Introduction
Reward dysfunction plays a pivotal role in the pathogenesis and progression of depression (Hagele et al., Reference Hagele, Schlagenhauf, Rapp, Sterzer, Beck, Bermpohl and Heinz2015). Anhedonia, which refers to diminished pleasure and/or reactivity to normally rewarding activities, is a core feature of depression and a hallmark of major depressive disorder (MDD) (Greenberg et al., Reference Greenberg, Chase, Almeida, Stiffler, Zevallos, Aslam and Phillips2015; Keedwell, Andrew, Williams, Brammer, & Phillips, Reference Keedwell, Andrew, Williams, Brammer and Phillips2005; Treadway & Zald, Reference Treadway and Zald2011). Reward dysfunction in depression manifests as decreased motivation to seek for rewards and difficulties in experiencing positive affect, which may lead to physiological and behavioral problems such as reduced energy, loss of interest, loss of appetite, and reinforcement learning deficits (Bishop & Gagne, Reference Bishop and Gagne2018; Eshel & Roiser, Reference Eshel and Roiser2010; Forbes, Shaw, & Dahl, Reference Forbes, Shaw and Dahl2007; Kumar et al., Reference Kumar, Waiter, Ahearn, Milders, Reid and Steele2008; Robinson, Cools, Carlisi, Sahakian, & Drevets, Reference Robinson, Cools, Carlisi, Sahakian and Drevets2012; Rothkirch, Tonn, Kohler, & Sterzer, Reference Rothkirch, Tonn, Kohler and Sterzer2017). In the past two decades, the neural mechanisms of depression-induced reward dysfunction have been the focus of numerous studies (Sankar et al., Reference Sankar, Yttredahl, Fourcade, Mickey, Love, Langenecker and Hsu2019; Satterthwaite et al., Reference Satterthwaite, Kable, Vandekar, Katchmar, Bassett, Baldassano and Wolf2015). Generally speaking, neuroimaging studies have revealed hyporesponsivity of brain reward systems (especially frontostriatal networks) in both resting state and task-dependent conditions among MDD patients (Epstein et al., Reference Epstein, Pan, Kocsis, Yang, Butler, Chusid and Silbersweig2006; McCabe, Cowen, & Harmer, Reference McCabe, Cowen and Harmer2009; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Schaefer, Putnam, Benca, & Davidson, Reference Schaefer, Putnam, Benca and Davidson2006) as well as healthy people at a high risk of depression (Forbes & Dahl, Reference Forbes and Dahl2012; McCabe, Woffindale, Harmer, & Cowen, Reference McCabe, Woffindale, Harmer and Cowen2012; Monk et al., Reference Monk, Klein, Telzer, Schroth, Mannuzza, Moulton and Ernst2008). Parallel findings have also been discovered by event-related potential (ERP) research on depression (for a review, see Proudfit, Reference Proudfit2015). Most notably, an ERP component feedback-related negativity (FRN; see below for details) becomes smaller among depressed individuals compared to healthy controls, which is understandable regarding the association of this component with dopaminergic reward activity (Foti & Hajcak, Reference Foti and Hajcak2009; Nelson, Perlman, Klein, Kotov, & Hajcak, Reference Nelson, Perlman, Klein, Kotov and Hajcak2016; Proudfit, Bress, Foti, Kujawa, & Klein, Reference Proudfit, Bress, Foti, Kujawa and Klein2015). However, it should be pointed out that some other studies have observed intact reward processing in depression (or in some of its specific subtypes; see Foti, Carlson, Sauder, & Proudfit, Reference Foti, Carlson, Sauder and Proudfit2014; Moutoussis et al. Reference Moutoussis, Rutledge, Prabhu, Hrynkiewicz, Lam, Ousdal and Dolan2018; Rutledge et al. Reference Rutledge, Moutoussis, Smittenaar, Zeidman, Taylor, Hrynkiewicz and Dolan2017). To our knowledge, studies to date have predominantly utilized monetary reward paradigms (i.e. winning a nominal amount of money in laboratory tasks), which might preclude a broader understanding of anhedonia severity in depression across reward types (Ait Oumeziane, Jones, & Foti, Reference Ait Oumeziane, Jones and Foti2019; Sharma et al., Reference Sharma, Satterthwaite, Vandekar, Katchmar, Daldal, Ruparel and & Wolf2016).
The concept of ‘reward’ is not a homogeneous construct (Nestler & Carlezon, Reference Nestler and Carlezon2006; Sescousse, Caldu, Segura, & Dreher, Reference Sescousse, Caldu, Segura and Dreher2013), including not only hedonic reinforcement (monetary and material gains) but also social information that has rewarding properties (e.g. social approval, social belonging, and social agency; see Ruff & Fehr, Reference Ruff and Fehr2014). Although there are many other kinds of stimuli (e.g. food, drink, and shelter) that are regarded as rewards (Sescousse et al., Reference Sescousse, Caldu, Segura and Dreher2013), this study focused on monetary and social rewards since they are most relevant in the depression literature (Forbes & Dahl, Reference Forbes and Dahl2012; Henriques & Davidson, Reference Henriques and Davidson2000). Monetary and social reward processing are phenomenally and neurologically divided (Goerlich et al., Reference Goerlich, Votinov, Lammertz, Winkler, Spreckelmeyer and Gossen2017; Gu et al., Reference Gu, Huang, Camilleri, Xu, Wei, Eickhoff and Feng2019; Morelli, Sacchet, & Zaki, Reference Morelli, Sacchet and Zaki2015) and are related to distinct behavioral characteristics (e.g. response accuracy, reaction time, and subjective rating) in diverse age groups according to developmental studies (Hardin, Schroth, Pine, & Ernst, Reference Hardin, Schroth, Pine and Ernst2007; Jazbec et al., Reference Jazbec, Hardin, Schroth, McClure, Pine and Ernst2006). For instance, 12-month-old infants' tendency to explore ambiguous situations is regulated more strongly by social rewards (e.g. caregiver's smile) compared to non-social rewards (Sorce, Emde, Campos, & Klinnert, Reference Sorce, Emde, Campos and Klinnert1985), while the concept of money may not develop before the age of 5 (Kohls, Peltzer, Herpertz-Dahlmann, & Konrad, Reference Kohls, Peltzer, Herpertz-Dahlmann and Konrad2009). On the neural level, both monetary and social rewards activate the midbrain dopaminergic system (Izuma, Saito, & Sadato, Reference Izuma, Saito and Sadato2008) and the prefrontal cortex (Lin, Adolphs, & Rangel, Reference Lin, Adolphs and Rangel2012), but the brain regions underlying social processes (e.g. empathizing) might also be needed for the encoding of social rewards, such as the temporoparietal junction (Liu et al., Reference Liu, Gu, Liao, Lu, Fang, Xu and Cui2020; Strombach et al., Reference Strombach, Weber, Hangebrauk, Kenning, Karipidis, Tobler and Kalenscher2015). Separating social from nonsocial rewards has been proven to be a valuable approach for clinical research (e.g. Gonzalez-Gadea et al. Reference Gonzalez-Gadea, Sigman, Rattazzi, Lavin, Rivera-Rei, Marino and Ibanez2016; Hanewald et al. Reference Hanewald, Behrens, Gruppe, Sammer, Gallhofer, Krach and Iffland2017). For example, Lee et al. (Reference Lee, Jimenez, Reavis, Horan, Wynn and Green2019) recently found that compared to non-social rewards, the neural sensitivity to social rewards reduced to a greater extent among patients with schizophrenia. Research in this direction has clinical implications by highlighting potential targets for treatment (Forbes & Dahl, Reference Forbes and Dahl2012). Because reward dysfunction and its related symptoms (e.g. anhedonia) are shared by diverse diagnostic categories (Husain & Roiser, Reference Husain and Roiser2018; Whitton, Treadway, & Pizzagalli, Reference Whitton, Treadway and Pizzagalli2015), differentiating various kinds of rewards helps identify unique (v. common) alterations in depression and thus facilitates more targeted interventions (Ait Oumeziane et al., Reference Ait Oumeziane, Jones and Foti2019).
