The growth hormone (GH) is synthesised and secreted by the anterior pituitary somatotroph cells and its secretion is tightly regulated, stimulated by growth hormone–releasing hormone (GHRH) and inhibited by somatostatin (SS or GHIH, growth hormone-inhibiting hormone), both secreted by the hypothalamus. The GH is released into systemic circulation and causes the secretion of the insulin like growth factor – 1 (IGF-1) in target tissues, it also causes a series of direct metabolic effects as it is both hyperglycaemic and lipolytic (Polkowska et al. Reference Polkowska, Wańkowska, Romanowicz, Gajewska, Misztal and Wójcik-Gładysz2011). As a consequence, the growth hormone is directly involved in animal processes such as metabolism (Barrera-Saldaña et al. Reference Barrera-Saldaña, Ascacio-Martínez, Sifuentes-Rincón, Arellano-Vera and Arbiza2010), growth (Hua et al. Reference Hua, Chen, Yu, Cai, Wu, Li, Zhang, Liang, Han, Geng, Shen, Xu and Yang2009), reproduction (Scaramuzzi et al. Reference Scaramuzzi, Murray, Downing and Campbell1999) and lactation (Baldi, Reference Baldi1999).
The GH gene spans 2·6 to 3·0 kbp in most mammalians and comprises five exons. In cattle, the growth hormone is synthesised by a single gene, the bGH (bovine GH) gene, mapped to chromosome 19 (19q22) (Fries, Reference Fries1993). A close relationship between genetic polymorphisms at the bGH gene and milk fat (Falaki et al. Reference Falaki, Gengler and Sneyers1996) and milk protein percentage (Lagziel et al. Reference Lagziel, Lipkin, Ezra, Soller and Weller1999) has been reported in this species. In addition, a nucleotide (nt) variation located on the third intron of bGH gene has been associated with milk yield (Zhou et al. Reference Zhou, Liu, Liu, Guo, Zhu and Wu2005). The ovine GH (oGH) gene has been mapped to chromosome 11 (11q25) (Ofir & Gootwine, Reference Ofir and Gootwine1997); significant associations between oGH genotypes and milk yield have been evidenced in Serra da Estrela, a Portuguese breed of sheep (Marques et al. Reference Marques, Santos, Carolino, Belo, Renaville and Cravador2006).
The caprine GH (gGH) gene, syntenic to bovine, has been mapped on 19q22. The caprine GH is a copy number variant (CNV), showing two different alleles: the Gh1 allele has a single copy of the gene (named GH1), while the Gh2 allele is duplicated and contains the GH2 and GH3 copies (Wallis et al. Reference Wallis, Lioupis and Wallis1998). Investigations on gGH gene genetic variability have highlighted significant correlations with: milk yield and protein percentage in the Portuguese Algarvia (Malveiro et al. Reference Malveiro, Pereira, Marques, Santos, Belo, Renaville and Cravador2001) and Serrana breeds (Marques et al. Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003); body weights in the Indian Sirohi breed (Kumar et al. Reference Kumar, Dixit, Gupta, Vyas and Kaur2011); litter size and superovulation response in Matou and Boer breeds (Zhang et al. Reference Zhang, Liu, Huang, Zeng, Xu, Wen and Yang2011).
The aim of this research was to investigate single-strand conformation polymorphisms (SSCP) in each of the five exons of the gGH gene, in order to establish the possible relationships between polymorphic patterns and milk production traits in the Sarda breed goat.
Materials and methods
Animals and sampling
One hundred primiparous lactating goats of Sarda breed were randomly chosen from one farm located in Sardinia (Italy). Six hundred goats, of which 110 primiparous, were reared on the farm, they were exclusively fed at pasture and hand-milked once daily. Length of lactation was 220 d for multiparous and 180 d for primiparous goats; average milk yield was 200 L, with a milk fat percentage of 4·7 and a protein percentage of 4·2, which are typical values for the breed (Pazzola et al. Reference Pazzola, Dettori, Carcangiu, Luridiana, Mura and Vacca2011, Reference Pazzola, Balia, Carcangiu, Dettori, Piras and Vacca2012). Productive parameters of a single lactation were monitored: at 45, 75, 105, 135 and 165 d after parturition, the daily milk yield was recorded and one individual milk sample was collected to determine fat and protein content, using an infrared spectrophotometer (Milko-Scan 133B; Foss Electric, DK-3400 Hillerød, Denmark) according to IDF 141C:2000 standard. A blood sample was collected from each animal for genomic DNA isolation (Puregene DNA Isolation Kit, Qiagen).
