Introduction
The Heteroptera (‘true bugs’) represent one of the mega-diverse insect groups (ca. 38,000 species worldwide), and occupy a wide diversity of ecological niches. Within the Heteroptera, the Miridae (‘plant bugs’) constitute the largest family comprising about 10,000 species that exhibit a broad range of feeding habits including herbivory, carnivory, and omnivory (Wheeler, Reference Wheeler2001). Commercially distributed predators, including Macrolophus pygmaeus and more recently Nesidiocoris tenuis, are among the most important arthropod natural enemies used in augmentative and conservational biological control (van Lenteren, Reference van Lenteren2011; Perdikis et al., Reference Perdikis, Fantinou and Lykouressis2011). These predatory bugs are widely employed to protect greenhouse crops (mainly tomato and eggplant) against the greenhouse whitefly Trialeurodes vaporariorum, the sweet potato whitefly Bemisia tabaci (both Hemiptera: Aleyrodidae), and the tomato borer Tuta absoluta (Lepidoptera: Gelechiidae).
Intracellular bacterial symbionts of arthropods are very diverse in both distribution within the insect's body and influence on their hosts (Zchori-Fein & Bourtzis, Reference Zchori-Fein and Bourtzis2011). Zindel et al. (Reference Zindel, Gottlieb and Aebi2011) have summarized the various ways such symbionts may dramatically affect all phases of augmentative biological control, from the mass rearing of natural enemies to actual efficiency in the field, by manipulating their host's biology. For example, bacteria may induce cytoplasmic incompatibility, which results in sterile eggs when a symbiont-infected male mates with an uninfected female. A population with a mixed infection would thus reproduce slower than one in which all members carry the symbiont or are symbiont-free. In addition, symbiotic microorganisms may protect their insect host against natural enemies such as parasitoids, pathogenic bacteria, fungi, and viruses, increase or decrease the survivorship of a natural enemy under extreme environmental conditions or influence the vectorial capacity of disease-vectoring arthropods (Zindel et al., Reference Zindel, Gottlieb and Aebi2011 and references therein).
In general, bacteria are referred to as ‘primary symbionts’ if they are maternally inherited and obligatory for the existence of their hosts, and ‘secondary symbionts’ if they are not required for the host's survival or reproduction. The latter nonetheless, may have profound effects on the biology and ecology of their hosts, ranging from reproductive manipulators to protectors against natural enemies (Zchori-Fein & Bourtzis, Reference Zchori-Fein and Bourtzis2011).
In many heteropteran families, specific bacterial symbionts belonging to the γ- and β-proteobacteria or actinobacteria are housed in crypts in the posterior region of the midgut (Glasgow, Reference Glasgow1914; Buchner, Reference Buchner1965; Fukatsu & Hosokawa, Reference Fukatsu and Hosokawa2002; Prado & Almeida, Reference Prado and Almeida2009; Hosokawa et al., Reference Hosokawa, Kikuchi, Nikon, Meng, Hironaka and Fukatsu2010; Kikuchi et al., Reference Kikuchi, Hosokawa and Fukatsu2011), in the lumen or on the epithelial walls of the midgut itself (Haas & König, Reference Haas and König1987; Kaltenpoth et al., Reference Kaltenpoth, Winter and Kleinhammer2009), or in specialized bacteriomes (Kuechler et al., Reference Kuechler, Renz, Dettner and Kehl2012; Matsuura et al., Reference Matsuura, Kikuchi, Hosokawa, Koga, Meng, Kamagata, Nikoh and Fukatsu2012). Interestingly, transitions between these different locations have occurred multiple times in the evolutionary history of the Heteroptera group (Matsuura et al., Reference Matsuura, Kikuchi, Hosokawa, Koga, Meng, Kamagata, Nikoh and Fukatsu2012). Most of the Heteroptera-associated symbionts are vertically transmitted by post-hatching transmission mechanisms such as egg-surface contamination, coprophagy, or the formation and deposition of special symbiont-containing capsules by the mother (Abe et al., Reference Abe, Mishiro and Takanashi1995; Hosokawa et al., Reference Hosokawa, Kikuchi, Meng and Fukatsu2005; Prado et al., Reference Prado, Rubinoff and Almeida2006; Kikuchi et al., Reference Kikuchi, Hosokawa and Fukatsu2007). In some cases, experimental elimination of the symbiotic bacteria has resulted in high mortality and reduced growth, indicating