Introduction
The production of circa 80% of the agricultural crops worldwide is dependent on, or significantly enhanced by, insect pollinators (Klein et al., Reference Klein, Vaissière, Cane, Steffan-Dewenter, Cunningham, Kremen and Tscharntke2007). This dependence has increased in the last decades essentially because of the growing proportion of pollinator-dependent crops and their relatively higher market values (Aizen et al., Reference Aizen, Garibaldi, Cunningham and Klein2008, Reference Aizen, Garibaldi, Cunningham and Klein2009; Gallai et al., Reference Gallai, Salles, Settele and Vaissière2009). Crops that rely heavily on insect pollination include hybrid seed crops which have been expanding over the last decades and now represent nearly 20% of the global crop production, with the share of vegetable hybrids growing at a rate of 8–10% per year (da Silva Dias, Reference da Silva Dias2014; Broussard et al., Reference Broussard, Mas, Howlett, Pattemore and Tylianakis2017). The commercial success of hybrid varieties lies in their vigour and productivity which is the result of a phenomenon called heterosis or hybrid vigour.
Production of hybrid seeds consists of crossing two genetically different parental lines, one of which is rendered male-sterile (androsterile) by hand-emasculation or genetic techniques so that seeds of the hybrid cultivar can only be obtained by means of controlled cross-pollination. Since hybrid seed crops require pollinators to move pollen from the fertile parental line (donor) to the androsterile line (recipient), they rely even more on pollinating insects than their open-pollinated counterparts (Broussard et al., Reference Broussard, Mas, Howlett, Pattemore and Tylianakis2017). Moreover, the selection process through which parental lines exhibiting the desired genetic characteristics are obtained, sometimes results in lines producing floral rewards such as pollen or nectar in less quantity or quality (Simpson and Neff, Reference Simpson and Neff1981; Schrieber et al., Reference Schrieber, Paul, Höche, Salas, Didszun, Mößnang, Müller, Erfmeier and Eilers2021). This can render one of the parental lines less attractive to pollinators than the other, which reduces the chances of successful cross-pollination and ultimately leads to lower yields of hybrid seeds (Bohart and Todd, Reference Bohart and Todd1961; Gaffney et al., Reference Gaffney, Allen and Brown2011). Besides, to maintain genetic purity and prevent contamination from outside pollen sources, hybrid seed production usually takes place inside insect or pollen-proof isolations that can vary in size (e.g. bags, cages, tunnels) (Brar et al., Reference Brar, Saini, Kaushik, Chauhan and Kamboj2020; Townson et al., Reference Townson, Virk and Senior2020). Managed pollinators (i.e. insect pollinators produced elsewhere to be released in the crops on demand) are then introduced in these isolations to cross-pollinate the parental lines.
Available managed pollinators commonly used in protected crops are limited to two groups of hymenopterans: bees (Apis sp., Megachile sp. and Osmia sp.) and bumblebees (Bombus sp.). However, inside small isolations, bees can be less effective and become aggressive (Ochiuzzi, Reference Ochiuzzi1999; Dag, Reference Dag2008; Evans et al., Reference Evans, Cutting, Jochym, Janke, Felman, Cross, Jacob and Goodwin2019). Furthermore, honeybees and bumblebees tend to discriminate between parental lines, preferring those producing more pollen or nectar, and this can have a detrimental effect on cross-pollination (Faulkner, Reference Faulkner1974; Erickson et al., Reference Erickson, Peterson and Werner1979; Kobayashi et al., Reference Kobayashi, Tsukamoto, Tanaka, Niikura and Ohsawa2010; Howlett et al., Reference Howlett, Lankin-Vega and Pattemore2015; Gaffney et al., Reference Gaffney, Bohman, Quarrell, Brown and Allen2018, Reference Gaffney, Bohman, Quarrell, Brown and Allen2019). Blowflies (Diptera: Calliphoridae), on the other hand, are not as selective with regard to floral rewards, meaning that chances of successful cross-pollination can be improved (Faulkner, Reference Faulkner1978; Currah and Ockendon, Reference Currah and Ockendon1984). For this reason, blowflies are currently being extensively used for pollination in various hybrid seed crops, especially vegetables, where their relative individual inefficiency is compensated by the sheer number of flies released (Schittenhelm et al., Reference Schittenhelm, Giadis and Rao1997; Howlett, Reference Howlett2012; Inouye et al., Reference Inouye, Larson, Ssymank and Kevan2015; Gabai et al., Reference Gabai, Vaissière, Blacquiere, Freitas, Allsopp, Chabert and Dag2018). Thus, when considering an increase in marketable hybrid seed yield, it has been shown that pollination is as important a factor as crop management and plant quality. In fact, insect pollination appears to be the weakest link, over fertilization and irrigation, for production of several hybrid seed crops (Fijen et al., Reference Fijen, Scheper, Boom, Janssen, Raemakers and Kleijn2018, Reference Fijen, Scheper, Vogel, van Ruijven and Kleijn2020).
Within the vegetable seed industry, Apiaceae seed crops such as carrot, celery and fennel are economically important, hence the numerous research and innovative breeding programmes that enable the development of new hybrid varieties every year (Li et al., Reference Li, Feng, Hou, Jiang, Xu, Wang, Liu, Wang and Xiong2020; Spurr and Lucas, Reference Spurr, Lucas, Emmanuel and Philipp2020; Chappell and Dunford, Reference Chappell, Dunford, Al-Khayri, Jain and Johnson2021; Palumbo et al., Reference Palumbo, Vannozzi and Barcaccia2021). Bringing these new varieties to the market can take 5–10 years and implies extensive use of managed pollinators in order to meet the commercial yield and quality requirements. Dipterans and hymenopterans are two of the main insect visitors in Apiaceae, with Diptera being more frequent than Hymenoptera in fennel, celery and carrot (Warakomska et al., Reference Warakomska, Kolasa and Wróblewska1986; Spurr, Reference Spurr2003; Chaudhary, Reference Chaudhary2006; Hogendoorn and Keller, Reference Hogendoorn and Keller2011). In celery, pollen-feeding hoverflies (Syrphidae) are some of the main pollinators of the cultivar rapaceum, although bees also visit its flowers (Warakomska et al., Reference Warakomska, Kolasa and Wróblewska1986). Chaudhary (Reference Chaudhary2006) found that wild pollinators were as much responsible for increasing fennel seed production as honeybees, suggesting that insects other than honeybees, hoverflies in particular, play a significant role in fennel pollination.
