Introduction
The surface of Mars today appears hostile to life as we know it. Liquid water, the principal requisite for life, is not stable on the planet's surface due to the low atmospheric pressure and surface temperatures. However, the Martian surface was not always as it is today. During the first hundreds of millions of years, Mars was likely warmer and wetter, and may have had a relatively thick CO2-dominated atmosphere (Baker et al. Reference Baker, Strom, Gulick, Kargel, Komatsu and Kale1991; Baker Reference Baker2001; Phillips et al. Reference Phillips2001; Fairén et al. Reference Fairén, Fernández-Remolar, Dohm, Baker and Amils2004). Liquid water could have been stable on the surface for extended periods of time, forming large standing bodies of water, rivers, and glaciers (Head et al. Reference Head, Kreslavsky, Hiesinger, Ivanov, Pratt, Seibert, Smith and Zuber1998; Clifford & Parker Reference Clifford and Parker2001). A planetary magnetic field shielded the surface from harmful Solar radiation (Acuña et al. Reference Acuña1999), and volcanic activity provided energy and heat in localized areas. During these time periods, the conditions on the surface may have been sufficiently benign for life to originate and evolve.
Alternative models have been proposed in which liquid water-related geological evidence on Mars can be explained as a consequence of spatially and temporally localized heat sources in a planet cold throughout its entire history, in which not even high atmospheric pressures would have been enough to raise the temperatures above the freezing point of pure water at a substantially lower early Solar luminosity (Colaprete & Toon Reference Colaprete and Toon2003). In such enduring, intensely cold and arid conditions, only punctuated events such as hydrothermally released groundwater (Griffith & Shock Reference Griffith and Shock1997), impact events (Segura et al. Reference Segura, Toon, Colaprete and Zahnle2002), or groundwater circulation (Gulick Reference Gulick2001) could have triggered liquid water release to the surface.
Whatever the scenario, after several hundred million years the atmosphere of Mars became thinner and the planet became increasingly colder and more arid. The planetary dynamo ceased and extremely dry and cold conditions persevered on the surface. These conditions have only been interrupted by sporadic, albeit catastrophic, flooding and ponding events in the northern plains, some of them probably lasting as long as several thousand years, and capable of forming bodies of water ranging in size from oceans to lakes (Fairén et al. Reference Fairén, Dohm, Baker, de Pablo, Ruiz, Ferris and Anderson2003), and by episodic climate fluctuations caused by large inclination changes of the axis of rotation of the planet (obliquity). The latter resulted in the deposition of alternating bright and dark layers on the northern polar cap, that are likely the result of differential dust deposition, ice formation and sublimation controlled by orbital fluctuations during the last 900 000 years (Laskar et al. Reference Laskar, Levrard and Mustard2002). During high obliquity periods the amount of Solar energy reaching the caps doubles, and induces the partial melting and sublimation of the northern cap. This can provide episodes of liquid water availability, and the potential for metabolic activity, in polar latitudes. The last such cycle occurred as recently as 5–10 million years ago (Laskar et al. Reference Laskar, Levrard and Mustard2002; Levrard et al. Reference Levrard, Foget, Montmessin and Laskar2004).
Just as the environmental histories of Earth and Mars diverged drastically after the first few hundred million years, so too would their life histories (Schulze-Makuch et al. Reference Schulze-Makuch, Irwin, Lipps, LeMone, Dohm and Fairén2005). Present-day conditions on the exposed surface of Mars are not suitable for life as we know it; however, life could be present today on Mars in liquid water in or beneath ice sheets, in ground water, or in protected habitats such as lava tubes, cracks and fissures, or caves. The potential for life would be enhanced in regions where elevated heat flow may occur, such as volcanic areas (e.g. the Tharsis and Elysium areas). Any Martian organisms would likely be cold-adapted microbes, which use inorganic chemical food sources adapted to a nutrient-poor environment, or life based on photosynthesis in selected habitats such as in fringe areas of polar ice.
Thus, for the last period of Martian history (Amazonian) the picture of a thriving biosphere emerges during high obliquity periods with relatively abundant liquid water, while life would be much less in evidence during the more common cold and dry periods (as is the case on present-day Mars). This is not unlike life on Earth today, which waits during a cold, long winter for the coming of warm spring days. Certainly, the waiting period on Mars would be much longer. However, there seems to be no essential limit on the waiting period, at least not for microbial organisms, as bacterial spores do not expend energy during their dormant stages. For example, Cano & Borucki (Reference Cano and Borucki1995) reported on the isolation of a viable strain of Bacillus sphaericus from an extinct bee trapped in 25–30 million-year-old amber, and Vreeland et al. (Reference Vreeland, Rosenzweig and Powers2000) claimed the isolation of a viable 250 million-year-old bacterium from a salt crystal in New Mexico. Thus, if organisms on Earth can remain viable for so long a time without even having the evolutionary pressure to do so, then there is no principal reason why Martian organisms could not have developed the capability to do the same.
