INTRODUCTION
Visual-spatial neglect (neglect) is a disabling neurological condition most commonly due to right brain damage, such as a stroke. Neglect is characterized by a failure to orient toward or respond to stimuli on the individual’s contralesional side of space, not due to a primary sensory or motor deficit (Heilman, Watson, & Valenstein Reference Heilman, Watson and Valenstein1993). This attentional disorder is common particularly in stroke patients with damage to a variety of areas in fronto-parietal networks in the right-hemisphere (Bartolomeo, Thiebaut De Schotten, & Chica, Reference Bartolomeo, Thiebaut De Schotten and Chica2012). The incidence of neglect can be as high as 82–85% during the acute phase and 69% in the early rehabilitation phase (Azouvi et al., Reference Azouvi, Samuel, Louis-Dreyfus, Bernati, Bartolomeo and Beis2002; Cherney, Halper, Kwasnica, Harvey, & Zhang, Reference Cherney, Halper, Kwasnica, Harvey and Zhang2001; Stone, Halligan, Marshall, & Greenwood, Reference Stone, Halligan, Marshall and Greenwood1998). Neglect is associated with poor rehabilitation outcomes including slower recovery, increased length of stay at rehabilitation facilities, and decreased independence in activities of daily living (Cherney et al., Reference Cherney, Halper, Kwasnica, Harvey and Zhang2001; Gillen, Tennen, & McKee, Reference Gillen, Tennen and McKee2005; Katz, Hartman-Maeir, Ring, & Soroker, Reference Katz, Hartman-Maeir, Ring and Soroker1999). Thus, assessment of neglect and its impact on everyday function is of clinical importance for the development of effective rehabilitation strategies and improving the health outcomes of stroke patients.
Many theoretical interpretations of neglect suggest a deficit in spatial attentional factors mediated by a wide-spread neuroanatomical network (Corbetta & Shulman, Reference Corbetta and Shulman2011; Danckert & Ferber, Reference Danckert and Ferber2006; Driver & Mattingley, Reference Driver and Mattingley1998; Halligan & Marshall, Reference Halligan and Marshall1994; Làdavas, Carletti, & Gori, Reference Làdavas, Carletti and Gori1994; Posner, Walker, Friedrich, & Rafal, Reference Posner, Walker, Friedrich and Rafal1984; Rafal, Reference Rafal1994; Vallar, Reference Vallar1998). Spatial representation is not unitary, however, and the presence or severity of deficits seen in neglect patients can vary depending on a variety of factors (Bowen, McKenna, & Tallis, Reference Bowen, McKenna and Tallis1999) with neglect subtypes reported depending on spatial region of testing (Buxbaum et al., Reference Buxbaum, Ferraro, Veramonti, Farne, Whyte, Ladavas and Coslett2004; Committeri et al., Reference Committeri, Pitzalis, Galati, Patria, Pelle, Sabatini and Pizzamiglio2006).
For example, Bisiach, Perani, Vallar, and Berti (Reference Bisiach, Perani, Vallar and Berti1986) reported a double dissociation between personal and reaching space, in which some patients exclusively demonstrated symptoms of neglect in personal space, while others only demonstrated symptoms in reaching space. In some patients, symptoms occurred in both spaces. This double dissociation has been replicated in subsequent studies (Beschin & Robertson, Reference Beschin and Robertson1997; Marangolo, Piccardi, & Rinaldi, Reference Marangolo, Piccardi and Rinaldi2003; Ortigue, Megevand, Perren, Landis, & Blanke, Reference Ortigue, Megevand, Perren, Landis and Blanke2006).
Others have sought to further divide space beyond the body into reaching distance (also called peripersonal space) and beyond reaching distance (also called extrapersonal space). Studies have noted double dissociations between neglect symptoms in these regions (Aimola, Schindler, Simone, & Venneri, Reference Aimola, Schindler, Simone and Venneri2012; Butler, Eskes, & Vandorpe, Reference Butler, Eskes and Vandorpe2004; Pitzalis, Di Russo, Spinelli, & Zoccolotti, Reference Pitzalis, Di Russo, Spinelli and Zoccolotti2001). In these studies, a variety of methods were used to assess neglect in peripersonal space: Verbally identifying visual targets (Butler et al., Reference Butler, Eskes and Vandorpe2004), completing paper-and-pencil tasks (Aimola et al., Reference Aimola, Schindler, Simone and Venneri2012), and indicating the center of lines using a laser pointer (Pitzalis et al., Reference Pitzalis, Di Russo, Spinelli and Zoccolotti2001). Neglect in extrapersonal space was assessed using the same peripersonal tasks adapted to extrapersonal space, either using a laser pointer or verbal naming to respond.