Social rewards contribute significantly to human functioning and behavior (Fehr & Camerer, Reference Fehr and Camerer2007; Gunaydin et al., Reference Gunaydin, Grosenick, Finkelstein, Kauvar, Fenno, Adhikari and Deisseroth2014) and are critical to understanding the development of depression (Morgan, Olino, McMakin, Ryan, & Forbes, Reference Morgan, Olino, McMakin, Ryan and Forbes2013; Olino, Silk, Osterritter, & Forbes, Reference Olino, Silk, Osterritter and Forbes2015). Severe expression of depression symptoms and syndromes have been linked to blunted response to social rewards and social feedback (i.e. social anhedonia; for a review, see Kupferberg, Bicks, & Hasler, Reference Kupferberg, Bicks and Hasler2016). Alterations in social reward processing reduce depressed individuals' motivation to engage in social interactions (Brinkmann, Franzen, Rossier, & Gendolla, Reference Brinkmann, Franzen, Rossier and Gendolla2014; He, Liu, Zhao, Elliott, & Zhang, Reference He, Liu, Zhao, Elliott and Zhang2019a), impair their social functioning (Hirschfeld et al., Reference Hirschfeld, Montgomery, Keller, Kasper, Schatzberg, Moller and Bourgeois2000; Kupferberg et al., Reference Kupferberg, Bicks and Hasler2016), and increase their vulnerability to social stress (Pegg et al., Reference Pegg, Ethridge, Shields, Slavich, Weinberg and Kujawa2019). From a developmental perspective, Davey, Yucel, and Allen (Reference Davey, Yucel and Allen2008) proposed that repeated failure or frustration of social rewards could lead to suppression of the reward system and eventually result in the emergence of depression in adolescence (see also Silk, Davis, McMakin, Dahl, & Forbes, Reference Silk, Davis, McMakin, Dahl and Forbes2012). Adolescents often deal with peer rejection, emotional failure, and social evaluative concerns with limited social skills (Steinberg & Morris, Reference Steinberg and Morris2001). Under the influence of these negative experiences, some adolescents might become pessimistic about the possibility of receiving social rewards, and thus withdraw from social activities to avoid interpersonal stress (Silk et al., Reference Silk, Davis, McMakin, Dahl and Forbes2012). Since adolescence is a critical period for the development of brain reward circuitry (Spear, Reference Spear2000), low anticipation of social rewards and its behavioral consequences (e.g. social avoidance) could significantly disturb this development process and further cause anhedonia in depression (Davey et al., Reference Davey, Yucel and Allen2008; Forbes & Dahl, Reference Forbes and Dahl2012). To our knowledge, there is a paucity of research investigating the neural representations of social reward processing in depression. Some recent studies suggest that depression severity is associated with reduced striatal activity (Enneking et al., Reference Enneking, Krussel, Zaremba, Dohm, Grotegerd, Forster and Dannlowski2019; Sharma et al., Reference Sharma, Satterthwaite, Vandekar, Katchmar, Daldal, Ruparel and & Wolf2016) and an attenuated FRN (Distefano et al., Reference Distefano, Jackson, Levinson, Infantolino, Jarcho and Nelson2018; Klawohn, Burani, Bruchnak, Santopetro, & Hajcak, Reference Klawohn, Burani, Bruchnak, Santopetro and Hajcak2020; Pegg et al., Reference Pegg, Ethridge, Shields, Slavich, Weinberg and Kujawa2019) in response to social rewards. Though these results show similar patterns with previous findings based on monetary reward (see above), more works should be done to examine whether the influence of depression on reward processing varies in its different stages.
The monetary incentive delay (MID) task, of which the reliability has been verified by numerous studies, is a classic paradigm for the research on reward processing in both healthy and clinical populations (for reviews, see Balodis & Potenza, Reference Balodis and Potenza2014; Gu et al. Reference Gu, Huang, Camilleri, Xu, Wei, Eickhoff and Feng2019; Wilson et al. Reference Wilson, Colizzi, Bossong, Allen, Kempton and Bhattacharyya2018). In each MID trial, participants first observe an incentive cue indicating the amount of potential reward (anticipation stage), then respond to a target stimulus as quickly as possible before the presentation of performance feedback (consumption stage; see Knutson et al., Reference Knutson, Westdorp, Kaiser and Hommer2000, Reference Knutson, Adams, Fong and Hommer2001 for details). One of its popular variants is the social incentive delay (SID) task, which provides socially relevant information (e.g. friendly faces) rather than monetary feedback (Rademacher et al., Reference Rademacher, Krach, Kohls, Irmak, Grunder and Spreckelmeyer2010; Spreckelmeyer et al., Reference Spreckelmeyer, Krach, Kohls, Rademacher, Irmak, Konrad and Grunder2009). Both the MID and SID tasks have contributed significantly to depression research (Arrondo et al., Reference Arrondo, Segarra, Metastasio, Ziauddeen, Spencer, Reinders and Murray2015; Knutson, Bhanji, Cooney, Atlas, & Gotlib, Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Smoski, Rittenberg, & Dichter, Reference Smoski, Rittenberg and Dichter2011; Stringaris et al., Reference Stringaris, Vidal-Ribas Belil, Artiges, Lemaitre, Gollier-Briant, Wolke, Vulser and & IMAGEN Consortium2015). In our opinion, comparing MID and SID data in the same sample would provide an opportunity to clarify: (1) whether depressed individuals' deficits in monetary and social reward processing show different patterns; (2) whether those differences appear in the anticipation or the consumption stage. The latter issue is worth noting considering that the neural correlates of reward anticipation and consumption differ to some extent (Liu, Hairston, Schrier, & Fan, Reference Liu, Hairston, Schrier and Fan2011; Oldham et al., Reference Oldham, Murawski, Fornito, Youssef, Yucel and Lorenzetti2018). Therefore, it is possible that domain-specific effects associated with depression selectively modulate the neural mechanisms of reward processing in specific stages (anticipation v. consumption).
The current study relied on the ERP technique since its high temporal resolution enables recognizing sub-stages within both anticipatory and consummatory reward processing (Ait Oumeziane et al., Reference Ait Oumeziane, Jones and Foti2019). This study focused on three ERP components to investigate anticipatory and consummatory reward processing, the significance of which have been confirmed by previous studies using both the MID and SID tasks (Ait Oumeziane, Schryer-Praga, & Foti, Reference Ait Oumeziane, Schryer-Praga and Foti2017, Flores et al., Reference Flores, Munte and Donamayor2015; Greimel et al., Reference Greimel, Bakos, Landes, Tollner, Bartling, Kohls and Schulte-Korne2018; Gu, Jiang, Kiser, Luo, & Kelly, Reference Gu, Jiang, Kiser, Luo and Kelly2017). Regarding the reward anticipation stage, we focused on the contingent negative variation (CNV), a sustained, negative-going component that reflects preparation processes for an event of interest (Rohrbaugh, Syndulko, & Lindsley, Reference Rohrbaugh, Syndulko and Lindsley1976; Walter, Cooper, Aldridge, McCallum, & Winter, Reference Walter, Cooper, Aldridge, McCallum and Winter1964). This fronto-central distributed component is elicited by a preceding signal and returns to baseline when the targeted event occurs (for a review, see Kononowicz & Penney, Reference Kononowicz and Penney2016). In the MID/SID context, researchers have used the CNV during cue presentation to investigate the anticipation of the target stimulus (Novak & Foti, Reference Novak and Foti2015 Novak, Novak, Lynam, & Foti, Reference Novak, Novak, Lynam and Foti2016). However, the sensitivity of the CNV to depression level is largely unclear. Regarding the consumption stage, we were most interested in two ERP components associated with outcome processing, that is, the FRN and the P3 (for a review, see San Martín, Reference San Martín2012). The FRN reaches its maximum approximately 200–300 ms after the onset of outcome feedback, being more negative-going for unfavorable (e.g. monetary losses) than favorable feedback (Gehring & Willoughby, Reference Gehring and Willoughby2002; Miltner, Braun, & Coles, Reference Miltner, Braun and Coles1997). As mentioned above, the FRN is widely considered as an ERP index of depression severity, such that it becomes less negative-going among depressed individuals compared to controls (Barch et al., Reference Barch, Whalen, Gilbert, Kelly, Kappenman, Hajcak and Luby2019; Brush, Ehmann, Hajcak, Selby, & Alderman, Reference Brush, Ehmann, Hajcak, Selby and Alderman2018; Foti et al., Reference Foti, Carlson, Sauder and Proudfit2014; Foti & Hajcak, Reference Foti and Hajcak2009). Finally, we also examined the P3 component, a positive-going waveform of which the emergence follows the FRN (Polezzi, Sartori, Rumiati, Vidotto, & Daum, Reference Polezzi, Sartori, Rumiati, Vidotto and Daum2010; San Martin, Appelbaum, Pearson, Huettel, & Woldorff, Reference San Martin, Appelbaum, Pearson, Huettel and Woldorff2013). This component has been associated with various cognitive functions across different research fields (Polich, Reference Polich2007; Polich & Criado, Reference Polich and Criado2006). Compared to the FRN, researchers suggest that the P3 component reflects a more deliberate stage of information integration (Gu et al., Reference Gu, Lei, Broster, Wu, Jiang and Luo2011; Wu & Zhou, Reference Wu and Zhou2009). A majority of studies reveal that the P3 amplitude is negatively correlated with depression level (Gangadhar, Ancy, Janakiramaiah, & Umapathy, Reference Gangadhar, Ancy, Janakiramaiah and Umapathy1993; Karaaslan, Gonul, Oguz, Erdinc, & Esel, Reference Karaaslan, Gonul, Oguz, Erdinc and Esel2003; Urretavizcaya et al., Reference Urretavizcaya, Moreno, Benlloch, Cardoner, Serrallonga, Menchon and Vallejo2003), though some others disagree (Feng et al., Reference Feng, Gu, Liang, Broster, Liu, Zhang and Luo2015; Zhang, He, Chen, & Wei, Reference Zhang, He, Chen and Wei2016; Zhang, Xie, He, Wei, & Gu, Reference Zhang, Xie, He, Wei and Gu2018).
In this research, we recruited participants with depressive symptoms (as well as normal controls) to examine their task performance and ERP patterns in both the MID and SID tasks. According to previous findings (see above), we expected to observe attenuated CNV, FRN, and P3 among individuals with depressive symptoms, indicating deficits in both reward anticipation and reward consumption. More importantly, we directly compared the effect of depression between the MID and SID tasks, so as to explore whether reward dysfunction associated with depressive symptoms is domain-general or domain-specific, and to determine whether the domain-specific effects (social v. nonsocial) occur in the anticipation or the consumption stage.
