Introduction
Host specificity is a measure of the number and phylogenetic diversity of hosts a parasite can infect at a particular stage in its life cycle (Poulin et al. Reference Poulin, Krasnov and Mouillot2011). Specialists have high host specificity and are only able to infect one or a few hosts, whereas generalists have low host specificity and infect a broad range of species (Poulin et al. Reference Poulin, Krasnov and Mouillot2011). The degree of host specificity a parasite displays has a direct bearing on its ability to utilize different hosts if a preferred host becomes difficult to obtain, as well as the parasite's success in utilizing any single host species (Poulin et al. Reference Poulin, Krasnov and Mouillot2011). Host specificity was historically difficult to evaluate in larval helminths due to the inability to accurately identify species using morphological characteristics (De León and Nadler, Reference De León and Nadler2010; Perkins et al. Reference Perkins, Martinsen and Falk2011). To address this problem, molecular data have been increasingly utilized to obtain accurate identifications of larval parasite species, including trematodes within fish hosts (De León and Nadler, Reference De León and Nadler2010; Locke et al. Reference Locke, McLaughlin and Marcogliese2010; De León et al. Reference De León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016; Soldánová et al. Reference Soldánová, Georgieva, Roháčová, Knudsen, Kuhn, Henriksen, Siwertsson, Shaw, Kuris, Amundsen, Scholz, Lafferty and Kostadinova2017).
The use of molecular data has led to a better understanding of trematode diversity and life cycles as previously considered conspecific or unknown larval forms are often identified as separate ‘cryptic species’ and/or linked to other larval and adult forms (Poulin, Reference Poulin2011). Improved species identification reveals potential inaccuracies in our understanding of how trematode species utilize hosts. While molecular data are an increasing component of trematode studies, many are at the taxonomic level and few molecular studies examine large numbers of trematodes from different infection sites within hosts (Locke et al. Reference Locke, Al-Nasiri, Caffara, Drago, Kalbe, Lapierre, McLaughlin, Nie, Overstreet, Souza, Takemoto and Marcogliese2015; Blasco-Costa et al. Reference Blasco-Costa, Cutmore, Miller and Nolan2016). Given the prevalence of cryptic species and that larval trematodes often infect multiple locations within their hosts, current estimates of infection site preferences/specificity are likely inaccurate (Hoffman, Reference Hoffman1999; Poulin, Reference Poulin2011). Infection site preference is a key aspect of trematode evolution, transmission, and virulence, and accurately assessing infection locale preferences is necessary for understanding host–parasite interactions and how these parasites impact ecosystems (Locke et al. Reference Locke, McLaughlin and Marcogliese2010; Reference Locke, Al-Nasiri, Caffara, Drago, Kalbe, Lapierre, McLaughlin, Nie, Overstreet, Souza, Takemoto and Marcogliese2015; Mladineo et al. Reference Mladineo, Bott, Nowak and Block2010; Herrmann and Poulin, Reference Herrmann and Poulin2011).
Posthodiplostomum minimum is a strigeid digenean trematode which utilizes a complex three-host life cycle (Hoffman, Reference Hoffman1999). Adult worms occur in fish-eating birds, cercariae develop in physid snails, and metacercariae, commonly known as white grub, encyst in fish tissues (Spall and Summerfelt, Reference Spall and Summerfelt1969; Hoffman, Reference Hoffman1999). White grub has been reported from several tissues of multiple species of freshwater fishes and is a concern for fisheries biologists due to infections causing detrimental effects on host health (Klak, Reference Klak1940; Hoffman, Reference Hoffman1958, Reference Hoffman1999; Meade and Bedinger, Reference Meade and Bedinger1967; Grizzle and Goldsby, Reference Grizzle and Goldsby1996; Pracheil and Muzzall, Reference Pracheil and Muzzall2010). Two subspecies have been generally recognized: P. minimum minimum which infects fishes of the family Cyprinidae and P. minimum centrarchi which infects fishes of the family Centrarchidae (Hoffman, Reference Hoffman1999). Historically, larval P. minimum were classified as generalists with low host specificity due to the lack of morphological variation among host species (Hoffman, Reference Hoffman1999; Locke et al. Reference Locke, McLaughlin and Marcogliese2010). Molecular-based studies performed to date have revealed that there are at least eight cryptic species of Posthodiplostomum metacercariae infecting freshwater fishes (Moszczynska et al. Reference Moszczynska, Locke, McLaughlin, Marcogliese and Crease2009; Locke et al. Reference Locke, McLaughlin and Marcogliese2010; Stoyanov et al. Reference Stoyanov, Georgieva, Pankov, Kudlai, Kostadinova and Georgiev2017), at least six of which can infect centrarchids (Locke et al. Reference Locke, McLaughlin and Marcogliese2010). One of these, Posthodiplostomum sp. 3, has been described as P. centrarchi (Stoyanov et al. Reference Stoyanov, Georgieva, Pankov, Kudlai, Kostadinova and Georgiev2017). While these species did not infect all hosts equally, sample sizes were too small (n ⩽ 6 for all parasite species except spp. 3 and 4) to infer host-specificity within Centrarchidae (Locke et al. Reference Locke, McLaughlin and Marcogliese2010). Based on infections in bluegill (Lepomis macrochirus) vs white crappie (Pomoxis annularis) and literature reviews, Lane et al. (Reference Lane, Spier, Wiederholt and Meagher2015) suggested that P. minimum is more of a host specialist than previously recognized and that P. m. centrarchi is a Lepomis specialist lacking host preferences outside of the genus. Given the molecular evidence for multiple Posthodiplostomum ‘minimum’ species within centrarchids, we refer to metacercariae in this study as Posthodiplostomum and identify specific species when appropriate.
Within fish hosts, Posthodiplostomum metacercariae can infect several organs and tissues. Lane et al. (Reference Lane, Spier, Wiederholt and Meagher2015) detected higher kidney infections in L. macrochirus than P. annularis and attributed it to potential ‘spill over’ from saturated livers. Given the evidence for multiple Posthodiplostomum species infecting freshwater fishes, it is possible that infection site preferences also differ. For example, Locke et al. (Reference Locke, McLaughlin and Marcogliese2010) recorded different Posthodiplostomum spp. from ‘viscera’ and ‘musculature’. Careful examinations of infection sites combined with molecular data are currently needed to determine if infection site preferences differ among Posthodiplostomum species.
Given how widespread and common Posthodiplostomum infections are within freshwater fishes, their potentially damaging effects on hosts, and the ecological and recreational importance of many of their hosts, it is important to understand the infection dynamics of this species complex parasitizing centrarchid fishes. The purpose of this study was to determine host and tissue specificity of P. minimum in 11 species of sympatric centrarchids using necropsy and molecular data. We compared three measures of parasitism (prevalence, intensity and abundance) to determine differences in host infection rates and the structuring of infections within the family Centrarchidae. Molecular data were used to determine the number of Posthodiplostomum species present and their distributions within host tissues and confirm host species identifications. In addition, the geographic genetic structure of the two most common Posthodiplostomum species was investigated.
Materials and methods
Host collections and measures
Eleven species of centrarchid fishes from three genera were collected from May to October in 2014 and 2015 throughout the Illinois portion of the Ohio River Drainage, including the main channel of the Ohio River and seven of its tributaries (Table 1, Fig. 1). Fish were collected during pre-existing long-term monitoring surveys. Ohio River and Wabash River sites were sampled using pulsed DC electrofishing and collections in the remaining six tributaries used AC electrofishing. Site locations within the Ohio River and the Wabash River were selected using the create random points tool in ArcGIS (ESRI, 2015). All other tributaries were sampled at fixed locations approximately one mile from the confluence with the Ohio River. In the field, fish were identified using morphological characteristics described by Pflieger (Reference Pflieger1997). All fish were measured and weighed, sacrificed in the field and frozen individually until dissection in the laboratory.