Although symptoms of neglect have been shown to vary across spatial regions, few clinical assessment tools examine neglect outside of peripersonal space. Stand-alone tasks administered in personal space have been developed to assess personal neglect (also referred to as “body representational neglect” by some authors), such as the Fluff Test (Cocchini, Beschin, & Jehkonen, Reference Cocchini, Beschin and Jehkonen2001), the Vest Test (Glocker, Bittl, & Kerkhoff, Reference Glocker, Bittl and Kerkhoff2006), and the Comb and Razor Test (Beschin & Robertson, Reference Beschin and Robertson1997). All tasks require patients to interact with stimuli in personal space and show good reliability. However, these measures still attend to only one spatial region.
Batteries of tasks have been created to examine the occurrence of neglect symptoms in different spatial regions, although it is difficult to compare between tasks that are not equated for difficulty or test requirements. For example, to assess the occurrence of various neglect subtypes, Buxbaum and colleagues (Reference Buxbaum, Ferraro, Veramonti, Farne, Whyte, Ladavas and Coslett2004) administered subtests of the Behavioral Inattention Test (BIT; paper-and-pencil tasks completed in peripersonal space) and a modification of the Fluff Test. These tasks differed not only in their number of target stimuli but also in the nature of their stimuli, and even in the method by which the task was completed (written vs. motor response). Thus, performance differences observed between tasks could be attributed to factors other than neglect, such as task difficulty or a specific motor deficit.
Through a comprehensive literature review, Menon and Korner-Bitensky (Reference Menon and Korner-Bitensky2004) identified 62 assessment tools used to examine symptoms of neglect. Of these, they identified only one that measured symptoms across all three spatial regions: the Catherine Bergego Scale (CBS). In the initial validation study of the CBS (Azouvi, Reference Azouvi1996) and subsequent study of its psychometric properties (Azouvi et al., Reference Azouvi, Olivier, De Montety, Samuel, Louis-Dreyfus and Tesio2003), the CBS was compared to conventional neuropsychological assessment tasks and showed increased specificity for identifying symptoms of neglect. The CBS requires approximately 20–40 min to administer and involves direct observation of a patient post-stroke as they complete 10 daily living activities. Although the Kessler Foundation Research Centre has developed a standardized protocol (Chen, Hreha, Fortis, Goedert, & Barrett, Reference Chen, Hreha, Fortis, Goedert and Barrett2012), it is difficult to specifically define neglect subtypes in these spatial regions, given the global functional demands of the test.
Since Menon and Korner-Bitensky’s (Reference Menon and Korner-Bitensky2004) comprehensive review other functional assessment tools have been identified and developed, such as the Árnadóttir OT-ADL Neurobehavioral Evaluation (Gardarsdóttir & Kaplan, Reference Gardarsdóttir and Kaplan2002) and the Activities of Daily Living battery (Eschenbeck et al., Reference Eschenbeck, Vossel, Weiss, Saliger, Karbe and Fink2010). Although Eschenbeck and colleagues (2010) discussed the need to assess neglect symptoms in different spatial regions, neither of the above-mentioned measures allow the assessor to compare performance on tasks standardized and matched across these regions. The Sunnybrook Neglect Assessment Procedure (Leibovitch, Vasquez, Ebert, Beresford, & Black, Reference Leibovitch, Vasquez, Ebert, Beresford and Black2012) has also been developed as a bedside battery to meet the need for early assessment of neglect, yet measures neglect in peripersonal space exclusively. Similarly, stand-alone tasks exist to assess extrapersonal neglect only (e.g., The Dublin Extrapersonal Neglect Assessment; Cunningham, O’Rourke, Finlay, & Gallagher, Reference Cunningham, O’Rourke, Finlay and Gallagher2017).
Given the behavioral evidence supporting a dissociation of neglect symptoms experienced in different spatial regions and the lack of current standardized clinical measures to assess neglect severity specifically across these regions, we have developed a novel measure to assess symptoms of neglect matched by stimuli and task difficulty across personal, peripersonal, and extrapersonal space: the Halifax Visual Scanning Test (HVST). The goal of the current studies was to provide initial construct validity for this assessment measure by examining performance on the HVST in a sample of stroke patients in comparison to conventional and functional outcomes. In addition, these studies sought to accumulate healthy adult normative data to create a basis for evaluating stroke-related impairment.
STUDY I: COMPARISONS OF THE HVST TO CONVENTIONAL TASKS AND WHEELCHAIR NAVIGATION
Navigating while walking or using a wheelchair is often impaired in individuals with neglect (Turton et al., Reference Turton, Dewar, Lievesley, O’Leary, Gabb and Gilchrist2009) and collisions while walking or using a wheelchair were found to be one of the most sensitive functional outcomes during the CBS validation (Azouvi, Reference Azouvi1996). The purpose of Study I was to assess the construct validity of the HVST by comparing this novel assessment tool to a conventional measure for neglect (the BIT) and a functional task (wheelchair navigation) in both stroke patients and healthy adult controls.
