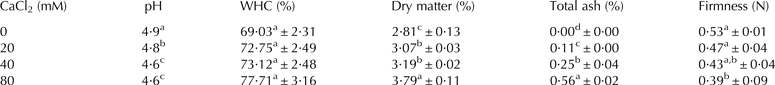Whey proteins in addition to high nutritive value have important functional characteristics including heat-induced and cold-set gelation, as well as assembly into various nano- and micro-scaled structures. The assembly of whey proteins into fibrils, microgels and soluble aggregates (Nicolai et al. Reference Nicolai, Britten and Schmitt2011) has received considerable attention by the food, nutraceutical and pharmaceutical industries (Donato et al. Reference Donato, Schmitt, Bovetto and Rouvet2009). Whey protein assemblies’ morphology is governed by the repositioning of partially unfolded protein molecules to a more stable state so that a balance between attractive hydrophobic interactions and repulsive electrostatic interactions is set (Veerman et al. Reference Veerman, Sagis, Heck and van der Linden2003b; van der Linden & Foegeding, Reference van der Linden, Foegeding, Kasapis, Norton and Ubbink2009). Whey proteins are fibrillated into long semi-flexible filaments with micro length and nano diameter when heated in very acidic (conventional pH ≈ 2·0) solution of low ionic strength (Akkermans et al. Reference Akkermans, van der Goot, Venema, van der Linden and Boom2008a). Remarkable electrostatic repulsion amongst the charged whey protein molecules at aforementioned condition results in unidirectional growth of protein aggregates rather than being packed in latitude. Strength and stability of the fibrils are conferred by a highly ordered cross-β core structure (Hughes & Dunstan, Reference Hughes, Dunstan, Kasapis, Norton and Ubbink2009; Nicolai & Durand, Reference Nicolai and Durand2013).
Flexible whey protein nanofibrils can entangle and yield viscous dispersion. The fibrils may also gel at low protein concentrations (Akkermans et al. Reference Akkermans, van der Goot, Venema, van der Linden and Boom2008b; Loveday et al. Reference Loveday, Su, Rao, Anema and Singh2011). Veerman et al. (Reference Veerman, Baptist, Sagis and van der Linden2003a) gelled β-lactoglobulin nanofibrils solution of 2% protein content by pH neutralisation with strong alkali and subsequent addition of CaCl2. Whey proteins might alternatively be gelled by adding (mineral) acid and/or salt into an already heat-denatured whey protein solution to screen up the electrostatic repulsion amongst protein molecules and induce their aggregation (Sadeghi et al. Reference Sadeghi, Madadlou and Yarmand2014). Accordingly, cold-set whey protein (nanofibrils) gels contain essentially mineral ions because of the acids and/or alkalis used in nanofibrils formation and gelation processes.
Cold-set gels of whey proteins have gained considerable attention as carriers of bioactive ingredients such as antioxidants, vitamins, bioactive peptides and probiotics (Chen et al. Reference Chen, Remondetto and Subirade2006; Garti & McClements, Reference Garti and McClements2012). It is well known that the physicochemical properties of (bioactive) biomacromolecules and the rate of bacterial growth are affected by the presence of salt ions in the surrounding aqueous solution (Lund et al. Reference Lund, Vacha and Jungwirth2008; Sedlák et al. Reference Sedlák, Stagg and Wittung-Stafshede2008). It is hence of interest to fabricate whey protein gels with zero or minimum content of mineral acids and alkalis. The aim of the present study was, therefore, to fabricate protein gels with minimum ash content and no added salt from whey protein nanofibrils by removing through dialysis the acid employed already for pH adjustment. The influence of calcium chloride presence on the techno-functional properties of nanofibrils gel was also investigated by CaCl2 enriching the ash-depleted pre-gel nanofbrils solution.
Materials and methods
Materials
Whey protein isolate (WPI) with 92% protein content was a kind gift from Arla Food Ingredients (Viby J, Denmark). Calcium chloride (CaCl2), glutaraldehyde and hydrochloric acid (HCl) were purchased from Merck (Darmstadt, Germany). Distilled water was used throughout the study.
