Introduction
Epidemiological research points towards a link between the use of cannabis and the increased risk of developing a psychotic illness, in a dose-dependent manner (Arseneault et al. Reference Arseneault, Cannon, Murray, Poulton, Caspi and Moffitt2002; Zammit et al. Reference Zammit, Allebeck, Andreasson, Lundberg and Lewis2002; Moore et al. Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). However, cannabis affects individuals differently and not everyone who uses it develops psychosis. The basis of this variable sensitivity is unclear, as is the location where Δ9-tetrahydrocannabinol (THC), the main compound of the plant, mediates its psychotogenic effects. Individuals with a predisposition to psychosis who might be particularly vulnerable to its adverse effects were indicated by a positive family history of psychosis (McGuire et al. Reference McGuire, Jones, Harvey, Williams, McGuffin and Murray1995), a schizotypal personality (Stirling et al. Reference Stirling, Barkus, Nabosi, Irshad, Roemer, Schreudergoidheijt and Lewis2008), the presence of subclinical psychotic features (Henquet et al. Reference Henquet, Krabbendam, Spauwen, Kaplan, Lieb, Wittchen and van Os2004), being at ultra-high risk for psychosis (Peters et al. Reference Peters, de Koning, Dingemans, Becker, Linszen and de Haan2009) or carrying specific genes (Caspi et al. Reference Caspi, Moffitt, Cannon, McClay, Murray, Harrington, Taylor, Arseneault, Williams, Braithwaite, Poulton and Craig2005; van Winkel et al. Reference van Winkel2011; Bhattacharyya et al. Reference Bhattacharyya, Atakan, Martín-Santos, Crippa, Kambeitz, Prata, Williams, Brammer, Collier and McGuire2012a).
Elucidating which behavioural and biological factors confer greater risk for psychosis in cannabis users is crucial, because as the availability of plants with higher THC content has increased, so have the related health risks (Degenhardt et al. Reference Degenhardt, Coffey, Carlin, Swift, Moore and Patton2010; Cascini et al. Reference Cascini, Aiello and Di Tanna2011). Although relatively few individuals develop a full-blown psychotic illness after cannabis use, a larger number (between 15 and 51%) experience transient psychotic symptoms lasting from a few hours to a few days, as a result of cannabis use (Thomas, Reference Thomas1996; Green et al. Reference Green, Kavanagh and Young2003; D'Souza et al. Reference D'Souza, Perry, MacDougall, Ammerman, Cooper, Wu, Braley, Gueorguieva and Krystal2004, Reference D'Souza, Sewell and Ranganathan2009; Morrison et al. Reference Morrison, Zois, McKeown, Lee, Holt, Powell, Kapur and Murray2009). It is not yet known if there is a continuum of risk between those who become transiently psychotic (TP) and those who develop an enduring psychotic illness in relation to cannabis use. However, it would be both logical and ethically feasible to study the effects of THC in healthy individuals by comparing those who experience transient psychotic symptoms due to cannabis intoxication with those who do not. Findings may inform research on the mechanisms underlying psychotic symptoms per se, as well as examining behavioural and neurobiological mechanisms that increase the potential risk to an individual.
Although there are a growing number of neuroimaging studies that have examined the acute effects of THC administration on brain function (Martín-Santos et al. Reference Martín-Santos, Fagundo, Crippa, Atakan, Bhattacharyya, Allen, Fusar-Poli, Borgwardt, Seal, Busatto and McGuire2010), including those from our group (Borgwardt et al. Reference Borgwardt, Allen, Bhattacharyya, Fusar-Poli, Crippa, Seal, Fraccaro, Atakan, Martín-Santos, O'Carroll, Rubia and McGuire2008; Fusar-Poli et al. Reference Fusar-Poli, Crippa, Bhattacharyya, Borgwardt, Allen, Martín-Santos, Seal, Surguladze, O'Carroll, Atakan, Zuardi and McGuire2009; Bhattacharyya et al. Reference Bhattacharyya, Fusar-Poli, Borgwardt, Martín-Santos, Nosarti, O'Carroll, Allen, Seal, Fletcher, Crippa, Giampietro, Mechelli, Atakan and McGuire2009, Reference Bhattacharyya, Morrison, Fusar-Poli, Martín-Santos, Borgwardt, Winton-Brown, Nosarti, O'Carroll, Seal, Allen, Mehta, Stone, Tunstall, Giampietro, Kapur, Murray, Zuardi, Crippa, Atakan and McGuire2010; Winton-Brown et al. Reference Winton-Brown, Allen, Bhattacharrya, Borgwardt, Fusar-Poli, Crippa, Seal, Martín-Santos, Ffytche, Zuardi, Atakan and McGuire2011), to date none of these studies has examined the effects of THC according to psychotic symptom outcome.