Among hoverflies, species of the Eristalinae subfamily (Diptera: Syrphidae, subfamily Eristalinae) are frequent visitors of Apiaceae crops including anise (El-Berry et al., Reference El-Berry, Gawaad, Moustafa and El-Gayar1974), carrot (Bohart and Nye, Reference Bohart and Nye1960; Spurr, Reference Spurr2003; Pérez-Bañón et al., Reference Pérez-Bañón, Petanidou and Marcos-García2007; Gaffney et al., Reference Gaffney, Allen and Brown2011, Reference Gaffney, Bohman, Quarrell, Brown and Allen2018; Hogendoorn and Keller, Reference Hogendoorn and Keller2011), coriander (Ambrosino et al., Reference Ambrosino, Luna, Jepson and Wratten2006; Bendifallah et al., Reference Bendifallah, Louadi and Doumandji2013; Sharma et al., Reference Sharma, Bakshi, Thakur and Devi2016; Shivashankara et al., Reference Shivashankara, Srivastava, Subbanna, Kumar and Sandip2016; Bhowmik et al., Reference Bhowmik, Sarkar, Sen and Bhadra2017; Usman et al., Reference Usman, Amin, Saqib, Syed Fahad Shah and Aziz2018; Wojciechowicz-Zytko, Reference Wojciechowicz-Zytko2019), eryngo (Babaei et al., Reference Babaei, Asghar Fathi, Gilasian and Barimani Varandi2018), fennel (Sihag, Reference Sihag1986; Chaudhary, Reference Chaudhary2006; Gama et al., Reference Gama, Bruno R de, Quirino, Ramalho and Júnior2013; Bharti et al., Reference Bharti, Ahlawat, Sharma, Singh, Jitender and Singh2015; Ahmad et al., Reference Ahmad, Pathania and Kumar2019; Skaldina, Reference Skaldina2020) and parsnip (Jogesh et al., Reference Jogesh, Zangerl, Stanley and Berenbaum2013). In addition, the potential of eristaline hoverflies for pollination of several vegetable seed crops, both in open field and protected environments, has already been studied (Jarlan et al., Reference Jarlan, De Oliveira and Gingras1997; Rader et al., Reference Rader, Cunningham, Howlett and Inouye2020). Like blowflies, hoverflies do not seem to discriminate as much as hymenopterans between parental lines. Indeed, in hybrid carrot, hoverflies – including Eristalinae – did not show a preference towards either fertile or male sterile parental lines, whereas honeybees foraged preferentially on fertile plants (Spurr, Reference Spurr2003; Hogendoorn and Keller, Reference Hogendoorn and Keller2011; Gaffney et al., Reference Gaffney, Bohman, Quarrell, Brown and Allen2018). Hence hoverflies have been proposed as managed pollinators for several vegetable seed crops (Jauker and Wolters, Reference Jauker and Wolters2008; Howlett and Gee, Reference Howlett and Gee2019; Cook et al., Reference Cook, Voss, Finch, Rader, Cook and Spurr2020).
Recently, two eristaline hoverfly species have become commercially available as alternative managed pollinators: Eristalis tenax L. (Queenfly®, Polyfly) and Eristalinus aeneus Scopoli (Goldfly®, Polyfly) (Cook et al., Reference Cook, Voss, Finch, Rader, Cook and Spurr2020; Pekas et al., Reference Pekas, De Craecker, Boonen, Wäckers and Moerkens2020; Osterman et al., Reference Osterman, Aizen, Biesmeijer, Bosch, Howlett, Inouye, Jung, Martins, Medel, Pauw, Seymour and Paxton2021). The latter species, very abundant in the Mediterranean area (Dirickx, Reference Dirickx1994), is well adapted to the hot and dry climatic conditions that characterize the flowering period of most Apiaceae seed crops, especially in Southern Europe.
The objective of this study was to assess the pollination ability of E. aeneus as managed pollinator of hybrid celery (Apium graveolens L.) and fennel (Foeniculum vulgare Mill.) seed crops (both plant species belonging to the Family Apiaceae). To that aim, we evaluated the seed production and adhesion of pollen grains to flower stigmas comparing two densities of hoverflies in isolation cages. In addition, pollination ability of the blowfly Lucilia sericata (Meigen), a species commonly used for pollination in some seed crops, was also evaluated in celery.
Materials and methods
Biological material and study site
The pollination trials on celery and fennel hybrid seed crops were carried out at the University of Córdoba (Campus de Rabanales, Córdoba, SW Spain) during spring–summer of 2019 and 2020. Meteorological data during the study period were obtained from the Agroclimatic Station of Cordoba, IFAPA Centro Alameda del Obispo, sited 9 km from the experimental area. In 2019, the average temperature (±s.d.) during the trial was 24.06 ± 2.24°C (max.: 32.68 ± 2.87°C; min.: 14.81 ± 2.92°C); and in 2020 it was 24.83 ± 2.98°C (max.: 33.58 ± 3.89°C; min.: 15.66 ± 2.23°C).
The celery seeds were kindly provided by the Spanish company Diamond Seeds S.L. Celery plants were grown from seeds in individual pots of 25 × 25 × 29 cm, sowed each year in October and transplanted in experimental isolation cages in December. The fennel seeds used in the trial were kindly provided by Innovación Vegetal Mediterránea S.L. Fennel plants were grown in a seedbed and transplanted into the soil inside a screenhouse at the same time as the celery plants in 2019. However, unlike celery, the same fennel plants were used for both years as they were maintained in the screenhouse until completion of the 2020 season.
Both species of pollinators were released as pupae. Eristalinus aeneus were provided by Polyfly S.L. (Goldfly®) and L. sericata used in the celery trial were sourced from Koppert España S.L.U. (Natupol Fly®). Releases of pollinators started in May, at the beginning of the flowering period, and continued until mid-end of June for both crops.