that the symbionts play an important role in the host insect's fitness (Huber-Schneider, Reference Huber-Schneider1957; Abe et al., Reference Abe, Mishiro and Takanashi1995; Fukatsu & Hosokawa, Reference Fukatsu and Hosokawa2002; Kikuchi et al., Reference Kikuchi, Hosokawa and Fukatsu2007; Nikoh et al., Reference Nikoh, Hosokawa, Oshima, Hattori and Fukatsu2011; Tada et al., Reference Tada, Kikuchi, Hosokawa, Musolin, Fujisaki and Fukatsu2011; Salem et al., Reference Salem, Kreutzer, Sudakaran and Kaltenpoth2013). Although vitamin provisioning has been demonstrated as a possible function conferred by the symbionts of bedbugs (Hosokawa et al., Reference Hosokawa, Kikuchi, Nikon, Meng, Hironaka and Fukatsu2010), and evidence has been presented of environmentally acquired symbionts conferring resistance to pesticides (Kikuchi et al., Reference Kikuchi, Hayatsu, Hosokawa, Nagayama, Tago and Fukatsu2012), the symbiont-provided benefits for the hosts remain unknown or speculative in most cases (Nikoh et al., Reference Nikoh, Hosokawa, Oshima, Hattori and Fukatsu2011).
Although N. tenuis and M. pygmaeus are currently the most effective natural enemies of many pests, their ability to feed on the crop plants themselves, in addition to the pest insect, can cause serious damage and significant loss when prey becomes scarce (Sanchez & Lacasa, Reference Sanchez and Lacasa2008; Castañé et al., Reference Castañé, Arnó, Gabarra and Alomar2011). This undesirable trait poses a major constraint for their broad usage in biological pest-control programs worldwide. Exploring the possible involvement of symbionts in sustaining omnivory represents a major first step toward the manipulation of gut bacterial symbionts and is expected to facilitate their broad application. The array of bacterial symbionts associated with M. pygmaeus has been characterized (Machtelinckx et al., Reference Machtelinckx, Van Leeumen, Vanholme, Gehesquiere, Dermauw, Vandekerkhove, Gheysen and De Clercq2009, Reference Machtelinckx, Van Leeumen, Van De Wiele, Boon, De Vos, Sanchez, Nannini, Gheysen and De Clercq2012). Those authors reported that Wolbachia manipulates the insect's reproduction by causing severe cytoplasmic incompatibility (Machtelinckx et al., Reference Machtelinckx, Van Leeumen, Vanholme, Gehesquiere, Dermauw, Vandekerkhove, Gheysen and De Clercq2009). In addition, they found that M. pygmaeus harbors two Rickettsia species, most closely related to Rickettsia bellii and Rickettsia limoniae (Machtelinckx et al., Reference Machtelinckx, Van Leeumen, Van De Wiele, Boon, De Vos, Sanchez, Nannini, Gheysen and De Clercq2012). Tissue-specific analyses revealed only Rickettsia in the gut tissue, whereas both Rickettsia and Wolbachia were observed in the ovaries. Life history experiments showed no significant influence of any of the symbionts on predators’ fitness traits, such as nymphal development and fecundity (Machtelinckx et al., Reference Machtelinckx, Van Leeumen, Van De Wiele, Boon, De Vos, Sanchez, Nannini, Gheysen and De Clercq2012). It should be noted that under mass rearing conditions, the sex ratio of N. tenuis is 1:1, with no obvious signs of reproductive manipulations (S. Steinberg, unpublished data).
The hypothesis underlying the current research was that symbiotic bacteria influence the feeding habits of N. tenuis, an omnivorous bug that is common along the Mediterranean coast on vegetable crops, including tomato (Tavella & Goula, Reference Tavella and Goula2001; Sanchez et al., Reference Sanchez, Martinez-Cascales and Lacasa2003). To establish a basis for testing this hypothesis, the symbiotic complex associated with N. tenuis was studied, with a specific focus on the bacteria's localization and dynamics throughout the different insect life stages.
Material and methods
Insect origin and rearing
Nesidiocoris tenuis originated from a bio-organic tomato field in Hama'ayanot Valley, in Northeast Israel. It has been mass-reared since 2010 at BioBee Sde Eliyahu Ltd, Israel. The rearing system consisted of tomato seedlings as an oviposition substrate and plant food source. In addition, insects were fed frozen eggs of the Mediterranean flour moth Ephestia kuehniella or of the Mediterranean fruit fly Ceratitis capitata (European registered patent no. 2456324). Rearing was maintained under conditions of 27±2 °C, 70% relative humidity and a 16:8 h light/dark photoperiod.