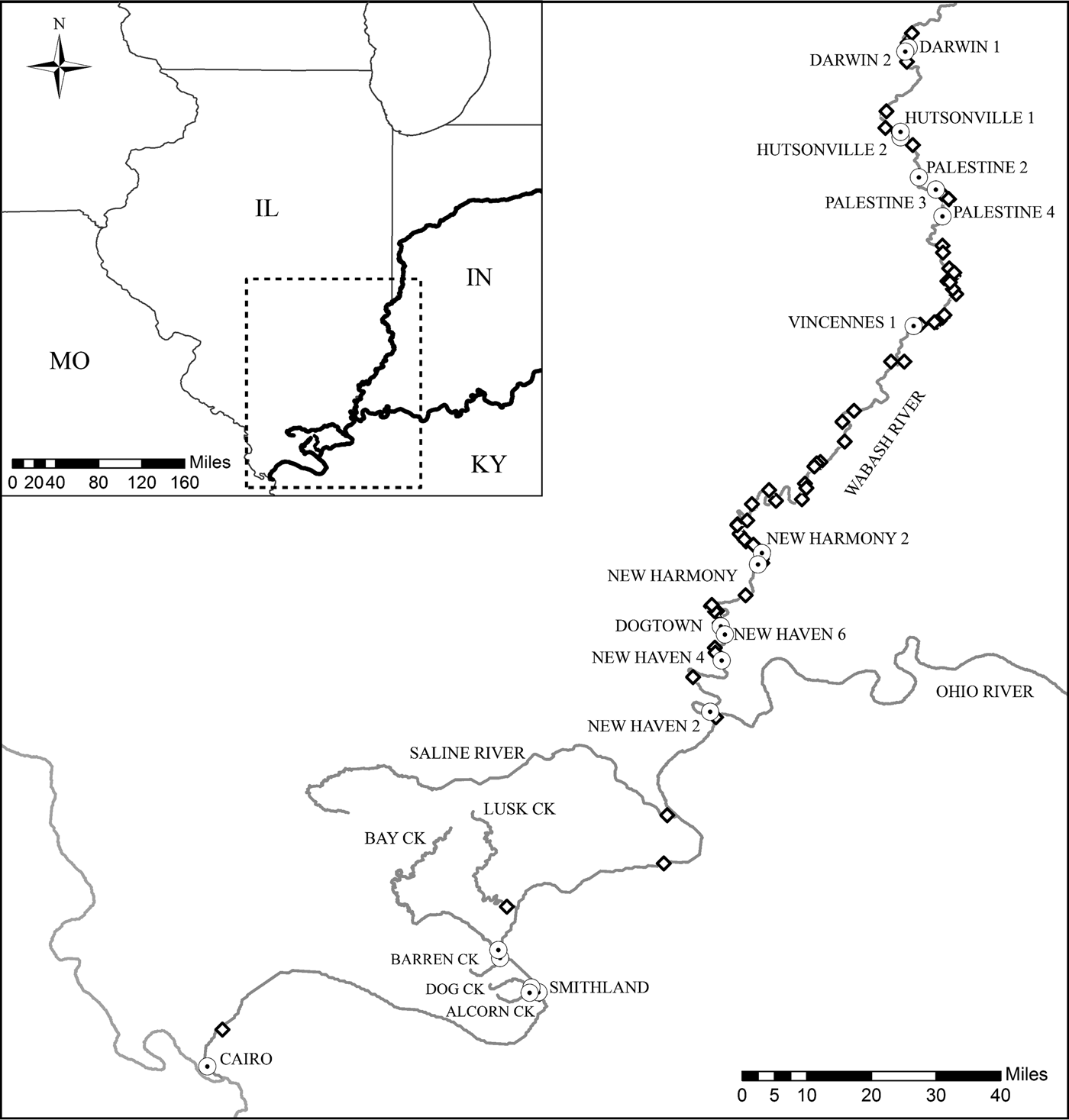
Fig. 1. Map of the Illinois portion of the Ohio River drainage with sampling localities. Labelled sampling localities denoted by a white circle with a black dot on the map are locations where Posthodiplostomum metacercariae were utilized for genetic analyses. Unlabelled sites denoted by a diamond are locations where hosts were collected and necropsied for infection analyses only.
Table 1. Common name, scientific name, sample size, range of host total length (mm), range of host age (year) and host sex (F – female, M – male, U – unknown) for the 11 centrarchid species collected from the Illinois portion of the Ohio River drainage

Host samples were allowed to thaw for at least 12 h at 4 °C before dissection. Sagittal otoliths were removed from the neurocranium, cleaned in a deionized water bath, and placed in a 1.5 mL microcentrifuge tube to dry. After a drying period of at least 3 weeks, otoliths were embedded in epoxy and multiple transverse sections were cut from each using a Buehler Isomet® low-speed saw (Buehler Limited, Lake Bluff, Illinois; Quist et al. Reference Quist, Pegg, DeVries, Zale, Parrish and Sutton2012). Cross-sections were placed in immersion oil on a contrasting background and viewed under a stereomicroscope (Leica Microsystems Inc., Buffalo Grove, Illinois). In a blind fashion, two independent readers estimated the age of each fish by counting the number of annuli on each section. Readers resolved discrepancies with a consensus age. Host sex was determined by examination of the gonads. Often sex in juvenile hosts could not be identified, so sex was classified as unknown. Five Lepomis hosts (one L. megalotis, one L. cyanellus, two L. gulosus and one L. macrochirus) were likely sexually mature (greater than age 1), but due to the status of the reproductive tissue after spawning, sex was unknown.
Posthodiplostomum collections and measures
Visceral organs (heart, kidney, liver and spleen), and tissue next to the neurocranium and the first two vertebrae (head) were removed from the body cavity. Metacercarial cysts from each of the five infection sites were counted by compressing tissues in saline between two slides and viewed with a dissection microscope. Visceral organs >0.10 g were sectioned into multiple sections to ensure visibility. A random subset of metacercriae from each of the infected anatomical locations was placed in a small dissection dish with saline where they were released from their cysts and then stored in 70% ETOH for DNA analyses. Dissection equipment was cleaned between organ necropsies to prevent contamination of genetic material.
Prevalence (percentage of hosts infected with Posthodiplostomum at any locality), mean abundance (average number of Posthodiplostomum metacercariae in all hosts, uninfected and infected) and mean intensity (average number of Posthodiplostomum metacercariae per infected host) were calculated for each fish host, following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Due to small sample sizes in some host species, infection analyses were performed at the taxonomic level of genus. Overall prevalence (Posthodiplostomum at any locality) and tissue-specific prevalence were analysed using logistic modelling and chi-square analyses with host genera and host age as factors. Pomoxis hosts were excluded from all remaining analyses due to the absence of infected hosts. Intensity data were log10 transformed to meet the assumption of normality and analysed using an ANOVA with Tukey's post hoc analyses with host genera and infection locality as factors. To visualize the structure of infections within each host genera, relative abundance within each infection locality was plotted for each host using the Bray–Curtis distance metric with non-metric multidimensional scaling (NMDS) in the metaMDS function. Differences in the structure of Posthodiplostomum infections between host genera were quantified using a permutational MANOVA in the Adonis function. All analyses were conducted in R with an α-value of 0.05 (R Development Core Team, 2016).