Method
Participants
A convenience sample of 15 right-hemisphere stroke inpatients from a tertiary-care rehabilitation unit were recruited for the study (stroke group). Ten were later classified as having left neglect (67%), and five were classified as not exhibiting symptoms of neglect (see Results). The occurrence of neglect in this sample was consistent with other prevalence estimates of neglect in an early rehabilitation setting (69%; Cherney et al., Reference Cherney, Halper, Kwasnica, Harvey and Zhang2001). Sixteen adults living independently in the community were also recruited for the study (control group).
Participants were required to have normal or corrected-to-normal visual acuity and normal reading ability. Participants were excluded if they had a neurological or extrapyramidal motor system disorders (except for stroke in the stroke group), peripheral motor impairment that would interfere with wheelchair performance (except for contralesional hemiparesis in the stroke group), or severe arthritis.
Data from two controls were eliminated from analyses. The first was eliminated due to the presence of bilateral upper limb weakness (grip strength, L:R=5.5:6.5 lb; average L:R grip strength in the control group=29.6:32.9 lb) that could potentially interfere with wheelchair mobility. The second control was eliminated because their performance on the measure of overall intellectual functioning was more than two standard deviations below the range of the group (Wechsler Abbreviated Scale of Intelligence [WASI]; see Measures). This left 14 individuals remaining in the control group. See Table 1 for detailed participant information.
Table 1 Study I: Participant demographic and baseline testing information

Note. *Indicates a statistically significant difference (two-tailed) at α=0.01
a Mean (SD).
b Raw score.
c Percentile.
Measures
Halifax Visual Scanning Test (HVST)
The HVST was developed to characterize subtypes of neglect by measuring participant’s visual scanning in three spatial regions. Subtests were created with a focus on matching the stimuli across these regions. Stimuli consisted of words with four or more letters, one to three syllables, and a word frequency of more than 25 appearances per million (Thorndike & Lorge, Reference Thorndike and Lorge1972). Each list included 12 natural object nouns, 12 man-made object nouns, and 12 non-words. The three lists were equated as closely as possible for word frequency and the number of syllables per word. These words were printed among distracter symbols (stars) and were presented at three different distances matched for visual angle: personal space (on a shirt worn by the participant in a seated position; Shirt subtest), peripersonal space (on a sheet of paper on a table placed 62.5 cm directly in front of the participant; Table subtest), and extrapersonal space (on a board mounted on a wall 250 cm away from the participant; Wall subtest). Words were roughly organized in four columns centered on the participant’s midline, with each column containing three natural object nouns, three man-made object nouns, three non-words, and eight stars. No words were repeated across the three spatial regions. Images of the HVST subtests are depicted in Figure 1.
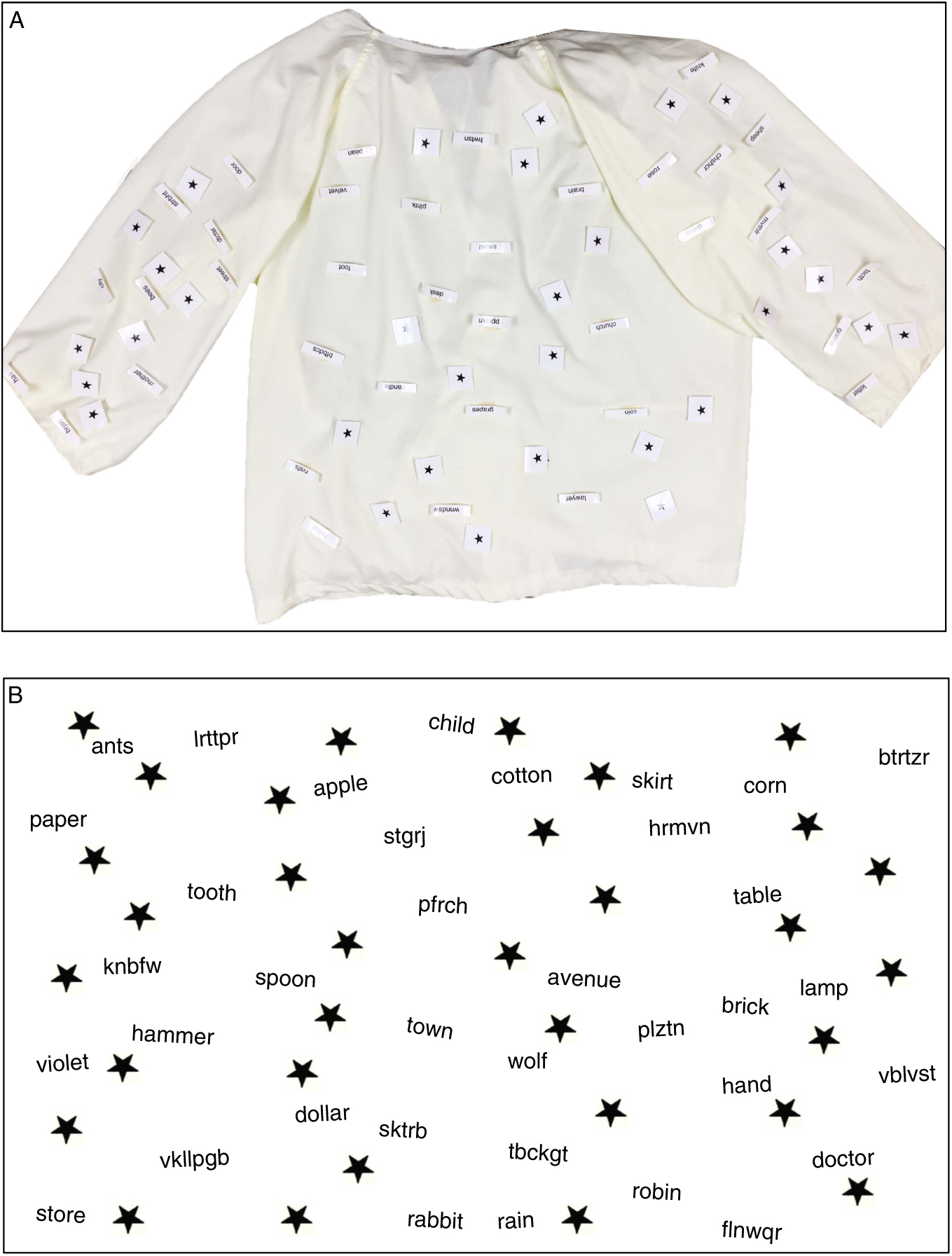