Sequence-based characterization of Rickettsia in N. tenuis
Characterization of the microbial community
Denaturing gradient gel electrophoresis (DGGE) was used to characterize the microbial community of N. tenuis. Live adults from the mass-reared colony were placed in 96% alcohol, and seven of them were individually ground in lysis buffer as described by Frohlich et al. (Reference Frohlich, Torres-Jerez, Bedford, Markham and Brown1999), and the lysate was used as a template for polymerase chain reaction (PCR). That reaction was conducted using the primers 27F and 907R which target the most known bacteria, under conditions that permit the amplification of the 16S rRNA gene from the most known bacteria (Muyzer et al., Reference Muyzer, Hottentrager, Teske, Wawer, Akkermans, van Elsas and de Bruijn1996) (table 1). Because only Wolbachia could be detected when the resulting products were sequenced (data not shown), a semi-nested PCR was conducted. Using 1 μl of the product as a template for the second reaction, with primers 341F-GC clamp (40-nucleotide, GC-rich sequence) and 907R (table 1). DNA of B. tabaci served as a positive control, and the negative control samples were sterilized water. Reactions were performed in a 25-μl volume containing 3 μl of the template DNA lysate, 10 pmol of each primer, and 1 unit of ready mix (APEX 2X Red Taq mix, Genesee Scientific).
Table 1. PCR primer sets used in this study.

A 5 μl aliquot of the PCR mix was subjected to agarose gel electrophoresis, and the remaining 20 μl containing the amplified DNA fragments was then subjected to DGGE analysis using the following conditions: separation on a 6% (w/v) acrylamide gel (acrylamide-N,N'-methylenebisacrylamide, 37.5:1) prepared in 1X Tris–acetate–EDTA buffer with a denaturing gradient ranging from 20 to 60%. Polymerization was carried out with N,N,N′,N″-tetramethylethylenediamine (0.09%, v/v) and ammonium persulfate (0.04%, w/v). Electrophoresis for separation of PCR fragments was performed at 70 V and 60 °C for 20 h. After electrophoresis, gels were incubated in ethidium bromide solution (250 ng/ml−1) for 10 min, rinsed in distilled water, and photographed under UV illumination (data not shown). Bands were cut from the gel, eluted, cloned into a vector, and sequenced (as described by Caspi-Fluger et al., Reference Caspi-Fluger, Inbar, Mozes-Daube, Mouton, Hunter and Zchori-Fein2011).
Screening for Rickettsia abundance
To study the frequency of Rickettsia in N. tenuis, the symbiont was screened by PCR. About 50 individuals from the mass-rearing facility at BioBee, and 65 individuals collected in tomato fields between January and July 2013 (table 2) were ground separately in lysis buffer (Frohlich et al., Reference Frohlich, Torres-Jerez, Bedford, Markham and Brown1999) and checked by PCR with species-specific primers for the 16S rRNA gene of Rickettsia (table 1). Thirty-five E. kuehniella eggs from the batch used for feeding the predator in mass rearing were also tested for the presence of Rickettsia to avoid the possibility of false-positive reactions due to gut content after feeding with the eggs. Negative controls were sterilized water and DNA of the whitefly B. tabaci that does not harbor Rickettsia, and positive controls were DNA of whiteflies harboring the bacterium.
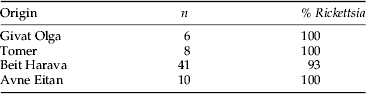
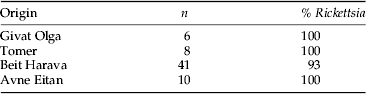
Table 2. Origin and Rickettsia infection rates in N. tenuis collected in tomato fields in Israel in 2013.
