites was 3.1 ± 1.0 mean herb abundance was 10.3 ± 3.7, and mean herb height was 42.4 ± 24.6 cm (Figure 5 and Table S2). The mean tree canopy height was 14.2 ± 5.7 m and mean canopy cover was 43.6% ± 27.0; the percentage canopy cover was higher in broadleaved forest nests than in coniferous forest. The mean litter cover was 56.0% ± 26.0, mean rock cover 26.9% ± 27.1 and mean bare ground 17.1% ± 17.2 was observed across the nests (Figure 6 and Table S3).
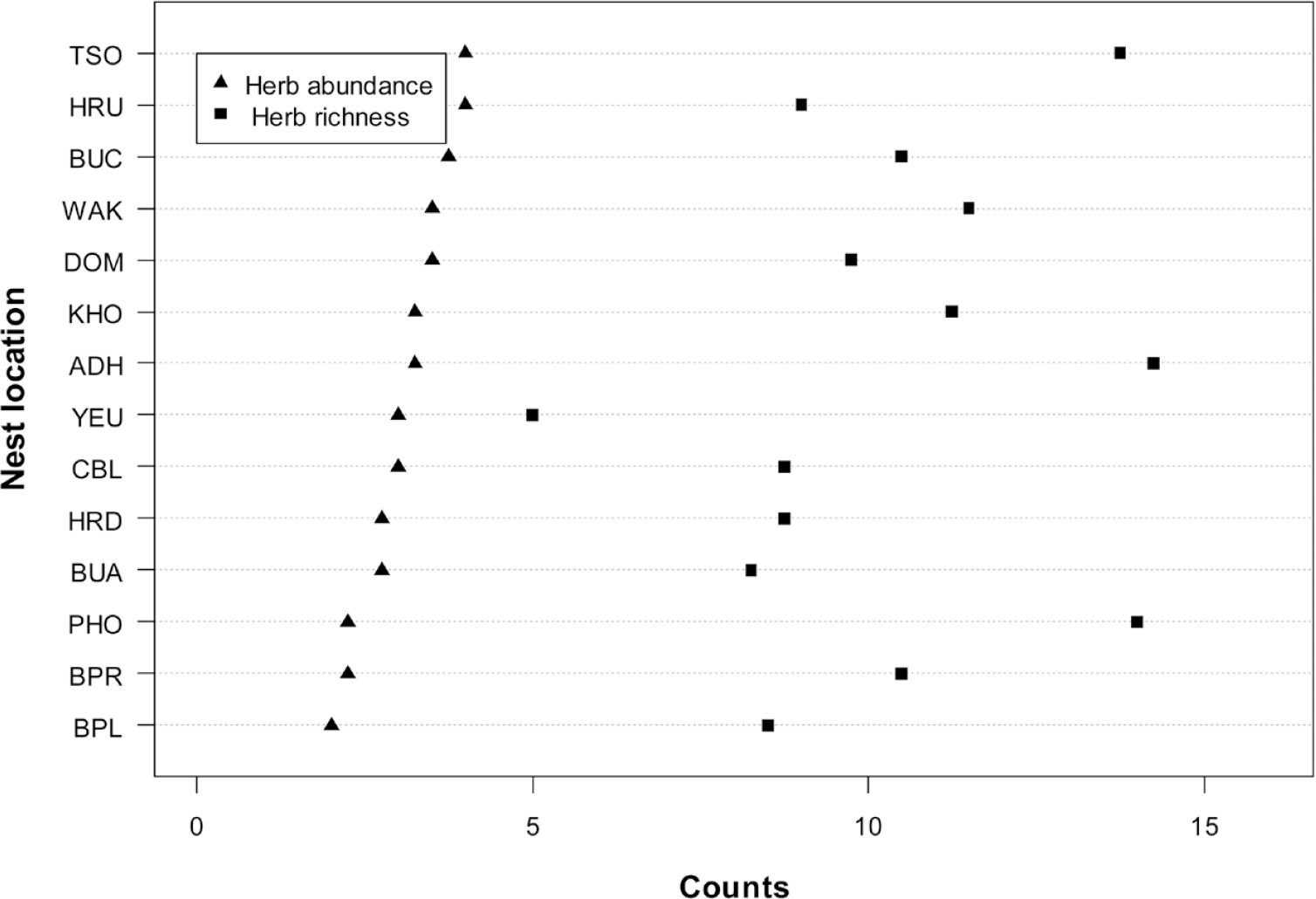
Figure 5. The mean herb richness and herb abundance of White-bellied Heron nest sites in Bhutan, 2018.