Fig. 1 A: Shirt worn by participants to measure Personal neglect. B: Image used for producing the Table and Wall subtests. Stimuli in each spatial region were matched in word frequency, length, and frequency of word type. To receive materials to use the HVST, please contact the corresponding author.
Participants were instructed to “look all over the [shirt, table, or wall] and read aloud as many real words as you can find” in a 2-min time limit. Thus, they were asked to visually scan each region of space (personal, peripersonal, or extrapersonal) to find and read the words. The total score was the number of words read aloud by the participant (maximum=24 words in each space). HVST administration requires approximately 10 min.
Behavioral Inattention Test (BIT)
The BIT is a battery of six paper-and-pencil tasks used to assess attention post-stroke (Wilson, Cockburn, & Halligan, Reference Wilson, Cockburn and Halligan1987). The BIT is a recommended and commonly used tool for the assessment of neglect symptoms due to its established cutoff values for impairment and its comprehensive assessment of neglect in peripersonal space (Halligan, Cockburn, & Wilson, 1991; Intercollegiate Stroke Working Party, 2012; Salter et al., 2013).
The three visual scanning subtests of the BIT were used for comparison: Star Cancellation, Letter Cancellation, and Line Crossing. These subtests involve crossing out stars or letters among distractors, or crossing out lines that are randomly oriented across a piece of paper. Scores for each task are the total number of target items successfully crossed out.
Baseline neuropsychological screening
Additional baseline neuropsychological tests were administered and scored according to standardized instructions to screen for dementia and to characterize the neuropsychological profile of the stroke group in relation to the control group. The test battery included: (1) a measure of overall intellectual functioning - the WASI (Wechsler, Reference Wechsler1999), (2) measures of attention and working memory - Digit Span Forwards and Backwards (Wechsler, Reference Wechsler1987), (3) a measure of visual-spatial ability - Judgment of Line Orientation (Benton, Hamsher, Varney, & Spreen Reference Benton, Hamsher, Varney and Spreen1983), and (4) Grip strength.
Wheelchair obstacle course
The wheelchair obstacle course was based on a method described by Webster and colleagues (Reference Webster, Cottam, Gouvier, Blanton, Beissel and Wofford1989). Participants were asked to guide their wheelchair along a 61-m course twice, once in each direction. The course contained three right and three left turns. The boundaries were marked by rope, held by pylons at roughly eye level when sitting in a wheelchair (see Figure 2). Twelve common objects (e.g., chairs, garbage cans, caution pillars) served as obstacles placed equally frequently on either the right or left side of the course. The course was wide enough to allow space between an obstacle and the course boundary (81 cm—the width of a standard door frame). Participants were videotaped as they navigated through the course for the blind scoring of errors. Errors recorded included direct hits (contact of the frontal plane of the wheelchair with obstacles) and sideswipes (contact with the side plane of the chair), as previously defined by Webster and colleagues (Reference Webster, Cottam, Gouvier, Blanton, Beissel and Wofford1989).

Figure 2 Illustration of the wheelchair obstacle course.