Experimental set-up and design
Pollination in celery
The trial in celery was performed in eight isolation cages of 7.2 m2 (1.9 m in height) consisting of a metallic parallelepipedal structure covered with insect-proof mesh. Each cage contained six celery plants from a segregating population for androsterility; three of them with androsterile genotype (aa) (hereinafter referred to as ‘S’) and three of them with fertile genotype (Aa) (referred to as ‘F’).
The pollination treatments were: (a) high density of E. aeneus (40 individuals/m2, 48 ind./plant, i.e. 288 pupae per cage and release); (b) low density of E. aeneus (20 ind./m2, 24 ind./plant, i.e. 144 pupae per cage and release); (c) L. sericata at the recommended commercial density for Apiaceae seed crops (375 ind./m2, 450 ind./plant, i.e. 2700 pupae per cage and release); and (d) control treatment without release of pollinators.
We considered the start of flowering period as the moment when the flowers in the primary umbels of either the F or S plant genotypes started to open. Eristalinus aeneus pupae were introduced in the cages every 2 weeks during the flowering period (three releases in total). Pupae of L. sericata were introduced weekly during flowering, with a total of six releases. High and low densities of E. aeneus and the frequency of release of hoverflies used in the trial were calculated after preliminary trials performed at Polyfly S.L. (M. Sánchez, personal communication). Introductions of L. sericata followed the recommended dose and frequency of release indicated for Natupol Fly in Apiaceae (https://www.koppert.es/natupol-fly/). Both hoverfly and blowfly pupae were introduced in the centre of each cage, in their release cardboard boxes or in open plastic containers, respectively.
Two cages were assigned per treatment. The control treatment was included in the celery trial as a measure of pollination without release of managed pollinators, to be compared with the other three treatments in which hoverflies or blowflies were introduced. All cages were set-up in the open air and equally exposed to accidental access by wild pollinators. During the trial, some wild flies, wasps and bees were seldom found flying over the celery plants in all cages, with a relatively low frequency, and similar for all treatments. No stable populations or wild hymenopterans nests were found inside the cages in the celery trial.
Pollination in fennel
The trial in fennel was performed in four 19 m2 cages (2.2 m in height) isolated with insect-proof mesh and set-up with a metallic structure inside an insect-proof screenhouse (136 m2, 2.8 m tall). Each cage contained ten fennel plants; five of them from a cytoplasmic male sterile line (S) and five from a fertile line (F).
The pollination treatments were: (a) high density of E. aeneus (40 ind./m2, 76 ind./plant, i.e. 760 pupae per cage and release); (b) low density of E. aeneus (20 ind./m2, 38 ind./plant, i.e. 380 pupae per cage and release); and (c) control treatment without release of managed pollinators. The hoverfly pupae were introduced every 2 weeks in the cages, for a total of three releases during the flowering period. As in the celery trial, the high and low densities of E. aeneus and the frequency of release for hybrid fennel seed crops used in the fennel trial were calculated after preliminary trials performed at Polylfy S.L. (M. Sánchez, personal communication). Hoverfly pupae were introduced in their cardboard release boxes in the centre of each compartment, between the two rows of F and S plants, from the start of the flowering period (once the flowers in the primary umbels of either plant genotypes started opening).
Two cages were assigned per treatment, whereas the control treatment consisted of ten fennel plants (five of them S, and five F) placed outside of the cages, inside the same screenhouse. Therefore, wild pollinators had easier access to the control plants than to the plants in the hoverfly treatments, since the latter were isolated with an extra mesh enclosure. The control treatment was included in the fennel trial as a measure of pollination by wild pollinators, without release of managed pollinators, as compared to the other two treatments in which hoverflies were introduced at two different densities. During the trial, wild pollinators (flies, wasps and bees) were found flying over the fennel plants, with relatively higher frequency on the control plants inside the screenhouse than in the four compartments. However, no stable populations or nests of wild hymenopteran pollinators were found inside the fennel screenhouse.
Seed collection and evaluation of pollen adhesion
Apiaceae plants grow a central leading stem with one primary umbel, and several secondary umbels on lateral branches, with tertiary umbels also developing on branches arising from the secondary branches and so on (Peterson, Reference Peterson1991). These plants can produce quaternary and even fifth order umbels, depending on the plant density (Falzari et al., Reference Falzari, Menary and Dragar2005). The number of umbels per plant depends on the species, cultivar and ecological factors (Nemeth and Szekely, Reference Nemeth and Szekely2000). In turn, each umbel is composed of several umbellets, held to the umbel by stems called ‘rays’. Finally, each umbellet is made of several small flowers. Here we used the term ‘sample branch’ to name any of the lateral stems (not the central one) holding secondary umbels and subsequent order branches and umbels (tertiary, quaternary and fifth).
Seed production was evaluated from sample branches collected from celery and fennel plants in July of both years. In the celery trial, one branch per each of the three androsterile (S) genotype plants and one branch per each of the three fertile (F) genotype plants were sampled from each cage. In the fennel trial, three branches from three of the five S plants and three branches from three of the five F plants were also sampled at random from each cage. In 2019, production of fennel seeds was almost null due to an irrigation failure during the peak of high temperatures in the flowering period, so it could not be included in the analysis, hence only production of seeds from 2020 was evaluated in fennel.
For initial drying, paper bags containing the collected sample branches were kept inside a greenhouse from the end of July to mid-September. Subsequently, the collected branches were desiccated during 24 h in a laboratory oven at 60°C. After dehydration, the number of umbels per sample branch (considering secondary and subsequent order umbels from each branch) was counted and umbels were detached from the branch. Seeds were manually separated from the umbels and debris material was removed. Hollow seeds were excluded after sieving. Finally, the resulting seeds were weighed with an analytical balance (Sartorius Entris®).