Figure 6. The mean percentage canopy cover, litter cover, bare ground and rock cover of White-bellied Heron nest sites in Bhutan, 2018.
Nest-site preference
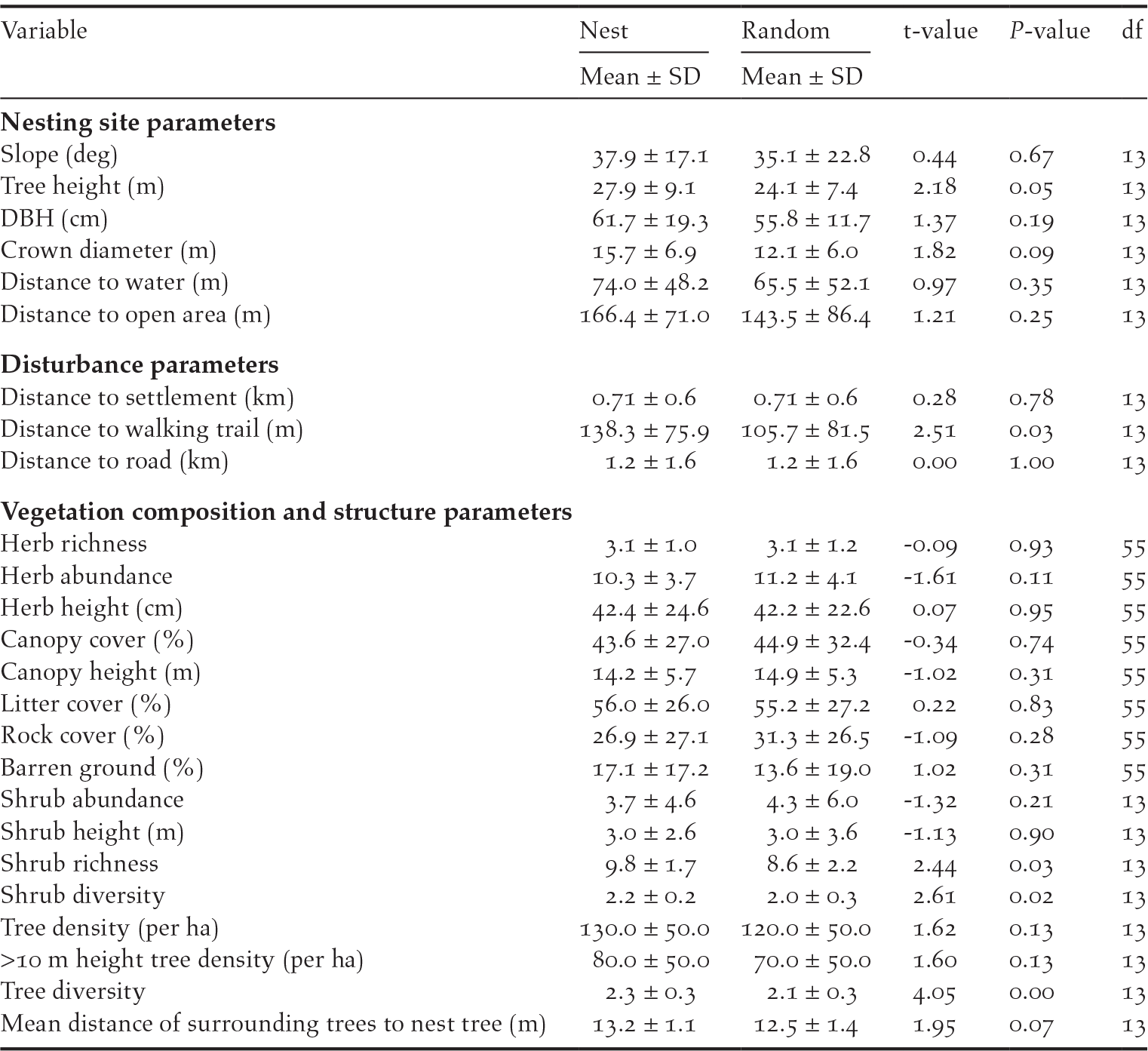
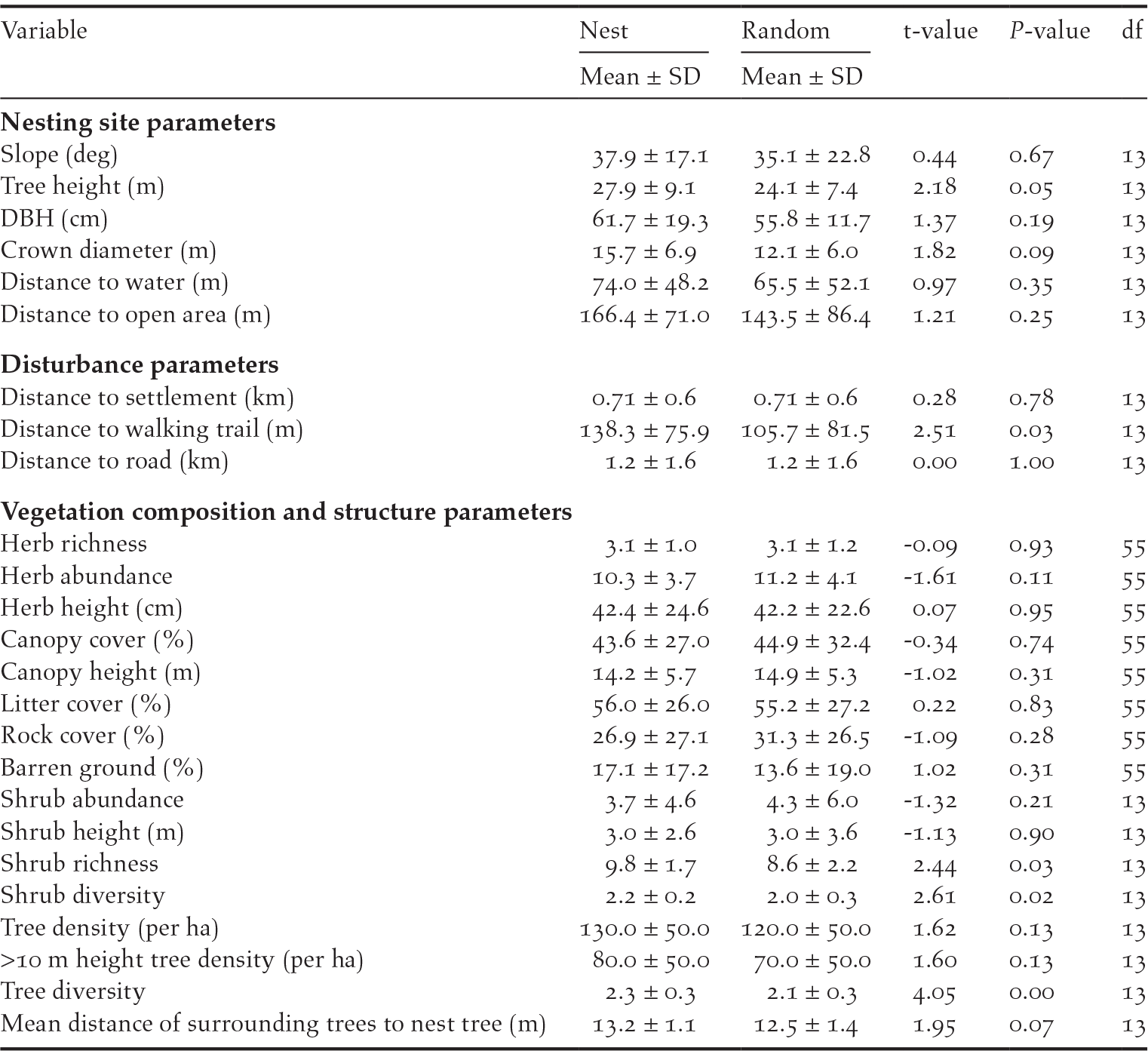
Looking at the current nesting pattern, WBH prefers to nest on the tallest (11/14, P = 0.06, df = 1) of the available trees in the selected site (Table 1). Data also suggest that the height of the surrounding trees increases linearly with increasing distance from the nesting tree (slope β = 0.41, P < 0.00) indicating a preference for a lone tree with clear visibility of the surroundings. There is also uniformity in selection of aspect of the nesting landscape with preference to east (north-east, east or south-east) facing landscapes (16/20, P = 0.01, df = 1) and also, a significant number of nests (14/19, P = 0.07, df = 1) were uniformly aligned towards east, north-east, or south-east directions. When comparing nest site characteristics with a paired random site (Table 3), height of the nest tree (t = 2.18, P = 0.05, df = 13), crown diameter (t = 1.82, P = 0.09, df = 13) and distance from the walking trail (t = 2.51, P = 0.03, df = 13) were found to be significantly different at 90% level (alpha = 0.1). Data suggest a preference for taller trees with a larger crown diameter, which are also away from a walking trail or disturbance. There is also an indication that aggregation of trees plays a key role in site selection with preference for sites with sparsely aggregated trees (t = 1.95, P = 0.07, df = 13). However, the data also suggest a preference for sites with greater overall tree density (t = 1.62, P = 0.13, df = 13) and greater density of trees taller than 10 m height (t = 1.60, P = 0.13, df = 13). The data also suggest a statistically significant difference in herb abundance (t = -1.61, P = 0.11, df = 55), shrub richness (t = 2.44, P = 0.03, df = 13), shrub diversity (t = 2.61, P = 0.02, df = 13) and tree diversity (t = 4.05, P = 0.00, df = 13). The above differences notwithstanding, the data also suggest many site characteristics of the nest site and the random locations within 100–120 m from the nests were similar in the landscape. Boxplots of all habitat variables are shown in Figure S1.
Table 3. Mean ± SD of habitat variables of White-bellied Heron nesting sites and random locations with results from paired sample t-test (t-value, P-value & df) in Bhutan 2018.
Discussion
Finding an active nest in 2003 was a remarkable moment in the history of WBH conservation. Had no such discovery been made then, perhaps the story of WBH conservation in Bhutan and the world would have been different today. Even though there are no records of breeding in other range countries, there are 3–5 active breeding pairs in Bhutan (RSPN 2018). In sixteen years, the WBH have reproduced 38 times from 20 nests producing at least 76 juveniles, although only 22–30 birds have been recorded during subsequent population surveys conducted annually by the RSPN (Pradhan et al. Reference Pradhan, Acharja, Tshering and Lhendup2017, RSPN 2018). Although the WBH nests have been found along the slopes on tall chir pine trees for a long time, this research was first systematic and detailed evaluation of the characteristics of all known nests in Bhutan.
This research found that site selection of WBH nests was associated with several biotic and abiotic factors, most notably vegetation structure, aspect, site visibility, access to a water body or feeding sites and lack of disturbance. Looking at the pattern of 22 nest sites, the bird had a significant preference for nesting on east-facing aspects and also built nests facing east among the tallest trees. Preference for the east could potentially be associated with heat requirement and exposure to the sun during incubation. This assumption can also be related to the observations by Pradhan et al. (Reference Pradhan, Norbu and Frederick2007) that the bird was witnessed rolling the eggs periodically and consistently changing posture facing the direct sun during incubation. In mountainous terrain, all the nests were found along river valleys where shadows could limit exposure to the sun, therefore facing east would potentially have an advantage. It could be that east-facing nest in east-facing landscapes increase the time of exposure to direct sun as it receives heat from the rising sun until it sets in the west, minimising exposure to shade. Further detailed observations of breeding and incubation behaviour and climatic parameters are essential to gain a better understanding of breeding and nesting ecology.
