Introduction
It is now well established that the maternal milieu during pregnancy, including stress, mental illness, lifestyle factors and substance use, is critical in determining long-term offspring health and disease outcomes. Seminal research from David Barker and his team in the 1980s has associated infant mortality and cardiovascular-related deaths within lower income regions of the United KingdomReference Barker and Osmond1, which initiated subsequent investigation eventually forming the Developmental Origins of Health and Disease (DOHaD) hypothesis. This hypothesis has determined that changes to maternal physiology, placental function and altered exposure of the foetus to key nutrients and hormones result in predictive adaptive responsesReference Barker2-Reference McMillen and Robinson4. These adaptations are often observed in critical organs such as the foetal kidney, heart and brain, as well as the placenta, and are essential for continued in utero development and survivalReference Dorey, Pantaleon, Weir and Moritz5,Reference Gluckman, Hanson, Gluckman and Hanson6 . This often occurs in a sex-specific manner and is suggested to be regulated by the placenta (reviewed in by Clifton et al. and Sundrani et al.Reference Clifton7,Reference Sundrani, Roy, Jadhav and Joshi8 ). Following birth, however, these adaptations may result in altered physiological responses in offspring, impairing offspring health and increasing the risk of developing diseases in later lifeReference McMillen and Robinson4,Reference Warner and Ozanne9,Reference Langley-Evans10 . These include highly prevalent non-communicable diseases related to cardiovascular, metabolic, renal and respiratory function.
Of relevance to this review is the evidence of developmental programming of mental illness, which has been observed in numerous studies (recently reviewed by O’Donnell et al.Reference O’Donnell and Meaney11). Low birth weight, the traditional surrogate marker of an in utero perturbation, is associated with a higher risk of psychopathology, such as attention deficit disorder (ADHD)Reference Breslau and Chilcoat12-Reference Sucksdorff, Lehtonen and Chudal14, impaired executive function, working memoryReference Aizer and Currie15 and childhood emotional reactivityReference Schlotz and Phillips16,Reference Räikkönen, Pesonen, Roseboom and Eriksson17 . These outcomes have been shown to preface increased frequency and severity of depressive symptomsReference Costello, Worthman, Erkanli and Angold18, and anxiety disorders. Several studies have indicated that low birth weight is also associated with several more severe mental health outcomes, including psychosis-like symptoms, schizophrenia and a propensity to develop substance use disordersReference Abel, Wicks and Susser19.
Specific maternal conditions or perturbations that contribute to such outcomes include maternal stress, immune response and infection, hypoxia, undernutrition, maternal depression and anxiety, obesity and metabolic condition, as well as drug use such as tobacco. As outlined in Table 1, these perturbations are associated with reactive behaviour, externalising symptoms including attention deficit disorders, inhibition, impaired executive functioning and inhibition, as well as more debilitating psychiatric illnesses such as anxiety, major depressive disorder, bipolar disorders and schizophrenia.
Table 1. A non-exhaustive list of maternal perturbations and conditions associated with adverse offspring mental health
Another common exposure during pregnancy associated with mental illness, albeit often neglected in the context of DOHaD, is alcohol consumption. Psychiatric outcomes in offspring prenatally exposed to alcohol are often similar to those of other perturbations, including ADHD, behavioural problems, lower IQ, increased frequency and severity of depressive symptoms, and psychosis-like symptoms. This overlap suggests that the programming of mental illness may be a multifactorial combination of several perturbations, and it is, therefore, essential to consider alcohol not only in combination with these but also in the context of DOHaD.
Alcohol consumption during pregnancy
The implications of high-dose alcohol consumption on foetal development have been recognised, with vast evidence supporting the teratogenic impacts on neurological and behavioural development. The outcomes included foetal alcohol spectrum disorder (FASD) with foetal alcohol syndrome (FAS) are at the severe end of the spectrum. These conditions are highly prevalent with a recent meta-analysis performed by Popova et al. suggests the prevalence of FAS to be 14.6 per 10 000Reference Popova, Lange and Shield82. However, since this first identification of FAS, the umbrella term of FASD has been adopted to include all diagnostic criterion of FAS, including partial foetal alcohol syndrome (pFAS), alcohol-related birth defects and alcohol-related neurodevelopmental disorder (ARND)Reference Bower and Elliott83. FASD is a complex condition to diagnose, regardless of the guidelines in many countries which describe only two specific categories: FASD with three sentinel facial features and FASD with less than three sentinel facial featuresReference Bower and Elliott83. As such, epidemiological research has determined the prevalence of FASD as greater than overt FAS, with conservative estimates of 5% in the United StatesReference May, Chambers and Kalberg84, and up to 19% in some regions of Australia impactedReference May, Chambers and Kalberg84-Reference Fitzpatrick, Latimer and Olson86.