Response inhibition, the ability to suppress irrelevant acts, is a function that is impaired in cannabis users, since they make more inhibitory errors (Hester et al. Reference Hester, Nestor and Garavan2009; Ramaekers et al. Reference Ramaekers, Kauert, Theunissen, Toennes and Moeller2009; Battisti et al. Reference Battisti, Roodenrys, Johnstone, Pesa, Hermens and Solowij2010a). It is also relevant to patients with schizophrenia who are reported to perform slowly in various response inhibition tasks (Enticott et al. Reference Enticott, Ogloff and Bradshaw2008; Huddy et al. Reference Huddy, Aron, Harrison, Barnes, Robbins and Joyce2009) and have poor error awareness (Turken et al. Reference Turken, Vuilleumier, Mathalon, Swick and Ford2003). Furthermore, this group shows abnormal frontostriatal activation during inhibition tasks (Rubia et al. Reference Rubia, Russell, Bullmore, Soni, Brammer, Simmons, Taylor, Andrew, Giampietro and Sharma2001a). A well-established response inhibition paradigm used in imaging studies is the go/no-go task, which involves the activation of the inferior frontal cortex (IFC), dorsolateral prefrontal cortex (DLPFC), inferior parietal cortices and anterior cingulate gyrus (ACG) (Rubia et al. Reference Rubia, Russell, Overmeyer, Brammer, Bullmore, Sharma, Simmons, William, Giampetro, Andrew and Taylor2001b; Simmonds et al. Reference Simmonds, Pekar and Mostofsky2008).
The neuroimaging findings regarding the effect of cannabis on response inhibition are inconclusive due to methodological variations. Two studies report significantly lower activation in regular cannabis users, relative to non-users, within the ACG and diffuse bilateral activity in the DLPFC (Gruber & Yurgelun-Todd, Reference Gruber and Yurgelun-Todd2005; Hester et al. Reference Hester, Nestor and Garavan2009). Another study reports increased response in the right DLPFC, bilateral medial frontal, inferior and superior parietal lobules in cannabis users even after 28 days of monitored abstinence (Tapert et al. Reference Tapert, Schweinsburg, Drummond, Paulus, Brown, Yang and Frank2007). In our previous study on response inhibition in participants who had seldom used cannabis, but were challenged with oral THC relative to placebo, THC was shown to attenuate activation in the right IFC and ACG and precuneus bilaterally (Borgwardt et al. Reference Borgwardt, Allen, Bhattacharyya, Fusar-Poli, Crippa, Seal, Fraccaro, Atakan, Martín-Santos, O'Carroll, Rubia and McGuire2008).
In the present study, we supplemented our previous sample by recruiting additional participants, using exactly the same criteria and methodology, to investigate brain activation in those who experienced transient psychotic symptoms after THC administration, compared with those who did not. The administration of cannabidiol, in addition to THC and placebo, is not included in this paper, as it is not relevant to the investigation in question. We hypothesized that participants who developed transient psychotic symptoms with THC would show differential activation relative to those that did not experience psychotic symptoms in brain regions that have previously been implicated in the pathophysiology of psychosis, such as the prefrontal, medial temporal and ventral temporal cortex.
Method
Design
This was a double-blind, placebo-controlled within-subject study, with a 1-month interval between scans. The order of drug administration was pseudo-randomized so that equal numbers followed each drug sequence. The Joint South London and Maudsley National Health Service and Institute of Psychiatry Research Ethics Committee approved the protocol. Each subject provided informed consent and was given extensive written and verbal information about the effects of cannabis, including psychotic symptoms.
Participants
All 21 participants were healthy, native English-speaking, right-handed males. The majority (90.5%) were white British. Their ages ranged from 20 to 42 years. All of them had used cannabis on no more than 25 occasions in their lifetime and none had used cannabis in the previous 3 months.
Criterion for inclusion into the TP group was made post hoc, on the basis of those who scored 3 or more on at least three items of the Positive and Negative Syndrome Scale (PANSS) positive subscale (Kay et al. Reference Kay, Fiszbein and Opler1987) at 2-h measurements. D'Souza et al. (Reference D'Souza, Perry, MacDougall, Ammerman, Cooper, Wu, Braley, Gueorguieva and Krystal2004), in their THC challenge study, had used the same criterion previously. Participants who scored below these thresholds were classed as ‘non-psychotic’ (NP). We identified 11 who met the criteria for transient psychosis. All completed the scanning procedure, except for one who became too anxious to stay in the scanner. Therefore, the behavioural and symptomatic data are based on 11 TP participants and the imaging data on 10.