Procedure
As the control participants were not familiar with navigating in a wheelchair and wheelchair expertise in the stroke group may not have been consistent, all participants completed a practice day in the wheelchair course. On this day, participants also completed the cognitive assessment measures. The three word-sets were counterbalanced among the three HVST subtests. Participants completed the wheelchair obstacle course again, within 1 week of their practice run. The experimenter recorded the number and location of collisions (i.e., direct hits and side swipes) that the participants made with their wheelchair. Participants in the stroke group completed the same obstacle course again before their inpatient discharge, approximately 30 days after their initial testing session (Time 2).
Our stroke participants had moderate to severe hemiparesis, and thus used only their unaffected side for wheelchair navigation. To equate method of navigation between groups, controls were only allowed to use their right arm and leg to complete the course and their left arm was braced as a reminder. Control participants were allowed to practice navigating turns using a slalom-like series of three pylons placed 1.5 m apart until they felt comfortable (10 min time limit), before completing their practice day on the wheelchair course.
Procedures were in accord with the ethical standards of the Capital Health District Health Authority Research Ethics Board. As multiple comparisons were used to examine the numerous variables of interest, a more conservative alpha of 0.01 was used to determine statistical significance when comparing groups.
Results
Participant demographics
The majority of participants in both groups were male and right-handed. In the stroke group, time since stroke ranged from one to four months. Comparisons between groups using either Mann-Whitney U or chi-square tests, as applicable, revealed that the control and stroke groups did not differ in age, education, frequency of right-handedness, or gender. The two groups did not differ in estimated premorbid IQ (WASI Vocabulary). The control group had significantly stronger grip strength on their left side, compared to the stroke group. There was no difference between groups on their right side (see Table 1).
Visual scanning tasks
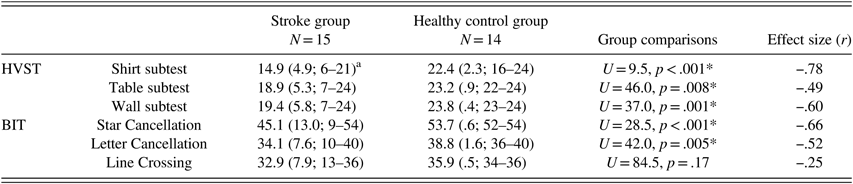
The BIT Line Crossing, Letter, and Star Cancellation subtests were used for comparison with the three HVST subtests. The BIT cancellation tasks are visual scanning tasks completed in peripersonal space exclusively. Performance on all visual scanning tasks was compared between groups using two-tailed Mann-Whitney U tests, given the non-normal distribution (see Table 2). Although the control group had higher raw scores on all visual scanning tasks compared to the stroke group, performance on the BIT Line Crossing subtest did not significantly differ between stroke patients and controls.
Table 2 Performance on HVST subtests and BIT cancellation subtests in Study I

Note. *Indicates a statistically significant group difference (two-tailed) at α=0.01, Mann-Whitney U test.
a Mean correct (SD; range of scores). Maximum possible scores: HVST subtests=24, BIT Star Cancellation=54, BIT Letter Cancellation=40, BIT Line Crossing=36.
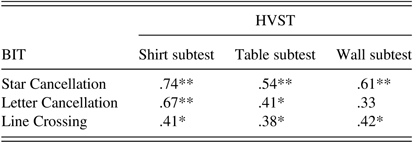
Non-parametric Spearman’s correlations were calculated between each of the HVST subtests and the three cancellation subtests of the BIT. Most HVST subtests correlated significantly with each of the three BIT cancellation subtests (r’s ranged from 0.38 to 0.74). However, the HVST Wall subtest was not correlated with the BIT Letter Cancellation (r=0.33; see Table 3).
Table 3 Correlations between the HVST subtests and the three BIT cancellation subtests for all participants (N=29) in Study I

*Significant spearman correlation (two-tailed) at α=.05.
**Significant spearman correlation (two-tailed) at α=.01.
As the stimuli for the HVST are organized in four columns, additional analyses were completed comparing the location of HVST errors (rightmost, middle-right, middle-left, and leftmost columns), within the different spatial regions (Shirt, Table, Wall subtests), between control and stroke group participants. A 4 (Column) × 3 (Spatial Region) × 2 (Group) repeated-measures analysis of variance was run. There was a significant main effect of Column, however, this was qualified by a significant Column by Group interaction [F(2.21, 59.65)=7.49, p=.001; effect size (ηp2)=0.22]. For the control group, participant’s scores remained consistent across columns (from left to right: M=5.57, 5.74, 5.90, 5.93). For the stroke group, however, participant’s scores increased as they moved from left to right (M=3.29, 4.56, 4.80, 5.09), consistent with symptoms of neglect.