Methods
Participants
In a mental health screening of Shenzhen University, the Self-Rating Depression Scale (SDS: Zung, Reference Zung1965) and the Beck Depression Inventory Second Edition (BDI-II: Beck, Steer, and Brown, Reference Beck, Steer and Brown1996) were administered to approximately 2000 undergraduate students. The norms of the SDS suggest that a score >0.5 (range = 0.25–1.0) indicates mild depression, while those of BDI-II suggest that a score >13 (range = 0–63) indicates mild depression. Accordingly, individuals who scored higher than 0.5 on the SDS and higher than 13 on the BDI-II were determined as having a mild depressive state. The key exclusion criterion was any lifetime Axis I disorders other than depression according to Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP: First, Gibbon, & Spitzer, Reference First, Gibbon and Spitzer2002). Healthy controls were also recruited from the original sample, who had scores of SDS < 0.5, BDI-II < 13, and were screened with SCID-I/NP to exclude any lifetime Axis I disorders including depression. For all the participants, the exclusion criteria also included: (1) seizure disorder; (2) a history of head injury with possible neurological sequelae; (3) self-reported prior use of any psychoactive drugs especially medication for depression; (4) current alcohol or drug dependence.
Among the students who met the above criteria, 80 individuals (40 with mild depression and 40 controls) were invited to participate in the formal experiment. All participants were medical-free at the time of the experiment. As shown in Table 1, no significant difference was found between these two groups with respect to gender, age, and handedness. Written informed consent was obtained prior to the experiment. The study was approved by the Ethics Committee of Shenzhen University.
Table 1. Demographic characteristics of the participants (mean & standard deviation)

BDI-II, the Beck Depression Inventory Second Edition; STAI-T, the Trait form of Spielberger's State-Trait Anxiety Inventory; RSAS, the Revised Social Anhedonia Scale; SR, the subscale of Sensitivity to Reward of the Sensitivity to Punishment and Sensitivity to Reward Questionnaire. *p < 0.05, **p < 0.01, ***p < 0.001.
Self-reported measures
On the day of the experiment, each participant was required to complete four questionnaires: (1) the BDI-II measures depressive symptoms, with a high score indicating a high level of depressive tendency (range = 0–63); (2) the Trait form of Spielberger's State-Trait Anxiety Inventory (STAI-T: Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983) measures trait anxiety, with a high score indicating a high level of trait anxiety (range = 20–80); (3) the Revised Social Anhedonia Scale (RSAS: Eckblad, Chapman, Chapman, & Mishlove, Reference Eckblad, Chapman, Chapman and Mishlove1982) measures social anhedonia, with a higher score indicating less enjoyment from and need for social contact (range = 0–40); (4) the ‘Sensitivity to Reward’ subscale of the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ: Torrubia, Avila, Moltó, & Caseras, Reference Torrubia, Avila, Moltó and Caseras2001) measures reward sensitivity, with a higher score indicating a higher level of sensitivity to reward (range = 0–24). The results of these measures from the two groups (on the day of the experiment) are reported in Table 1.
Procedure and experimental design
The current study relied on the electroencephalography (EEG) technique since its high temporal resolution enables recognizing sub-stages within both anticipatory and consummatory reward processing (Ait Oumeziane et al., Reference Ait Oumeziane, Jones and Foti2019). Prior to EEG recording, the individual threshold of visual reaction time was assessed with a simple reaction time task (averaged across 40 trials) to set up the difficulty level of the formal task for each participant.
The formal task consisted of two MID and two SID blocks, the sequence of which was randomized across participants. In both the MID and SID blocks (Fig. 1), each trial began with a cue (i.e. anticipation stage) indicating a small (‘I’) or large (‘II’) amount of potential reward for 500 ms, followed by a delay for a random duration ranging from 2 to 4 s. After that, participants were required to press the space button on the keyboard as quickly as possible upon the presentation of a white square (target) to gain reward. If participants' response time was shorter than the duration of target presentation, the ongoing trial would be labeled as a ‘hit’ trial; otherwise, it would be labeled a ‘miss’ trial. The presentation time of the target was initially set according to the average performance in the simple reaction time task (see above) for each participant and then was slightly adjusted (±10 ms) in a trial-by-trial manner so as to keep the hit rate at approximately 50% (Ait Oumeziane et al., Reference Ait Oumeziane, Schryer-Praga and Foti2017; Landes et al., Reference Landes, Bakos, Kohls, Bartling, Schulte-Korne and Greimel2018). After responding to the target, each participant received monetary or social feedback (i.e. consumption stage) for 1 s (see below for details). Each MID or SID block consisted of 40 small-reward trials and 40 large-reward trials. Inter-trial interval was 1 s. The formal task (320 trials in total) lasts for approximately 35 min.
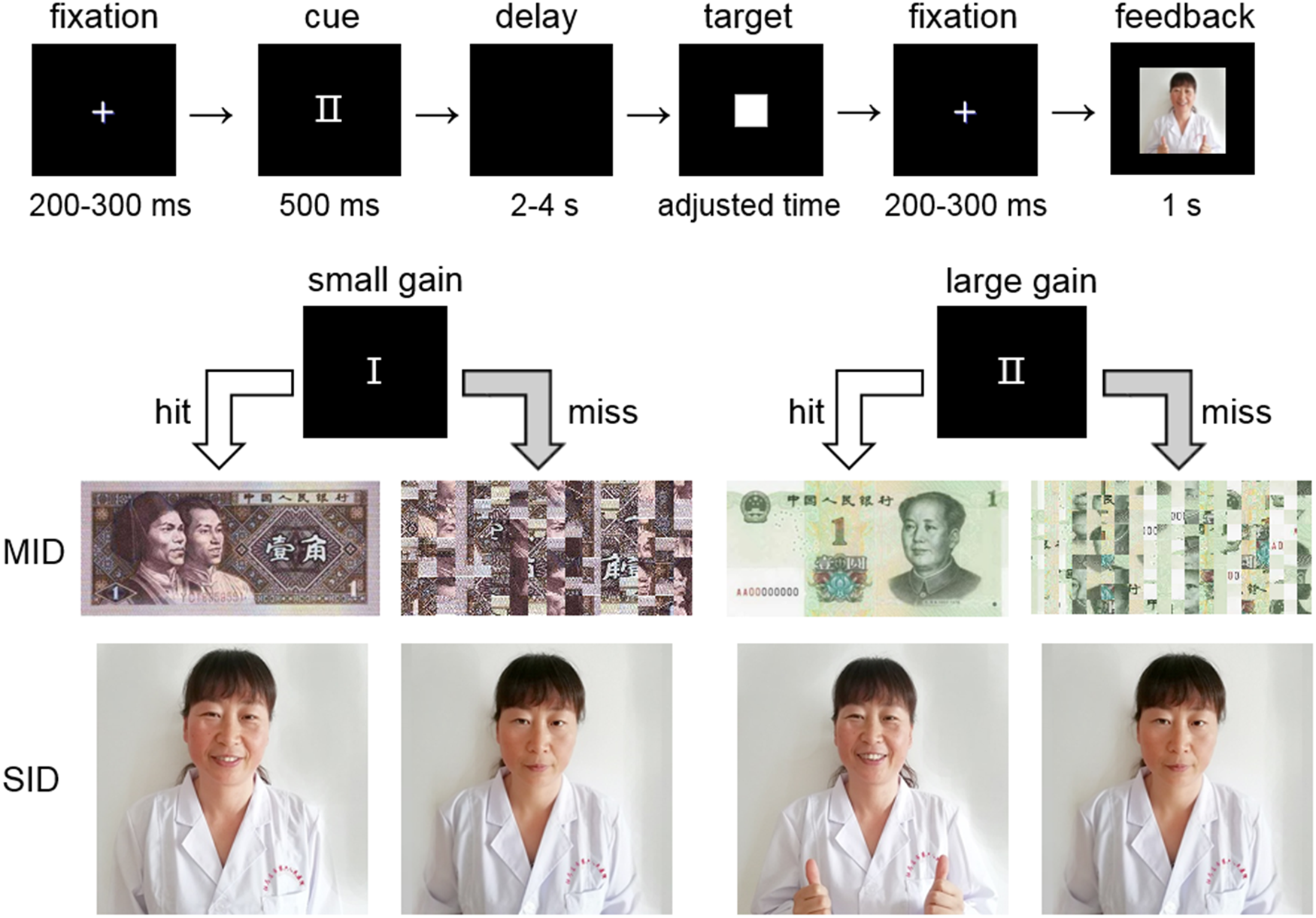
Fig. 1. Illustration of the experimental design. Upper panel: an exemplar trial of the social incentive delay (SID) task. Lower panel: the correspondence between cues and feedback (for hits or misses) in the monetary incentive delay (MID) and SID tasks.
Regarding the MID blocks, participants received a picture of ¥0.1 Chinese yuan (approximately US$0.015) after successfully hitting the target but a scrambled picture of ¥0.1 (indicating no monetary gain) after missing that target in each trial of the small-reward condition; meanwhile, the feedback was a picture (or scrambled picture) of ¥1 (approximately $0.15) in the large-reward condition. Regarding the SID blocks, participants received a picture showing a person with a smiling face after they successfully hit the target but a person with a neutral facial expression after they missed that target in each trial of the small-reward condition; meanwhile in the large-reward condition, feedback for hits was a picture showing a person who smiled and gave a thumbs up while feedback for misses was a person with neutral facial expression.