Participants were carefully screened and the details of the procedures can be found in the supplementary material. They were asked to abstain from any illicit drug use during the study period, from alcohol and coffee 24 and 12 h before, respectively, and cigarettes on the morning of each session, as well as receiving a urine drug screening prior to scans.
Procedure
Participants were examined at the start of each session and their pulse and blood pressure were monitored. They were given identical-looking red gelatine capsules of either 10 mg of THC (99.6% pure; THC-Pharm, Germany) or placebo (flour). Both participants and researchers were blind to the content of the capsules. The dose of THC was selected on the basis of previous research (Chesher et al. Reference Chesher, Bird, Jackson, Perrignon and Starmer1990; Curran et al. Reference Curran, Brignell, Fletcher, Middleton and Henry2002; Gray et al. Reference Gray, Hart, Christie and Upadhyaya2008) to produce an effect on regional brain activation without prominent intoxication. Even though oral administration is known to indicate an erratic absorption and inter-subject variability (Grotenhermen, Reference Grotenhermen2003), it was the preferred method in this study in order to produce a slow peaking plasma level for the duration of the imaging session (Lemberger et al. Reference Lemberger, Axelrod and Kopin1971; Ohlsson et al. Reference Ohlsson, Lindgren, Wahlen, Agurell, Hollister and Gillespie1980).
Behavioural ratings
The behavioural effects were evaluated at baseline (before drug administration), +1 h (immediately before scanning), +2 h (immediately after scanning) and at +3 h time points by using the Visual Analogue Mood Scale (VAMS), State-Trait Anxiety Inventory (STAI), Addiction Research Centre Inventory (ARCI), Analogue Intoxication Scale (AIS), Cambridge Depersonalization Scale and PANSS. Further information on these scales is available in the supplementary material.
As the focus of this paper is to explore the differences between those who become TP under THC and those who do not, we mainly evaluated the baseline and 2-h measurements, when the peak intoxication is experienced following oral administration. The 3-h measurements are also presented in the graphs.
Researchers stayed with the participants until all their symptoms disappeared. In all cases symptoms had resolved spontaneously within 2–3 h. No psychopathological symptoms were reported in follow-up checks the next day, and at 1 week and 1 month later.
Functional MRI paradigm – go/no-go
Participants practised the go/no-go task prior to scanning to ensure familiarity. The task involves motor response inhibition and selective attention. Subjects are required to either execute or inhibit a motor response according to the visual cues presented on a screen. The task is described in detail in the supplementary material.
Behavioural analyses
Data were recorded on SPSS version 20.0 (SPSS, Inc., USA) and analysed using Stata 11 (StataCorp LP, USA). Descriptive statistics were used to summarize the baseline variables. Age, years of education, and cannabis, cigarette, alcohol and other drug use were compared between the two groups using t tests (or equivalent non-parametric Mann–Whitney U tests or Fisher's exact test). A multilevel model was used to assess the effect of THC on each outcome measure, with subject included as a random effect and time as a fixed effect. A second multilevel model assessed the difference between the TP and NP groups. The distribution of each measure was assessed and no gross violations of normality were found, thus making transformations of the data unnecessary. Non-parametric methods are not advisable in this situation as they are unable to handle missing data. The multi-level models used in our analysis are less restrictive regarding missingness assumptions.
When investigating task performance, two further multilevel models were run. The first included the main effect of drug only, while group effect and its interaction with drug were also added in the second.
Image acquisition
Images were acquired on a 1.5-T Signa (GE, USA) system at the Maudsley Hospital, London. T2*-weighted images were acquired with a repetition time (TR) of 1.8 s, echo time (TE) of 40 ms, flip angle 90° in 16 planes (7 mm thick), parallel to the anterior commissure–posterior commissure line. To facilitate anatomic localization of activation, a high-resolution inversion recovery image dataset was also acquired, with 3-mm contiguous slices and an in-plane resolution of 3 mm (TR 16000 ms, inversion time 180 ms, TE 80 ms).
Data processing and analysis
A complete description of image analysis including pre-processing and non-parametric statistical modelling can be found in the supplementary material. A non-parametric approach (XBAM v4; http://www.brainmap.co.uk) was used to analyse the imaging data, as this method does not assume that the population distribution is Gaussian. It is difficult to test this assumption with neuroimaging data in small groups, and, when tested, is often found to be violated (Rabe-Hesketh et al. Reference Rabe-Hesketh, Bullmore and Brammer1997; Thirion et al. Reference Thirion, Pinel, Tucholka, Roche, Ciuciu, Mangin and Poline2007). Instead, this approach uses median statistics to control outlier effects and employs permutation rather than normal theory-based inference as recommended by Hayasaka & Nichols (Reference Hayasaka and Nichols2003). The test statistic is computed by standardizing for individual difference in residual noise before embarking on second-level, multi-subject testing, using robust permutation-based methods, employing a mixed-effects method. The group activation maps for each task condition were computed for THC and placebo by determining the median sum of squares ratio at each voxel and then compared using non-parametric repeated-measures analysis of co-variance, with a voxelwise threshold of p = 0.05. The clusterwise threshold was set such that the total number of false-positive clusters per brain volume was <1 per map and the p value at which this occurred is reported.