We present here a case for life on Mars based on the key assumption that liquid water has been present in diverse amounts on the surface of the planet during different times. We review the last 40 years of astrobiology-relevant research on Mars, discuss some of the findings in view of current knowledge, and conclude with perspectives of life and possible microorganisms that are consistent with the observations made to date.
The changing environment on Mars
The first billion years: liquid water and environmental diversity
The geological history recorded on the Martian surface spans nearly 4.6 billion years (Jakosky & Phillips Reference Jakosky and Phillips2001). During the first billion years, the physico-chemical conditions on the surface were remarkably different from those of today, and were mainly controlled by a surface and subsurface global hydrological cycle, large-scale meteoritic bombardment and massive volcanic activity.
From a biological perspective, the availability of liquid water in these early stages was the only constraint on the evolution of life on Mars, given the presence of various types of energy sources including sunlight, reduced mineral compounds and volcanic activity. Evidence of liquid water flowing and ponding on the surface of the planet covers most of the Martian landscape (Fig. 1). The scale and diversity of the Martian hydrological regime is evidenced by the variety of aqueous environments that have been identified. These include ocean-related landforms (Parker et al. Reference Parker, Gorsline, Saunders, Pieri and Schneeberger1993; Head et al. Reference Head, Kreslavsky, Hiesinger, Ivanov, Pratt, Seibert, Smith and Zuber1998; Clifford & Parker Reference Clifford and Parker2001; Fairén et al. Reference Fairén, Dohm, Baker, de Pablo, Ruiz, Ferris and Anderson2003) including a large plain surrounding the north pole that resembles a sediment-filled ocean basin with true shoreline features (Fig. 2; Fairén et al. Reference Fairén, Dohm, Baker, de Pablo, Ruiz, Ferris and Anderson2003; Ruiz et al. Reference Ruiz, Fairén, Dohm and Tejero2004; Perron et al. Reference Perron, Mitrovica, Manga, Matsuyama and Richards2007), anastomosing and meandering rivers and deltas (Malin & Edgett Reference Malin and Edgett2003), massive layered outcrops interpreted as water-deposited sediments (Malin & Edgett Reference Malin and Edgett2000a), cross-stratification in sedimentary outcrops (Squyres et al. Reference Squyres2004), and water-related mineralogies extending over regional scales (Squyres et al. Reference Squyres2004; Hynek Reference Hynek2004; Arvidson et al. Reference Arvidson, Poulet, Bibring, Wolff, Gendrin, Morris, Freeman, Langevin, Mangold and Bellucci2005; Poulet et al. Reference Poulet, Bibring, Mustard, Gendrin, Mangold, Langevin, Arvidson, Gondet and Gómez2005), and contemporary surface-water runoff (Malin & Edgett Reference Malin and Edgett2000b; Heldmann & Mellon Reference Heldmann and Mellon2004; Heldmann et al. Reference Heldmann, Toon, Pollard, Mellon, Pitlick, McKay and Andersen2005; Malin et al. Reference Malin, Edgett, Posiolova, McColley and Noe Dobrea2006). However, alternative models exist that invoke melting snow, gas fluidization, dry flows, Martian wind, or base surges during volcanic activity or meteorite impacts as formation mechanisms for some of these features.

Fig. 1. Evidence of liquid water flowing and ponding on the surface of Mars. Top left: Layered sediments in Hellas Planitia, probably deposited on the floor of an ancient lake. Top right: Gullies in a crater wall. Bottom: Inverted relief of fossilized river channels forming a fan-like structure. Scale bars are 500 m. Pictures courtesy of NASA/JPL/University of Arizona.
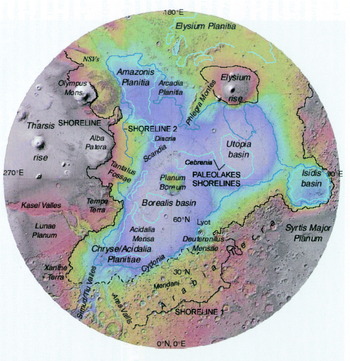
Fig. 2. Topographic shaded relief map of the northern hemisphere of Mars constructed from Mars Orbiter Laser Altimeter (MOLA) data showing major geographic features of the northern hemisphere, including possible oceanic shorelines. Polar Stereographic projection; scale varies with latitude. Modified from Fairén et al. (Reference Fairén, Dohm, Baker, de Pablo, Ruiz, Ferris and Anderson2003).