Mental health outcomes associated with prenatal alcohol exposure
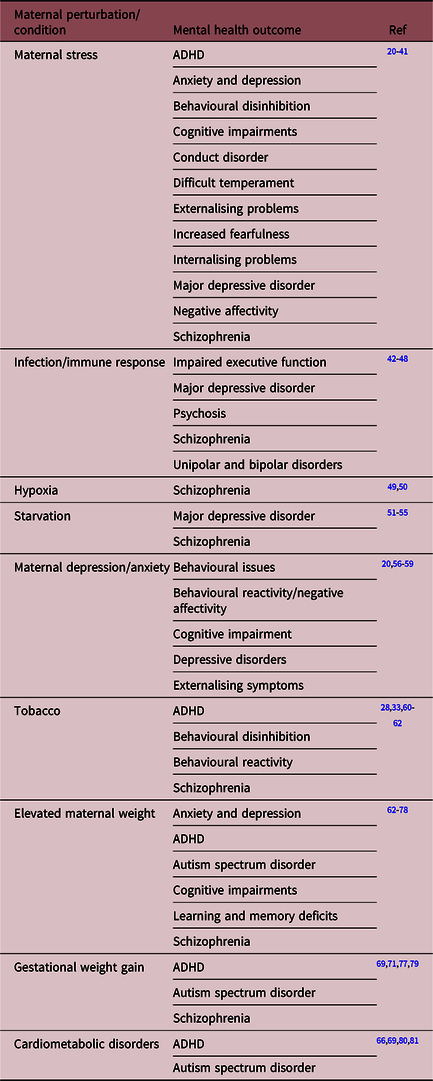
Individuals exposed to high levels of alcohol prenatally demonstrate many developmental deficits, including altered brain structure, craniofacial abnormalities, cardiac defects and in utero growth restrictionReference Jones and Smith87,Reference Jones, Smith, Ulleland and Streissguth88 . Although there are also significant implications of alcohol exposure during early postnatal life, this review will focus only on those studies investigating the outcomes of prenatal exposure. Once born, children exposed to prenatal alcohol may display developmental delays, behavioural difficulties and a range of externalising problems, including behavioural, impulse control and hyperactivityReference Jones, Smith, Ulleland and Streissguth88-Reference Riley, Infante and Warren90. However, secondary to these are several internalising issues, with studies revealing that 70%–90% of adults diagnosed with FAS or FASD also display psychiatric conditions including depression, anxiety and mood disordersReference Popova, Lange and Shield82,Reference Streissguth, Barr and Kogan91 . A recent study performed by Ipsiroglu et al. determined that within a cohort (n = 40) of individuals with diagnosed FASD and/or prenatal alcohol history, 95% displayed disruptive behaviour or externalising disorders and 73% demonstrated anxiety and mood disorders. This was associated with several neurodevelopmental presentations and altered sleep behaviourReference Ipsiroglu, Wind, Hung and Berger92. This finding is in support of a study by O’Connor et al. of 23 children prenatally exposed to alcohol, where it was determined that 87% developed a psychiatric illness and 61% of these developed a mood disorder such as major depressive disorderReference O’Connor, Shah and Whaley93. Another small clinical study from this group demonstrated that binge alcohol consumption at 16 and 30 weeks’ gestation resulted in children displaying conduct disorderReference Niclasen, Andersen, Strandberg-Larsen and Teasdale94. Other clinical studies have demonstrated that females exposed to alcohol (inclusion criteria of a diagnosis of FAS, pFAS, ARND, suspected exposure or awaiting diagnosis) during pregnancy suffer greater social difficulties and hyperactivity than malesReference Rasmussen, Becker, McLennan, Urichuk and Andrew95,Reference Schonfeld, Paley, Frankel and O’Connor96 . Psychiatric and behavioural deficits in children have been associated with binge alcohol exposure during pregnancy (greater than five standard drinks, at least once every 2 weeks)Reference Bailey, Delaney-Black and Convington97-Reference Streissguth, Barr and Sampson99, a finding that has also been observed in young adultsReference Barr, Bookstein and O’Malley100, suggesting a persistent deficit in mental health.