Establishment of Rickettsia sp. identity
To identify the newly discovered Rickettsia, the genotypic scheme suggested by Fournier et al. (Reference Fournier, Dumler, Greub, Zhang, Yimin and Raoult2003) was followed. Two Rickettsia genes – gltA and 16S rRNA – were sequenced and the presence of the rickettsial outer membrane protein (rOmp) encoded by the gene OmpA was determined. 16S rRNA, gltA, and OmpA were chosen because they are conserved genes that are commonly used for bacterial classification in general and for that of Rickettsia in particular. An attempt was made to amplify fragments of the three genes from the insect lysate with specific primer combinations (table 1) by PCR. Reactions were performed in a 25 μl volume containing 3 μl of the template DNA lysate, 10 pmol of each primer, and ready mix Taq DNA polymerase. PCR products were stained with SafeView™ (NBS Biologicals) and visualized on a 1.2% agarose gel. PCR products of gltA and 16S rRNA were cloned into the pGEM T-Easy plasmid vector (Promega) and transformed into Escherichia coli, and two colonies from each plate were randomly picked and sequenced. Sequencing was performed for each gene, and data obtained from all six replicates (three individuals × two colonies) had no detectable differences, and were used to create consensus sequences. These sequences were compared to known sequences in databases using the BLAST algorithm in NCBI (Nucleotide collection (nr/nt).
The sequences have been deposited in the GenBank database under accession numbers KF646707 and KF646706.
Rickettsia multiplication rate
To study Rickettsia dynamics during host development, the bacterial densities were assessed using real-time quantitative PCR. About ten N. tenuis individuals from each of five developmental stages (1–2, 2–3, 3–4, 5, adults) were collected directly into 96% ethanol. Amplification of Rickettsia gltA from N. tenuis samples was performed using 1X Absolute™ QPCR SYBR Green ROX mix (Thermo Scientific) and 5 pmol of each primer (table 1). N. tenuis 18S rRNA was used as an internal standard for data normalization (table 1). To validate the data, each gene was amplified in duplicate in each of ten biologically independent replicates. The cycling conditions were: 15 min activation at 95 °C, 40 cycles of 15 s at 95 °C, 1 min at 60 °C. Standard curves were drawn using standard plasmid samples for Rickettsia gltA at concentrations of 102, 103, 104, 105, and 106 copy μl−1. An ABI Prism® 7000 Sequence Detection System (Applied Biosystems) and accompanying software were used to analyze the real-time quantitative PCR data. The ratios, corresponding to bacterial cycle threshold (CT) minus the host 18S rRNA CT (relative density), were calculated according to the instructions in User Bulletin 2: ABI PRISM 7700 sequence Detection System. Because of non-normal distribution of the data, we used a nonparametric Kruskal–Wallis test.
Rickettsia localization in N. tenuis body
To determine whether Rickettsia is vertically transmitted in N. tenuis, fluorescence in situ hybridization (FISH) was applied. Approximately 3-week-old adult females were dissected in physiological saline and then placed in Carnoy`s fixative (Sakurai et al., Reference Sakurai, Koga, Tsuchida, Meng and Fukatsu2005). FISH was performed with symbiont-specific 16S rRNA probe for Rickettsia as described by Gottlieb et al. (Reference Gottlieb, Ghanim, Chiel, Gerling, Portnoy, Steinberg, Tzuri, Horowitz, Belausov, Mozes-Daube, Kontsedalov, Gershon, Gal, Katzir and Zchori-Fein2006). Stained samples were whole mounted and viewed under a 1X-81 Olympus FluoView 500 confocal microscope. Specificity of detection was confirmed using no-probe staining. To assess the possible association of Rickettsia with the gut, adult males and females were dissected in physiological saline, and their digestive tracts were removed. The organs were then processed as described above, but Wolbachia-specific 16S rRNA probe was also applied (Gottlieb et al., Reference Gottlieb, Ghanim, Chiel, Gerling, Portnoy, Steinberg, Tzuri, Horowitz, Belausov, Mozes-Daube, Kontsedalov, Gershon, Gal, Katzir and Zchori-Fein2006).