Molecular methods
Genetic confirmation of host fish species whose Posthodiplostomum were used for molecular analyses was conducted by extracting DNA from approximately 0.5 cm2 of host fin tissue and amplifying a portion of the cytochrome c oxidase subunit I (COI) barcode gene using the PCR primers VF2_t1 and FR1d_t1 for Lepomis or FishF2-t1 and FishR2_t1 for Micropterus and Pomoxis (modified from Walsh et al. Reference Walsh, Metzger and Higuchi1991; Ivanova et al. Reference Ivanova, Zemlak, Hanner and Hebert2007) (see Supplementary File 1 for details of molecular methods). To further investigate potential Micropterus punctulatus misidentification, one additional mitochondrial gene, NADH dehydrogenase subunit 2 (ND2), and two nuclear regions: internal transcribed spacer second intron (ITS2) and fourth intron of the calmodulin gene (CaM) were analysed following Breden et al. (Reference Breden, Ptacek, Rashed, Taphorn and Figueiredo1999), Presa et al. (Reference Presa, Pardo, Martínez and Bernatchez2002) and Chow and Takeyama, (Reference Chow and Takeyama2000), respectively. Purified PCR products were sequenced using PCR primers at the DNA Analysis Facility at Yale University. NCBI BLASTN searches were conducted to determine if morphological identifications of hosts matched resources available in GenBank.
Posthodiplostomum metacercariae were selected for DNA analyses based on host species and infection site. Our goal was to analyse several metacercariae from each host and infection site, when possible. We were not able to identify Posthodiplostomum species prior to DNA analyses. DNA was extracted from individual metacercariae and a portion of the COI gene was amplified using the forward primer Plat-diploCOX1F with either the reverse primer Plat-diploCOX1R (Moszczynska et al. Reference Moszczynska, Locke, McLaughlin, Marcogliese and Crease2009) or the reverse primer RevComp-JB3: 5′-ATAAACCTCAGGATGCCCAAAAAA-3′ (Keeney unpublished, the reverse complement of primer JB3, Bowles et al. Reference Bowles, Blair and McManus1995). In addition, the ribosomal internal transcribed spacer region 1 (ITS1) was amplified from a subset of white grub representing all major COI clades using the primers BD1 and 4S (Bowles and McManus, Reference Bowles and McManus1993).
Clustal W (Thompson et al. Reference Thompson, Higgins and Gibson1994) as implemented in MEGA7 (Kumar et al. Reference Kumar, Stecher and Tamura2016) was used to align Posthodiplostomum DNA sequences for both COI and ITS1. NCBI BLASTN searches were conducted to determine if confirmation of hosts and identification of Posthodiplostomum species were possible based on resources available in GenBank. Bayesian phylogenetic analyses were conducted for COI and ITS1 separately using MrBayes 3.2 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Maximum-likelihood analyses were conducted for COI and ITS1 separately using MEGA7 (Kumar et al. Reference Kumar, Stecher and Tamura2016). For each analysis, 1000 bootstrap replicates were conducted and phylogenetic trees were visualized using FigTree v1.3.1 (Rambaut, Reference Rambaut2009). Sequences from previously identified Posthodiplostomum species that most closely matched those in the present study were included in phylogenetic analyses for species identification (see Supplementary File 1 for sequence and outgroup information). Uncorrected p distances were calculated within and among major Posthodiplostomum clades using MEGA7 with all insertion/deletions treated as single nucleotide differences for ITS1.
Genetic population structure was examined with COI haplotypes for Posthodiplostomum spp. 3 and 8 using Bayesian clustering and Analysis of Molecular Variance (AMOVA) methods. Spatial Bayesian clustering of individual haplotypes was performed using BAPS v6.0 (Corander et al. Reference Corander, Waldmann and Sillanpää2003; Cheng et al. Reference Cheng, Connor, Sirén, Aanensen and Corander2013) with the estimated number of populations (k) allowed to vary from 1 to 20. AMOVA analyses at the sample site level were performed using Arlequin v3.5.2.2 (Excoffier and Lischer, Reference Excoffier and Lischer2010) and incorporated the most appropriate model of sequence evolution available (TN93 + G, with gamma shape parameter = 0.22). Because clonal replicates from the same first intermediate host can accumulate within second intermediate host fish, additional AMOVA analyses were performed with identical haplotypes from the same host fish removed. While identical COI haplotypes do not necessarily indicate identical genetic clones, comparison of results with and without within-host identical haplotypes provides insight as to whether or not any genetic differentiation is influenced by identical haplotypes within hosts.
Results
Host demographics
We collected and necropsied a total of 359 centrarchids from three genera and 11 species. This included 186 Lepomis hosts, 152 Micropterus hosts and 21 Pomoxis hosts. Sample sizes and demographic characteristics of the host species are in Table 1. Age structures were similar among species, with 91% of the hosts being younger than age 3. Largemouth bass and spotted bass were the only two species with age estimates equal to or greater than age 4, but these cohorts only accounted for 2% of the host sample. Because 34% of the hosts were young of year, sex was classified as unknown for 128 of 359 hosts.
Posthodiplostomum infections
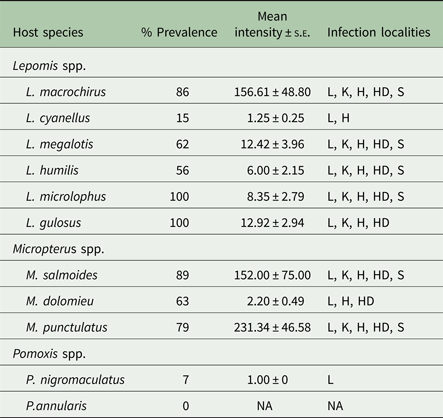
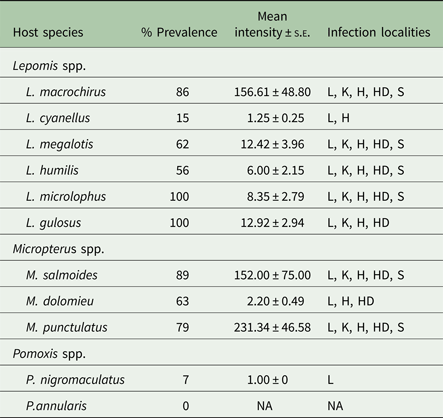
A total of approximately 32 000 Posthodiplostomum metacercariae were recovered. Prevalence, mean intensity ± s.e., and infection localities for each host species are found in Table 2. Prevalence was greater than 55% for all host species, except black crappie, green sunfish and white crappie. Total mean intensity exceeded 150 in bluegill, largemouth bass and spotted bass, but was <15 for all remaining host species. Metacercariae were recovered from all five tissues in bluegill, largemouth bass, longear sunfish, orangespotted sunfish, redear sunfish and spotted bass. Black crappie, green sunfish and smallmouth bass were the only species that were not infected in all localities. The liver was the only infection site that was infected in all species. Metacercariae were not found in the mesentery surrounding the gonads or the gastrointestinal tract in any of the examined hosts.
Table 2. Prevalence, mean intensity ± standard error and infection localities of Posthodiplostomum from 11 centrarchid species from the Illinois portion of the Ohio River Drainage.
L, liver; K, kidney; H, heart; HD, head; S, spleen.
Overall prevalence varied with host genera (χ 2 = 48.80; df = 2, 358; P < 0.001) and host age (χ 2 = 105.63, df = 5, 358 P < 0.001). There was no significant genus × age interaction effect in the model. Overall prevalence was higher in Micropterus and Lepomis hosts compared with Pomoxis hosts. Only 5% (1/21) of all Pomoxis hosts were infected with Posthodiplostomum, whereas the other two genera displayed a prevalence >65%. Host age had a significant effect on Posthodiplostomum prevalence, with younger fishes less likely to be infected. Of all uninfected hosts, 86% were young of year or yearlings. Conversely, <5% of fish from all age classes greater than age 2 were uninfected. Similar to the first logistic model, tissue prevalence varied significantly by host genera (χ 2 = 58.04; df = 1, 1689; P < 0.001) and infection locality (χ 2 = 149.89; df = 4, 1689; P < 0.001). There was a significant genus × locality interaction effect in the model (χ 2 = 68.50; df = 4, 1689; P < 0.001). The liver displayed the highest prevalence of all infection localities, followed by the heart, then the kidney, then the head, and finally the spleen. Micropterus hosts displayed higher prevalences in all sites except the heart when compared with Lepomis hosts.