Importantly, clinical studies have also demonstrated an association between low-dose and/or early exposure and mental health outcomes, regardless of a FAS or FASD diagnosis. A study performed by Sayal et al. investigated sex-specific mental health outcomes in children following a single dose of binge alcohol after the first trimester; this increased child behavioural problems, including emotional and conduct difficulties, hyperactivity and inattention, as determined by the strengths and difficulties questionnaireReference Sayal, Heron and Golding101. A subsequent study concluded that low-level alcohol exposure during the first 18 weeks of pregnancy resulted in higher levels of mental health deficits in female, but not male offspringReference Sayal, Heron, Golding and Emond102. Similarly, O’Connor and team demonstrated that 9% of children exposed to low-dose alcohol (one or fewer drinks per drinking occasion) at some point during pregnancy, displayed depressive symptomsReference O’Connor and Kasari103. This group also determined that a binge exposure during the first trimester is associated with increased prevalence of mental health problems in male children at 7 years of ageReference Niclasen, Nybo Andersen, Teasdale and Strandberg-Larsen104. Similarly, Alvik et al. showed that this same binge dose during the first 4 weeks of pregnancy is associated with a 3- to 5-fold increase in the likelihood of children displaying a problematic temperament and sleeping problemsReference Alvik, Torgersen, Aalen and Lindemann105, as well as behavioural issues measured by the strengths and difficulties questionnaireReference Alvik, Aalen and Lindemann106. Greater social disinhibited behaviours, including seeking strangers and unfamiliar situations, have been observed in children exposed to binge doses in early pregnancyReference Nulman, Rovet and Kennedy107.
A recent investigation of the impacts of first-trimester prenatal alcohol exposure (greater than one drink per day) demonstrated that at 22 years of age, those exposed showed greater symptoms of alcohol use disorderReference Goldschmidt, Richardson, De Genna and Day108. These results align with numerous studies that show increased behavioural problems, attention disorder, internalising and externalising symptoms, as well as altered social behaviour, sleeping patterns, social difficulties, anxiety and depression occurring at both low- and high-dose alcohol, regardless of the period during pregnancy exposure occurred. These findings, summarised in Table 2, suggest the permanence of changes associated with prenatal alcohol exposureReference Day, Helsel, Sonon and Goldschmidt109 and provide provocative evidence that even relatively low-dose alcohol exposure can programme mental illness and behavioural difficulties, irrespective of an overt FAS or FASD diagnosis.
Table 2. A non-exhaustive list of the impact of prenatal alcohol exposure on offspring mental health outcomes in humans
Animal models of prenatal alcohol exposure and mental illness
For the reasons listed above, animal models are essential in understanding the impact of prenatal alcohol exposure, providing the capacity to control dosage closely, as well as minimising variables and limitations innate in human research, such as the impact of maternal nutrition, genetics and social demographics. Furthermore, this allows the identification of mechanistic changes that may underlie mental health-like changes in offspring. The earliest studies using animal models, pre-dating the classification of FAS and FASD, were performed in the early 1890 s, where studies demonstrated death following prenatal alcohol exposure in guinea pigsReference Stockard and Craig113 and central nervous system malformations in zebrafishReference Stockard114.