Preparation and gelification of protein nanofibrils
WPI was dissolved in distilled water at concentration of 40 mg/ml and sodium azide (0·1 mg/ml) was added as antimicrobe. The protein solution was stirred at 25 °C for 2 h and pH was adjusted on 2·0 with 6 M HCl. This was followed by incubation of protein solution at 4 °C for 12 h in order to assure protein hydration. The solution was subsequently filtered through 0·22 µm sterile syringe filters and heated under stirring in a water bath at 80 °C for 4 h. After heat treatment, the sample was rapidly cooled to room temperature by tap water. Whey protein nanofibrils solution was gently poured into dialysis membranes (D9527, 27 mm average diameter, 14000 Da typical molecular weight cut-off, Sigma-Aldrich Co., Germany) and mounted perpendicularly inside the beakers filled with distilled water containing sodium azide. The fibrils solution was dialyzed against the distilled water for 67 h at room temperature while being stirred gently. The water was refreshed every 3 h during day times. At the final round of dialysis which lasted 17 h, different levels of CaCl2 (0, 20, 40 and 80 mM) were added to dialysis water in order to infiltrate gradually into the protein fibrils solution. The exit of hydronium ions out of nanofibrils solution and concomitant entrance of water molecules into the solution mitigated the acid content of protein nanofibrils solution and augmented pH toward the pI of whey proteins. This resulted in slow gelation of nanofibrils solution.
Protein nanofibrils shape and size
Protein nanofibrils solution was diluted in distilled water with pH 2·0 at the final concentration of 0·1 mg/ml. For atomic force microscopy (AFM) imaging, 10 µl of the diluted dispersion was spread on glass slide and left to dry under ambient conditions. The specimen was scanned using an atomic force microscope (NanoWizard, JPK Instrument AG, Germany). Fibrils size was determined by processing AFM images with JPK data processing software version 3.4·15 by averaging data taken from many images for statistical analysis.
Texture of gel samples
The firmness of protein nanofibrils gel samples and influence of CaCl2 concentration in dialysis water on the firmness of gels was studied by measuring the maximum force needed to penetrate a distance of 10 mm using a texture analyser (M350-10CT, Testometric, Lancashire, UK). Gels with 30 mm diameter and 40 mm height were penetrated with a cylindrical stainless steel probe (diameter 13 mm) at a constant speed of 0·25 mm/s.
The storage (G’) and loss (G″) moduli of nanofibrils gel samples were measured on a rheometer (Malvern Instruments Ltd., Worcestershire, UK) using a parallel plate geometry (diameter 25 mm, gap 2 mm). Specimens were sheared at frequency range of 0·01–10 Hz (constant strain of 1%) and at temperature of 20 °C.
Fourier transform infrared (FTIR) spectroscopy
In order to investigate the alterations occurred in functional groups of whey proteins due to gelation process, FTIR spectra of gels were acquired with a Perkin Elmer 2000 FT-IR spectrometer (Perkin Elmer Co., MA, USA) from 450 to 4000 cm wavenumber range. Samples were dried and mixed with potassium bromide powder and then pressed into a tablet. The spectra of samples were plotted using OPUS software (Bruker Optics Inc, Billerica, USA).
Microstructure of gel samples
Small pieces of gels (1 cm3) were cut with a razor blade and fixed overnight in 2·5% glutaraldehyde. Fixed gel specimens were dehydrated through immersion in a graded aqueous alcohol series (30, 40, 60, 70, 90 and 100% v/v ethanol) for 60 min each time. The specimens were subsequently cut into very thin slices and air dried at room temperature. This was followed by coating of a thin layer of gold onto specimens within a sputter-coater (SBC12, KYKY Technology Development Ltd, China) and microscopic imaging with a scanning electron microscope (SEM, KYKY-EM3200, KYKY Technology Development Ltd, China) operated at 26·0 kV. Photomicrographs were recorded on at 10 000 and 20 000 magnifications.
Water-holding capacity (WHC) and ash content of gels
The WHC of nanofibrils gel samples was determined using a centrifugation procedure (Kuhn et al. Reference Kuhn, Cavallieri and Cunha2011). Small pieces of gels (3·5–4 g) were gently cut with a sharp blade and weighed. The gel pieces were placed in 15 ml centrifuge tubes and centrifuged (Universal 320, Hettich Zentrifugen, Germany) at room temperature at 1500 g for 10 min. The WHC was expressed according to the following equation:
where waterreleased is the amount of water in grams released from gel after centrifugation and watergel is the amount of water in the intact gel (before centrifugation). To measure the ash content of nanofibrils gel samples, 1 g of each sample was heated at 500 °C for 4 h.
Statistical analysis
Samples were prepared in duplicate and data obtained from the experiments carried out in triplicate were subjected to one-way analysis of variance (ANOVA) by SPSS (ver. 16, IBM software, NY, USA) software. Duncan's test was used to determine any significant differences among the mean values at a P level of 0·05.