Results
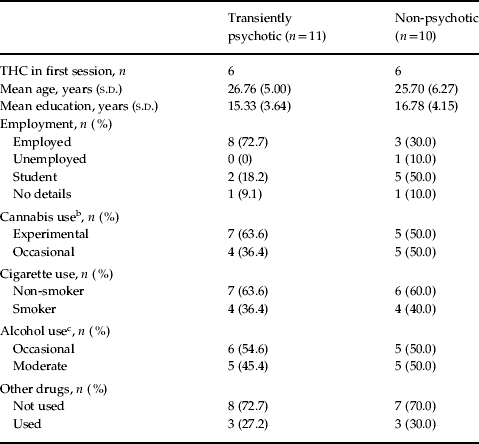
Of the 12 participants receiving THC in the first session and placebo in the second, six were classified as being in the TP group. The order of drug administration was reversed in the remaining nine participants, of whom five were subsequently included in the TP group. There was no evidence of an order effect, and no significant group differences with respect to age or years of education (all p > 0.1). Out of 21, 13 did not smoke. A total of eight participants were current tobacco smokers, but only two smoked more than 10 cigarettes per day and both of these were in the NP group. Fisher's exact test showed no significant difference between the two groups in terms of cigarette smoking, cannabis, alcohol and other drug use (all p > 1.00) (Table 1).
Table 1. Participants' sociodemographic and substance-use comparisonsa

THC, Δ9-Tetrahydrocannabinol; s.d., standard deviation; df, degrees of freedom.
a No order effect (χ2 = 0.06, df = 1, p = 0.80) and no significant group differences with respect to age (Mann–Whitney U test: Z = − 0.78, p = 0.44), years of education (t = 0.79, df = 16, p = 0.44) and with Fisher's exact test: use of cigarette smoking (p = 1.00), cannabis (p = 0.67), alcohol (p = 1.00) and other drug use (p = 1.00).
b Experimental cannabis use = less than 10 times. Occasional use = 10–25 times, in lifetime.
c Occasional alcohol use = drinking at weekends, social events. Moderate use = drinking at least three times per week.
Symptom data
In all participants, a significant change in the level of the following outcome measures was observed 2 h after the administration of THC: STAI (p < 0.001), ARCI (p < 0.001), VAMS tranquillization subscale (p = 0.007), AIS (p < 0.001) and each of the PANSS subscales (p ⩽ 0.001) (Fig. 1). For each of these measures an increase in score was observed, with the exception of VAMS tranquillization, which was lower at 2 h. The differences observed between baseline and 2 h were only significant when participants received THC, rather than placebo.

Fig. 1. Comparison of behavioural measures over time, in the Δ9-tetrahydrocannabinol (THC) condition: (a) Spielberger's State-Trait Anxiety Inventory; (b) Addiction Research Centre Inventory; (c) Analogue Intoxication Scale; and (d) Visual Analogue Mood Scale (VAMS) tranquillization category. Data are means, with standard errors represented by vertical bars. Measurements were taken just before drug administration at baseline (0) and repeated 1, 2 and 3 h after drug administration. When conditioning on THC, a comparison of transiently psychotic and non-psychotic at 2 h after drug administration showed a significant difference in the VAMS tranquillization category only (p = 0.03).
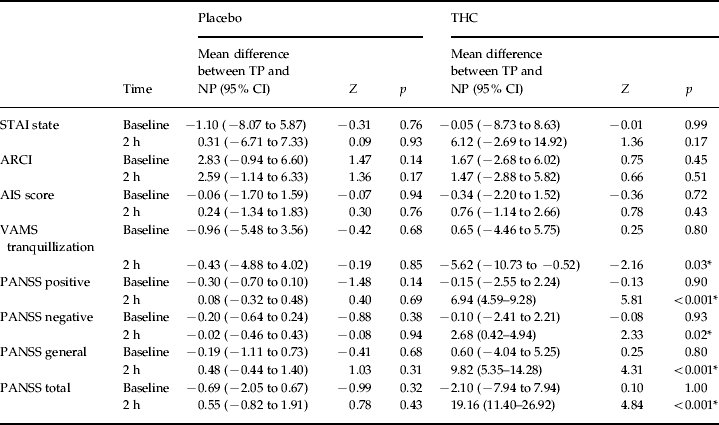
There was no significant difference between the TP and NP groups on any symptom measure at baseline or after placebo administration. However, 2 h after the administration of THC, there was a significant difference between the groups for VAMS tranquillization (p = 0.031), PANSS negative (p = 0.020) PANSS positive, general and total subscales (all p ⩽ 0.001); no significant difference was found for the other behavioural scales (Table 2, Fig. 2).