The WBH has a noticeable preference for nesting on tall trees with high visibility of the surroundings and ample open space to fly in and out. The current pattern also suggests that access to feeding sites and proximity to threats or potential disturbance like walking trails and settlements also significantly influence nest site selection. High nests with low undergrowth and greater distance from the nest to the adjacent trees suggest an adaptation against predation. Although there are no records of direct predation, small mammals are presumed to pose significant threats to breeding herons (RSPN 2009b). Site examination of two unsuccessful nests of 2018 along the Punatsangchhu suggests potential predation by small mammals. There were abundant dropping of small frugivorous mammals and monkeys on both the abandoned nests and surroundings, suggesting possible predation or intrusion of small mammals with breeding herons. This finding indicates the disadvantage of nesting on broad-leaved species with high foliage and rich undergrowth, which we surmise may increase the chances of predation and breeding failure. No such evidence was observed in the past from nests built on tall chir pine trees with low undergrowth and high visibility, which drove the assumption that the WBH was adapted to breed only on chir pine trees (RSPN 2006, 2008). It was also hypothesized that high load-bearing capacity, elasticity, and resin secretion of chir pine growing on windy slopes provided a safer breeding site for herons and protection against insects and avian predators (RSPN 2006, 2012). However, two breeding pairs successfully bred and fledged five juveniles in 2018 from broad-leaved trees (Khandu et al. 2018), implying that successful breeding in broad-leaved forests cannot be ruled out. The primary risk of nesting in the chir pine dominated forests is that these tall trees along the valleys are prone to forest fires and falling in windstorms. The RSPN’s records indicate that forest fires burned two nests and high winds destroyed one. Although the high hilltops and big Chir pines with high visibility are safer breeding sites for the bird, a large heron with a wide wingspan also requires considerable energy (Royal Society for Protection of Nature Reference Pradhan and Frederick2011) to fly high during long breeding seasons. We speculate that the breeding herons may be inclined to nest on elevated sites near rivers, in order to both minimise energy loss and increase the accessibility to feeding sites during the time of scarce food supply for both breeding birds and juveniles. It was also observed that the majority of older nests were located along tributaries and smaller rivers, while newer ones were along the main rivers but closer to confluences. This is another unique nesting site preference to overcome feeding site limitations during the monsoon. The fledging stage of the heron’s breeding coincides with the monsoon in Bhutan, where the rivers are flooded and remain turbid for several weeks or even months. However, smaller streams and tributaries are clearer and can recede quickly. By preferring to nest along the tributaries, smaller rivers, or near the confluence, the nesting birds ensure enough feeding sites within a convenient distance during changes in water turbidity.
The WBH was observed to be highly sensitive to disturbance and threats (RSPN 2008, 2012, Reference Pradhan and Frederick2011). The statistically meaningful difference in mean distance to the nearest walking trail between the nest and the paired random location, as well as the apparent preference by the heron to be further away from the trails, indicates sensitivity by WBH to perceived threats of human presence. The minimum flush distance in feeding habitat to human presence has been estimated at 150 m (Royal Society for Protection of Nature Reference Pradhan and Frederick2011). It is likely that the distance is even greater for nesting habitat.
Looking at the past nesting records, the number of nests reused had also significantly decreased over the recent years from up to four times in the past to mostly single-use from 2016 onwards. It was also observed that the birds had abandoned almost all of the older nesting habitats. No successful nests have been discovered in Phochhu, Zawa, Adha, Nangzhina, and Harachhu since 2015. These were once the most abundantly used nesting sites (RSPN 2006, 2008, 2009a, 2009b, 2010, 2012, 2015).
Human-caused disturbances in nesting and feeding habitats are one of the major threats to the WBH. The RSPN’s population and nesting data from previously occupied habitats indicate that WBH are intolerant of rafting, picnicking, and other disturbance along the foraging sites. The local communities at Phochhu associate the population decline to an increase in the incidences of rafting and riverside recreation activities in the area in recent years. Although up to five WBH were regularly sighted in Phochhu before 2012, only one is infrequently sighted today. The LCSG member at Zawa attributes the drastic decrease in the number of WBH sightings to the road and bridge construction at Digchhu. Similarly, the population in Adha and Harachhu has declined after the beginning of new road construction and mega construction work at the Harachhu-Punatsangchhu confluence. Forest fires are another direct threat to both breeding parents and juveniles. In 2016, an incubating pair abandoned the nest after a major forest fire outbreak in the area, and no active nest has been located there since. Overall, WBH habitats are shrinking at an exponential rate due to human encroachment. The riverine habitats are transforming at an alarming pace with an increasing number of hydropower projects under construction. The fish populations, on which the WBH depends are also under threat due to unsustainable and illegal harvesting. These are expected to impact resource availability and isolate one micro-population from another, which would affect breeding and genetic viability of the extremely low surviving populations.