Wheelchair navigation performance
Direct hits and sideswipes were scored by blinded observers uninformed about group membership. Overall, the stroke group made significantly more errors compared to the control group for both direct hits (effect size r=−0.67) and sideswipes (effect size r=−0.54; see Table 4). Although the stroke group did decrease in their number of total direct hits and total sideswipes between the two time-points (pre- and post-rehabilitation), this difference was not significant [direct hits: paired t(11)=0.83; p=0.42; side swipes: paired t(11)=1.35; p=.20].
Table 4 Wheelchair obstacle course performance

a Mean (SD).
*Indicates a statistically significant different (two-tailed) at α=0.01.
To examine the association of our novel visual scanning measures with a functional measure of performance, Spearman’s correlations were calculated between the HVST and performance on the wheelchair course at Time 1 for all participants (see Table 5). Significant correlations were found between wheelchair direct hits and all HVST subtests (Shirt r=−0.67; p≤.01; Table r=−0.39; p≤.05; Wall r=−0.55; p≤.01). Wheelchair side swipes correlated significantly with the Shirt (r=−0.54; p≤.01) and Table (r=−0.59; p≤.01) subtests, but not the Wall subtest (r=−0.26).
Table 5 Correlations between the HVST and wheelchair course performance for all participants in Study I

*Significant spearman correlation (two-tailed) at α=.05.
**Significant spearman correlation (two-tailed) at α=.01.
STUDY II: DEVELOPING A HVST NORMATIVE DATABASE
Results from Study I suggested that the HVST may be an informative measure for assessing the functional prognosis of neglect rehabilitation in addition to currently used assessment measures for neglect. As such, further development of the HVST was deemed appropriate. The purpose of Study II was to collect further normative data on the HVST in healthy adults to establish cutoffs for impairment that could be then applied to stroke patients. An additional cancellation subtest (i.e., Figure Cancellation) commonly used to detect neglect in stroke patients was also administered for further comparison with the HVST.
Method
Participants
Healthy adults were recruited from the community using online advertisements as well as through a volunteer contact list. Participants did not receive compensation for participating in the study. Participants were required to: (1) be 45+ years of age, (2) have self-reported normal or corrected-to-normal vision, and (3) be able to read English (from self-report). Participants were excluded if their self-reported health history indicated that they had: (1) experienced a stroke, (2) received a diagnosis of dementia or another neurological disorder/disease, or (3) if they received a score of 23 or lower on the Montreal Cognitive Assessment (MoCA). An exclusion cutoff score of 23 or lower was used given its increased specificity for correctly identifying older adults without mild cognitive impairment (Luis, Keegan, & Mullan, Reference Luis, Keegan and Mullan2009).
Although 27 participants were originally recruited, eight were removed from the study based on the following criteria: improper procedures (n=5), not meeting the minimum required score on the MoCA for inclusion (n=2), and poor vision (n=1). The study concluded with 19 participants in total.
Measures
Figure Cancellation subtest (Sunnybrook Neglect Assessment Procedure; SNAP)
The Figure Cancellation test from the SNAP was included for further comparison of the HVST with another standard visual scanning measure. The SNAP is a short assessment battery designed to be administered to patients at their bedside (Leibovitch et al., Reference Leibovitch, Vasquez, Ebert, Beresford and Black2012). The Figure Cancellation subtest requires participants to cross out 60 designated targets among distractor symbols.
Montreal Cognitive Assessment (MoCA)
The MoCA (www.mocatest.org) is a validated short screening tool used to detect MCI (Luis et al., Reference Luis, Keegan and Mullan2009; Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead, Collin and Chertkow2005; Smith, Gildeh, & Holmes, Reference Smith, Gildeh and Holmes2007). The measure totals to 30 points and examines seven cognitive domains: visual-spatial/executive abilities, naming, attention, language, abstraction, delayed recall, and orientation.
Halifax Visual Scanning Task
See Study I Materials for a full description of this task.
Procedure
Participants were invited for a one-time testing session at the Nova Scotia Rehabilitation and Arthritis Centre (Halifax, Nova Scotia). The session began with two screening measures that were later used to confirm eligibility for inclusion: a brief demographic and health history questionnaire, and the MoCA. Participants then completed two measures of visual scanning: The Figure Cancellation subtest of the SNAP and the HVST.
Procedures were in accord with the ethical standards of the Nova Scotia Health Authority Research Ethics Board. As multiple comparisons were used to examine the numerous variables of interest, a more conservative alpha of 0.01 was used to determine statistical significance.
Results
Participant demographics
The Study I control group and the Study II participants were combined to form a normative database for the HVST (see Table 6 for combined participant demographic information; see Table 7 for combined visual scanning task performance).
Table 6 Healthy adult normative database demographic information

a Mean (SD).
Table 7 Comparison of healthy adult normative database participants on visual scanning tasks

Note. Mean (SD; range of scores).
a Study I participants, BIT Star Cancellation; Study II participants, SNAP Figure Cancellation.