Fig. 2. Comparison of Positive and Negative Syndrome Scale (PANSS) subscales, between the transiently psychotic and non-psychotic groups under Δ9-tetrahydrocannabinol (THC) and placebo conditions: (a) positive symptoms; (b) negative symptoms; (c) general psychopathology; and (d) total score. Data are means, with standard errors represented by vertical bars. A comparison of transiently psychotic and non-psychotic participants 2 h after drug administration showed significant differences in the PANSS negative subscale (p = 0.02) and a highly significant difference in all other subscales (all p ⩽ 0.001). Note that the y-axes have different scales in the graphs.
Table 2. Comparison of symptom scales between TP and NP groups at both baseline and 2 ha

TP, Transiently psychotic; NP, non-psychotic; THC, Δ9-tetrahydrocannabinol; CI, confidence interval; STAI, State-Trait Anxiety Inventory; ARCI, Addiction Research Centre Inventory; AIS, Analogue Intoxication Scale; VAMS, Visual Analogue Mood Scale; PANSS, Positive and Negative Syndrome Scale.
a Due to small sample size, multilevel model analyses were performed separately for THC and placebo. The only significant difference between the groups was seen in VAMS tranquillization (p = 0.03) and all PANSS subscales: PANSS negative (p ⩽ 0.02) and the PANSS positive, general and total subscales (all p ⩽ 0.001).
* p < 0.05.
Physiological measures
Under the THC condition, there was no evidence of a difference in heart rates between the two groups either at baseline or 2 h after drug administration. However, when looking at the effect of the drug across all participants, heart rate was significantly increased at 2 h after administration of either THC (p ⩽ 0.001) or placebo (p = 0.002). There were no significant differences between either systolic or diastolic blood pressure in the two groups either at baseline or 2 h after administering THC. Graphs of physiological measures are provided in the supplementary material.
Task performance
There was a non-significant trend suggesting that THC increased inhibition errors among all participants (p = 0.066). A significant interaction was found between group and drug condition (p = 0.002). Inhibition errors were significantly higher in the TP group than in the NP group (p < 0.001), but only when participants received THC. No significant differences were found for mean reaction time to ‘go’ trials between the THC and placebo conditions. A table on task performance is provided in the supplementary material.
Neuroimaging results
Task effect
Under the placebo condition, no-go relative to oddball trials were associated with activation in the right ACG, prefrontal cortex and right middle temporal gyrus (MTG) independent of group, but with a less conservative significance threshold contrast (p < 0.025; uncorrected for <1 false-positive cluster).
Main effect of drug
During no-go compared with oddball trials, across all subjects, THC increased activation in the hippocampus, the tail of the caudate nucleus and the insula in the right hemisphere, relative to placebo. There were no areas where THC was associated with reduced activation relative to placebo.
Main effect of group
During no-go compared with oddball trials, independent of drug, the TP group showed less activation than the NP group in the right MTG (p < 0.005; corrected for <1 false-positive cluster) and the vermis of the cerebellum (p < 0.005; corrected for <1 false-positive cluster). There were no areas where the TP group showed greater activation than the NP group (Fig. 3).

Fig. 3. Trait differences. Analysis of all subjects, independent of drug condition, showed significant differences between the two groups in two regions. (a) Crosshair showing that activation in the right middle temporal gyrus is attenuated in the transiently psychotic (TP) group in comparison with the non-psychotic (NP) group (TP <NP, p < 0.007, corrected for <1 false-positive cluster). (b) Crosshair showing that activation in the vermis of the cerebellum is attenuated in the TP group in comparison with the NP group (TP <NP, p < 0.005, corrected for <1 false-positive cluster). The left side of the brain is shown on the left side of the images. All coordinates in Talairach (Tal) space.
Group × drug interaction
There was a significant interaction (p < 0.01; corrected for <1 false-positive cluster) between the effects of drug and group in the left parahippocampal gyrus (PHG), MTG, superior temporal gyrus (STG) and in the region spanning the right cerebellum and adjacent fusiform gyrus. In all of these regions, relative to placebo, THC significantly attenuated activation in the TP group, whereas it increased it in the NP group. Relative to placebo, THC also increased activation in the right MTG in the TP group (p < 0.01; corrected for <1 false-positive cluster), but it attenuated activation in the NP group (Fig. 4).