Samples of umbellets from the primary umbel (i.e. from the primary stem) were collected from celery and fennel plants and preserved in glass vials with 70% alcohol. For celery, six umbellets per cage (one per plant) were collected twice during full blooming. For fennel, ten umbellets per cage (one per plant) were also collected twice during flowering. Pollen–pistil interactions were evaluated in five flowers from each sample umbellet. Flowers were stained with aniline blue (Martin, Reference Martin1959), and the observations were made immediately afterwards using an epifluorescence microscope. Aniline blue methodology was chosen because it is a simple and widely used technique to allow visualization of adhered and germinated pollen grains and pollinic tubes (Abdelgadir et al., Reference Abdelgadir, Johnson and Van Staden2012; Vieira et al., Reference Vieira, de Santana, Alves, da Silva Ledo and Souza2015). The number of pollen grains adhered to both stigmas was counted for each flower, and the proportion of pollinated flowers with pollen adhered to two stigmas, from the total of flowers with pollen adhesion, was also recorded for both crops.
Statistical analysis
In celery, seed production was analysed using the computer environment R (R-Core Team, Vienna, Austria, version 4.0.2). Seed weights were summed for same-genotype plants in each cage to avoid pseudo-replication. Seed production of celery as weight of seeds from three branches (‘Weight3b’ hereinafter) was analysed using three factorial generalized linear models with Gaussian error. The full model for celery data included ‘number of umbels’ as covariable, and ‘year’ (2 years), ‘treatment’ (four treatments) and ‘genotype’ (two genotypes) as independent variables. Including ‘number of umbels’ as covariable accounted for potential effects on the dependent variable caused by differences in the number of umbels per branch between treatments, which could be reflecting differences in plant growth. Full models were initially run, and higher-order interactions were sequentially removed from the model when they were not significant, and the new model yielded a lower AIC, following a backward elimination procedure. When applicable, post-hoc comparisons were done with Tukey tests.
Similarly, in fennel, seed weights were summed for same-genotype plants in each cage (three branches from three different plants per cage) and in the control plants (three branches from three different plants); and the dependent variable was weight of seeds from three branches (Weight3b). Since the number of replicates was low (two for each of the hoverfly treatments and one for the control without release of pollinators), we compared seed production of fennel between treatments and within genotypes using χ 2 tests. The number of umbels per branch was compared between treatments and genotypes with χ 2 test to detect possible differences in plant growth between treatments. When multiple comparisons were done, α values for significance were corrected using the sequential Bonferroni method.
In both celery and fennel data sets, adhesion of pollen grains to stigmas and percentage of flowers with pollen grains adhered to two stigmas from the total of flowers with pollen adhesion were compared between treatments using χ 2 tests, within S and F plant genotypes. In celery, pairwise comparisons between treatments were also made with χ 2 tests. Significance in multiple comparisons was corrected via the sequential Bonferroni method.
Results
Celery
The mean weight of seeds per branch (g), mean number of umbels per branch and mean weight of seeds per umbel (mg) (±s.e.m.) obtained in celery plants are shown for each treatment in 2019 and 2020, for androsterile (S) and fertile (F) plant genotypes separately, and for both genotypes together (Table 1).
Table 1. Results of seed production in celery: mean weight of seeds per branch (in grams), mean number of umbels per branch and mean weight of seeds per umbel (mg) (±s.e.m.) for androsterile (S), and fertile (F) plant genotypes separately, and for both genotypes together (Both), obtained in 2019 and 2020

Production of celery seeds, measured as weight of seeds per three branches (Weight3b), was positively correlated with the number of umbels per branch (LR χ 2 = 21.43, d.f. = 1, P < 0.001; Table 2, Fig. 1(a)). In general, seed production was higher in 2020 than in 2019 (LR χ 2 = 94.79, d.f. = 1, P < 0.001; Table 2, Fig. 1(b)); and the genotype affected seed production, with F plants obtaining higher Weight3b than S plants (LR χ 2 = 8.79, d.f. = 1, P < 0.003; Table 2, Fig. 1(d)). Moreover, there were significant differences in seed production between pollinator treatments (LR χ 2 = 8.15, d.f. = 3, P = 0.043; Table 2, Fig. 1(c)). Plants pollinated with high density of hoverflies showed significantly higher Weight3b than the control without pollinators (HD: 3.97 ± 0.92 g (mean Weight3b ± s.e.m.), C: 2.81 ± 1.02 g; z = −2.67, P < 0.038 Tukey contrasts; Fig. 1(c)). However, production with low density of hoverflies and with blowflies were not significantly different from high density of hoverflies (LD: 3.07 ± 1.12 g; LS: 3.12 ± 1.04 g, both P > 0.160, Fig. 1(c)), nor from the control without release of pollinators (both P > 0.918; Tukey contrasts).

Fig. 1. Colour online. Production of hybrid seeds in celery, as mean weight of seeds per three branches (Weight3b) (in grams, ±s.e.m.). Effect of number of umbels per branch (a), year (b), pollination treatment (c) and plant genotype (d). Weight3b was significantly different for points accompanied by different letters, after submitting data to generalized lineal models, and using Tukey tests for post-hoc analyses (b–d).
Table 2. Results of the generalized linear models (analyses of deviance, type III) applied to celery data
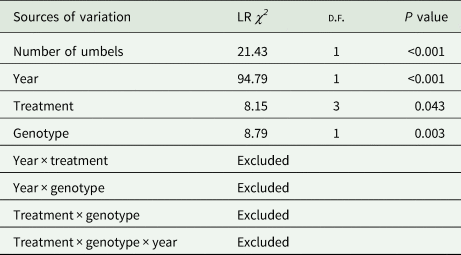
Explanatory variables were year (2019 and 2020), treatment (high density of hoverflies, low density of hoverflies, Lucilia sericata and control) and genotype (fertile and androsterile); and the response variable was weight of celery seeds per three branches (Weight3b). Number of umbels per branch was included in the model as covariable.