Host genus and host tissue also had significant effects on transformed intensity data (Genera: F 1,674 = 62.90, P < 0.001; Locality: F 4,674 = 9.34, P < 0.001). There was a significant genus × locality interaction effect in the model (F 4,674 = 11.52, P < 0.001). Overall, Micropterus hosts were infected with higher total intensities than Lepomis hosts. Micropterus hosts displayed significantly higher mean intensities in the head (Micropterus – 112.38 ± 21.84, Lepomis – 6.97 ± 1.84), liver (Micropterus – 99.42 ± 18.44, Lepomis – 27.88 ± 8.12) and spleen (Micropterus – 21.58 ± 4.08, Lepomis – 2.61 ± 0.66) than Lepomis hosts. Conversely, mean intensities in the heart (Micropterus – 9.08 ± 2.33, Lepomis – 14.90 ± 3.63) and kidney (Micropterus – 30.84 ± 6.24, Lepomis – 38.75 ± 13.47) were higher in Lepomis hosts than Micropterus hosts, but differences were not significant in post hoc analyses.
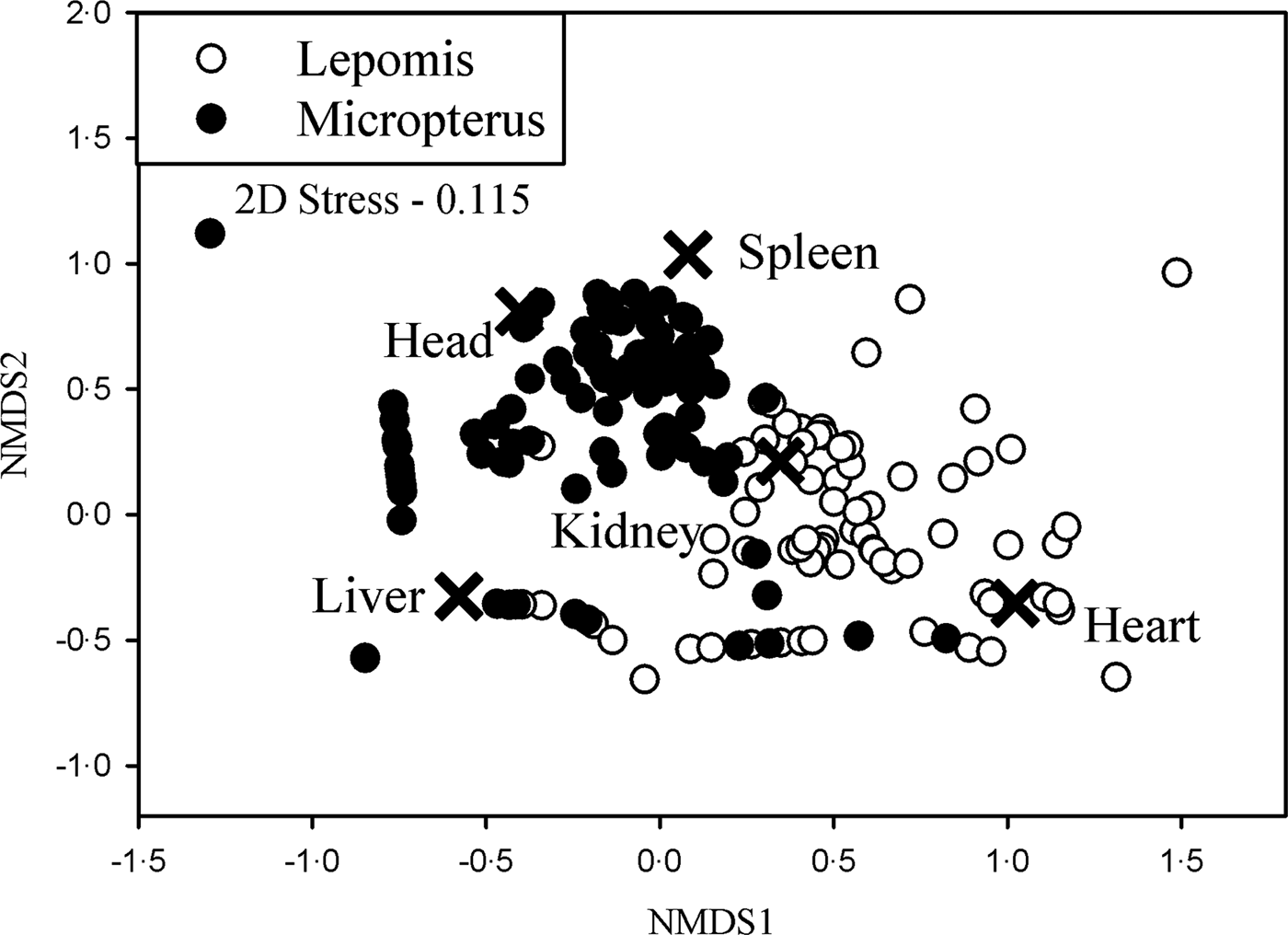
The structure of Posthodiplostomum infections varied with host genus. Host genus explained 8.0% of the variation in the dataset (F 1,242 = 20.92, P < 0.001). In the NMDS plot, Micropterus and Lepomis clusters separated spatially indicating different Posthodiplostomum infection structures within each host genera (Fig. 2). The majority of Micropterus infections were characterized by infections in the spleen and head, while the majority of Lepomis infections were characterized by infections in the kidney and heart. Few infections were uniquely identified by infections in the liver because this was the most commonly infected locality in both genera.

Fig. 2. NMDS plot of relative Posthodiplostomum abundances plotted by the five infection localities and separated by host genera. Organ scores (indicated by an ×) represent the influence of infection locality on the ordination.
Host species genetic confirmation
COI sequences of host fish supported morphological identifications for all Lepomis species, Pomoxis nigromaculatus, Micropterus salmoides and Micropterus dolomieu (99–100% similarity). Three out of four M. punctulatus COI sequences showed the highest similarity to M. salmoides (100% similarity; next closest species 97% similarity; M. cf. punctulatus 95% similarity), and one individual showed potential heteroplasmy for M. salmoides and M. punctulatus/dolomieu COI haplotypes by having double peaks for the appropriate nucleotides at all sites distinguishing these species. Identical M. punctulatus results were obtained with ND2. Three out of four M. punctulatus matched M. salmoides with 100% similarity and several Micropterus species with 99% similarity for ITS2 and the fourth M. punctulatus was heterozygous at four of the 11 nucleotides that differed between our M. salmoides and M. dolomieu. Per cent similarities cannot be accurately assessed for this individual without haplotype information for the mutations. The additional nuclear genes lacked divergence among species. Taken together, these results support the identification of all host species, with the possible exception of M. punctulatus. Given the presence of tooth patches on their tongues and mitochondrial association with M. salmoides, the M. punctulatus in our study are potentially hybrids (Godbout et al. Reference Godbout, Aday, Rice, Bangs and Quattro2009).