Fig. 4. Interaction between the transiently psychotic (TP) and non-psychotic (NP) groups and drug conditions [Δ9-tetrahydrocannabinol (THC) versus placebo]. Plots (a), (b) and (c) show that the administration of THC attenuated activation in the left parahippocampal gyrus/fusiform gyrus (a crosshair), left middle temporal gyrus/superior temporal sulcus (b crosshair) and right cerebellum/fusiform gyrus (c crosshair) in the TP group, whilst it increased activation in the same region in the NP group (p = 0.01). Plot (d) shows that THC modulated activation by increasing it in the right middle temporal gyrus (d crosshair) in the TP group, whilst it attenuated it in the NP group (p = 0.01). Data are means indexed by the mean sum of squares ratio, with standard errors represented by vertical bars. The left side of the brain is shown on the left side of the images. All coordinates in Talariach (Tal) space. a In the NP group THC did the reverse activity in these regions.
Discussion
We used functional magnetic resonance imaging to investigate differential response to oral THC in a group of healthy, seldom cannabis users and compared the behavioural and imaging findings of those who developed transient psychotic symptoms with those who did not. We found significant differences between the two groups in the effects of THC in the left PHG, STG, MTG and cerebellum, where THC decreased activation in TPs, but increased it in NPs. In the right MTG the reverse happened; THC increased activation in the TPs and decreased it in the NPs. This was accompanied by a higher error rate in the TPs, relative to the NP group, during THC condition. TPs also showed less activation than the NPs in the right MTG and the vermis of the cerebellum, independent of THC.
Symptomatic effects of THC
As the groups were defined in terms of their psychotic experiences following THC, it is not surprising that they differed in their PANSS scores. Due to the small sample size, however, formal corrections for multiple testing were not possible. Instead we lowered the significance level from 5% to 1% at which the PANSS positive, general and total scores remained significant, and the negative subscale showed a trend (p = 0.02). Therefore the negative scale result needs to be treated with caution. Even though THC significantly affected most measures in all participants, there were remarkably few significant differences in the levels of mood, anxiety and intoxication between the two groups. This may suggest that the differential sensitivity to the effects of THC was particularly and specifically related to psychotic symptoms. We cannot exclude the possibility that the absence of differences between the two groups in NP symptoms was due to limited statistical power. However, that seems unlikely, as the groups differed significantly not just on psychotic symptom severity, but also in terms of another behavioural measure: response inhibition errors.
In terms of the acute effects of THC, our findings are in line with other challenge studies in which healthy volunteers who received THC, either orally or intravenously, experienced a broad range of transient positive psychotic, negative psychotic and cognitive symptoms (Curran et al. Reference Curran, Brignell, Fletcher, Middleton and Henry2002; D'Souza et al. Reference D'Souza, Perry, MacDougall, Ammerman, Cooper, Wu, Braley, Gueorguieva and Krystal2004; Morrison et al. Reference Morrison, Zois, McKeown, Lee, Holt, Powell, Kapur and Murray2009). These studies also found that psychotic symptoms were not correlated with anxiety symptoms following THC. Significant increases in pulse rate occurred both in THC and placebo conditions, possibly due to the experimental conditions.
Task performance
We found a process-specific effect of THC on the main inhibitory measure of the task (commission/inhibition errors), but not on the executive process of the task (mean reaction time to ‘go’ trials). Across all participants THC increased inhibition errors at a trend-level of significance. Furthermore, there was a significant group × drug interaction on this measure, where TPs made significantly more commission errors than NPs. This finding cannot be related to performance differences, as we modelled only the correct trials. The findings show, that the effect of THC on impairing go/no-go task performance is specific to the inhibitory process and that this effect is more pronounced in those who develop transient psychosis. Our participants seldom used cannabis, whilst previously both occasional and heavy users of cannabis have been shown to have increased reaction time with the stop signal task, which is considered to be indicative of poor impulse control to single doses of THC (Ramaekers et al. Reference Ramaekers, Kauert, Theunissen, Toennes and Moeller2009). The finding of inhibition deficits in TPs is comparable with those reported in people with schizophrenia and bipolar disorder during the go/no-go task (Kiehl et al. Reference Kiehl, Smith, Hare and Liddle2000; Fleck et al. Reference Fleck, Kotwal, Eliassen, Lamy, DelBello, Adler, Durling, Cerullo and Strakowski2011). Higher impulsivity, inability to suppress irrelevant acts and being unaware of making errors are likely to originate from a poorly coordinated response inhibition system and may be associated with the formation of some of the psychotic symptoms.