There were four sets of pictures for both the MID (paper or coin money in its front or backside) and SID (two actors and two actresses) blocks. Forty individuals with a low level of depressive tendency (Table 1), who were not involved in the formal experiment, were invited to assess the ‘liking’ and ‘wanting’ ratings of the rewarding pictures on a 1-to-7 point scale (Table 2). Their results showed that the ‘liking’ and ‘wanting’ scores of these pictures were only sensitive to the reward magnitude factor (large > small: F (1,39) = 34.7, p < 0.001 for ‘liking’ rating; F (1,39) = 60.5, p < 0.001 for ‘wanting’ rating), whereas neither reward category (monetary v. social) nor the interaction of two factors was significant (Fs ⩽ 2.1, p ⩾ 0.151).
Table 2. ‘Liking’ and ‘wanting’ ratings of rewarding pictures on a 1-to-7 point scale (mean & standard deviation)

A high score indicates a high level of liking or wanting.
Each participant was encouraged to maximize his/her performance prior to the task. For the MID blocks, his/her monetary gains in ‘hit’ trials were accumulated into the final payment; for the SID blocks, he/she was told that task performance was online assessed by strangers in the picture with an assumption that successfully hitting the target indicates a high level of cognitive ability. Unbeknownst to each participant, these strangers were four volunteers who provided consent for their portraits to be used for scientific purposes. After the whole task, each participant was asked if he/she believed that his/her cognitive ability was evaluated by other persons during the SID blocks. All the participants reported that they believed in the cover story.
EEG recording and analysis
Brain electrical activity was recorded by a 32-channel wireless amplifier with a sampling frequency of 250 Hz (NeuSen.W32, Neuracle, Changzhou, China). Data were on-line recorded referentially against left mastoid and off-line re-referenced to average activities over the scalp. EEG data were collected with electrode impedances kept below 10 kΩ. Ocular artifacts were removed from EEGs using a regression procedure implemented in NeuroScan software (Scan 4.3: NeuroScan, Inc., Herndon, VA).
This study focused on the anticipation and the consumption stage of reward processing, corresponding to the ERPs evoked by MID/SID cues and feedback. The recorded EEG data were filtered (half-amplitude cutoff: 0.1–30 Hz) and segmented beginning 200 ms prior to stimulus onset. The cue-evoked ERP epochs lasted from −200 ms to 2.5 s while the feedback-evoked epochs lasted from −200 ms to 800 ms. All epochs were baseline-corrected with respect to the mean voltage over the 200 ms preceding stimulus onset, followed by averaging for each experimental condition. Epochs containing artifacts with peak-to-peak deflection exceeding ± 100 μV were rejected.
The electrode sites and time window for each ERP component were selected before data analysis based on prior knowledge. For cue-evoked ERPs, this study focused on the CNV elicited by small (‘I’) and large-reward cues (‘II’) in the MID and SID blocks. The mean amplitude of the CNV was calculated using the arithmetic average of the electrode sites in the mid-frontocentral area (including Fz, FCz, FC1, and FC2) within a time window of 750–2500 ms post cue onset (Chronaki, Soltesz, Benikos, & Sonuga-Barke, Reference Chronaki, Soltesz, Benikos and Sonuga-Barke2017). For feedback-evoked ERPs, we focused on the FRN and P3 elicited by feedback for hits or misses in the MID and SID blocks. The mean amplitude of the FRN was calculated using the arithmetic average, also at electrode sites in the mid-frontocentral area (i.e. Fz, FCz, FC1, and FC2), within a time window of 200–300 ms post feedback onset (Gu et al., Reference Gu, Jiang, Kiser, Luo and Kelly2017; Zhu, Wang, Gao, & Jia, Reference Zhu, Wang, Gao and Jia2019). Finally, the mean amplitude of the P3 was calculated using the arithmetic average at electrode sites in the mid-centroparietal area (i.e. Pz, P3, P4, CP1, and CP2) within a time window of 200–400 ms post feedback onset (Greimel et al., Reference Greimel, Bakos, Landes, Tollner, Bartling, Kohls and Schulte-Korne2018).
Since an ERP experiment usually has many measurements (e.g. the amplitude and latency of multiple ERP components on multiple electrode sites), the heightened likelihood of false-positive findings is a potential issue for statistical analysis; for this concern, reducing the number of factors in a given ANOVA (i.e. dimensionality reduction) would be helpful (Luck & Gaspelin, Reference Luck and Gaspelin2017). For this purpose, this study used the difference wave approach to measure the FRN amplitude, that is, the FRN evoked by neutral feedback (miss trials) minus the FRN evoked by positive feedback (hit trials) (Hajcak, Moser, Holroyd, & Simons, Reference Hajcak, Moser, Holroyd and Simons2007; Holroyd & Krigolson, Reference Holroyd and Krigolson2007; Holroyd, Pakzad-Vaezi, & Krigolson, Reference Holroyd, Pakzad-Vaezi and Krigolson2008). In the same vein, this study measured the P3 amplitude as the difference wave (Kogler, Sailer, Derntl, & Pfabigan, Reference Kogler, Sailer, Derntl and Pfabigan2017; Lei et al., Reference Lei, Wang, Wang, Wang, Lou and Li2019), that is, the P3 evoked by positive feedback (hit trials) minus the P3 evoked by neutral feedback (miss trials). Using this approach to measure feedback-related ERPs may also help removing the potential influence of unfamiliarity (Becker, Smith, & Schenk, Reference Becker, Smith and Schenk2017; Conde, Goncalves, & Pinheiro, Reference Conde, Goncalves and Pinheiro2015; Cycowicz, Reference Cycowicz2019): in our opinion, it was possible that unfamiliarity has affected feedback processing in both hit trials and miss trials of the SID because participants were unfamiliar with strangers' faces (while they were supposed to be familiar with monetary pictures).
Statistics
Descriptive data were presented as mean ± standard deviation, unless otherwise mentioned. The significance level was set at 0.05. Repeated-measures ANOVA was performed on behavioral and ERP measurements, with reward category (monetary v. social) and reward magnitude (small v. large) as the two within-subject factors, and group (depressive v. control) as the between-subjects factor. Regarding the strong relationship between anxiety and depression (Stavrakaki & Vargo, Reference Stavrakaki and Vargo1986), and that individual anxiety level might also influence social and monetary reward processing (Bishop & Gagne, Reference Bishop and Gagne2018; Luo et al., Reference Luo, Wu, Broster, Feng, Zhang and & Luo2014), we considered the trait anxiety score (measured by STAI-T) as a covariate. Significant interactions were analysed using simple effects model.
Results
Behavioral data
Hit rate
The main effect of reward magnitude was significant (F (1,77) = 14.4, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.157): the hit rate in the large-reward condition (0.537 ± 0.115) was higher than that in the small-reward condition (0.458 ± 0.118). The interaction of reward magnitude × group (F (1,77) = 18.5, p < 0.001, $\eta _p^{2\;\;}$
= 0.157): the hit rate in the large-reward condition (0.537 ± 0.115) was higher than that in the small-reward condition (0.458 ± 0.118). The interaction of reward magnitude × group (F (1,77) = 18.5, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.194) was also significant: while large rewards were associated with a higher hit rate in the control group compared to the depressive group (F (1,77) = 4.0, p = 0.048, $\eta _p^{2\;\;}$
= 0.194) was also significant: while large rewards were associated with a higher hit rate in the control group compared to the depressive group (F (1,77) = 4.0, p = 0.048, $\eta _p^{2\;\;}$![]() = 0.050; control v. depressive = 0.562 ± 0.116 v. 0.512 ± 0.108), this group difference did not reach significance in the small-reward condition (F < 1; 0.451 ± 0.123 v. 0.466 ± 0.112).
= 0.050; control v. depressive = 0.562 ± 0.116 v. 0.512 ± 0.108), this group difference did not reach significance in the small-reward condition (F < 1; 0.451 ± 0.123 v. 0.466 ± 0.112).
Finally, the three-way interaction of reward category × reward magnitude × group was significant (F (1,77) = 5.3, p = 0.024, $\eta _p^{2\;\;}$![]() = 0.064; Fig. 2a). To break down this three-way interaction, we tested the two-way interaction of reward magnitude × group in the MID and SID blocks separately. In the MID blocks, only the main effect of reward magnitude was significant (F (1,77) = 7.7, p = 0.007, $\eta _p^{2\;\;}$
= 0.064; Fig. 2a). To break down this three-way interaction, we tested the two-way interaction of reward magnitude × group in the MID and SID blocks separately. In the MID blocks, only the main effect of reward magnitude was significant (F (1,77) = 7.7, p = 0.007, $\eta _p^{2\;\;}$![]() = 0.091; small v. large reward = 0.442 ± 0.118 v. 0.551 ± 0.111); meanwhile in the SID blocks, not only the main effect of reward magnitude (F (1,77) = 6.53, p = 0.013, $\eta _p^{2\;\;}$
= 0.091; small v. large reward = 0.442 ± 0.118 v. 0.551 ± 0.111); meanwhile in the SID blocks, not only the main effect of reward magnitude (F (1,77) = 6.53, p = 0.013, $\eta _p^{2\;\;}$![]() = 0.078) but also the interaction of reward magnitude × group (F (1,77) = 33.5, p < 0.001, $\eta _p^{2\;\;}$
= 0.078) but also the interaction of reward magnitude × group (F (1,77) = 33.5, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.303) were significant: while large social rewards were associated with a higher hit rate in the control group compared to the depressive group (F (1,77) = 7.9, p = 0.006, $\eta _p^{2\;\;}$
= 0.303) were significant: while large social rewards were associated with a higher hit rate in the control group compared to the depressive group (F (1,77) = 7.9, p = 0.006, $\eta _p^{2\;\;}$![]() = 0.093; control v. depressive = 0.564 ± 0.113 v. 0.483 ± 0.108), this group difference did not reach significance in the small-social-reward condition (F < 1; 0.464 ± 0.118 v. 0.486 ± 0.113).