A total of 960 flowers of celery was processed to evaluate pollen adhesion to stigmas (Fig. 2(a)). Adhesion of pollen grains to flower stigmas (average number of flower grains adhered to a single flower) was different between treatments and genotypes (χ 2 = 21.25, P < 0.001, Fig. 3(a)). In flowers from S-genotype plants, a higher number of pollen grains were adhered to stigmas in the high-density treatment as compared to the control and blowflies treatment, whereas the low-density treatment was not significantly different from the other three treatments (HD: 1.97 ± 0.20 grains (mean ± s.e.m.), LD: 0.77 ± 0.11, LS: 0.97 ± 0.10, C: 0.47 ± 0.07) (Table 3, Fig. 3(a)). In flowers from F-genotype plants, a significantly higher number of pollen grains was adhered to stigmas in the high-density treatment, followed by low-density, the blowfly and the control treatment (HD: 3.75 ± 0.36 grains, LD: 2.67 ± 0.26, LS: 1.46 ± 0.17, C: 1.16 ± 0.12), although the difference with the latter treatment was not significant (Table 3, Fig. 3(a)).

Fig. 2. Colour online. Image of celery (a) and fennel (b) pistils from sterile flowers under fluorescence microscopy after dissection of flowers and dyeing with aniline blue. Arrows point to adhered and germinated pollen grains on the stigma.

Fig. 3. (a) Mean total number of pollen grains (±s.e.m.) adhered to flower stigmas in celery, and (b) percentage (%) of flowers with pollen adhered to two stigmas (from the total of flowers with adhered pollen grains) for each pollinator treatment and plant genotype in celery. Bars accompanied with different letters within each plant genotype were significantly different (χ 2 tests, significance corrected via the sequential Bonferroni method).
Table 3. Pairwise comparisons of adhesion of pollen grains to stigmas in celery flowers between treatments within each plant genotype

HD, high density of hoverflies; LD, low density of hoverflies; LS, Lucilia sericata; C, control without release of pollinators.
*P values with * were significant with α corrected by sequential Bonferroni correction for multiple comparisons.
Among flowers showing pollen adhesion, the percentage of those with pollen grains adhered to two stigmas in the S genotype was significantly higher in the hoverfly high-density treatment (82%), followed by the low-density (36%) and blowfly (15%) treatments; while the control without release of pollinators (17%) was not different from the latter two treatments (χ 2 = 178.13, P < 0.001; Table 4, Fig. 3(b)). Within the F plant genotype, the percentage of flowers with pollen adhesion in two stigmas was significantly higher in high density of hoverflies (98%), followed by low density and blowflies, without significant differences between these two (56 and 45%, respectively), and then by the control without release of pollinators (18%) (Table 4, Fig. 3(b)).
Table 4. Pairwise comparisons of percentage of celery flowers with pollen adhered to two stigmas, from the total of flowers with adhered pollen between treatments within each plant genotype

HD, high density of hoverflies; LD, low density of hoverflies; LS, Lucilia sericata; C, control without release of pollinators.
*P values with * were significant with α corrected by sequential Bonferroni correction for multiple comparisons.
Fennel
The mean weight of seeds per branch (g), mean number of umbels per branch and mean weight of seeds per umbel (mg) (±s.e.m.) obtained in fennel plants are shown for each treatment in 2020, separately for sterile and fertile plants, and for both genotypes together (Table 5). In 2019, the production of seeds was practically nil mainly because of an unforeseen failure in the watering system affecting some of the isolation cages during the highest temperatures occurred in the flowering period, hence the decision to exclude that year's seed production data from the statistical analysis.
Table 5. Results of seed production in fennel (year 2020): mean weight of seeds per branch in grams, mean number of umbels per branch and mean weight of seeds per umbel (mg) (±s.e.m.) for androsterile (S) and fertile (F) plant genotypes separately, and for both genotypes together (Both)

In 2020, no significant differences were found in the number of umbels per branch between treatments and plant genotypes (χ 2 = 1.47, d.f. = 2, P > 0.05). Therefore, we assumed similar plant growth between treatments. Production of seeds (Weight3b) was different between pollinator treatments and genotypes (χ 2 = 7.55, d.f. = 2, P < 0.05). For F genotypes, high density of hoverflies showed higher seeds production than low density (χ 2 = 42.82, d.f. = 1, P < 0.025, α corrected by sequential Bonferroni) and control plants without release of pollinators (χ 2 = 35.31, d.f. = 1, P < 0.025, α corrected by sequential Bonferroni), whereas no differences were found between the low density of hoverflies and control plants (χ 2 = 1.73, d.f. = 1, P > 0.05) (Weight3b for F plant genotypes: HD: 14.96 ± 2.61 g (mean Weight3b ± s.e.m.); LD: 6.01 ± 1.49 g; C: 5.59 g; Fig. 4). Within the S plant genotypes, there were no significant differences in production of seeds between treatments (χ 2 = 5.143, d.f. = 2, P > 0.05) (Weigh3b for S plant genotypes: HD: 6.17 ± 0.77 g; LD: 4.92 ± 0.59 g; C: 4.41 g; Fig. 4).

Fig. 4. Mean production of seeds in fennel (Weight3b) (in grams, ±s.e.m.). Bars accompanied with different letters within each plant genotype were significantly different (χ 2 tests, significance corrected via the sequential Bonferroni method).
A total of 800 fennel flowers was processed to evaluate pollen–pistil interactions (Fig. 2(b)) in the two hoverfly treatments. Adhesion of pollen grains to stigmas in the control treatment was not measured because wild pollinators (dipterans and hymenopterans) found their way into the screenhouse more frequently than in the compartments with release of hoverflies. Indeed, wild pollinators were spotted in the control plants during every visit, and they appeared to occur more than inside the mesh compartments.
Adhesion of pollen grains to individual stigmas was higher with high density of hoverflies than with low density (HD: 6.58 ± 0.49 grains (mean ± s.e.m.); LD: 4.29 ± 0.39 grains; χ 2 = 92.20, d.f. = 1, P < 0.001); both within F (10.7 v. 7.9 grains per flower; χ 2 = 6.36, d.f. = 1, P = 0.012) and S plant genotypes (2.4 v. 0.7 pollen grains; χ 2 = 21.72, d.f. = 1, P < 0.001) (Fig. 5(a)).

Fig. 5. (a) Mean total number of pollen grains (±s.e.m.) adhered to flower stigmas in fennel and (b) percentage (%) of flowers with pollen adhered to two stigmas (from the total of flowers with adhered pollen grains) for each pollinator treatment and plant genotype in fennel. Bars accompanied with different letters within each plant genotype were significantly different (χ 2 tests, P < 0.001).