Neural effects of go/no-go task
Even though in our previous study (Borgwardt et al. Reference Borgwardt, Allen, Bhattacharyya, Fusar-Poli, Crippa, Seal, Fraccaro, Atakan, Martín-Santos, O'Carroll, Rubia and McGuire2008) relative to placebo, THC attenuated activation in the right inferior frontal gyrus and the ACG, here we found that the activation of the specific motor response inhibition network occurred only with a lenient threshold. However, consistent with the results of previous studies (Borgwardt et al. Reference Borgwardt, Allen, Bhattacharyya, Fusar-Poli, Crippa, Seal, Fraccaro, Atakan, Martín-Santos, O'Carroll, Rubia and McGuire2008; Bhattacharyya et al. Reference Bhattacharyya, Morrison, Fusar-Poli, Martín-Santos, Borgwardt, Winton-Brown, Nosarti, O'Carroll, Seal, Allen, Mehta, Stone, Tunstall, Giampietro, Kapur, Murray, Zuardi, Crippa, Atakan and McGuire2010), we have again found that THC significantly increased activation in the right hippocampus, tail of the caudate and insula. While the latter two are key areas of inhibition, the hippocampus is not (Chambers et al. Reference Chambers, Garavan and Bellgrove2009). These findings suggest increased brain-processing effort during an inhibition task in a more widespread manner involving brain regions other than the specific response inhibition network, as has been reported previously in subjects who use cannabis on a regular basis (Tapert et al. Reference Tapert, Schweinsburg, Drummond, Paulus, Brown, Yang and Frank2007; Roberts & Garavan, Reference Roberts and Garavan2010). Our findings extend those previous findings by showing that the up-regulation effect of these areas is already observed in people who use cannabis seldomly and further support the view that THC may be disrupting the neural mechanisms involved with this task. Alternative neuroanatomic recruitment such as involvement of the STG, MTG and cerebellum have also been reported in a number of studies carried out on patients with bipolar disorder and schizophrenia during response inhibition tasks (Fleck et al. Reference Fleck, Kotwal, Eliassen, Lamy, DelBello, Adler, Durling, Cerullo and Strakowski2011; Hughes et al. Reference Hughes, Fulham, Johnston and Michie2012).
Group effect
The two groups differed inherently in terms of their task-related activation in the right MTG and the vermis of the cerebellum, independent of THC, which were reduced in the TPs. This is an interesting finding which implies a trait difference between the groups. As we excluded those with personal and family history of psychosis, it is unlikely that this finding reflects these factors. Additionally, the task we used does not normally involve the right MTG or the cerebellum. We can tentatively suggest that the differences we found may reflect a more general difference in participants' vulnerability to transient psychosis or to inhibitory dyscontrol and could be related to variations in single nucleotide polymorphisms that are associated with an increased risk of psychosis. However, our sample was not large enough to investigate this. Some recent studies focusing on early identification of psychosis have reported that the right MTG is implicated in at-risk or high-risk groups (Fusar-Poli et al. Reference Fusar-Poli, Broome, Matthiasson, Woolley, Johns, Tabraham, Bramon, Valmaggia, William and McGuire2010; Meijer et al. Reference Meijer, Schmitz, Nieman, Becker, van Amelsvoort, Dingemans, Linszen and de Haan2011). Grey matter loss in the cerebellum amongst first-onset psychosis patients has also been shown in a recent meta-analysis (Fusar-Poli et al. Reference Fusar-Poli, Radua, McGuire and Borgwardt2011). Other supporting evidence for the involvement of this region to genetic vulnerability to psychosis is found in a recently reported study, when a significant three-way interaction between two susceptibility genes implicated in glutamate transmission (G72 and DAAO) and the diagnosis of psychosis was detected at the right MTG (Mechelli et al. Reference Mechelli, Fusar-Poli, Papagni, Tognin, Kambeitz, Fu, Picchioni, Walshe, Toulopoulou, Bramon, Murray and McGuire2012).