Posthodiplostomum species identification and genetic structure
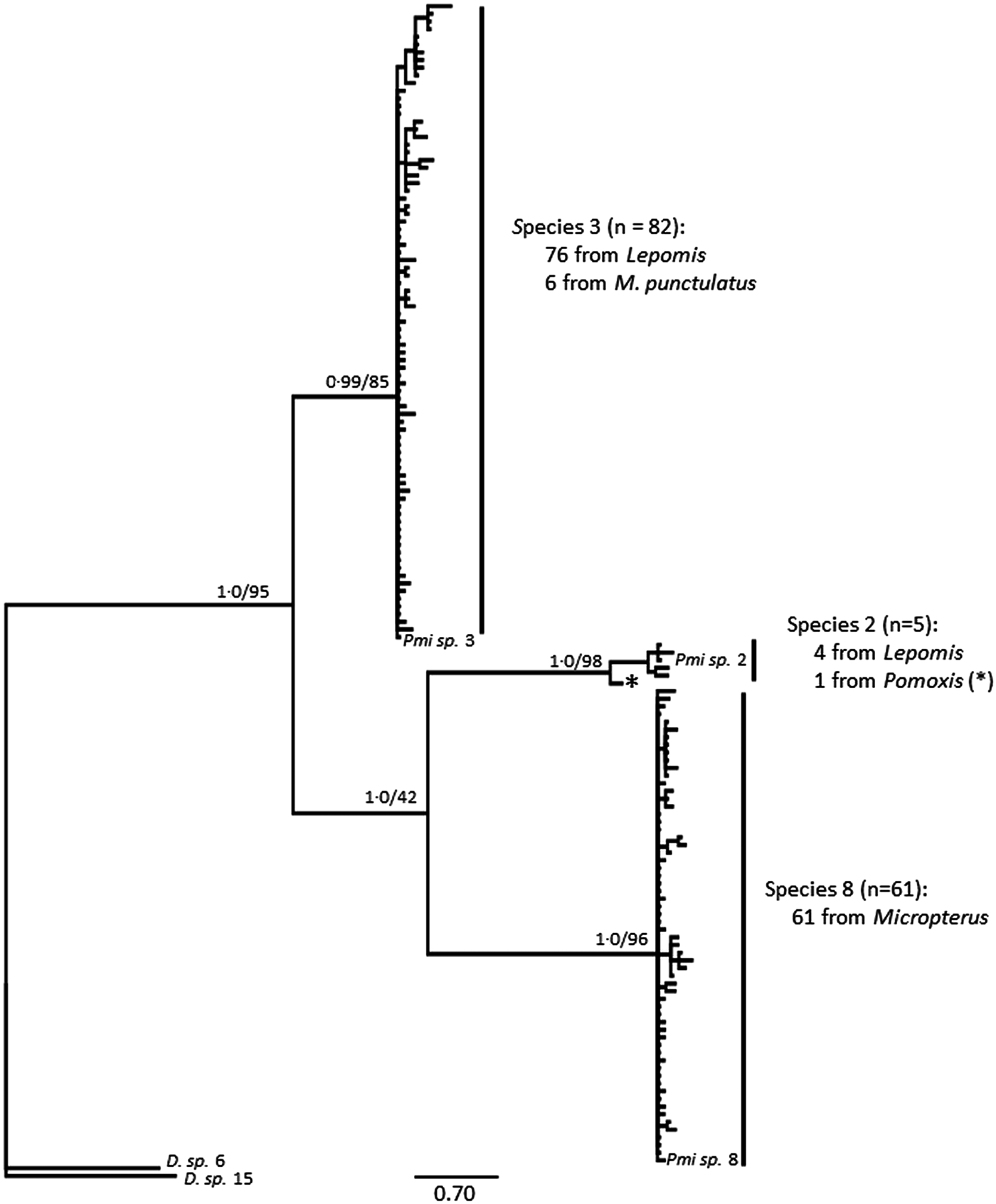
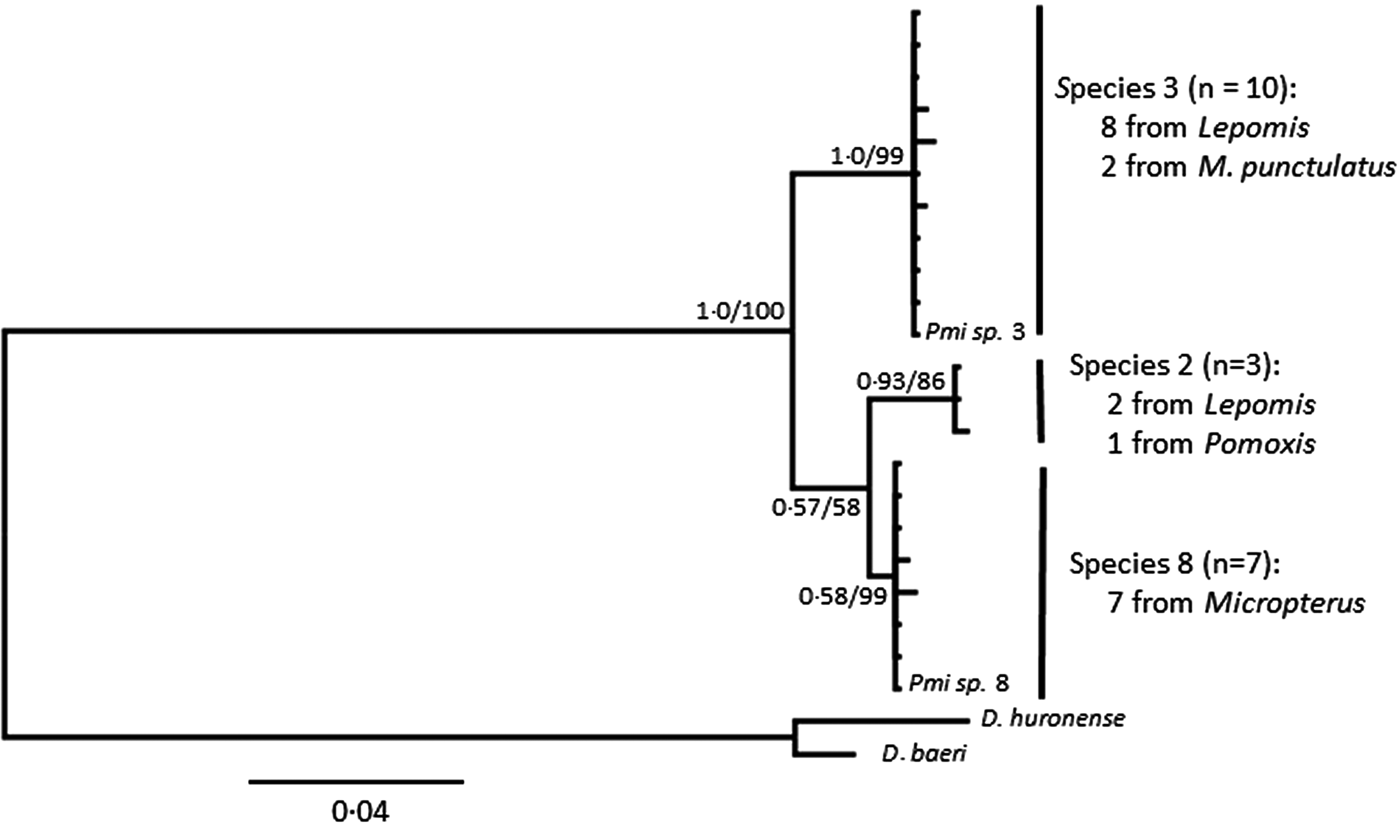
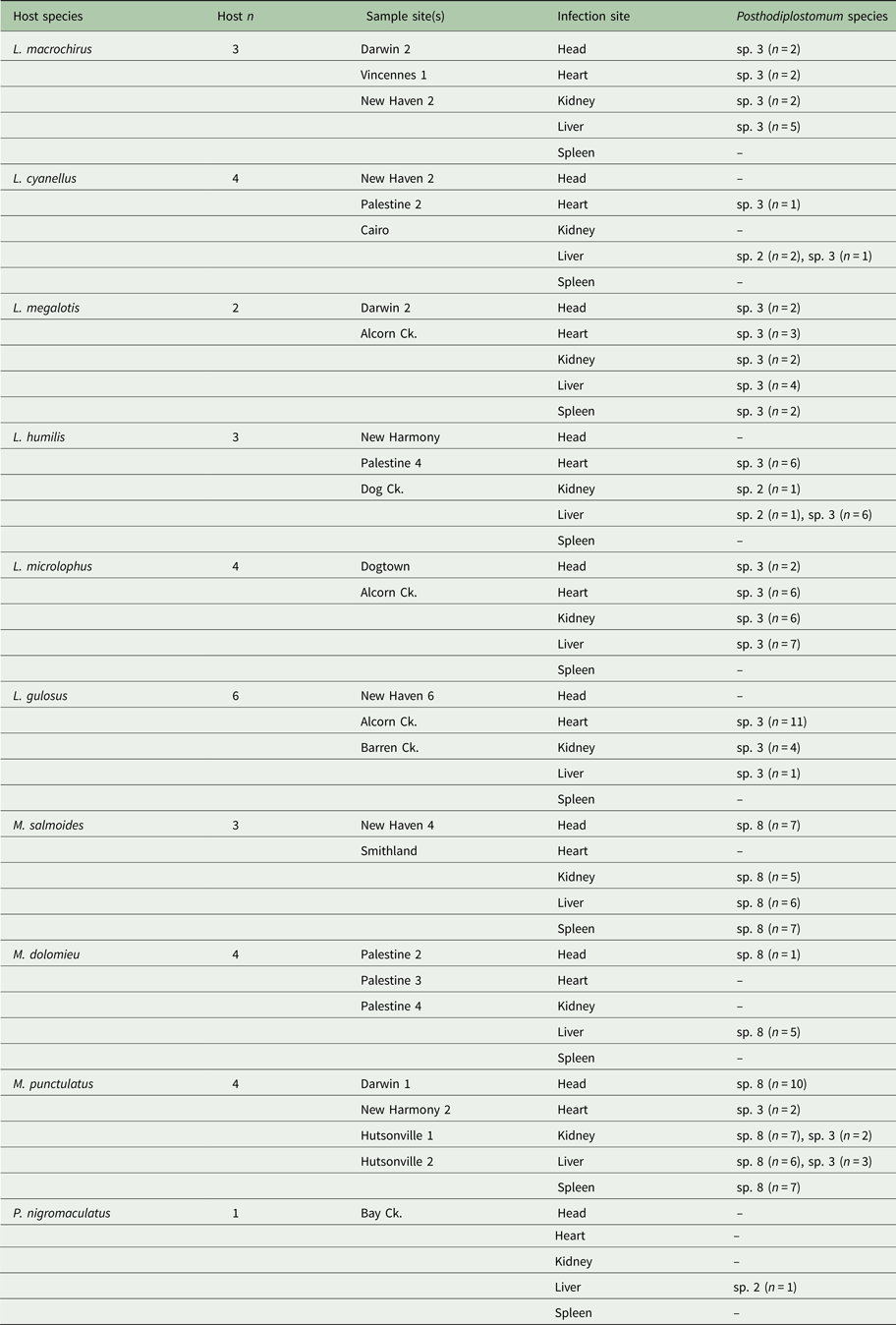
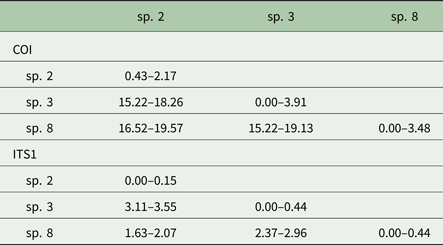
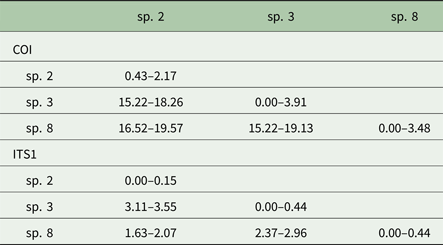
Posthodiplostomum from a total of 34 host fish representing 10 host species were sampled for DNA sequencing. One to 13 individual Posthodiplostomum were analysed from each fish and parasites were analysed from one to five infection sites per fish species (Table 3). A portion of the COI up to 514 nucleotides long was sequenced for 148 Posthodiplostomum metacercariae, producing 87 unique COI haplotypes (GenBank Accession #s MG873355-MG873441). Maximum-likelihood and Bayesian analyses produced identical tree topologies with three relatively well-supported clades. Initial NCBI Blast results for COI identified members of the three clades as belonging to species Posthodiplostomum sp. 3 (100% similarity), sp. 2 (100% similarity) and sp. 8 (99% similarity) and were supported by phylogenetic analyses (Fig. 3). While divergence within species was typically minimal, it is noteworthy that the single individual analysed from Pomoxis was divergent from sp. 2 recovered from Lepomis (denoted with * on Fig. 3). An approximately 680 bp sequence including portions of the ITS1 region and 5.8S rRNA was analysed from 20 Posthodiplostomum representing the major clades identified with COI, producing 10 different sequences (GenBank accession #s MG857103-MG857112). For