Finally, the percentage of flowers with pollen adhered to two stigmas from the total of flowers with pollen adhesion was different between hoverfly densities and plant genotypes (χ 2 = 141.61, d.f. = 3, P < 0.001). For genotype F, the percentage of flowers with pollen adhered to two stigmas was higher with high density of hoverflies (92%) than with low density (60%) (χ 2 = 11.42, d.f. = 1, P < 0.001). Likewise, for genotype S, the percentage of flowers with pollen adhered to two stigmas was higher with high density (49%) than with low density of hoverflies (18%) (χ 2 = 19.07, d.f. = 1, P < 0.001) (Fig. 5(b)).
Discussion
Effects of hoverfly densities on seed production
Eristalinus aeneus confirmed its ability to effectively pollinate celery hybrid seed crops in our trial. Indeed, the release of hoverflies at high density increased production of seeds as compared to plants without released pollinators, for both androsterile and fertile plant genotypes. In fennel, production of seeds increased with hoverflies released at high density as compared to plants without released hoverflies, although this difference was only significant for fertile and not for sterile plant genotypes. Therefore, our results suggested that E. aeneus is also able to effectively pollinate hybrid fennel seed crops, but higher densities may be necessary to significantly increase seed production in sterile plant genotypes.
Considering both years, high density of hoverflies increased seed production in celery by +49% (S and F lines combined), while low density accounted for a +5% increase with respect to the control (Fig. 1(c)). However, in 2019, the year with lower seed set overall, the differences between seed production obtained in the hoverfly and control treatments were considerably more pronounced than in 2020 (HD: +456% in 2019 v. +13% in 2020; LD: +19% in 2019 v. +4% in 2020; Table 1). In the fennel trial, high density of hoverflies significantly increased seed production by +111%, while low density only accounted for +9% when compared to the control treatment (S and F genotypes combined, Table 5). These increases in seed production obtained with hoverfly pollinators relative to the control without pollinators are within the range of increments found in other studies involving Apiaceae seed crops. For instance, Schittenhelm et al. (Reference Schittenhelm, Giadis and Rao1997) found that seed production in carrot (Daucus carota) was 64% higher with pollinators than without; Ahmad et al. (Reference Ahmad, Pathania and Kumar2019) also reported that the seed set increased by 538% in carrot when umbels were left to open pollination as compared to caged umbels. Likewise, Sihag (Reference Sihag1986) found that carrot and fennel seed production was significantly higher in open than in caged plots, with increases of 336 and 178% respectively for each crop. Chaudhary (Reference Chaudhary2006) reported that honeybees and open pollination increased fennel seed production by 413 and 475% respectively, when compared to a treatment without insect pollination.
Nevertheless, these references considered control plants or flowers with complete pollinator exclusion, which means the increase in seed production obtained with pollinators is expected to be higher than in our trials, where control plants were exposed to pollinators intrusions. As explained previously, in the celery trial, wild pollinators were seldom found in all cages, with a similar incidence in the control and in the other cages with released managed pollinators. In fennel, control plants located under the screenhouse, but outside of the compartments, were more readily accessible and consequently visited more frequently by wild pollinators than the plants in the compartments of the hoverfly treatments. However, in both trials, the released pollinators were more abundant than wild pollinators, and this resulted in the relative increase in seed production found with high density of hoverflies as compared to the controls.
Previous studies in several Brassicaceae seed crops comparing different pollinators densities in isolation cages highlighted that seed production is positively correlated with the density of pollinators. For example, Schittenhelm et al. (Reference Schittenhelm, Giadis and Rao1997) and Steffan-Dewenter (Reference Steffan-Dewenter2003) found that, in Brassica sp., the seed weight produced per plant increased with increasing pollinator density (blowflies L. sericata and C. vicina for the former, mason bee Osmia rufa for the latter). Also, Jauker et al. (Reference Jauker, Bondarenko, Becker and Steffan-Dewenter2012) indicated that in oilseed rape, Brassica napus, fruit set and number of seeds per pod increased with increasing pollinator density, although these effects were stronger in the mason bee than the hoverfly treatment; and seed set of mustard (B. rapa) increased significantly when the number of pollinators was higher (Atmowidi et al., Reference Atmowidi, Buchori, Sjafrida, Suryobroto and Hidayat2007). Similarly, Ohsawa and Namai (Reference Ohsawa and Namai1987) found that the percentage of seed set increased linearly when the density of the hoverfly Eristalis cerealis was increased from one to four individuals per plant. In our trials, for both crops, seed production was higher when the hoverflies were released at high density (40 ind./m2), as compared to the low density (20 ind./m2). However, in fennel, the difference in seed production between the two hoverfly densities was found to be significant only for fertile plant genotypes. In celery, no differences in seed production were found between high and low density of hoverflies, nor between low density of hoverflies and the control treatment. The differences between hoverfly densities tested in our trials (24 v. 48 ind./plant in celery and 38 v. 76 ind./plant in fennel) might not have been enough to show a clear effect of pollinator densities in seed production. Nonetheless, effects of different hoverfly densities were observed in pollen adhesion for both crops.
Higher seed production was obtained in the F than in the S plant genotypes in both our celery and fennel trials. This might be the reason why a significant effect of hoverfly density in the production of seeds was only found in F fennel plants, and not in S plant genotypes. A higher production of seeds in F plants is consistent with the fact that chances of successful pollination by insects are greater within F than S lines. Indeed, Apiaceae fertile flowers open progressively, following a fixed sequence, and produce viable pollen available during long term. This means that repeated insect floral visits on the same F plant can facilitate self-pollination. In contrast, for sterile plants, pollen must necessarily come from a fertile plant, and this transfer must occur during the effective pollination period (EPP) of both plant genotypes (Chassaigne-Ricciulli et al., Reference Chassaigne-Ricciulli, Mendoza-Onofre, Córdova-Téllez, Carballo-Carballo, San Vicente-García and Dhliwayo2021). Thus, the efficiency of pollen transfer and the implementation of an adequate pollination design (i.e. ratio of fertile to sterile plants, sowing and transplanting dates), enabling flowering synchronization of parental lines, is critical to achieve satisfactory cross-pollination. Besides, other factors such as fertilization and irrigation during the flowering period can also influence pollinators visitation rates and subsequent pollen dispersal (Fijen et al., Reference Fijen, Scheper, Vogel, van Ruijven and Kleijn2020).