= 0.093; control v. depressive = 0.564 ± 0.113 v. 0.483 ± 0.108), this group difference did not reach significance in the small-social-reward condition (F < 1; 0.464 ± 0.118 v. 0.486 ± 0.113).
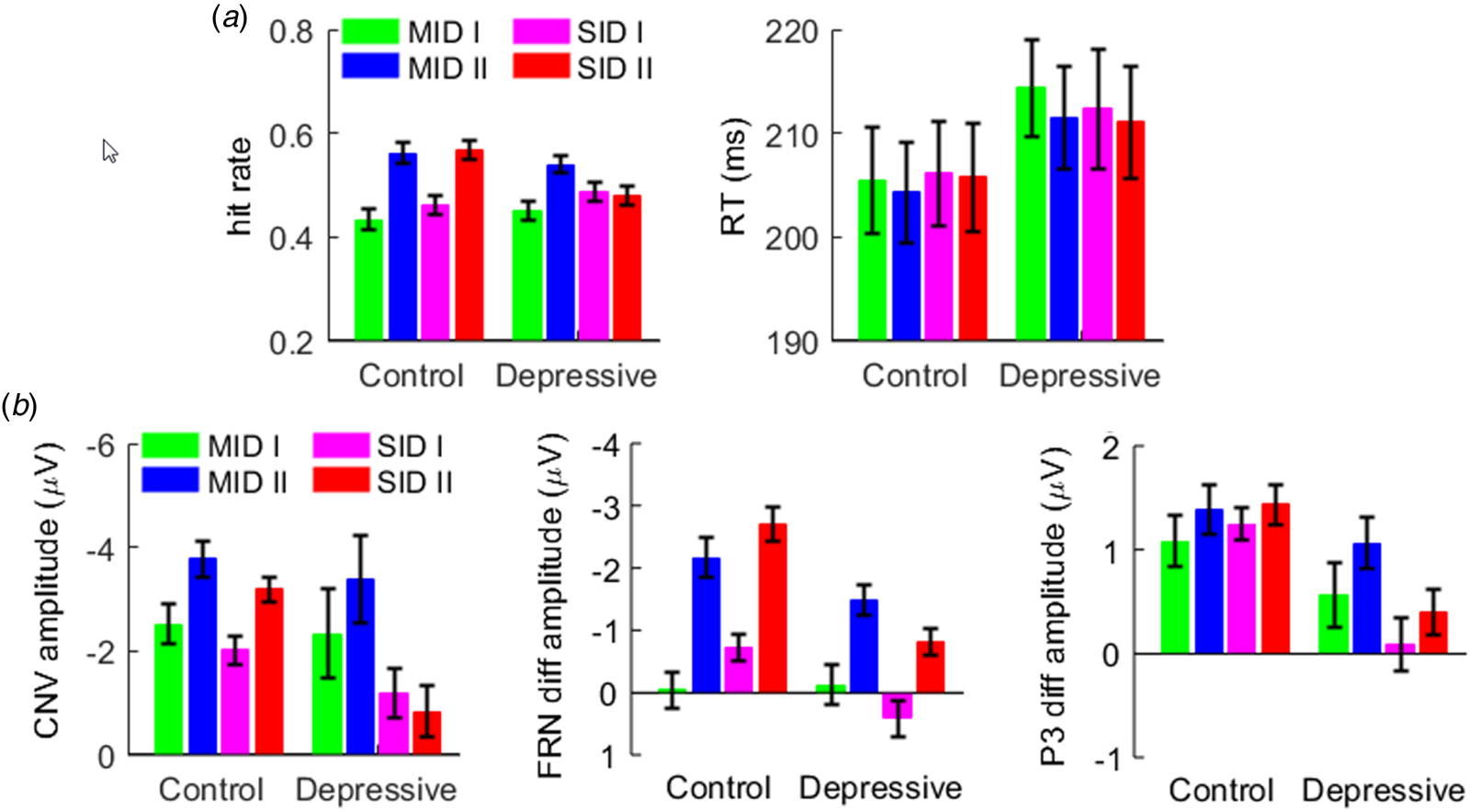
Fig. 2. The results of behavioral and event-related potential indexes. (a) The hit rate and reaction time in the depressive and control groups. (b) The amplitudes of the contingent negative variation (CNV), feedback-related negativity (FRN), and P3 in two groups. Error bars indicate one standard error.
Reaction time (RT)
The RT was averaged across hit trials in each condition (He, Zhang, Muhlert, & Elliott, Reference He, Zhang, Muhlert and Elliott2019b). We defined the outlier threshold as mean ± 1.96 standard deviation (95% confidence interval). According to this criterion, two participants per group were removed from the datasets as outliers. After these outliers were removed, the RT data were normally distributed (i.e. all the p values of the K-S tests were larger than 0.05). For the outlier-removed RT datasets (n = 38 per group), the main effect of reward magnitude was marginally significant (F (1,73) = 3.91, p = 0.052, $\eta _p^{2\;\;}$![]() = 0.050): the RT for large rewards (213.5 ± 22.7 ms) showed a tendency to be shorter than that for small rewards (214.8 ± 23.7 ms). No other main or interaction effects were significant (Fig. 2a).
= 0.050): the RT for large rewards (213.5 ± 22.7 ms) showed a tendency to be shorter than that for small rewards (214.8 ± 23.7 ms). No other main or interaction effects were significant (Fig. 2a).
ERPs
Cue-evoked CNV
The main effect of reward magnitude was significant (F (1,77) = 7.20, p = 0.009, $\eta _p^{2\;\;}$![]() = 0.086): the CNV following large-reward cues (−2.80 ± 3.58 μV) was larger (i.e. more negative-going) than that following small-reward cues (−2.02 ± 3.53 μV).
= 0.086): the CNV following large-reward cues (−2.80 ± 3.58 μV) was larger (i.e. more negative-going) than that following small-reward cues (−2.02 ± 3.53 μV).
The three-way interaction of reward category × reward magnitude × group was significant (F (1,77) = 4.14, p = 0.045, $\eta _p^{2\;\;}$![]() = 0.051; Figs 2b and 3). To break down this three-way interaction, we tested the two-way interaction of reward magnitude × group in the MID and SID blocks separately. In the MID blocks, only the main effect of reward magnitude was significant (F (1,77) = 5.76, p = 0.019, $\eta _p^{2\;\;}$
= 0.051; Figs 2b and 3). To break down this three-way interaction, we tested the two-way interaction of reward magnitude × group in the MID and SID blocks separately. In the MID blocks, only the main effect of reward magnitude was significant (F (1,77) = 5.76, p = 0.019, $\eta _p^{2\;\;}$![]() = 0.070; small v. large reward = −2.43 ± 4.24 v. −3.58 ± 4.02 μV). However in the SID blocks, not only the main effects of group (F (1,77) = 6.92, p = 0.010, $\eta _p^{2\;\;}$
= 0.070; small v. large reward = −2.43 ± 4.24 v. −3.58 ± 4.02 μV). However in the SID blocks, not only the main effects of group (F (1,77) = 6.92, p = 0.010, $\eta _p^{2\;\;}$![]() = 0.083; control v. depressive = −2.63 ± 1.71 v. −0.99 ± 3.08 μV), but also the interaction of reward magnitude × group were significant (F (1,77) = 5.83, p = 0.018, $\eta _p^{2\;\;}$
= 0.083; control v. depressive = −2.63 ± 1.71 v. −0.99 ± 3.08 μV), but also the interaction of reward magnitude × group were significant (F (1,77) = 5.83, p = 0.018, $\eta _p^{2\;\;}$![]() = 0.070): while large-reward cues evoked a larger CNV in the controls than in the depressive group (F (1,77) = 10.9, p = 0.001, $\eta _p^{2\;\;}$
= 0.070): while large-reward cues evoked a larger CNV in the controls than in the depressive group (F (1,77) = 10.9, p = 0.001, $\eta _p^{2\;\;}$![]() = 0.124; control v. depressive = −3.14 ± 1.49 v. −0.89 ± 3.19 μV), this group difference did not reach significance for small-reward cues (F (1,77) = 2.46, p = 0.121, $\eta _p^{2\;\;}$
= 0.124; control v. depressive = −3.14 ± 1.49 v. −0.89 ± 3.19 μV), this group difference did not reach significance for small-reward cues (F (1,77) = 2.46, p = 0.121, $\eta _p^{2\;\;}$![]() = 0.031; −2.13 ± 1.73 v. −1.08 ± 2.99 μV).
= 0.031; −2.13 ± 1.73 v. −1.08 ± 2.99 μV).