ITS1, maximum-likelihood and Bayesian tree topologies were identical, resolving three clades. These clades matched those found for COI. Support for each clade was relatively high, with the exception of Bayesian support for the ‘species 2’ clade (0.58). This clade was well supported with maximum-likelihood analysis (99% bootstrap support). The relationship between sp. 2 and 8 was not well supported with maximum-likelihood analyses for COI and ITS1 and with Bayesian analysis for ITS1 (Figs. 3 & 4). Pairwise p-distances ranged from 0.00 to 3.91% for COI and 0.00 to 0.44% for ITS1 within species and 15.22 to 19.57% for COI and 1.63 to 3.55% for ITS1 among species (Table 4).

Fig. 3. Bayesian topology of phylogenetic relationships of Posthodiplostomum COI sequences. Nodal support is Bayesian support values/maximum-likelihood bootstrap values. Outgroups are Diplostomum spp. 6 (GenBank Accession # KX037901.1) and 15 (# KR271125.1), and Posthodisplostomum sp. 2 (# HM064797.1), sp. 3 (# HM064800.1) and sp. 8 (# HM064876.1) are included as references. The Posthodiplostomum sp. 2 recovered from Pomoxis nigromaculatus is indicated with an ‘*’.

Fig. 4. Bayesian topology of phylogenetic relationships of Posthodiplostomum ITS1 sequences. Nodal support is Bayesian support values/maximum-likelihood bootstrap values. Outgroups are Diplostomum huronese (GenBank Accession # AY123044.1) and D. baeri (# AY123042.1) and Posthodisplostomum sp. 3 (# HM064951.1) and sp. 8 (# HM064962.1) are included as references.