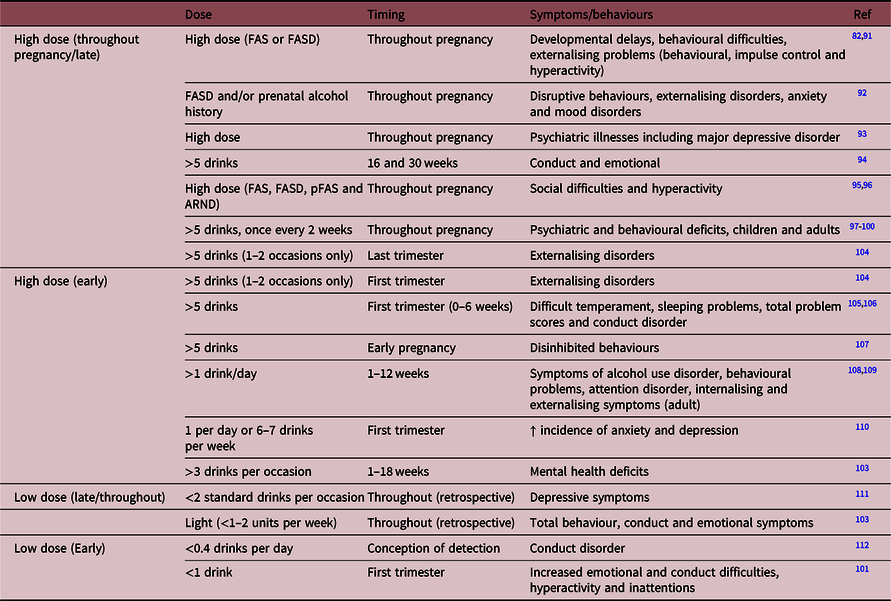
Since this time, numerous studies have supported knowledge that prenatal alcohol exposure results in mental-illness-like behaviour, including but not limited to anxiety- and depressive-like, altered social interactions and learning/memory deficits, utilising a battery of behavioural paradigms in rodents (summarised in Table 3). Models of high-dose alcohol administration have demonstrated extensive evidence of mental-illness-like phenotypes. One such study treated Sprague–Dawley rat dams with 4.3 g/kg ethanol (blood alcohol level [BAL]: 155 ± 8 to 195 ± 11 mg/dl, liquid diet), followed by pup treatment with 4 g/kg ethanol (BAL not recorded, liquid diet) during the weaning period, was commonly considered comparable to the third trimester of brain development in humans and demonstrated that offspring displayed increased depressive- and anxiety-like phenotypes in a range of testsReference Brocardo, Boehme and Patten115. Similarly, treating Wistar rat dams with 2.9 g/kg (a BAL of 420 mg/dl, intravenous injection administration) from embryonic day (E) 7 to birthReference Wieczorek, Fish, O’Leary-Moore, Parnell and Sulik116 and C57/Bl6J mice with 14.01 g/kg (BAL not reported, liquid diet) throughout gestation demonstrated similar behavioural changes in offspringReference Bond and Di Giusto130.
Table 3. A summary of the impact of prenatal alcohol exposure on offspring mental-illness-like outcomes in rodent models
NR = not reported.
These outcomes are also observed with lower dose alcohol exposure, whereby moderate alcohol throughout gestation (6.37% v/v from E7 to E20, BAL of 176.6 ± 15.5 to 192.2 ± 11 mg/dl [reported in WeinbergReference Weinberg117], liquid diet) in the Sprague–Dawley rat has also been associated with anxiety- and depressive-like signsReference Hellemans, Sliwowska, Verma and Weinberg118. A low dose of 6% v/v ethanol treatment (30 mg/dl, gavage administration) to Sprague–Dawley rat dams throughout pregnancy demonstrated that offspring display a sustained anxiety-like phenotype at both 6 and 12 months of ageReference Cullen, Burne, Lavidis and Moritz126. Animal models have also consolidated the evidence of altered social behaviour, with several studies demonstrating changes in both social interaction and play behaviour. Hellemans and colleagues showed that 6.37% v/v (BAL of 176.6 ± 15.5 to 192.2 ± 11 mg/dL [reported in WeinbergReference Weinberg117], liquid diet) alcohol exposure from E7 to E20 programmed hyperactivity in both male and female Sprague–Dawley rat offspring but reduced social interaction in malesReference Hellemans, Verma and Yoon121, whereas another study using this dose but treating from E6 to E20 resulted in increased social interaction in female rats compared to male Long–Evans ratsReference Meyer and Riley131. More recently, Holman et al. identified that male offspring of Sprague–Dawley dams exposed to this dose of alcohol throughout pregnancy (with a gradual increase of dosage in a liquid diet early in pregnancy of E1 diet containing 66% control and 34% ethanol diet; E2 diet containing 34% control and 66% ethanol diet; E3 to E22 diet containing 100% ethanol diet, final BAL of 95.85 ± 10.79 to 135.5 ± 50.8 mg/dL) displayed delays in adolescent male offspring social behaviour, deficits in recognition memory and were less effective at engaging and responding in playful interaction with control ratsReference Holman, Ellis, Morgan and Weinberg122,Reference Holman, Baglot, Morgan and Weinberg123 . Similarly, a study treating Long–Evans rat dams with 5% v/v ethanol (BAL of 84 ± 5.5 mg/dL, liquid diet) throughout gestation resulted in altered social interaction in female but not male offspringReference Hamilton, Akers and Rice127. These findings corroborate clinical studies which demonstrate that children with FASD struggle with a wide range of social interactions and are at a higher risk of social ostracism, impacting social development, cognitive success and later mental illnessReference Streissguth, Barr and Kogan91,Reference Olson, Feldman, Streissguth, Sampson and Bookstein132 .