Electron microscopy was used to verify the results obtained by FISH. Ovaries and digestive tracts were dissected from adults as described above, immediately placed in phosphate buffered saline (PBS, pH 7.4) and fixed in 2.5% glutaraldeyde and 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2.5 h at room temperature. The tissues were then rinsed four times, 10 min each, in cacodylate buffer and post-fixed and stained with 1% osmium tetroxide and 1.5% potassium ferricyanide in 0.1 M cacodylate buffer for 1 h. Tissues were then washed four times in cacodylate buffer followed by dehydration in increasing concentrations of ethanol (30, 50, 70, 80, 90, and 95% for 10 min each step), followed by 100% anhydrous ethanol three times, 20 min each, and propylene oxide two times, 10 min each. Following dehydration, the tissues were infiltrated with increasing concentrations of Agar 100 resin in propylene oxide, consisting of 25, 50, 75, and 100% resin for 16 h each step. The tissues were then embedded in fresh resin and allowed to polymerize in an oven at 600 °C for 48 h. Tissues embedded in blocks were sectioned with a diamond knife on an LKB 3 microtome and ultrathin sections (80 nm) were collected onto 200 Mesh, thin copper bar grids. The sections on grids were sequentially stained with uranyl acetate and lead citrate for 10 min each and viewed with a Tecnai 12 transmission electron microscope (TEM) 100 kV (Phillips, Eindhoven, the Netherlands) equipped with MegaView II CCD camera and Analysis® version 3.0 software (SoftImaging System GmbH, Münstar, Germany).
Results
Sequence-based characterization of Rickettsia in N. tenuis
Characterization of the microbial community
The DGGE analysis performed on the 16S rRNA gene resulted in only two bands (data not shown). The two 550-bp long sequences of the bands extracted from the gel showed high sequence similarities to known bacteria. One was identical to Wolbachia described from the Japanese beetle Curculio okumai 16S rRNA (AB604659) and the other highly resembled (99%) various bellii-group Rickettsia 16S rRNA, such as strain 369L42-1 (NR036774), OSU 85-389 (CP000849), and RML 369-C (CP000849) (numbers in parentheses refer to NCBI accession numbers).
Screening for Rickettsia abundance
Results of the species-specific PCRs showed that all 50 individual N. tenuis originating from the mass-rearing facility carried Rickettsia, but that the symbiont could not be detected in any of the 35 E. kuehniella eggs tested. Rickettsia was also virtually fixed in Israeli field populations, where over 95% of the 65 individuals screened tested positive for its presence (table 2).
Establishment of Rickettsia sp. identity
The combination of primers used yielded a nearly complete (1469 bp) sequence of the 16S rRNA gene which exhibited the highest sequence similarity to R. bellii (99%) found in the tick species Dermacentor variabilis (U11014). The use of specific primers for gltA resulted in a 1,111-bp long sequence showing 99% similarity to the citrate synthase gene of Rickettsia associated with M. pygmaeus (HE583221) and 96% similarity to a Rickettsia endosymbiont of B. tabaci gltA (DQ077708). The OmpA gene could not be detected by PCR using specific primers.
Rickettsia multiplication rate
Rickettsia relative densities were measured at the various developmental stages of N. tenuis using real-time quantitative PCR. The analysis showed an increase in Rickettsia densities during early stages of the life cycle, with constantly low numbers in nymphal instars 1–4 relative to the amounts found in the 5th instar and adults (fig. 1, Kruskal–Wallis test, P<0.001). Rickettsia densities increased 4- to 12-fold as the insect reached maturity (fig. 1).

Fig. 1. Mean(±SE) relative amounts of Rickettsia at N. tenuis developmental stages: instars 1–5 and adults (A). Rickettsia quantification was evaluated in terms of gltA CT minus N. tenuis 18S rRNA cycle threshold. Values correspond to the average of 8–10 individuals per line.
Rickettsia localization in N. tenuis body
FISH analysis showed that in the ovaries, Rickettsia are concentrated in the germarium, at the tip of the ovarioles, but not inside the mature oocytes (fig. 2A). This result was partially supported by TEM, where the bacteria could not be detected anywhere but the germarium (fig. 3D, E). Both confocal microscopy and TEM revealed the presence of high numbers of bacteria in the gut. The FISH analysis suggested that Rickettsia is distributed in the gut lumen along the digestive tract (fig. 2B2), while Wolbachia can be detected in the epithelial cells surrounding that organ (fig. 2C1–C6). TEM observations supported both the intra- and extra-cellular localization of bacteria (fig. 3A–C), but did not allow distinguishing between Rickettsia and Wolbachia because of their similar size and shape.