Neglect in Different Spatial Regions
Performance on the HVST was compared between the normative database and the stroke group from Study I using two-tailed Mann-Whitney U tests. Participants in the normative database had significantly higher performance scores on all HVST subtests compared to the stroke group (all p<.001).
The normative database was used to establish cutoff scores for impairment on the HVST. Given the skewed nature of the healthy adult performance on the HVST subtests (i.e., many healthy adults had perfect scores for each column), it was decided to use the lower range of scores to establish cutoffs for abnormal performance. An abnormal score thus was defined as any score less than or equal to the lowest score obtained in the normative database.
Individual data from all participants in the stroke group of Study I were then examined for the presence of neglect in each spatial region using the newly established HVST cutoff criteria (shown in Table 8). Given these criteria, 14 of 15 individuals were classified as having neglect in at least one spatial region on the HVST. Of the 14 individuals with neglect symptoms, 5 showed symptoms of neglect in all spatial regions, 3 showed neglect in two spatial regions (peripersonal/extrapersonal space, n=2; personal/extrapersonal space, n=1), and 4 showed neglect in only one spatial region (personal space, n=1; peripersonal space, n=3).
Table 8 HVST and BIT performance for each stroke group participant

Note. Participants are organized in ascending order according to BIT total score. Cutoff scores for impairment are indicated in parentheses. Shaded-cells indicate subtests in which the participant was classified as exhibiting neglect. See text for definition of impairment. Age is reported in years. * Indicates participant had a visual field deficit.
F=female, M=male.
Individual data from all participants in the stroke group were also examined using the established cutoff criteria for the BIT (shown in Table 8). Neglect was defined as having a score at or below the cutoff for impairment (Wilson et al., Reference Wilson, Cockburn and Halligan1987) on one or more of the three cancellation subtests. Ten participants in the stroke group met these criteria for exhibiting symptoms of neglect.
When comparing the BIT Line Crossing, Letter, and Star Cancellation subtests to performance on the HVST, four participants exhibited symptoms of neglect on the HVST subtests that were not identified by the BIT tasks (see Table 8).
GENERAL DISCUSSION
The purpose of the current studies was to evaluate a new measure, the Halifax Visual Scanning Test, intended to quantify neglect subtypes in three spatial regions: personal, peripersonal, and extrapersonal space. All three HVST subtests were found to correlate with both traditional visual scanning tests administered (medium to strong relationships; Cohen, Reference Cohen1992), with one exception. The association among tests of neglect has been shown to be variable, either due to the attentional variability inherent in neglect symptoms or to the possibility that different tests are measuring different aspects of neglect.
For example, previous validation work on the CBS similarly compared performance on their functional assessment for neglect in different spatial regions to the BIT (Luukkainen-Markkula, Tarkka, Pitkänen, Sivenius, & Hämäläinen, Reference Luukkainen-Markkula, Tarkka, Pitkänen, Sivenius and Hämäläinen2011). Luukkainen-Markkula and colleagues (Reference Luukkainen-Markkula, Tarkka, Pitkänen, Sivenius and Hämäläinen2011) found only 5 of the 10 CBS tasks to correlate with at least 1 of the BIT tasks, despite both the BIT and CBS being established measures for neglect assessment. Variability in performance on neglect measures was also reported by Azouvi and colleagues (Reference Azouvi, Samuel, Louis-Dreyfus, Bernati, Bartolomeo and Beis2002), who administered seven paper-and-pencil assessment tasks for neglect and the CBS to a large sample of stroke patients (n=206). Stroke patient’s performance varied on both the paper-and-pencil tasks and behavioral measures of the CBS, meeting criteria for neglect on some subtests and not others.
Our findings demonstrate that the HVST similarly captures both unique and overlapping symptoms when compared to other standard measures. While a strong relationship was observed between some subtests of the HSVT and BIT, HVST subtests that were less related to these conventional tasks may be identifying additional aspects of neglect not captured by the BIT.
When examining performance on the wheelchair obstacle course, all three HVST subtests were associated with wheelchair navigation outcomes. Webster and colleagues (Reference Webster, Cottam, Gouvier, Blanton, Beissel and Wofford1989), who sought to better understand the impact of neglect on wheelchair navigation, recruited both stroke patients who demonstrated symptoms of neglect on two standard paper-and-pencil tests and stroke patients who did not demonstrate neglect. When participants then completed a wheelchair task, the two groups did not differ in their number of sideswipe wheelchair collisions on the lefterrors consistent with the more general effects of right-hemisphere stroke). Observations such as these suggest that conventional paper-and-pencil tasks may fail to capture the functional subtleties of neglect, demonstrating the importance of developing new measures that are associated with functional outcomes.