Results and discussion
Size and shape of protein nanofibrils
An exemplar AFM image of whey protein nanofibrils is shown in Fig. 1. Fibrils were of linear shape with 2–5 µm length and 10–20 nm diameter. These results are in agreement with those reported by Veerman et al. (Reference Veerman, Sagis, Heck and van der Linden2003b) for β-lactoglobulin fibrils with contour length range of 2–7 µm formed through heating of protein solution at 80 °C for 10 h. Bolder et al. (Reference Bolder, Hendrickx, Sagis and van der Linden2006) suggested that mixing of β-lactoglobulin with other whey proteins, as present in commercial whey protein isolate preparations, does not change the fibrils morphology.

Fig. 1. AFM image of whey protein nanofibrils formed on prolonged heating at pH 2·0.
Rheology of gels
The pH of whey protein nanofibrils solution increased with diminishing acid content during dialysis against water. This resulted in progressive reduction of the electrostatic repulsion amongst nanofibrils. At pH values close to the isoelectric point, the gel network started to form gradually. The formed gel was self-standing, visually opaque (Fig. 2) and depleted in ash. Veerman et al. (Reference Veerman, Baptist, Sagis and van der Linden2003a) observed no gel formation at CaCl2 concentrations less than 10 mM added directly to a 2% β-lactoglobulin fibrils solution with pH 7·0 and pointed out the necessity of establishment of salt bridges for gel network formation at the neutral pH. Bolder et al. (Reference Bolder, Hendrickx, Sagis and van der Linden2006) found that transparent strong gels are formed upon heating of whey protein nanofibrils solutions at protein concentrations above 6 wt %. The main mechanism of sol–gel transition in the present study was hydronium ions ejection and increment of pH toward the pI of proteins which formed a cold-set opaque hydrogel. By adding CaCl2 to the last round of dialysis water, diffusion of hydronium ion out of the fibrils solution was disturbed and decelerated. Table 1 reports that the pH values of gels formed via dialysis against waters enriched with CaCl2 were lower than that of the ion-free sample. When CaCl2 was added into the dialysis water, chloride ions that diffused into the acid-mitigated fibrils solution neutralised the oppositely charged proteins and induced gel formation process via reducing the electrostatic repulsion amongst the gel-forming protein nanofibrils at pHs relatively far from the pI of proteins.

Fig. 2. Cold-set gel of whey protein nanofibrils (4% w/w) formed through acid mitigation.
Table 1. Effect of CaCl2 level on properties of fibrilar gels induced by acid mitigation

Means within the same column with different superscripts differ significantly (P < 0·05)
Figure 3 shows the G′ and G″ values of whey protein nanofibrils gel samples as function of oscillation frequency. For all samples, G′ was greater than G″ throughout the whole frequency range. This indicates that whey protein nanofibril gels had a dominant elastic nature (Vardhanabhuti et al. Reference Vardhanabhuti, Foegeding, McGuffey, Daubert and Swaisgood2001). The higher the CaCl2 concentration in dialysis water, and the higher the dry matter content of gels the lower was the gels pH (Table 1) and G′. A very similar trend is observed for gel firmness results (Table 1). It is concluded that gelation pH played a much more significant role in dictating the firmness of gels than dry matter content. Gelation at pH values less than pI minimised the contribution of Ca2+-mediated cross-linking of protein fibrils in gel formation. Also, it is argued that chloride ions although neutralised the counter charged protein domains and finally established a gel network, influenced negatively the hydrophobic interactions and hydrogen bonding among nanofibrils leading to gels with weaker structures. Chloride is a low-charge-density ion (chaotrope) and tends to stick to the hydrophobic surfaces. This strongly reduces the hydrophobic interactions among the elements covered with chloride (Zangi et al. Reference Zangi, Hagen and Berne2007; Beauchamp & Khajehpour, Reference Beauchamp and Khajehpour2012). Furthermore, since Cl– is a very good proton acceptor (Kovács & Varga, Reference Kovács and Varga2006), it might form N–H⋯Cl– hydrogen bonds and prevent amide groups from forming additional intermolecular N–H⋯O=C hydrogen bonds. This is turn would promote the formation of hydrogen bonds between protein CO groups and neighbour water molecules as tabulated (Table 1) as the higher (although indifferent statistically) WHC values of gels with higher ash contents. Another factor that contributed to higher WHC in the gel samples with non-zero ash content was the strongly hydrated calcium ions which interacted with non-charged regions of protein chains by site-specific interactions such as cation-π interactions (Loveday et al. Reference Loveday, Su, Rao, Anema and Singh2012).