Fig. 2. FISH of N. tenuis ovaries (A) and gut (B, C). A – one ovary with several ovarioles. Rickettsia (red) is concentrated in the germarium, at the tip of the ovary. M – mature oocyte; D – developing oocyte; white arrow – germarium; black arrow – lateral oviduct (bar=200 μm). B, C – Rickettsia (red) are found in the gut lumen, whereas Wolbachia (blue) can be seen in the tissue surrounding the gut. B1 – light microscope; B2 – confocal images of both bacteria overlaid on the light microscope picture (bar=200 μm). C – high magnification of the gut. Wolbachia (blue) are concentrated in gut epithelial cells, while Rickettsia (red) are inside the gut. C1–C6 represent serial Z sections of 2 μm from 2 to 12 μm, respectively (bar=20 μm).

Fig. 3. Transmission electron micrographs of bacteria in N. tenuis. A–C Gut tissue. A – bacteria embedded in gut tissue and free in the gut lumen (bar=10 μm); B – magnification of the black square in ‘A’ showing bacteria embedded in gut tissue (bar=1 μm); C – bacteria free in the gut lumen (bar=2 μm). Black arrowheads – bacteria in gut lumen; black arrows – bacteria embedded in gut tissue; white arrows – microvilli; white asterisk – mitochondria. D–E ovaries. Bacteria can be seen in the germarium at the tip of N. tenuis ovary. D – the tip of the N. tenuis ovary (bar=5 μm); E – enlargement of the black square in D (bar=1 μm). Black arrowheads – bacteria found within the germarium; black arrows – nuclei of the follicle cells; white asterisk – mitochondria.
Discussion
Only Rickettsia and Wolbachia could be detected by the DGGE analysis. Similar results have been reported from two other mirid species, but while M. pygmaeus was found to be a host for two Rickettsia species, R. limoniae and R. bellii, only R. limoniae could be found in Macrolophus caliginosus.
Fournier et al. (Reference Fournier, Dumler, Greub, Zhang, Yimin and Raoult2003) suggested that a bacterium can be described as the genus Rickettsia if it shares >98.1% sequence similarity of the 16S rRNA gene and >86.5% similarity of the gltA gene of any known Rickettsia species, and therefore these two genes were sequenced to identify the Rickettsia found in N. tenuis. As the 16S rRNA and gltA genes of the symbiont found in N. tenuis exhibited 99% similarity with the previously described R. bellii and Rickettsia sequenced from M. pigmaeus, respectively, that bacterium could be considered a member of the genus Rickettsia. The absence of the ompA gene (according to the PCR analysis) classifies the Rickettsia into the ancestral group, together with R. bellii, a rickettsial species which is well documented in sap-sucking phytophagous insects (Gottlieb et al., Reference Gottlieb, Perlman, Chiel, Zchori-Fein, Zchori-Fein and Bourtzis2011).
The density of Rickettsia, as measured by real-time quantitative PCR, was the highest in the 5th instars and adults, indicating that the symbiont multiplies as the insect reaches maturity. A similar trend has been reported in other insects. For example, in the pea aphid Acyrthosiphon pisum, the population of Rickettsia increases during nymphal growth, reaching a plateau in 10-day-old adults (Sakurai et al., Reference Sakurai, Koga, Tsuchida, Meng and Fukatsu2005). The combined results suggest that although the association with Rickettsia may influence profoundly on the mirid hosts, different Rickettsia species may have similar effects.
Screening for Rickettsia in both field-collected and lab-reared individuals revealed its presence in almost all samples, and suggested that it is nearly fixed in the population. This tight association may indicate that the symbiont plays an essential role in the host, but what this role remains vague. Two possibilities can be envisioned.
Rickettsia is a reproductive manipulator
Localization studies demonstrated that in N. tenuis, Rickettsia is found in the ovarian tissue, and is probably vertically transmitted from the mother to her offspring via the egg (figs 2A and 3D–E) These observations are in agreement with the results of Machtelinckx et al. (Reference Machtelinckx, Van Leeumen, Van De Wiele, Boon, De Vos, Sanchez, Nannini, Gheysen and De Clercq2012), who found Rickettsia to be localized in the oocytes of M. pygmaeus and M. caliginosus using PCR. Those authors could not detect any fitness costs to Rickettsia colonizing the oocytes of the Macrolophus species studied. In N. tenuis, Rickettsia was found in all developmental stages by PCR, and in adult females it was observed in the germarium. If Rickettsia plays a role in reproduction, e.g., manipulating the insect's reproduction to enhance its own transmission, then it is expected to be in close proximity to the developing oocytes, as was found here (fig. 2A). Further support for this hypothesis may come if the symbiont is found to cause cytoplasmic incompatibility in its insect host. However, experiments designed to address this issue are challenging because of the need to differentiate the Wolbachia effect from the one exerted by Rickettsia.