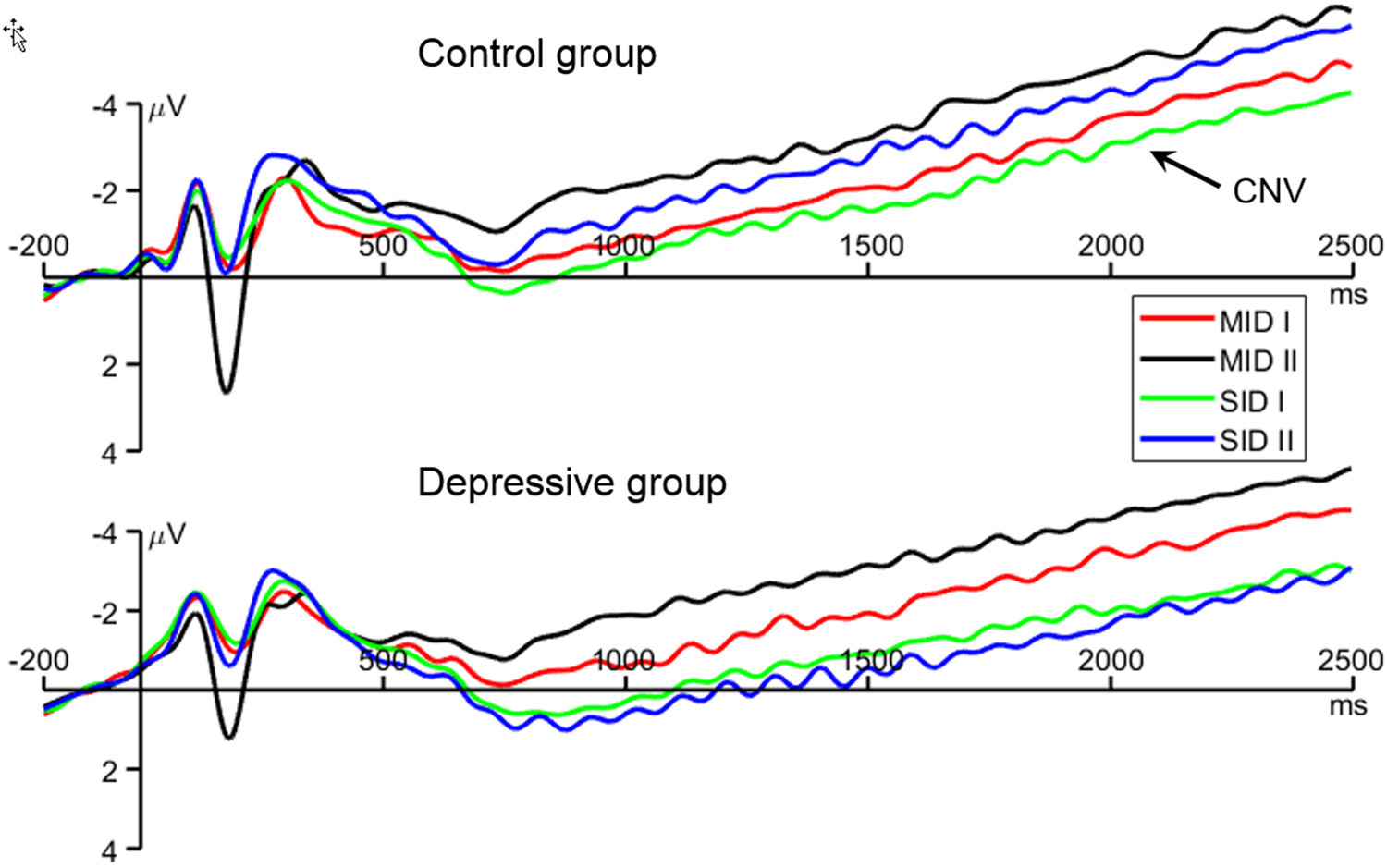
Fig. 3. Grand-average waveforms of the contingent negative variation (CNV). MID: monetary incentive delay task; SID: social incentive delay task; I: small cue; II: large cue. The waveforms were averaged across the electrode sites of Fz, FCz, FC1, and FC2.
Seeing that the results of hit rate and CNV amplitude showed a similar pattern (specifically, the reward category × reward magnitude × group interaction), we tested the two-tailed Pearson correlation between these two indexes. This analysis showed that the hit rate and CNV amplitude was positively correlated in each condition (r = 0.335 ~ 0.648, p = 0.035 ~ 0.0005; Table 3).
Table 3. Correlation between hit rate and CNV amplitude (n = 40).

*p < 0.05, **p < 0.01, ***p < 0.001.
Feedback-evoked FRN
The FRN was measured as the mean amplitude of the difference wave between miss and hit trials (see the Methods section). The main effect of group was significant (F (1,77) = 15.0, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.163): the FRN amplitude was larger (i.e. more negative-going) in the control group (−1.65 ± 2.04 μV) than in the depressive group (−0.26 ± 1.85 μV). The main effect of reward magnitude was significant (F (1,77) = 12.1, p = 0.001, $\eta _p^{2\;\;}$
= 0.163): the FRN amplitude was larger (i.e. more negative-going) in the control group (−1.65 ± 2.04 μV) than in the depressive group (−0.26 ± 1.85 μV). The main effect of reward magnitude was significant (F (1,77) = 12.1, p = 0.001, $\eta _p^{2\;\;}$![]() = 0.136): the FRN following large rewards (−1.79 ± 1.81 μV) was larger than that following small rewards (−0.12 ± 1.82 μV).
= 0.136): the FRN following large rewards (−1.79 ± 1.81 μV) was larger than that following small rewards (−0.12 ± 1.82 μV).
The interaction of reward category × group was significant (F (1,77) = 10.5, p = 0.002, $\eta _p^{2\;\;}$![]() = 0.120; Figs 2b and 4a). While social rewards evoked a larger FRN in the controls than in the depressive group (F (1,77) = 33.1, p < 0.001, $\eta _p^{2\;\;}$
= 0.120; Figs 2b and 4a). While social rewards evoked a larger FRN in the controls than in the depressive group (F (1,77) = 33.1, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.301; control v. depressive = −2.09 ± 1.83 v. 0.17 ± 1.70 μV), this group difference did not reach significance for monetary rewards (F (1,77) = 1.02, p = 0.316, $\eta _p^{2\;\;}$
= 0.301; control v. depressive = −2.09 ± 1.83 v. 0.17 ± 1.70 μV), this group difference did not reach significance for monetary rewards (F (1,77) = 1.02, p = 0.316, $\eta _p^{2\;\;}$![]() = 0.013; −1.20 ± 2.20 v. −0.70 ± 1.95 μV). Finally, the interaction of reward magnitude × group was significant (F (1,77) = 4.26, p = 0.042, $\eta _p^{2\;\;}$
= 0.013; −1.20 ± 2.20 v. −0.70 ± 1.95 μV). Finally, the interaction of reward magnitude × group was significant (F (1,77) = 4.26, p = 0.042, $\eta _p^{2\;\;}$![]() = 0.052; Figs 2b and 4a). While the FRN amplitude was larger (i.e. more negative-going) in the control group than in the depressive group, this group effect was more significant in the large-reward condition (F (1,77) = 20.6, p < 0.001, $\eta _p^{2\;\;}$
= 0.052; Figs 2b and 4a). While the FRN amplitude was larger (i.e. more negative-going) in the control group than in the depressive group, this group effect was more significant in the large-reward condition (F (1,77) = 20.6, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.211; control v. depressive = −2.64 ± 1.87 v. −0.94 ± 1.52 μV) than in the small-reward condition (F (1,77) = 7.16, p = 0.009, $\eta _p^{2\;\;}$
= 0.211; control v. depressive = −2.64 ± 1.87 v. −0.94 ± 1.52 μV) than in the small-reward condition (F (1,77) = 7.16, p = 0.009, $\eta _p^{2\;\;}$![]() = 0.085; −0.66 ± 1.67 v. 0.42 ± 1.93 μV).
= 0.085; −0.66 ± 1.67 v. 0.42 ± 1.93 μV).