Table 3. Host species and Posthodiplostomum sampled for genetic analyses
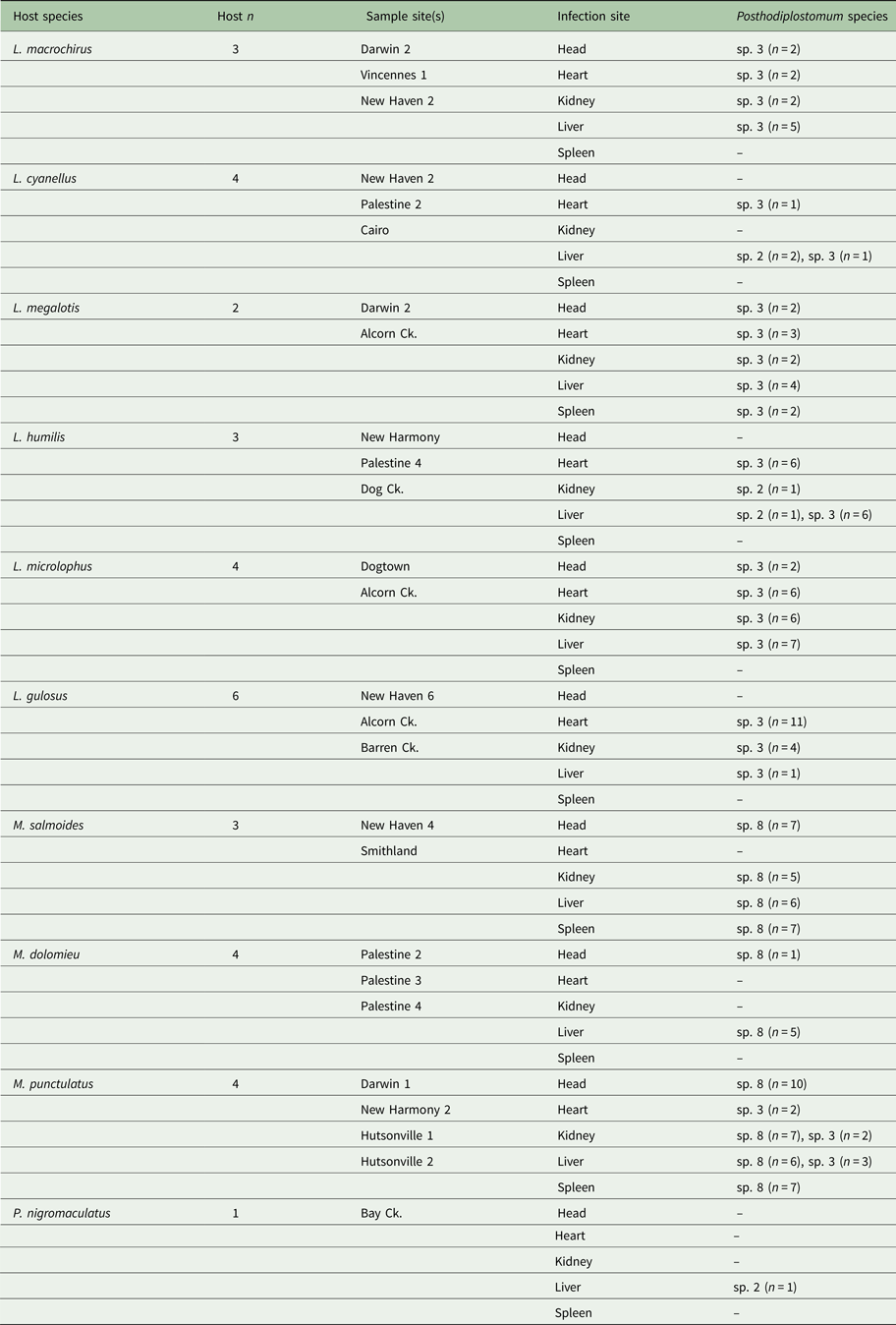
The number of hosts sampled (Host n), sample site, sites of infection, and Posthodiplostomum analysed from each infection site are listed for each host species. Sample sites refer to Fig. 1.
Table 4. COI and ITS1 p-distance values (%) within (diagonal) and among Posthodiplostomum species
The optimal number of spatial clusters recovered by BAPS was k = 1 for Posthodiplostomum species 3 and 8, suggesting a lack of geographic structure. AMOVA analyses at the sample site level including all haplotypes produced ΦST = 0.060, P = 0.042 and ΦST = 0.003, P = 0.409 for species 3 and 8, respectively. Removal of identical haplotypes within the same host fish (n = 13) produced ΦST = 0.029, P = 0.206 and ΦST = −0.021, P = 0.658 for species 3 and 8, respectively.
Host and infection site distribution
Out of the 148 Posthodiplostomum analysed, 82 were species 3, 61 were species 8, and 5 were species 2. All 61 Posthodiplostomum sp. 8 were recovered from Micropterus hosts and all three Micropterus species were infected with this parasite (Table 3). Seventy-six (93%) of the Posthodiplostomum sp. 3 analysed were from Lepomis hosts and six (7%) were recovered from M. punctulatus. All six Lepomis species examined were infected with Posthodiplostomum sp. 3. Four (80%) out of the five Posthodiplostomum sp. 2 were from Lepomis hosts and one was found in Pomoxis nigromaculatus. Looking at hosts, 95% of the parasites analysed from Lepomis were species 3 and 5% were species 2, 91% of the parasites analysed from Micropterus were species 8 and 9% were species 3 (all from M. punctulatus) and the only white grub recovered from P. nigromaculatus was a single species 2. Posthodiplostomum sp. 3 was recovered from all five tissues sampled but was rare in the spleen and the head. It was common in the heart (Table 3). Posthodiplostomum sp. 8 was recovered in approximately equal numbers from all infection sites except the heart and was common in the head (Table 3). Four of the five Posthodiplostomum sp. 2 were found in the liver and one was recovered from the kidney.
Discussion
We combined infection data and molecular species identification to determine the diversity, host specificity and tissue site specificity of Posthodiplostomum species in centrarchid fishes from the Ohio River drainage. Prevalence of Posthodiplostomum infection was relatively high (>50%) in all species examined, except L. cyanellus and the two Pomoxis species. It is unclear whether the low prevalence in Pomoxis was due to host specificity or small sample size. Lane et al. (Reference Lane, Spier, Wiederholt and Meagher2015) reported >50% prevalence of white grub in P. annuaris from a eutrophic lake, but this species was rare in the lotic habitats sampled in this study. Lepomis cyanellus was common in our study system but prevalence was much lower than in other Lepomis species, implying a species-specific effect. This pattern was consistent with the summary of Lane et al. (Reference Lane, Spier, Wiederholt and Meagher2015) and findings from our studies in the Sangamon River in Illinois in which prevalence in bluegill (L. macrochirus) was over 90% but L. cyanellus were rarely infected (Boone, unpublished data).
Highest mean intensities were seen in L. macrochirus, M. salmoides and M. punctulatus (>150 vs. <15 in all other hosts). Differences in intensity between Lepomis and Micropterus are likely influenced by differences in age structures. Micropterus hosts displayed higher mean intensities in the liver, head and spleen compared with Lepomis spp., which is likely explained by the older age structure and larger organs in the Micropterus host sample. However, despite having smaller visceral organs and a younger age structure, Lepomis hosts displayed larger mean intensities in the heart and the kidney compared with Micropterus spp., suggesting genera-specific infection preferences.
Prevalence increased with fish host age, consistent with older fish having greater opportunities to encounter cercariae and long-lived infections (Hoffman, Reference Hoffman1958). Fish size may have been a factor, but was not included in our model (Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015). Micropterus salmoides and M. punctulatus samples contained older fish than M. dolomieu but >90% of individuals from all species were less than 3. High prevalence but very low mean intensity in M. dolomieu is consistent with comparable exposure rates among Micropterus species, at least early in life, but differential levels of resistance. Although the sample size was small, it is possible that M. dolomieu mounts an effective immune response that can prevent superinfections but does not eliminate encysted metacercariae. Likewise, the higher mean intensity in L. macrochirus was not due to age differences among Lepomis hosts. In fact, both L. microlophus and L. gulosus had higher prevalence values than L. macrochirus, but much lower mean intensity. High prevalence in L. microlophus is consistent with their feeding extensively on snails (Pflieger, Reference Pflieger1997) creating high exposure rates to free-swimming ceracariae, but low mean intensity argues for resistance in this host as well. Infection rates in L. gulosus are not as easily explained by diet influencing exposure. We do not know when these fish were infected, so they may be infected at a high rate when young and then not accumulate additional parasites. Overall, our findings confirm that Posthodiplostomum is quite successful at parasitizing Lepomis and Micropterus in our study area. Differential levels of infection among fish hosts suggest that Posthodiplostomum varies in its ability to parasitize individual host species within each genus and/or fish hosts vary in their immunologic resistance (Poulin et al. Reference Poulin, Krasnov and Mouillot2011).