Fig. 3. Storage modulus (a) and loss modulus (b) of whey protein nanofibrils gels prepared through dialysis against water with different concentrations of CaCl2: 0 mM (○), 20 mM (●), 40 mM (■) and 80 mM (▲).
Chemical interactions
The amide I and II bands are the two most prominent vibrational bands of the protein backbone. The amide I band (1600–1700 cm) is due to C=O stretch vibrations of the peptide linkages (approximately 80%), while the amide II, absorbing near 1550 cm, derives mainly from in-plane NH bending (40–60% of the potential energy) and from the CN stretching vibration (18–40%). The exact frequency of the amide I and II absorptions depends on the nature of hydrogen bonding involving the C=O and NH moieties (Jackson & Mantsch, Reference Jackson and Mantsch1995; Kong & Yu, Reference Kong and Yu2007; Sadeghi et al. Reference Sadeghi, Madadlou and Yarmand2014). The bands appeared in the FTIR spectrum of ion-free gel (Fig. 4, sample donated a) at 1641 and 3292 cm attributed to the C=O and NH stretching vibrations, respectively. The bands at 1531 and 1232 cm contain contributions from NH bending vibrations. As the ash content of gels increased (follow the samples donated a, b, c and d in Fig. 4), the band at 3292 cm (NH stretching) shifted to lower wavenumbers with a concomitant decrease in intensity. As well, the bands at 1531 and 1232 cm (NH bending) slightly shifted to higher wavenumbers whilst the band at 1641 cm (CO stretching) remained unchanged. Barth (Reference Barth2007) mentioned that the wavenumber of bands involving bending vibrations increases on the formation of hydrogen bond, since hydrogen bonding produces an additional restoring force for bending vibrations but decreases the restoring force of stretching vibrations and lowers the wavenumber of the bands related to this type of vibration. It is concluded that the samples prepared through dialysis against waters enriched with CaCl2 possessed stronger hydrogen bonding in the gel network compared with the ash-free counterpart. FTIR results (Fig. 4) suggest that the enhanced hydrogen bonding in the ash-loaded samples was not due to interaction of N–H with C=O but because of interactions of either N–H or O–H groups as proton donors with hydrogen-bond acceptors other than CO. Halide anions are very good proton acceptors, being capable of forming very strong hydrogen bonding interactions (Kovács & Varga, Reference Kovács and Varga2006). It is hypothesised that chloride ions by forming N–H⋯Cl– hydrogen bond prevented the amide from forming additional intermolecular N–H⋯O=C bonds. The CO groups therefore formed hydrogen bonds with neighbour water molecules. The higher the chloride content in nanofibrils gel, the more extensive was the hydrogen bonding of CO with water i.e. higher water holding capacity (Table 1).

Fig. 4. FTIR spectra of whey protein nanofibrils gels prepared through dialysis against water with different concentrations of CaCl2: 0 mM (a), 20 mM (b), 40 mM (c) and 80 mM (d).
Gel microstructure
Figure 5 shows exemplar SEM micrographs of ion-free and ion-loaded whey protein nanofibrils gel samples. The ion-free gel had a compact structure in comparison with ion-loaded counterpart, which explains the more firmness and elasticity as well as less water holding capacity of the former. The presence of chloride ions in the gel network led to a more porous microstructure. In spite of the capability of Cl– to neutralise the positively charged groups and reduce the repulsive forces, the ion weakened the intermolecular interactions responsible for keeping aggregates close together. This resulted in a more open matrix with more pores. On the other hand, calcium ions in the gel network have failed to effectively fulfil bridging with carboxylate groups of side chains at such pH value. This microstructural feature justifies the lower forces needed to penetrate a given distance into the ash-loaded gels.

Fig. 5. SEM micrographs of whey protein nanofibrils gels prepared through dialysis against water of 0 mM CaCl2 (S1) and of 40 mM CaCl2 (S2).
Conclusion
Whey protein nanofibrils solution transformed to a soft viscoelastic gel with zero ash content upon dialysis against distilled water which removed ions and allowed fibrils to entangle. Calcium chloride enrichment of the last round of dialysis water caused Ca2+ and Cl‒ ions diffusion into the ash-depleted fibrils pre-gel solution, leading to lower firmness but increased water holding capacity for the resulting gels. It was suggested that chloride ions weakened the intermolecular interactions among the proteins and calcium ions did not have a strengthening effect on the gel network. The protein hydrogel with zero ash content is a potential candidate for conveying bioactive molecules that are sensitive to ions for example, those drugs that show decreased therapeutic impact due to binding with ions.
The authors wish to thank the Iranian National Science Foundation (INSF) for financial support of the project No. 93031573.