GH gene analysis
The five exons of the gGH gene were amplified by PCR using the primer pairs and conditions described by Marques et al. (Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003). SSCP analysis was carried out on a D-Code Universal Mutation Detection System (BioRad): 2·5 μl of each amplification product was added to 7·5 μl of Stop Solution (1 mg/ml xylene-cyanol, 1 mg/ml bromophenol blue, 10 mm EDTA in 80% deionised formamide). Samples were denatured at 94 °C for 5 min and then chilled on ice. The total volume was loaded into polyacrylamide gels and then run following the conditions described by Marques et al. (Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003). Products were visualised with SYBR Gold staining (Invitrogen, Carlsbad, CA). From one to three DNA fragments showing the same SSCP pattern, were sequenced in both directions, in order to establish the molecular basis of SSCP differences. Sequencing was achieved with an Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA), after purification with the ChargeSwitch® PCR Clean-Up Kit (Invitrogen, Carlsbad, CA, USA).
Cloning
The caprine GH gene was entirely amplified using the primer pair GH1F -5′-CAGAGACCAATTCCAGGATC-3′/GH5R-5′-TAATGGAGGGGATTTTTGTG-3′ to obtain DNA amplicons of about 1,7 kbp, from four goats showing different SSCP banding patterns. PCR reaction was performed in a final volume of 25 μl, containing 25–50 ng genomic DNA, 16 pmol each primer, 1·5 mm MgCl2, 200 μm dNTPs, 1×PCR buffer (20 mm Tris-HCl pH 8,4 and 50 mm KCl) and 1 U Taq DNA polymerase (Platinum; Invitrogen). The PCR program consisted of a denaturing step at 94 °C for 5 min, followed by 35 cycles [94 °C (30 s), 62 °C (30 s), 72 °C (30 s)] and a final elongation step at 72 °C for 5 min. The amplification product was cloned into pCR2·1 cloning vector and then transformed into chemically competent Escherichia coli One Shot TOP10 cells (Invitrogen Life Technologies). The recombinant plasmids were purified with Qiagen Plasmid Midi Kit (Qiagen GmbH, Hilden, Germany) prior to sequencing in both directions using the same primers utilised for exon PCR amplification.
Analysis of the sequencing data was performed using BioEdit (Hall, Reference Hall1999) and allele frequencies were measured with POPGENE V1·32 (Yeh et al. Reference Yeh, Yang and Boyle2000). The nucleotide changes described were named according to the recommendations of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/). Alibaba 2·1 software program, with the TRANSFAC database (http://www.gene-regulation.com/pub/programs/alibaba2) were used to identify differences in putative transcription factor binding sites.
Statistical analysis
A repeated measures GLM procedure was used to analyse the effects of the SSCP profiles on milk traits (Minitab statistical software; Minitab release 13·32, Minitab Inc. 2000, State College, PA). The model used for all variables was:
where y ijk is the variable (milk yield, fat percentage, protein percentage), μ is the general mean, g i is the random effect of the genotype (i = 3 for exon 1; i = 2 for exon 2; i = 5 for exon 3; i = 6 for exon 4 and i = 3 for exon 5), S j is the fixed effect of the stage of lactation (j = 5), gS ij is the interaction effect and e ijk is the error effect. Only genotypes with frequency higher than 5% were included in the statistical model and model effects were considered significant at P<0·05; multiple comparisons of the means were performed using the Bonferroni's method. Only genotypes present in more than 3% of the sample group were considered.
Results
Sequence analysis
Analysis of conformational polymorphism of caprine GH gene exons 1–5, including flanking regions of exons 1 and 5, revealed differing banding patterns (Fig. 1). The number of polymorphic profiles identified for each DNA fragment, and the genotype combination of nucleotide changes detected after sequencing, are shown in Table 1. SSCP of the DNA amplicon containing exon-1 revealed three different migration patterns, all displaying four bands. Exon-2 showed two SSCP configurations. Six different polymorphic schemes were evidenced at exon-3, characterised by a number of bands ranging between 2 (pattern VI) and 6 (pattern IV). Sequencing revealed that three SSCP patterns (I, II, IV) had two or more heterozygous genotypes, the others had homozygous genotypes, with patterns III and V showing the same sequence. Exon-4 proved to be the most polymorphic, with 9 different conformations, characterised by a number of bands between 4 (pattern I) and 10 (pattern VIII). Exon-4 SSCP profiles showed one (pattern I, II), two (II*, III, IV) or three (V, VI, VII) heterozygous genotypes after sequencing, only pattern IX displayed a homozygous genotype combination. Patterns II and II* had the same banding scheme, but showed a different sequence, then all the subjects with profile II were sequenced, revealing that pattern II* was unique. The DNA fragment including exon-5 had five different polymorphic patterns: I, III and IV showed different combinations of homozygous genotypes.