Enhanced seed production following an increase in pollinator densities may come as a result of more crosses between lines and exacerbated competition for resources when more pollinators are released in a confined environment. Jauker et al. (Reference Jauker, Bondarenko, Becker and Steffan-Dewenter2012) described a greater foraging effort with high densities of pollinators, but other studies also pointed out that overcrowding in cages may lead to pollen overexploitation and flower damages, which could hinder the production of seeds (Mesquida et al., Reference Mesquida, Renard and Pierre1988). Combining several species of pollinators exhibiting complementary flower-visiting behaviour could be a better approach to improve fruit and seed set than just increasing pollinator abundance (Klein et al., Reference Klein, Steffan-Dewenter and Tscharntke2003). However, in our study, we were not able to measure the effect of density on the pollinator's activity and behaviour (number and duration of floral visits, number of crossings between plant genotypes). More research is needed to verify whether seed production could be further improved by increasing the number of hoverflies and defining more accurately the optimal density for pollination of Apiaceae seed crops like celery and fennel.
Effect of hoverfly densities on pollen adhesion
Pollen adhesion in celery and fennel followed the same pattern found for seed production as both parameters evaluated (number of pollen grains adhered to stigmas per flower, and proportion of flowers with pollen adhered to two stigmas) increased with higher density of hoverflies, in both S and F lines; although the difference in number of pollen grains adhered to stigmas was not significant for the S celery plants.
In celery, pollen adhesion with low density of hoverflies was not different from the control, although the percentage of flowers with pollen in two stigmas for F plants was higher with low density of hoverflies than in the control. Also, the number of adhered pollen grains did not show significant differences between the two hoverfly densities in S celery lines, but it was higher with high density for F plants. This suggests that the treatment with low density of hoverflies likely suffered pollination deficit due to suboptimum density of pollinators, and that a density of 40 ind./m2 or higher is probably more adequate for E. aeneus to efficiently cross-pollinate this crop (Fig. 3).
In fennel, pollen adhesion was significantly higher with high than with low density of hoverflies, both in the F and S lines (Fig. 5). The fact that pollen adhesion increased with higher density of hoverflies also in S lines indicates that more effective cross-pollination occurred with higher density of hoverflies, therefore suggesting an increase of effective pollination by hoverflies. However, in this trial, with this pollination design and with densities of 20 and 40 ind./m2, E. aeneus did not perform enough crossings during the EPP to achieve significant differences between treatments in seed production.
Potential advantages of hoverflies as pollinators and comparison with blowfly L. sericata
Taking into account the 2 years of the celery trial, seed production with the blowfly L. sericata was not different from production with hoverflies at high nor at low density, for any plant genotypes, and it was also not different from the control without release of pollinators. With respect to the blowfly treatment, high density of hoverflies increased seed production by +35% (S and F lines combined), while low density accounted for a decrease of −5% (Fig. 1(c)), although these differences were not statistically significant. However, pollen adhesion with hoverflies (high and low densities) was higher than with blowflies in F plant genotypes, and it was significantly higher with high density of hoverflies than with blowflies in S plants.
Although in our trial we did not monitor the foraging activity of E. aeneus and L. sericata, we could appreciate more systematic and prolonged floral visits with the hoverflies than with blowflies. Similarly, in two different celery cultivars (A. graveolens var. rapaceum and A. graveolens var. dulce) E. aeneus was found to perform more frequent visits than another blowfly species, Protophormia terraenovae (Sáez et al., Reference Sáez, Pérez-Bañon, Driessen and Rojo2017). This foraging activity of hoverflies, with continuous and lengthier floral visits has been shown to increase the probability of pollen transfer and pollination as a result (Rader et al., Reference Rader, Howlett, Cunningham, Westcott, Newstrom-Lloyd, Walker, Teulon and Edwards2009; Ne'eman et al., Reference Ne'eman, Jürgens, Newstrom-Lloyd, Potts and Dafni2010).
Considering pollen load, the literature shows that most eristaline hoverflies transport more pollen on their bodies than other dipteran species in Apiaceae crops. In carrot, E. tenax was found to have a higher pollen load than Calliphora vicina and L. sericata (4353 grains v. 1930 and 1493, respectively) (Pérez-Bañón et al., Reference Pérez-Bañón, Petanidou and Marcos-García2007). Moreover, Gaffney et al. (Reference Gaffney, Bohman, Quarrell, Brown and Allen2018) noticed that E. tenax pollen load was independent of the line from which the specimens were collected in (F or S carrot plants), suggesting these hoverflies forage across rows. This study also reported that E. tenax carried more pollen grains on their body than other flies (with >80% of samples with less than 10 pollen grains). Likewise, in a 2-year trial involving another Apiaceae, Angelica sylvestris, Niemirski and Zych (Reference Niemirski and Zych2011) found the most significant pollen loads were carried by Eristalis sp. as compared to Lucilia sp. (2006: 579 v. 158; 2007: 144 v. 89 pollen grains). In other seed crops, the pollen load and pollen deposition capacity of E. tenax was also found to be greater than that of blowflies. In Allium sp., E. tenax showed pollen deposition similar to honeybees and bumblebees, and greater than Calliphora stygia and L. sericata (18.8 pollen grains per stigma by E. tenax v. 14.1 and 2.0 pollen grains by C. stygia and L. sericata, respectively) (Howlett et al., Reference Howlett, Evans, Pattemore and Nelson2017). In Brassica sp., pollen load of E. tenax was greater than that of C. stygia (8183 v. 6256 pollen grains), and pollen deposition by E. tenax was also higher than by blowflies (107 grains deposited by E. tenax v. 61 grains by C. stygia) (Howlett et al., Reference Howlett, Walker, Rader, Butler, Newstrom-Lloyd and Teulon2011). Thus, both the pollen carrying capacity and the foraging behaviour of eristaline hoverflies could have increased pollen adhesion in the celery trial, and explain why it was found to be higher with E. aeneus than with L. sericata.