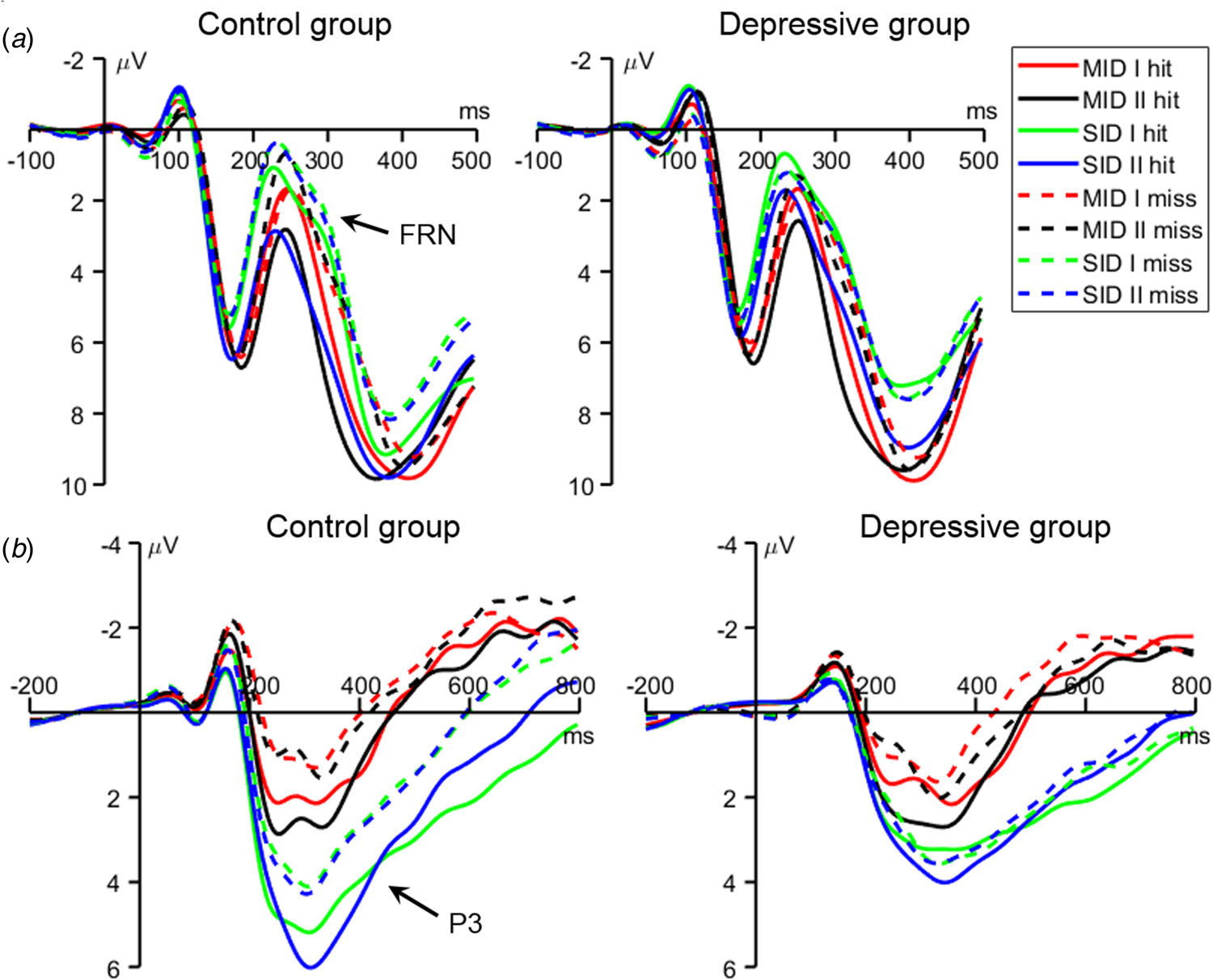
Fig. 4. Grand-average waveforms of the feedback-related negativity (FRN; upper panels) averaged across the electrode sites of Fz, FCz, FC1, and FC2, and the P3 (lower panels) averaged across the electrode sites of Pz, P3, P4, CP1, and CP2.
Feedback-evoked P3
The P3 was measured as the mean amplitude of the difference wave between hit and miss trials. The main effect of group was significant (F (1,77) = 7.78, p = 0.007, $\eta _p^{2\;\;}$![]() = 0.092): outcome feedback evoked a larger (i.e. more positive-going) P3 amplitude in the control group (1.42 ± 1.35 μV) than in the depressive group (0.40 ± 1.68 μV).
= 0.092): outcome feedback evoked a larger (i.e. more positive-going) P3 amplitude in the control group (1.42 ± 1.35 μV) than in the depressive group (0.40 ± 1.68 μV).
Furthermore, the interaction of reward category × group was significant (F (1,77) = 4.66, p = 0.034, $\eta _p^{2\;\;}$![]() = 0.057; Figs 2b and 4b). While social rewards evoked a larger P3 in the controls than in the depressive group (F (1,77) = 16.1, p < 0.001, $\eta _p^{2\;\;}$
= 0.057; Figs 2b and 4b). While social rewards evoked a larger P3 in the controls than in the depressive group (F (1,77) = 16.1, p < 0.001, $\eta _p^{2\;\;}$![]() = 0.173; control v. depressive = 1.50 ± 1.10 v. 0.08 ± 1.51 μV), this group effect did not reach significance for monetary rewards (F (1,77) = 1.82, p = 0.182, $\eta _p^{2\;\;}$
= 0.173; control v. depressive = 1.50 ± 1.10 v. 0.08 ± 1.51 μV), this group effect did not reach significance for monetary rewards (F (1,77) = 1.82, p = 0.182, $\eta _p^{2\;\;}$![]() = 0.023; 1.34 ± 1.56 v. 0.72 ± 1.79 μV).
= 0.023; 1.34 ± 1.56 v. 0.72 ± 1.79 μV).
Discussion
To clarify whether reward dysfunction associated with depressive symptoms is general across different reward types or domain-specific, we analysed the behavioral and ERP data from the MID and SID tasks. At the behavioral level, there was a significant three-way reward category × reward magnitude × group interaction, such that the depressive group showed a lower hit rate when anticipating large (but not small) social rewards compared to the controls, while there was no between-group difference when anticipating monetary rewards. The same pattern was replicated on the ERPs in the anticipation stage, such that the CNV elicited by large (but not small) social reward cues was smaller in the depressive group than the controls. Finally, the FRN and P3 elicited by the consumption of both monetary and social rewards were generally weaker in the depressive group than the controls, though the group difference only reached significance for social rewards. These findings indicate potentially domain-specific and domain-general deficits associated with depressive symptoms in reward processing.
While the CNV has been related to various cognitive processes (for reviews, see Macar & Vidal, Reference Macar and Vidal2004; van Rijn, Kononowicz, Meck, Ng, & Penney, Reference van Rijn, Kononowicz, Meck, Ng and Penney2011), most researchers agree that the main function of this component is to prepare for an upcoming internal or external stimulus (see the Introduction; see also Kononowicz & Penney, Reference Kononowicz and Penney2016). In the current study, participants with depressive symptoms showed a smaller CNV in the anticipation stage of large social rewards compared to the controls, indicating that they were not well-prepared to seek for that kind of reward. This interpretation has been strongly supported by our behavioral results, such that the CNV amplitude increased as a function of the hit rate (see also Boehm, van Maanen, Forstmann, & van Rijn, Reference Boehm, van Maanen, Forstmann and van Rijn2014; Elbert, Ulrich, Rockstroh, & Lutzenberger, Reference Elbert, Ulrich, Rockstroh and Lutzenberger1991), and that the hit rate for large social rewards was lower in the depressive group compared to the control group. Meanwhile, the small social reward condition was insensitive to depression level, possibly reflecting a ‘floor effect’ (see Yang, Zhou, Gu, & Wu, Reference Yang, Zhou, Gu and Wu2020 for similar results). In the reward anticipation stage, people should hold in mind their primary goals and the representation of future events (Davey et al., Reference Davey, Yucel and Allen2008). Our CNV results indicate that depressive symptoms are related to difficulties in conceiving of large social rewards, which could explain their reduced engagement in positive social activities (Setterfield, Walsh, Frey, & McCabe, Reference Setterfield, Walsh, Frey and McCabe2016).
Social context plays a pivotal role in the development and maintenance of depressive disorders including the MDD (Sankar et al., Reference Sankar, Yttredahl, Fourcade, Mickey, Love, Langenecker and Hsu2019; Sheeber, Hops, & Davis, Reference Sheeber, Hops and Davis2001). Depressive symptoms in youth are often caused by negative life events, including childhood maltreatment, peer rejection, and unfavorable social evaluation (Kendler, Hettema, Butera, Gardner, & Prescott, Reference Kendler, Hettema, Butera, Gardner and Prescott2003; Monroe, Rohde, Seeley, & Lewinsohn, Reference Monroe, Rohde, Seeley and Lewinsohn1999; Nolan, Flynn, & Garber, Reference Nolan, Flynn and Garber2003; Prinstein & Aikins, Reference Prinstein and Aikins2004; Silk et al., Reference Silk, Davis, McMakin, Dahl and Forbes2012). Over time, these social experiences could lead to interpersonal concerns and diminished pleasure from social interactions, which then precipitate and exacerbate depression (Allen et al., Reference Allen, Insabella, Porter, Smith, Land and Phillips2006; Allen & Badcock, Reference Allen and Badcock2003; Lee, Hankin, & Mermelstein, Reference Lee, Hankin and Mermelstein2010; Slavich, Tartter, Brennan, & Hammen, Reference Slavich, Tartter, Brennan and Hammen2014). As pointed out by Davey et al. (Reference Davey, Yucel and Allen2008), depressive episodes often result from the omission of anticipated social rewards; when a person predicts that his/her endeavors would be unsuccessful in achieving interpersonal goals, he/she may reduce the risk of wasting resources by withdrawing from the pursuit of social rewards. This idea explains why impairment in social functioning is a prominent feature of depression (Badcock & Allen, Reference Badcock and Allen2003), and why social withdrawal (a key symptoms of depression) is related to alterations in seeking social rewards and experiencing positive affect in anticipation of those rewards (Forbes, Reference Forbes2009; Hsu et al., Reference Hsu, Sanford, Meyers, Love, Hazlett, Walker and Zubieta2015; Silk et al., Reference Silk, Davis, McMakin, Dahl and Forbes2012). Thus, Pegg et al. (Reference Pegg, Ethridge, Shields, Slavich, Weinberg and Kujawa2019) suggested that compared to monetary reward, maladaptive responses to social reward is more relevant to understand anhedonia associated with depression (see also Copeland, Wolke, Angold, & Costello, Reference Copeland, Wolke, Angold and Costello2013; George, Blazer, Hughes, & Fowler, Reference George, Blazer, Hughes and Fowler1989; Slavich, Thornton, Torres, Monroe, & Gotlib, Reference Slavich, Thornton, Torres, Monroe and Gotlib2009). That said, experimental data supporting the relationship between depression and social reward anticipation are still limited (Ait Oumeziane et al., Reference Ait Oumeziane, Jones and Foti2019; Pegg et al., Reference Pegg, Ethridge, Shields, Slavich, Weinberg and Kujawa2019). Our CNV findings could be considered as neurophysiological evidence that the anticipation of social reward among depressed individuals is anomalous, confirming the importance of this stage of social reward processing to depressive symptoms; meanwhile, the anticipation of monetary reward might be relatively intact. In line with our observation, Pizzagalli et al. (Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009) reported that the effect of depression was much weaker in the anticipation stage than in the consumption stage during monetary reward processing. Further research could compare the CNV pattern between unipolar and bipolar depression since previous studies have reported dissociable differences in social valuation/processing between these two depressive disorders (Ehnvall et al., Reference Ehnvall, Mitchell, Hadzi-Pavlovic, Parker, Frankland, Loo and Perich2014; Ng & Johnson, Reference Ng and Johnson2013; Sharma et al., Reference Sharma, Satterthwaite, Vandekar, Katchmar, Daldal, Ruparel and & Wolf2016).