Fig. 1. SSCP patterns of exons 1–5 of Sarda goat GH gene.
Table 1. SSCP pattern frequencies and related genotype combination

† Nt: nucleotide positions corresponding to the genotype combination of each DNA fragment, related to the Acc. No. D00476
‡ –: information about nt position 1185 was not available
§ single nucleotide deletion
Sequencing of the differing SSCP profiles allowed the identification of 21 nucleotide changes compared with the reference sequence D00476 (Kioka et al. Reference Kioka, Manabe, Abe, Hashi, Yato, Okuno, Yamano, Sakai, Komano, Utsumi and Iritani1989) (Table 2). Out of these mutations, 14 were polymorphic and 7 monomorphic in the goat sample group analysed. Exon-1 did not show any sequence variation, however, a nucleotide change localised 3 bp upstream of the transcription initiation codon (c.-3A>G) was detected. Three nt variations were identified at exon-2, two of them were synonymous and monomorphic, and the third (c.104G>A) produced a deduced amino acid change (G35S). Exon-3 encompassed 5 nt changes, one causing a putative amino acid change (G89S). Five nt changes were identified at exon-4, three SNPs led to a deduced amino acid change (c.309G>A, c.399A>G and c.452C>T) and two were synonymous, one of which was monomorphic (c.445G>C). Analysis of the DNA segment including exon-5 revealed 6 synonymous single nucleotide polymorphisms (SNPs), four occurring in 100% of the goats tested. In addition, a polymorphic indel (c.*29_30insC) was detected in the 3′UTR region.
Table 2. Polymorphisms in the caprine GH gene of Sarda goats

† Nucleotide numbers (Nt. No.): nucleotide positions relative to gGH genomic DNA sequence D00476
‡ UTR: untranslated region
Cloning
The whole caprine GH gene of four subjects showing differing SSCP patterns was cloned and sequenced in order to reveal some of the possible haplotypes existing within the Sarda goat population. The sequences obtained were published with Acc No. GU355686-9, and haplotypes identified, for all the nt changes revealed after comparison with Acc. No. D00476, are shown in Table 3. The alignment of the 4 sequences led to the identification of three additional synonymous mutations (at exons-2 and 4), which had not arisen with the sole SSCP analysis, and to 22 nucleotide variations within the intervening sequences, including two indels, which add to the one detected in the 3′UTR region (Table 4).
Table 3. Haplotypes of the caprine GH gene
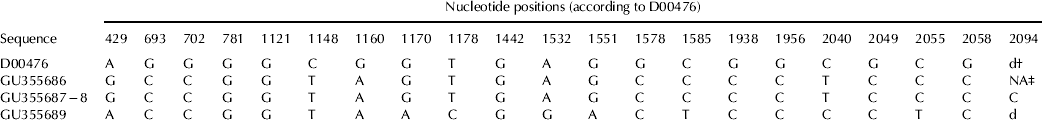
† Indicates deletion at nt 2094 (c.*29_30insC)
‡ NA, genotype information not available
Table 4. Nucleotide changes detected in gGH sequences Acc. No. GU355686-9

† Nucleotide numbers (Nt. No.): nucleotide positions relative to gGH genomic DNA sequence D00476
Association analysis
Table 5 shows the effects of genotype and stage of lactation on milk traits of 100 Sarda lactating goats. The stage of lactation significantly influenced (P<0·01) milk production and fat and protein percentage in a pattern that was typical of that reported for dairy goats lactation curve (Park et al. Reference Park, Juarez, Ramos and Haenlein2007), thus the mean values were not reported. There were no significant genotype×stage of lactation interaction effects on milk traits in this herd of goats (data not shown).