It is essential to recognise that alcohol exposure even confined to the periconceptional period, prior to neurological development, as well as models of alcohol restricted only to the first trimester, has a significant impact on programming mental illness outcomes. Mooney et al. demonstrated that following an intravenous treatment with 20% v/v (a BAL of 233 ± 7.5 mg/dL) alcohol on E7 only, male Long–Evans rat offspring had greater social interaction than those not exposed to alcoholReference Mooney and Varlinskaya124, potentially indicating hyperactivity and/or altered awareness of social cues. Periconceptional ethanol exposure in a Sprague–Dawley rat model (12.5% ethanol, a BAL of 180 ± 40 mg/dL, liquid diet), highly relevant due to the high incidence of unplanned pregnancy, has also been shown to programme sex-specific spatial memory deficits and anxiety-like behavioursReference Lucia, Burgess and Cullen125.
Although animal studies allow the capacity to control for several variables, as well as interrogation of molecular pathways, it is essential to acknowledge their limitations. Many behavioural paradigms are utilised to draw the same overarching mental-illness-like phenotype. However, protocols and animal handling vary between laboratories, as well as the interpretation of such protocols being subjective between experiments and individuals. Although this may explain discrepancies and variability in data, it also emphasises the necessity for greater human population investigation to understand the role of prenatal alcohol exposure in the aetiology of mental illness.
Pathways underlying mental health outcomes
The preclinical models of in utero alcohol exposure resulting in offspring behavioural changes are often associated with altered neurological pathways, including the limbic and neuroendocrine systems. Abnormal hippocampal and the hypothalamus–pituitary–adrenal axis (HPA) function are known to contribute to several cognitive and mental illness outcomes, including depression and stress dysregulationReference Pittenger and Duman133. Autopsies of individuals diagnosed with FASD have demonstrated central nervous system disorganisation, including deformities in the hippocampusReference Clarren, Alvord, Sumi, Streissguth and Smith134,Reference Autti-Rämö, Autti and Korkman135 , as well as gross microcephaly and migration errors (as reviewed by ClarrenReference Clarren and West136). Ramsay et al. demonstrated that cortisol was decreased in FASD children at 2 months of ageReference Ramsay, Bendersky and Lewis137, a result similarly observed in a study by Ouellet-Morin et al. with offspring at 19 months of age displaying a hyperactive response following the stress of an unfamiliar environmentReference Ouellet-Morin, Dionne and Lupien138. Conversely, other studies have demonstrated that cortisol concentrations in infants exposed to alcohol during gestation are higher under basal conditions, with a further increase following stressReference Jacobson, Bihun and Chiodo139,Reference Haley, Handmaker and Lowe140 .