In comparison to three conventional scanning measures, the three subtests of the HVST identified more stroke patients as exhibiting symptoms of neglect (14 patients vs. 10). Three of these patients had abnormal performance on the HVST peripersonal task that was not seen in the BIT subtests, also completed in peripersonal space. This difference could be due to the difference in task demands. The HVST draws more heavily on working memory due to the use of oral report. Thus, participants are required to remember which words they have already identified and read aloud, in order to know which words are remaining to be read. In contrast, participants cross out the target items on the BIT, and, therefore, do not have to keep track of which target items they have already identified. Spatial working memory deficits have been cited as a key element in neglect (Danckert & Ferber, Reference Danckert and Ferber2006); thus, the oral report methods of the HVST may make it more sensitive to neglect symptoms.
One other patient exhibited neglect symptoms exclusively in personal and extrapersonal space on the HVST, while performing in the normal range on the peripersonal tasks of both the BIT and HVST. These results are consistent with previous studies that have identified double-dissociations between symptoms of neglect in peripersonal and personal space (Beschin & Robertson, Reference Beschin and Robertson1997; Bisiach, Perani, Vallar, & Berti, Reference Bisiach, Perani, Vallar and Berti1986), as well as peripersonal and extrapersonal space (Aimola et al., Reference Aimola, Schindler, Simone and Venneri2012; Butler et al., Reference Butler, Eskes and Vandorpe2004; Pitzalis et al., Reference Pitzalis, Di Russo, Spinelli and Zoccolotti2001). Given that the HVST equates for task requirement and difficulty across spatial regions, the dissociations identified in the current study can be more confidently attributed to differences in visual scanning abilities in different spatial regions compared to previous research. The current data contribute to the literature highlighting the variability in symptom expression among those experiencing neglect. They further support the need for assessment measures to quantify neglect symptoms using multiple tests, including those targeting different spatial regions. Importantly, commonly used measures for assessing neglect may be missing symptoms that are expressed outside of peripersonal space, which would have negative implications with regards to rehabilitation planning for those individuals.
Recovery post stroke is a highly dynamic process and the present analyses provide only a snapshot of stroke patients during their recovery. We, therefore, are unable to comment on the recovery course of neglect symptoms in different spatial regions. The value that this additional assessment information may provide with regard to outcomes requires further consideration. Performance on the HVST appears to correlate with some functional measures, but more research and psychometric testing is needed to understand the value of this assessment to rehabilitation planning.
While the HVST can measure neglect symptoms in different spatial regions, the contribution of other underlying influences to performance is unknown (e.g., directional akinesia vs. inattention, working memory deficits, allocentric neglect). Future studies could explore these mechanisms, as well as examine the underlying brain mechanisms of neglect subtypes through more detailed lesion localization. Although the HVST advances our ability to test for symptoms of neglect across spatial regions, the reading and working memory demands may prove a challenge for some patients and limit the application of this test. Further development using different stimuli and detection methods may improve the applicability of the HVST to all stroke patients.
As explained by Buxbaum and colleagues (Reference Buxbaum, Ferraro, Veramonti, Farne, Whyte, Ladavas and Coslett2004), assessing for subtypes of neglect is the first step toward determining their effects on treatment outcomes. The HVST is easily administered in one session and performance is not affected by a motor deficit. These characteristics facilitate the integration of the HVST into clinical care and future research examining treatments for neglect to characterize patients by subtypes of neglect (i.e., personal, peripersonal, and extrapersonal neglect). This addition to treatment study outcomes may account for unique variability in treatment progress that is frequently observed in interventions for neglect post stroke.
Conclusions
The current analyses provide initial construct validity for a novel and sensitive assessment measure of subtypes of neglect in personal, peripersonal and extrapersonal space. These data further support the idea that symptoms of neglect can differ across these regions and demonstrate the need to assess neglect outside of peripersonal space. Although there is recognition that neglect symptoms can differ across spatial regions, standardized and validated measures are needed (Lindell et al., Reference Lindell, Jalas, Tenovuo, Brunila, Voeten and Hämäläinen2007). These analyses suggest that the HVST may fill this need. Proper assessment of neglect symptoms is necessary in planning rehabilitation strategies and identifying heterogeneity in individuals that may explain differences in treatment outcomes. Given the variation in neglect symptom expression, a multifactorial approach to neglect assessment for clinical and research purposes is needed (Azouvi et al., Reference Azouvi, Samuel, Louis-Dreyfus, Bernati, Bartolomeo and Beis2002). This novel measure shows potential to become an informative addition to the conventional assessment battery.
ACKNOWLEDGMENTS
The authors do not have financial conflicts of interest to disclose. This research was funded by the Heart and Stroke Foundation (to G.E.), the Canadian Institutes of Health Research (to G.E.), and the Nova Scotia Health Authority (to G.E., Translating Research into Care program). To receive materials to administer the HVST, please contact the corresponding author.