On the other hand, the blowfly pollination treatment in this trial consisted of releasing 375 flies/m2 every week, and this resulted in a total of 16 200 L. sericata pupae being introduced in each cage. In comparison, the hoverfly high- and low-density treatments only required 864 and 432 E. aeneus pupae respectively, which means the number of pollinators released was approximately 38 and 19 times less than in the blowfly treatment. Thus, the production of seeds per individual was considerably higher with hoverflies than with blowflies. Concretely, considering both years together, individual E. aeneus yielded 25.4 (high density) and 35.8 (low density) times, the production of seeds per L. sericata individual. Such adjustment of seed yields to a ‘per insect’ basis has sometimes been used to measure more accurately the insect intrinsic pollination efficiency (Jahns and Jolliff, Reference Jahns and Jolliff1991; Schittenhelm et al., Reference Schittenhelm, Giadis and Rao1997; Jauker et al., Reference Jauker, Bondarenko, Becker and Steffan-Dewenter2012).
Similarly to what has been observed with the seed production, the highest number of celery pollen grains adhered to stigmas was found with high density of hoverflies (2.86 pollen grains), followed by low density (1.72), blowflies (0.97) and finally the control (0.83). This pattern appears to be in line with the explanation that pollen adhesion is directly related to pollination effectiveness which subsequently affects seed set and yield. In our study, the pollination efficiency in celery was found to be higher with hoverflies at both densities tested than with L. sericata, in spite of the higher number of blowflies introduced in the isolations. Thus, our results indicate that, in celery, E. aeneus can have a greater pollinator potential than L. sericata.
Managed pollinators used in Apiaceae crops until now, such as honeybees, bumblebees and blowflies, present certain drawbacks. The temporal dioecism (i.e. Apiacieae flowers undergo a male phase followed by a female phase) may influence the behaviour of non-dipteran pollinators. These pollinators tend to discriminate between the sex of flowers, visiting only one phase, usually the male phase as they look for pollen (Niemirski and Zych, Reference Niemirski and Zych2011). Honeybees for instance can show preference towards parental lines offering better pollen or nectar resources, thereby reducing the chances of cross-pollination, whereas hoverflies do not seem to exhibit such discriminating behaviour (Spurr, Reference Spurr2003; Kobayashi et al., Reference Kobayashi, Tsukamoto, Tanaka, Niikura and Ohsawa2010). Furthermore, standard beehives are not suitable for pollination in small isolations (Ochiuzzi, Reference Ochiuzzi1999; Dag, Reference Dag2008; Evans et al., Reference Evans, Cutting, Jochym, Janke, Felman, Cross, Jacob and Goodwin2019). For spring–summer flowering crops, such as carrot, celery and fennel, the ability of certain hoverfly species like E. aeneus to continue being active at high temperatures can also be an advantage over bumblebees (Nayak et al., Reference Nayak, Rana, Bairwa, Singh and Bharthi2020; Descamps et al., Reference Descamps, Jambrek, Quinet and Jacquemart2021; Sánchez et al., Reference Sánchez, Velásquez, González and Cuevas2022). Indeed, E. aeneus can cope well with high temperatures (>30°C) and, unlike bumblebees, they do not need to spend time and energy ventilating a hive in order to cool it down and preserve the colony.
With regard to blowflies like L. sericata, these are not as efficient pollinators because they mostly search for nectar, which means they do not perform systematic flower visits. Besides, their ability to transfer pollen from one parental line to another is reduced compared to pollinators displaying more consistent foraging behaviour (Schittenhelm et al., Reference Schittenhelm, Giadis and Rao1997). Thus, to compensate their relative inefficiency, blowflies need to be released in very high numbers (several hundreds of flies per square meter, weekly) (Gabai et al., Reference Gabai, Vaissière, Blacquiere, Freitas, Allsopp, Chabert and Dag2018). This strategy, consisting of repeated mass introductions or inundative releases, is often considered obnoxious by the growers themselves. In fact, there have been reports of situations where blowflies escaping isolation cages or tunnels caused inconveniences to neighbouring inhabitants. Just as importantly, since several species of blowflies, including L. sericata, are considered problematic synanthropic insect pests that can cause allergies and myasis, their use as pollinators should be closely monitored (Khater et al., Reference Khater, Hanafy, Abdel-Mageed, Ramadan and El-Madawy2011; Muniz et al., Reference Muniz, Bedini, Sarrocco, Vannacci, Moura Mascarin, Fernandes and Conti2020; Fukutomi and Kawakami, Reference Fukutomi and Kawakami2021).
To our knowledge, this is the first time that the pollination ability of E. aeneus hoverflies has been evaluated in hybrid celery and fennel seed crops. The combined results obtained in celery for seed production and pollen adhesion, and the highest number of seeds obtained per individual hoverfly specimen, indicate that E. aeneus is a more efficient pollinator than L. sericata for this type of hybrid seed crop. Since the density of hoverflies was found to affect pollen adhesion and seed production, the number of hoverflies released could be adjusted to increase yields. More research is needed to establish the optimal density of hoverflies and adequate pollination protocols for different types of seed crops and production settings.
Conclusion
Altogether, our results show that the hoverfly E. aeneus can efficiently pollinate hybrid celery and fennel seed crops and that it could be used as an alternative managed pollinator for said crops in breeding and seed production activities.
Author contributions
JG and YV conceived the research; MS conducted the experiments; JG contributed the material; MS, BB and MM conducted statistical analyses; MS, BB and YV wrote the manuscript; YV secured funding; all authors read and approved the manuscript.
Financial support
This research has been partially supported by two grants from the Spanish Ministry of Science and Innovation (MICINN) awarded to Polyfly S.L.: A Torres Quevedo grant (PTQ-17-09043) and a Neotec grant (SNEO-20171067).
Conflict of interest
Two authors are employed by the company. Another author owns shares and is part of the Board of the company.
Ethical standards
Not applicable.