Animal models further corroborate the concept that prenatal alcohol exposure results in altered offspring HPA function and hyperactivity to stressors, with mental health-like phenotypes being displayed. Often this is, again, associated with neurological changes, with studies demonstrating neurological cell loss from a single exposure to alcohol, both early and later in pregnancyReference Bonthius and West141-Reference Cartwright and Smith144. Numerous studies utilising the Sprague–Dawley rat have demonstrated such changes, including comprehensive research carried out by Weinberg and colleagues. This group investigated HPA outcomes following prenatal exposure to moderate- to high-dose alcohol (6.37% v/v BAL ranging from E7 to E20, BAL of 176.6 ± 15.5 to 192.2 ± 1139.4 mg/dl) with offspring displaying elevated basal corticosterone and adrenocorticotropin hormone and HPA hyperactivity in response to stressors associated with several mental illness-like phenotypesReference Hellemans, Sliwowska, Verma and Weinberg118,Reference Weinberg, Sliwowska, Lan and Hellemans119,Reference Gabriel, Yu, Ellis and Weinberg145,Reference Glavas, Ellis, Yu and Weinberg146 . Other studies have also revealed that alcohol exposure results in changes to central regulatory gene expression profiles, in the limbic system including the hippocampusReference Burgess147-Reference Lam, Raineki, Ellis, Yu and Weinberg150 ventral tegmental area and the nucleus accumbensReference Dorey, Cullen and Lucia151. Furthermore, studies of chronic low-dose exposure to alcohol during pregnancy altered basolateral amygdala structureReference Cullen, Burne, Lavidis and Moritz126. Cudd and colleagues have further demonstrated changes in molecular pathways associated with the development of mental illness. Utilising the Suffolk sheep model, they treated dams with 1.75 g/kg ethanol intravenously, resulting in a BAL of 189 ± 19 mg/dL, and demonstrated changes within offspring hippocampus including density and volume of dentate gyrus granular cells and cerebellar Purkinje cell lossReference Ramadoss, Lunde, Piña, Chen and Cudd152,Reference Washburn, Ramadoss, Chen and Cudd153 . This group also observed significant changes to the HPA axis within the foetus, including increased circulating cortisol and adrenocorticotropic hormoneReference Cudd, Chen and West154. As the limbic system is a critical regulator of the HPA axis, with a direct connection to the hypothalamus and other HPA regulatory regions, these neurological changes may further contribute to altered neuroendocrine function.
However, the mechanism underlying how mental illness and altered behaviour occur following early exposure is one of discussion, considering that this exposure occurs prior to brain development. An emerging hypothesis and one with evidence from other studies of DOHaD is that early prenatal alcohol exposure impacts epigenetic processes, resulting in altered gene expression during development. Studies of in vitro embryo cultures treated with ethanol have demonstrated altered DNA methylation profiles and growth retardationReference Liu, Balaraman, Wang, Nephew and Zhou155,Reference Haycock and Ramsay156 . Kalisch-Smith et al. demonstrated that periconceptional alcohol exposure (12.5% v/v) altered blastocyst development including embryo-uterine communication and trophoblast differentiation, as well as resulting in reduced trophectoderm pluripotency and global hypermethylationReference Kalisch-Smith, Steane and Simmons157, indicative of inappropriate epigenetic reprogramming. Studies of alcohol exposure throughout pregnancy are associated with epigenetic modifications in several neurological pathways, such as within the HPA axis pathwaysReference Chen, Ozturk and Zhou158-Reference Ngai, Sulistyoningrum and O’Neill161. These findings suggest that other brain regions may also be epigenetically modified, as a mechanistic underpinning in the development of mental illness.
It is likely that alcohol consumption occurs alongside many other prenatal perturbations, including smoking, cardiometabolic conditions, obesity, socio-economic burden and maternal mental illness (Fig. 1) making it difficult to ascertain the impact of any of these factors in isolation. However, it may be hypothesised that the combination of perturbations may plausibly increase or extend the severity of offspring outcomes. Indeed, a wealth of studies have demonstrated that substance use disorder, as well as alcohol, in combination with smoking or illicit drug use, also results in adverse offspring mental health outcomesReference Richter and Richter110,Reference Hill, Lowers, Locke-Wellman and Shen162 . Furthermore, it has been stated that ‘fetal alcohol syndrome is not an equal opportunity defect’Reference Abel163, with studies showing that women in of higher socio-economic status are more likely to consume alcohol during pregnancy but having significantly lower rates of FASDReference Skagerstróm, Chang and Nilsen164. This is not to say that these children are not impacted by this consumption, but that there is a critical importance of other lifestyle factors in the severity of outcomes in mitigating overt outcomes. Therefore, it is essential to consider all elements of maternal health during pregnancy, rather than investigating risk factors independently. This must occur with an understanding that although in utero exposures may not reach the clinical threshold for concern, this does not imply that offspring are not impacted to an extent that warrants attention and support to increase their long-term quality of life.

Fig. 1. Numerous in utero perturbations, such as stress, maternal mental illness, body composition and nutritional status, may occur in combination with alcohol consumption and cause developmental programming in utero. This increases the risk of adverse offspring physiology and increases the risk of developing mental illness.