Molecular analyses of parasites identified three species separated by a minimum of 15% COI divergence corresponding to Posthodiplostomum spp. 2, 3 and 8 (Vilas et al. Reference Vilas, Criscione and Blouin2005; Moszczynska et al. Reference Moszczynska, Locke, McLaughlin, Marcogliese and Crease2009; Locke et al. Reference Locke, McLaughlin and Marcogliese2010; De León et al. Reference De León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016). While we only examined a subset of the metacercariae utilized for prevalence analyses, strong patterns emerged that likely reflect general trends. Posthodiplostomum sp. 3 and 8 are specialists for the host genera Lepomis and Micropterus, respectively, but infect multiple species within each genus. Despite limited sample sizes, Locke et al. (Reference Locke, McLaughlin and Marcogliese2010) also recovered these Posthodiplostomum species from the same host genera further supporting the high degree of host specificity detected. They also recovered sp. 3 from Ambloplites rupestris, demonstrating that this species can infect additional host genera. This species appears to specialize on Lepomis in our study region, but may utilize other hosts throughout its range and may use centrarchid hosts that were not included in our study (bantam sunfish Lepomis symmetricus, flier Centrarchus macropterus, pumpkinseed Lepomis gibbosus). Its presence in A. rupestris (Locke et al. Reference Locke, McLaughlin and Marcogliese2010) and exclusion from Pomoxis in the present study suggests that this species’ host-specificity does not reflect host phylogeny (Near et al. Reference Near, Bolnick and Wainwright2005); a pattern consistent with the literature (Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015). As parasites can utilize different hosts throughout their range (Hoberg and Brooks, Reference Hoberg and Brooks2008; Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015), a more thorough geographic investigation of host utilization by Posthodiplostomum spp. is warranted. However, it is clear in our study that species 3 utilizes Lepomis hosts preferentially over Micropterus hosts.
The only non-Lepomis host species infected with Posthodiplostomum species 3 in our study was M. punctulatus. Despite sequencing a small subset of white grub, this species was identified from three out of the four M. punctulatus examined. These were also the only hosts whose species identification was not positively supported with genetic data. These individuals possessed tooth patches on their tongues and lower jaw lines not extending past their eyes, characters typical of M. punctulatus and hybrids, but rare in M. salmoides (Godbout et al. Reference Godbout, Aday, Rice, Bangs and Quattro2009). The presence of these characters and genetic identification of likely M. salmoides maternal ancestry and ambiguous paternal ancestry suggest that these fish are M. salmoides × M. punctulatus hybrids. If these were misidentified M. salmoides, it would be an exceptional coincidence that they are the only M. salmoides from which species 3 was recovered. Hybridization in fishes can decrease host specificity by potentially altering immune mechanisms specific to each parental strain (Šimková et al. Reference Šimková, Davidova, Papousek and Vetesnik2013). In our system, hybridization may have allowed for parasitism by species that are not common in at least one of the host species (M. salmoides) and potentially both given its overall lack in Micropterus. Ecological differences could also produce differences in parasite–host specificity between M. punctulatus and other Micropterus (Dupont and Crivelli, Reference Dupont and Crivelli1988; Le Brun et al. Reference Le Brun, Renaud, Berrebi and Lambert1992). If M. punctulatus utilize different habitats, prey on different organisms, etc. that are more similar to Lepomis spp., they could be more likely to be exposed to Posthodiplostomum sp. 3. However, given that both M. punctulatus and M. salmoides are ecologically similar and often co-occur (Godbout et al. Reference Godbout, Aday, Rice, Bangs and Quattro2009), it is likely that they are exposed to similar parasites, supporting an altered host physiology. Host-specificity of Diplostomatid metacercariae in fishes is the result of physiological compatibility restraints between hosts and parasites and there is growing support for the importance of this in other fish metacercariae (Locke et al. Reference Locke, McLaughlin and Marcogliese2010; De León et al. Reference De León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016).
A single species 2 was the only white grub individual recovered from Pomoxis, with the remaining four individuals infecting Lepomis spp. It should be noted that two of the four metacercariae sequenced from L. cyanellus were also species 2 rather than the Lepomis specialist species 3 that dominated all other Lepomis infections. Species 2 may be a generalist but rare in our geographic area. Alternatively, it may be a Pomoxis parasite but not well adapted for transmission in a lotic system. Metacercariae species were not determined in the study by Lane et al. (Reference Lane, Spier, Wiederholt and Meagher2015), so we cannot compare with lentic systems.
Differences in infection site specificity were observed between the heavily infected host genera Micropterus and Lepomis and are consistent with the detection of different species being common in each genus. While the liver was heavily infected in both genera, white grub from Micropterus were more common in the spleen and head while Lepomis infections were more common in the kidney and heart. Therefore, both species 3 and 8 utilize the liver but differ in their utilization of other host tissues. Posthodiplostomum ‘minimum’ infect their host by burrowing through the skin. Once in the circulatory system, they preferentially travel to the liver and potentially utilize other organs as the liver becomes heavily parasitized (Hoffman, Reference Hoffman1958; Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015). Our data suggest that since each species would have equal access to host tissues, infection site differences may be based on some undetermined factors related to Posthodiplostomum species preferences and/or host-specific tissue susceptibility differences between Micropterus and Lepomis. Different Posthodiplostomum species often utilize different tissues within their hosts (Hoffman, Reference Hoffman1999; Kvach et al. Reference Kvach, Jurajda, Bryjová, Trichkova, Ribeiro, Přikrylová and Ondračková2017).
We did not detect strong evidence of genetic structure among geographic locations with either of the two common Posthodiplostomum species. This is not surprising given the relatively small geographic scale of our study and the utilization of avian definitive hosts by Posthodiplostomum spp. Trematodes typically lack innate mechanisms for distant geographic dispersal but can be dispersed by their hosts. Often, the most vagile host utilized will determine the extent of a trematode's genetic structure (Blasco-Costa and Poulin, Reference Blasco-Costa and Poulin2013). While initial sample site level AMOVA results for species 3 did suggest a very low but significant level of genetic differences (P = 0.042), results contradicted Bayesian analyses and genetic differences were not detectable when identical haplotypes were removed from individual hosts. Posthodiplostomum reproduces asexually within snail hosts producing large numbers of genetically identical cercariae (Hoffman, Reference Hoffman1958; Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015). While second intermediate fish are likely accumulating different cercariae from different snail hosts, some may occasionally be infected by multiple identical clones from a single snail as has been detected in other trematode second intermediate hosts (Rauch et al. Reference Rauch, Kalbe and Reusch2005; Keeney et al. Reference Keeney, Waters and Poulin2007). Their inclusion provides an incorrect estimate of the degree of genetic differences among sites. However, identical haplotypes are not necessarily genetic clones and species 3 did show larger ΦST value than species 8 after removal of identical haplotypes. Having now identified the host and tissue specificity of these species, further work focusing on their population genetics could reveal that differences in life histories, such as utilization of different hosts, effective population sizes, etc., are influencing their evolution.
In conclusion, our study contributes to the growing body of evidence that Posthodiplostomum infecting centrarchid fishes are a complex of several species. We have provided direct evidence that two different species are common in the genera Lepomis and Micropterus and utilize different tissues within these hosts. Utilization of a single host species in the non-targeted genus by one Posthodiplostomum species may be an example of a paratenic host or the result of host hybridization altering infection dynamics. Neither of the common Posthodiplostomum species displayed strong genetic structure likely due to their use of vagile bird hosts and the small geographic scale of our study, but differences may exist between them. Data from additional Posthodiplostomum species and study regions will shed further light on the transmission dynamics of this common and economically important species complex.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018000306
Acknowledgements
We thank all members of the fisheries and aquatic resources lab at Eastern Illinois University, Les Frankland of Illinois Department of Natural Resources and members of the fisheries programme at Southern Illinois University for helping in field collections. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
Financial support
This work was supported by U.S. Fish and Wildlife Federal Aid to Sportfish Restoration funds in conjunction with the Illinois Department of Natural Resources (F-186-R-04) and the Le Moyne College Student Research Committee.