Table 5. Analysis of variance showing the effects of genotype (G) and lactation stage (S) on milk traits
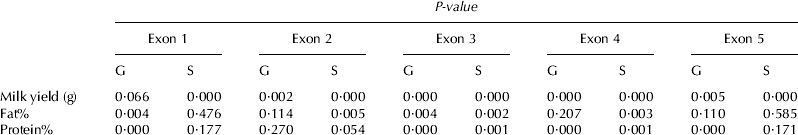
The mean values showing the effect of genotype on milk traits were presented in Table 6. SSCP analysis of the amplicon including exon-1 revealed that, on average, pattern I goats produced milk with the highest fat and protein percentage. Goats with pattern I at exon-2 showed higher milk yield than pattern II, but no differences were found for fat and protein content. The gGH exon-3 genotype significantly (P<0·01) influenced milk traits: animals with patterns III and IV had the highest milk yield; goats with pattern V showed higher fat percentages than goats with patterns I and IV, and goats with patterns I and V had higher protein percentages than goats with pattern IV. Goats with patterns V and VII at exon-4 showed the highest milk yield (P<0·01), and goats with pattern II (exon-4) had the highest protein percentage (P<0·01). Genotype at gGH exon-5 significantly (P<0·01) influenced milk traits: goats with pattern I had the lowest milk production, while goats with pattern III showed the lowest protein percentage. These results are preliminary and a large number of samples will be analysed in order to arrive at a final conclusion.
Table 6. Mean values (±sem) of milk traits associated with PCR-SSCP patterns of the gGH gene

Different letters in the same column indicate values significantly different; A, B, C = P < 0·01
Discussion
The SSCP variability of the caprine GH gene comprises two levels of complexity. The first is due to the different three-dimensional conformations of denatured DNA single strands, derived from sequence differences. The second is due to allelic polymorphism of the gGH gene (Wallis et al. Reference Wallis, Lioupis and Wallis1998), which could result in two (Gh1/Gh1), three (Gh1/Gh2) or four (Gh2/Gh2) gene copies, for diploid genome. At present, no information is available in the literature which allows selective amplification of the caprine GH1, GH2 or GH3 genes. Then, any polymorphism identified by SSCP is not attributable to any of the three genes, but indistinctly to the caprine GH gene.
The DNA regions corresponding to exons 1 and 2 showed the least SSCP variability, in agreement with Malveiro et al. (Reference Malveiro, Pereira, Marques, Santos, Belo, Renaville and Cravador2001) and Marques et al. (Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003), which detected only 2 patterns for each fragment in Portuguese Algarvia and Serrana goats, respectively. The present study has revealed that exon-1 was monomorphic. Analysis of the 5′UTR sequence (nt −1/−72) with Alibaba 2·1 software revealed the occurrence of a putative PEA3 transcription factor binding site (GTCCTGCTGAC, nt −13/−23), and showed that the nt change identified (c.-3A > G) apparently does not introduce any differences in the potential transcription factor binding sites of this region. The SNP detected at exon-2 (c.104G > A) causing the putative amino acid substitution G35S, may affect the functionality of the hormone, due to the shift from non-polar to polar uncharged amino acid. In fact, exon-2 encodes the N-terminal region of the mature peptide, which is involved in the binding sites with the two GH receptors, necessary to mediate its function (Juárez-Aguilar et al. Reference Juárez-Aguilar, Castro-Muñozledo, Guerra-Rodríguez, Reséndez-Pérez, Martínez-Rodríguez, Barrera-Saldaña and Kuri-Harcuch1999). This polymorphism has been revealed also in a population of Boer bucks where it has been associated with growth traits (Hua et al. Reference Hua, Chen, Yu, Cai, Wu, Li, Zhang, Liang, Han, Geng, Shen, Xu and Yang2009).
Six different SSCP profiles were detected at exon-3, in agreement with previous studies (Marques et al. Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003). Surprisingly, patterns III and V had the same sequence, with homozygote genotypes for five sites of variation. This prevents attribution of the observed banding differences to the occurrence of different haplotype combinations. But they may be due to more than one stable conformation of the same sequence (Rubio et al. Reference Rubio, Ayllon, Guerri, Pappu, Niblett and Moreno1996), or to nt variations not detected by sequencing, e.g. if one single nt variation occurred only once over three (Gh1/Gh2) or four (Gh2/Gh2) copies of the gGH gene, the relative peak in the sequencing chromatogram would not be sufficiently visible in terms of spacing, width or height, to be recognised as heterozygous, and it would be considered as background. Exon-3 showed the highest proportion of polymorphic loci (P = 1), defined as the number of polymorphic loci/total number of loci sampled (Frankham et al. Reference Frankham, Ballou and Briscoe2007), when compared with exon 4, where P = 0·8, and exons 2 and 5, where P = 0·33. The highest number of SSCP profiles was detected at exon-4, as reported for Serrana goats (Marques et al. Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003). The nt variations c.399A > G and c.452C > T, causing the amino acid substitutions D133G and R151W respectively, were polymorphic in Sarda goats, while they were monomorphic in dairy Jakhrana goats (Gupta et al. Reference Gupta, Pandey, Malik and Gupta2009). Five SSCP profiles were described at exon-5, lower than Jakhrana goats where 6 patterns have been observed (Gupta et al. Reference Gupta, Pandey, Malik and Gupta2009).