Limitations
Investigating outcomes associated with alcohol exposure during pregnancy is notoriously challenging, with limitations of collecting information about maternal alcohol consumption during pregnancy. The outcomes associated with specific doses, timing, frequency and type of exposure are not well characterisedReference O’Leary, Nassar and Zubrick165, making comparison across studies difficult. This often arises from clinical studies not providing a clear definition of what is considered as low-, moderate- or high-dose alcohol. Studies also utilise various methods of data collection, including self or medical report or retrospective reporting. This, in conjunction with the stigma associated with drinking during pregnancy, may suggest that dose and timing may be inaccurately recorded.
Similarly, mental health scoring in offspring, commonly young children, is often recorded by self-report, parent, guardian and teacher reports, meaning that personal discourse and bias may be present in the data collected. Studies also differ in the type and level of clinical thresholds for the diagnosis of symptoms, as well as the definition of internalising and externalising symptoms, and the fluidity in diagnostic tests of the years must also be considered. Furthermore, there is also a large amount of evidence associating other maternal factors, such as drug use, stress and maternal mental health with the development of offspring mental health, and is not always consistently considered across studies. These confounding factors impact the ability to determine the exact outcomes following prenatal exposure to low- and early-dose alcohol.
Utilising animal models provides the ability to regulate dose, timing and mode of alcohol exposure, as well as the ability to understand underlying physiological and behavioural mechanisms. However, there is often a lack of consistency in reporting of these controls, resulting in difficulty of cross-comparison with other animal models and with human studies. Furthermore, many studies provide the dose of alcohol consumption but do not report the blood alcohol concentrations. This is especially true for the ad libitum models where animals do not drink in a consistent pattern. The use of animal models to investigate behaviour associated with mental illness also has limitations. These studies have subjective interpretation and therefore may be inconsistent in identifying, measuring and reporting behaviours. Regardless, these have been successfully used the explore mental health outcomes and has provided an understanding of neurological circuitry in mental illness and several treatment avenues (reviewed in Milton and HolmesReference Milton and Holmes166)
Significance
Worldwide medical guidelines recommend that abstaining for alcohol consumption during pregnancy is the safest167,Reference Tan, Denny, Cheal, Sniezek and Kanny168 . However, a survey performed by the Foundation of Alcohol Research and Education in 2018 revealed that only 46% of Australian women were advised by a health professional during their pregnancy to modify their alcohol intake167,Reference Payne, Elliott and D’Antoine169 . Of these women, fewer than 15% adhere to the current guidelines presented by the National Health and Medical Research Council (NHMRC)Reference Payne, Elliott and D’Antoine169. For this reason, it is not surprising that such a high number of women admit to drinking at some point during pregnancy. Given that 50% of all pregnancies are unplannedReference Colvin, Payne, Parsons, Kurinczuk and Bower170,Reference Finer and Zolna171 , it is startling to consider that recent studies have revealed that 30%–56% of women worldwide consume alcohol early in trimester one (0–6 weeks), prior to pregnancy detection, and 27.9% of women continuing to drink at low levels throughout the remainder of their pregnancyReference Colvin, Payne, Parsons, Kurinczuk and Bower170,Reference Hutchinson, Youssef and McCormack172,Reference Muggli, O’Leary and Donath173 .
These statistics suggest that the public health message of alcohol abstinence in pregnancy is lacking. As this review has highlighted the adverse outcomes of alcohol consumption in low levels on the mental health and wellbeing of offspring, it is essential that mothers and medical practitioners are aware of any level of alcohol consumption during pregnancy, particularly when in combination with other developmental programming risk factors. FASD presents with a wide range of impairments, including neurodevelopmental, physiological and emotional, behavioural, cognitive, social and psychiatric. We emphasise the critical importance of considering the impact of prenatal alcohol exposure in the context of DOHaD, regardless of dosing and timing, and that extending research avenues and diagnostic criteria in place for FASD may allow for greater understanding and support for individuals experiencing mental illness.
Acknowledgements
None.
Conflicts of Interest
None.
Financial Support
This work was funded by the NHMRC of Australia (APP1078164). DJB is a recipient of an Australian Postgraduate Award scholarship. KMM is a Senior Research Fellow of the NHMRC.