Looking at the characteristics of current nesting habitats, an adaptive nest architecture is vital to withstand strong winds and other environmental hazards. The common characteristics observed in all nests were an adequate number of branches and platforms for the bird to take off, land, and perch during the long breeding season. The ample space and exposed branches also provided the practice ground for juveniles before they fledged. However, this research was limited by measurements from only one new nest on a broad-leaved tree, which was not representative of reused and old nests on chir pine trees. A more detailed assessment of nest architecture, stability and exposure and its contribution to breeding success is necessary to better understand the nesting ecology.
Although all known nests sites from 2003 to 2018 were included, this research was limited by the unavoidably small sample size of 22 sites to evaluate such a complex ecological process of nesting habitat selection and preferences. Another major drawback was the use of old nest sites, which had been abandoned several years earlier, as a proxy for site preference without baseline data on vegetation composition, structure, land-use, and other environmental parameters which do not exist for old nesting sites. While this research assumed that the sites had not changed significantly, it is difficult to envision how the sites appeared when the heron preferred to nest in that particular site. Timely documentation of habitat characteristics would improve the accuracy of such evaluations. If a similar study is conducted in the future, a plot size of at least 50 m diameter around the nest is recommended, because I found that 2 0m diameter was too small to assess actual tree density and spatial distribution of trees around the nests. Some of the nesting trees had crown diameter ∼15 m, almost covering the entire sampling plot. Also, having at least two random plots in vast and uniform landscapes would provide a better opportunity to distinguish the variation between the preferred site and surrounding habitat as Chir pine dominated habitats were found quite alike at ∼100 m distance. This research could neither evaluate the status and availability of nesting habitat nor explain the observed sudden change in nesting from chir pine to broad-leaved trees in 2018. This observation suggests either the WBH were gradually adapting to nest in a diverse environment, or that observers had missed nests in the broad-leaved forests in the past.
Finally, with fewer than five breeding pairs known from the entire range, the probability of locating an active nest is extremely low. Even with an exceptional habitat model developed, finding nests would remain extremely difficult because there are not enough birds to breed even if suitable habitats are available. When located, nest monitoring is essential to maximise the safety of existing pairs and nests. Protecting the site from hazards, avoiding disturbance, and maintaining safe feeding habitats and flight routes would all help WBH to continue breeding in their current locations. The current presence of WBH nests and feeding sites within a few hundred meters of human settlements symbolises the harmonious coexistence in the past and the future possibility. The small population at Phochu particularly has been sharing the short stretch of feeding site with grazing cattle and people living nearby for more than a decade. However, with the significant increase in disturbance due to rafting and riverside recreation, and decrease in the availability of safe foraging sites in recent years, the population has dwindled. Recreating safe feeding habitat with sufficient fish supply, reducing disturbance and direct conflicts would potentially ensure coexistence and revive the population. Moreover, providing artificial feeding ponds near nest sites during the breeding season in disturbed areas might improve nest reuse and brood size. The new record of successful breeding in broad-leaved forests also expands the search horizon for nests in future. Therefore, detailed research focused on habitat modelling, nest building mechanisms, breeding processes, and factors contributing to breeding failures and predators over the range of habitats would significantly enhance our current understanding of nesting ecology and natural threats to the population. Such research would also help understand the causes of population decline and fundamental challenges the bird is currently facing apart from risks due to habitat loss and anthropogenic pressures. Additionally, an assessment of nest materials, nest dimensions, architecture, and adaptation against hazards and predators would also contribute to planning and designing artificial nesting platforms in the proposed captive breeding facility and prioritising conservation initiatives.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S095927091900042X
Acknowledgements
This research was funded by the National Geographic Society (EC-415R-18) and Yale Tropical Resources Institute (TRI) Fellowship. I am thankful to the executive director and RSPN management for allowing me to conduct this research and providing logistics support while we were in the field. I extend my heartfelt gratitude to ecologist Rebecca Pradhan and my field crew Mr. Thinley Phuntsho, Mr. Pema Khandu, Mr. Tenzin Nima, Mr. Tshewang Lhendup and Mr. Sonam Tshering for their invaluable assistance in collecting data and sharing the data. Finally, I am indebted to Professor Timothy G. Gregoire and Dr. Simon Queenborough for unwavering guidance from developing the proposal to writing this manuscript.