Differential neurophysiological processing of THC
Our other main finding was that, as hypothesized, THC had a different effect on brain function in participants who developed transient psychotic symptoms from those who did not. These effects were evident in the left PHG, an area that has been implicated in the pathophysiology of psychosis in post-mortem (McDonald et al. Reference McDonald, Highley, Walker, Herron, Cooper, Esiri and Crow2000), neuropsychological (Marvel et al. Reference Marvel, Turner, O'Leary, Johnson, Pierson, Pnoto and Andreasen2007), volumetric (Witthaus et al. Reference Witthaus, Kaufmann, Bohner, Ozgurdal, Gudlowski, Gallinat, Ruhrmann, Brune, Heinz, Klingebiel and Juckel2009), functional (Wolf et al. Reference Wolf, Gur, Valdez, Loughead, Elliott, Gur and Ragland2007) and neurochemical (Stone et al. Reference Stone, Howes, Egerton, Kambeitz, Allen, Lythgoe, O'Gorman, McLean, Barker and McGuire2010) imaging studies. Effects in this region in relation to THC-induced psychosis are of particular interest because of the evidence that chronic cannabis use can impair memory (Battisti et al. Reference Battisti, Roodenrys, Johnstone, Respondek, Hermens and Solowij2010b). Our group had previously reported that THC increased parahippocampal activation bilaterally during an encoding task (Bhattacharyya et al. Reference Bhattacharyya, Fusar-Poli, Borgwardt, Martín-Santos, Nosarti, O'Carroll, Allen, Seal, Fletcher, Crippa, Giampietro, Mechelli, Atakan and McGuire2009) and attenuated it during an attentional salience task (Bhattacharyya et al. Reference Bhattacharyya, Crippa, Allen, Martín-Santos, Borgwardt, Fusar-Poli, Rubia, Kambeitz, O'Carroll, Seal, Giampietro, Brammer, Zuardi, Atakan and McGuire2012b). Furthermore, structural and functional changes in the parahippocampal region are frequently identified in relation to cannabis use (Lorenzetti et al. Reference Lorenzetti, Lubman, Whittle, Solowij and Yucel2010; Martín-Santos et al. Reference Martín-Santos, Fagundo, Crippa, Atakan, Bhattacharyya, Allen, Fusar-Poli, Borgwardt, Seal, Busatto and McGuire2010). The finding that attenuated left parahippocampal activity is observed only in the TPs, but not in the NPs, provides further support that this region may be implicated in psychoses.
Additional differences were evident in the left middle/superior temporal cortices and in the cerebellum, areas that are implicated as key regions in schizophrenia (for reviews, see Honea et al. Reference Honea, Crow, Passingham and Mackay2005; Smieskova et al. Reference Smieskova, Fusar-Poli, Allen, Bendfeldt, Stieglitz, Drewe, Radue, McGuire, Riecher-Rössler and Borgwardt2010; Jardri et al. Reference Jardri, Pouchet, Pins and Thomas2011). The STG, as well as the cerebellum, has been implicated in inhibitory control (Rubia et al. Reference Rubia, Smith, Brammer and Taylor2007). The increased inhibition error rate in the TP group together with the increased activation in these two inhibition-related areas may suggest that the TP group had to work harder to maintain their inhibitory capacity, which was still below the level of that in the NP group. Conversely, THC increased activation in the right MTG in the TPs, whilst it attenuated it in the NPs. It is interesting that this area is differentially activated between the groups whether or not THC was present. It is difficult to interpret the two findings in relation to one another as they involve different analyses involving the same region.
In all of these regions, the effect of THC on activation in the group that experienced psychotic symptoms was in the opposite direction to that in the group that did not develop psychotic symptoms. The underlying processes for this dissociated effect will require further research and replication. Interestingly, a ketamine challenge study with healthy volunteers also reported a compelling consistency between the task, region, symptom associations and those reported in patients with schizophrenia (Honey et al. Reference Honey, Corlett, Absolam, Lee, Pomarol-Clotet, Murray, McKenna, Bullmore, Menon and Fletcher2008).
To our knowledge, the present study is the first to demonstrate neurobiological differences that may contribute to the differential sensitivity to the psychotogenic effects of cannabis in healthy participants. Our findings imply that there is an association between individual variability in brain response and subsequent transitory psychotic symptom formation. Even though THC only transiently produced psychotic symptoms in some, the brain regions that were up-regulated are also those critically implicated in schizophrenia. Whilst acknowledging that transient psychosis is not the same as a full-blown psychosis, there may be varying degrees of risk in response to the psychotogenic effects of THC. How THC modulates specific brain regions can also provide information on symptom formation. Given the size of the problem universally, similar studies with larger samples are required to understand the basis of differential neural responses to THC to inform the ongoing public health debate about the risks of cannabis use, as well as leading to the development of interventions designed to reduce its use, particularly targeting those most at risk.
Limitations
This study has a modest sample size. Studies of this type are logistically difficult when participants, who seldom use cannabis, are asked to attend more than one study session. However, we have used non-parametric, repeated-measures analyses to obtain more robust findings in order to compensate for the low numbers (Brammer et al. Reference Brammer, Bullmore, Simmons, Williams, Grasby, Howard, Woodruff and Rabe-Hesketh1997; Bullmore et al. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer1999). The use of PANSS is another limitation, as this scale is not designed for transient psychosis, even though our participants experienced frank hallucinations and delusions temporarily.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291712001924.
Acknowledgements
The present study was supported by a Joint Medical Research Council/Priory clinical research training fellowship to S.B. and support from the Psychiatry Research Trust, UK. We are grateful to Glynis Ivin (Department of Pharmacology, the Maudsley Hospital), for the storing, blinding procedure and dispensing of the THC and placebo.
Declaration of Interest
None.