In addition, both the FRN and P3 were smaller in the depressive group compared to the controls, regardless of whether these components were elicited by monetary or social feedback (i.e. the main effect of the group). In our opinion, these results reveal a general deficit of reward sensitivity spanning monetary and social conditions, which is in line with previous literature that emphasizes the significance of blunted reward responsiveness to depression (Admon & Pizzagalli, Reference Admon and Pizzagalli2015; Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and & Stringaris2018; Pechtel, Dutra, Goetz, & Pizzagalli, Reference Pechtel, Dutra, Goetz and Pizzagalli2013; Rolls, Reference Rolls2016). However, it is important to note that the interaction effect further showed that the group difference was significant for social but not for monetary rewards. Similar findings have been reported very recently, that is, Ait Oumeziane et al. (Reference Ait Oumeziane, Jones and Foti2019) examined the ERPs in both the MID and SID tasks, but only found a unique association between depression score and the P3 elicited by social reward (see also Jin, Sabharwal, Infantolino, Jarcho, & Nelson, Reference Jin, Sabharwal, Infantolino, Jarcho and Nelson2019). One possible explanation is that when socially relevant and irrelevant information are represented in the same context, people (as social animals) tend to pay more attention on social information (Frazier et al., Reference Frazier, Strauss, Klingemier, Zetzer, Hardan, Eng and Youngstrom2017; Mesoudi, Whiten, & Dunbar, Reference Mesoudi, Whiten and Dunbar2006). However, this cognitive bias to social rewards might be suppressed among depressive individuals due to their pessimistic expectation (see above). Consequently, the difference between participants with depressive symptoms and normal controls became stronger in the social reward compared to the monetary reward condition. Further research is awaited to clarify whether there are domain-specific effects of depression in reward consumption.
Investigating the relationship between depression and reward-related aberrations with neuroscience methods has been fruitful (e.g. Bracht, Linden, & Keedwell, Reference Bracht, Linden and Keedwell2015; Heller et al. Reference Heller, Johnstone, Shackman, Light, Peterson, Kolden and Davidson2009; Naranjo, Tremblay, & Busto, Reference Naranjo, Tremblay and Busto2001) and provides important messages for depression prevention intervention (Burkhouse et al., Reference Burkhouse, Gorka, Klumpp, Kennedy, Karich, Francis and Phan2018). Altered processing of reward in depression may result in severe health consequences such as suicidal attempts (Marchand et al., Reference Marchand, Lee, Garn, Thatcher, Gale, Kreitschitz and Wood2011). Previous studies on depression have yielded mixed findings regarding neurobiologically and functionally distinct stages of reward processing that unfold in time dimension. Specifically, some studies suggest that depression is selectively associated with altered reward anticipation (McFarland & Klein, Reference McFarland and Klein2009; Sherdell, Waugh, & Gotlib, Reference Sherdell, Waugh and Gotlib2012; Ubl et al., Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015), but others have observed problems in both the anticipation and consumption stages among depressed individuals (Carl et al., Reference Carl, Walsh, Eisenlohr-Moul, Minkel, Crowther, Moore, Gibbs and Smoski2016; Forbes et al., Reference Forbes, Hariri, Martin, Silk, Moyles, Fisher and Dahl2009; Smoski et al., Reference Smoski, Felder, Bizzell, Green, Ernst, Lynch and Dichter2009; Watson & Naragon-Gainey, Reference Watson and Naragon-Gainey2010). By distinguishing between social and nonsocial rewards, we discovered both common and dissociable abnormalities of reward processing associated with depressive symptoms. Seeing that reward dysfunction is present in multiple psychiatric disorders, improving the knowledge about precise dysfunction in depression is critical to diagnostic and therapeutic efforts (Admon & Pizzagalli, Reference Admon and Pizzagalli2015). That is to say, recognizing depressed individuals' diverse responses to different kinds of rewards in different temporal stages could be indicative of efficient treatment targets to reduce the onset or recurrence of depression (Silk et al., Reference Silk, Davis, McMakin, Dahl and Forbes2012; Stoy et al., Reference Stoy, Schlagenhauf, Sterzer, Bermpohl, Hagele, Suchotzki, Schmack and Strohle2012). Indeed, a meta-analysis on depressive symptoms has confirmed that selective prevention programs targeted at specific risk are more effective than universal programs (Horowitz & Garber, Reference Horowitz and Garber2006).
Finally, some limitations of this study and potential future directions should be noted. First, this study did not include diagnosed patients. There has been some evidence supporting the continuity of depression in clinical and nonclinical populations (Flett, Vredenburg, & Krames, Reference Flett, Vredenburg and Krames1997); cognitive and behavioral correlates of depression differ quantitatively but may not qualitatively along a continuum of mild, moderate, and severe depression (Bradley, Mogg, & Millar, Reference Bradley, Mogg and Millar1996; Wierzbicki & Rexford, Reference Wierzbicki and Rexford1989). Still, follow-up studies are needed to verify the robustness of our findings in clinical samples. Second, due to resource limitation, we did not set up a third group with a medium level of depression. Therefore, it is undetermined whether the relationship between depression and monetary/social reward processing is non-linear (see also Gu, Ao, Mo, & Zhang, Reference Gu, Ao, Mo and Zhang2020). Third, the MID and SID feedback stimuli (pictures of money v. persons) were different in their level of visual complexity, which might be a potential confounding factor. However, recent studies have indicated that the ERPs from the MID and SID tasks are morphologically similar (Ait Oumeziane et al., Reference Ait Oumeziane, Schryer-Praga and Foti2017). Also, it is important that we observed dissociated effects of depression in the anticipation stage (using the same stimuli ‘I’ and ‘II’ as MID and SID cues), which were not contaminated by visual complexity. Moreover, since the ERP technique is unable to capture non-phase-locked neural activity (Kalcher & Pfurtscheller, Reference Kalcher and Pfurtscheller1995; Pfurtscheller & Lopes da Silva, Reference Pfurtscheller and Lopes da Silva1999), researchers could examine the effect of depression on non-phase-locked EEG synchronization and desynchronization associated with monetary and social reward processing (e.g. Gotlib, Reference Gotlib1998; Thibodeau, Jorgensen, & Kim, Reference Thibodeau, Jorgensen and Kim2006). Last but not least, our sample consisted of young people, which may limit the generalizability of the current findings. While it is still debated, recent studies suggest that anhedonia is more likely to be a state-like symptom in depression (e.g. Thomsen, Whybrow, & Kringelbach, Reference Thomsen, Whybrow and Kringelbach2015). Therefore, in our opinion, longitudinal investigations would be helpful to determine whether the reward processing patterns revealed in this study change over time (e.g. Burani et al., Reference Burani, Mulligan, Klawohn, Luking, Nelson and Hajcak2019). Seeing that social reward plays a more important role in adolescence than in adulthood, it would also be interesting to re-examine our ERP findings in older participants (see also Ethridge et al., Reference Ethridge, Kujawa, Dirks, Arfer, Kessel, Klein and Weinberg2017; Kujawa, Kessel, Carroll, Arfer, & Klein, Reference Kujawa, Kessel, Carroll, Arfer and Klein2017).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31970980, 31671173, 32071083, 31900757, 32020103008 and 31920103009), Guangdong Key Basic Research Grant (2018B030332001), Shenzhen Basic Research Project (JCYJ20180305124305294), Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions (2019SHIBS0003), the Major Program of the Chinese National Social Science Foundation (17ZDA324), and Youth Innovation Promotion Association CAS (2019088).
Author contributions
DZ conceived the experiment. JS, RB, FZ and YZ performed the experiment and collected data. DZ analyzed the data. DZ and RG wrote the manuscript. CF revised the manuscript.
Conflict of interest
The authors have declared that there is no conflict of interest in relation to the subject of this study.
Ethical standards
All procedures performed in this study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data and code availability
The data and code of this study would be available upon request and with approvals of College of Psychology and Sociology, Shenzhen University. More information on making this request can be obtained from the first author, Prof. Dandan Zhang (zhangdd05@gmail.com).