All the nucleotide changes detected in this study were biallelic, while c.309G > A and c.399A > G (exon-4) were reported to be triallelic in Black Bengal goats (Gupta et al. Reference Gupta, Ahlawat, Kumar, Gupta, Pandey and Malik2007). In addition, the silent mutation c.651C > T (exon-5), has been reported as c.651C > G, resulting in the amino acid change C215W, in Jakhrana goats (Gupta et al. Reference Gupta, Pandey, Malik and Gupta2009). This may be due to a different evolution of the three copies of the caprine GH gene (GH1, GH2, GH3), which may have caused sequence differences between geographically distant breeds.
All the monomorphic SNPs (7 out of 21) found in the goat sample group analysed indicate that new gGH gene haplotypes, different from Kioka et al. (Reference Kioka, Manabe, Abe, Hashi, Yato, Okuno, Yamano, Sakai, Komano, Utsumi and Iritani1989), occur in the Sarda goat, as confirmed by the alignment of sequences related to the 4 subjects cloned. Two new haplotypes were found: one was the same in three subjects (GU355686-8), which showed differing SSCP polymorphic patterns and intron SNPs, the second belonged to Acc. No. GU355689. Among the many nt changes detected in this study, a marked density of insertion/deletion events emerge: three indels over about 1785 bp, two of which were revealed based on the analysis of only four subjects. Indels are often linked with an increased mutation rate in the DNA region where they occur, and in the past, they were considered to be the cause of those mutations (Tian et al. Reference Tian, Wang, Zhang, Araki, Yang, Kreitman, Nagylaki, Hudson, Bergelson and Chen2008). Currently, a new hypothesis suggests that DNA repeat sequences, instead of indels, may be responsible for the accumulation of clusters of mutation in specific DNA regions, with indels occurring within or in proximity to the repeat sequences (McDonald et al. Reference McDonald, Wang, Huang and Leu2011). This might be applied in the case of the c.*29_30insC mutation, which can be considered as a contiguous indel, in fact it occurs immediately next to a CCCC homopolymer repeat. While c.455-33_32insC might be considered as a proximal indel, as it is located 6 nt upstream of a CTCTCT repeat, and c.454+54 _58delCCTGG does not fall into these categories, but is located in a 30 bp long region of DNA where 5 nt changes were detected, over the indel. The nucleotide changes identified in both coding and non coding regions of the caprine GH gene may have implications for its biological functions (Mattick, Reference Mattick2009) and then on all of those traits resulting from the physiological action of the growth hormone, including milk production and composition.
All DNA fragments analysed, except the one containing exon-1, affected milk yield, while in other goat breeds, a correlation was reported only for exons 4 and 5 (Algarvia goats, Malveiro et al. Reference Malveiro, Pereira, Marques, Santos, Belo, Renaville and Cravador2001), and for exons 2 and 4 (Serrana goats, Marques et al. Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003). SSCP profiles of the DNA fragments corresponding to exons 1 and 3, influenced milk fat percentage, in contrast with the aforementioned papers, which did not find any correlation. Exon-3 affected both milk yield and fat and protein percentage. All the DNA fragments analysed, except exon-2, affected the protein percentage, while Marques et al. (Reference Marques, Pereira, Marques, Santos, Belo, Renaville and Cravador2003) found correlations with this trait only for exons 1 and 2.
Conclusions
The genomic region corresponding to the caprine GH gene showed huge sequence variability, with nucleotide substitutions, insertion/deletions, and repeat sequences clustering together, which may provide useful information for the phylogeny of ruminants and, more importantly, might affect in different ways the productivity of animals.
Apparently each exon, except exon-1, affected milk yield, exons 1 and 3 influenced milk fat percentage, and all exons, except exon-2, had an effect on protein percentage, supporting previous results in livestock. The SSCP technique proved to be a good method for the screening of populations, keeping costs low, and giving an overview of the variability of the genes studied, however complex, which could be correlated with production traits. Nevertheless, an investigation aiming to highlight the sequence differences between the three allelic copies of the caprine GH gene, would allow attribution of the numerous mutations detected so far, to the single gGH alleles. The molecular information thus obtained, may be exploited for a more effective genetic selection for dairy traits in goats.
Research supported by the Fondazione Banco di Sardegna funds.