Rickettsia is a nutritional symbiont
In addition to the gonads, the presence of Rickettsia and/or Wolbachia in the gut was confirmed by both confocal and electron microscopy (figs 2B and 3). As detailed above, the presence of specific symbionts in various gut parts of a number of heteropteran insect families has been thoroughly documented (e.g., Glasgow, Reference Glasgow1914; Buchner, Reference Buchner1965; Haas & König, Reference Haas and König1987; Fukatsu & Hosokawa, Reference Fukatsu and Hosokawa2002; Kaltenpoth et al., Reference Kaltenpoth, Winter and Kleinhammer2009; Prado & Almeida, Reference Prado and Almeida2009; Hosokawa et al., Reference Hosokawa, Kikuchi, Nikon, Meng, Hironaka and Fukatsu2010; Kikuchi et al., Reference Kikuchi, Hosokawa and Fukatsu2011), but the lack of direct evidence can only allow us to speculate on their nutritional role. The first evidence of Rickettsia in Miridae was found in the nuclei and cytoplasm of midgut epithelial cells in Stenotus binotatus (Heteroptera: Miridae) (Chang & Musgrave, Reference Chang and Musgrave1970). Applying molecular tools, Machtelinckx et al. (Reference Machtelinckx, Van Leeumen, Van De Wiele, Boon, De Vos, Sanchez, Nannini, Gheysen and De Clercq2012) demonstrated the presence of that symbiont in the guts of both M. pygmaeus and M. caliginosus. A similar distribution pattern was recently reported in B. tabaci (Hemiptera: Aleyrodidae), where both the midgut cells and lumen were found to be heavily loaded with Rickettsia (Brumin et al., Reference Brumin, Levy and Ghanim2012). Those authors speculated that the symbiont has a role in its host's nutrition, and that it reaches the gut via oocyte and egg cells that give rise to intestinal tissue during embryonic development. It should also be noted that Rickettsia in B. tabaci belongs to the same well-defined bellii clade (Weinert et al., Reference Weinert, Werren, Aebi, Stone and Jiggins2009) as the one found in N. tenuis. Although Rickettsia are generally referred to as obligate intracellular bacteria, they frequently move inside and between host cells and tissues (Gottlieb et al., Reference Gottlieb, Perlman, Chiel, Zchori-Fein, Zchori-Fein and Bourtzis2011 and references therein). Their seemingly extracellular position in the gut lumen is intriguing. However, the fact they have been shown to be transferred from insects to plants, where they survive in the phloem and can be picked up by other insects through feeding (Caspi-Fluger et al., Reference Caspi-Fluger, Inbar, Mozes-Daube, Katzir, Portnoy, Belausov, Hunter and Zchori-Fein2012), gives further support to the observed localization pattern.
In N. tenuis, the high relative amounts of Rickettsia in adults could be associated with age-related nutritional differences in the insect. The ability of N. tenuis to develop to the 4th instar when solely phytophagous is limited (Urbaneja et al., Reference Urbaneja, Gervasio and Stansly2005), thus the high amount of Rickettsia in the 5th instar and adults might give the insect an advantage in utilizing the plant as a nutritional source. The fact that Rickettsia was found in the insect's gut supports the hypothesis of the bacterium as a nutritional symbiont. Rickettsia in the gut could hypothetically have come from the prey, but here this is highly unlikely, since PCR analysis showed that the prey (E. kuehniella eggs) does not contain Rickettsia.
Taken together, the newly discovered Rickettsia might have a reproductive, nutritional or other role in N. tenuis. Further investigations in these directions, such as differences between adults and instars harboring Rickettsia in utilizing only plant sources, as well as fitness and nutritional biology differences between N. tenuis lines with and without Rickettsia, might shed light on Rickettsia's role in this important biological control agent. The Wolbachia found in N. tenuis was not characterized; however, it is essential to study its role carefully since this symbiont is known as a major reproductive manipulator of many insects.
Acknowledgements
Funding for this research was provided by The Israel Science Foundation (grant no. 262/09), and ICA Israel, which supports research in agriculture, to E.Z.-F. The authors thank Eduard Belausov and Lilach Iasur-Kruh for technical help, and extend their gratitude to the three anonymous reviewers for their valuable comments.