Some of the valleys seem to have been exposed to recurrent water over a long period of time, eroding the landscape in successive stages, which extend from the Early Noachian (4.6–4.2 Gyr) to the late Hesperian (3.4–3 Gyr) (Ansan & Mangold Reference Ansan and Mangold2006). Some of these water-cut valleys have been buried beneath several meters of lava, as seems to be the case in Athabasca Valles (Jaeger et al. Reference Jaeger, Keszthelyi, McEwen, Dundas and Russell2007). Hemispheric oceans of water and ice were potentially formed in the northern lowlands (Clifford & Parker Reference Clifford and Parker2001), with more ice northbound and a huge northern polar cap almost the size of the Vastitas Borealis Formation (VBF), the final residue of the ancient Martian lowlands' ocean, formed by the frozen deposits of sediment-laden water from giant outflow channels. The ocean could have been frozen and dust-covered (Murray et al. Reference Murray2005). Preliminary results from the Mars Reconnaissance Orbiter, however, indicate the presence of ubiquitous boulders ranging up to about 2 m in diameter covering the VBF, questioning the presence of an ocean in that region (McEwen et al. Reference McEwen2007). Great lakes also formed within impact craters on the southern highlands, such as Argyre or Hellas (Wilson et al. Reference Wilson, Howard, Moore and Grant2007), and deeply incised valleys connected basins in an active hydrological cycle over the intercrater plains (Howard Reference Howard2007). Globally, large regions of the Martian crust underwent chemical alteration by water–rock interactions during the Noachian (e.g. Hynek Reference Hynek2004; Mustard et al. Reference Mustard, Poulet, Head, Mangold, Bibring, Pelkey, Fassett, Langevin and Neukum2007).
Many of these ancient hydrological landforms can now be linked to detailed mineral compositions, obtained by landers and orbiters in the past 10 years. This has revealed a relatively large variety of geochemical environments and aqueous processes that coexisted some time in the Martian past, or that evolved as a consequence of a dynamic planetary hydrology (Bibring et al. Reference Bibring2005; Gendrin et al. Reference Gendrin2005; Andrews-Hanna et al. Reference Andrews-Hanna, Phillips and Zuber2007; Bibring et al. Reference Bibring2007). Most of the Martian surface is composed of volcanic rocks and derived soils, over which aqueous solutions have acted to produce primary and secondary diagenetic deposits, with Mg (±Fe, Ca)-sulphates and Fe-oxides and hydroxides as the dominant phases (Fig. 3(a)) (Squyres et al. Reference Squyres2004; Bibring et al. Reference Bibring2005; Gendrin et al. Reference Gendrin2005; Bibring et al. Reference Bibring2007; Hurowitz & McLennan Reference Hurowitz and McLennan2007). Phyllosilicates, a different product of the aqueous alteration of igneous rocks, have also been detected in a restricted number of areas (Fig. 3(b)) (Bibring et al. Reference Bibring2005; Gendrin et al. Reference Gendrin2005), while olivine and pyroxene, minerals very susceptible to aqueous alteration, are found throughout the ancient southern highlands (Bibring et al. Reference Bibring2005; Gendrin et al. Reference Gendrin2005). Minor concentrations of these minerals have also been identified in areas of the northern lowlands, in close proximity to aqueous alteration products (Bibring et al. Reference Bibring2005; Gendrin et al. Reference Gendrin2005).

Fig. 3. Water-related mineralogies on Mars as seen by the Mars Recognizance Orbiter (MRO). Top: Phyllosilicates (bright) in Eberswalde Crater. Bottom: Sulphate deposits (bright) in the Aureum Chaos region. Scale bars are 500 m. Figures courtesy of NASA/JPL/University of Arizona.
These different mineral assemblages support the idea that the Martian surface was very heterogeneous during the first billion years, and that surface and near-surface aqueous processes were responsible for this diversity of geochemical environments. In addition, planetary-scale groundwater flows seem to have dominated a large part of the Martian hydrological history (Andrews-Hanna et al. Reference Andrews-Hanna, Phillips and Zuber2007). Groundwater flow and discharge on the surface could also have provided a variety of geochemical environments, such as transient evaporitic lakes and ponds, permanent saline and acidic/alkaline environments, freshwater lakes, inter-dune ponds and rivers.
This diversity of surface environments during the Noachian and Early Hesperian epochs was accentuated by massive volcanic activity, mainly related to the formation of the Tharsis region (Anderson et al. Reference Anderson, Dohm, Golembek, Haldemann, Franklin, Tanaka, Lias and Peer2000; Dohm et al. Reference Dohm, Ferris, Baker, Anderson, Hare, Strom, Barlow, Tanaka, Klemaszewski and Scott2001; Phillips et al. Reference Phillips2001). The resulting heavy loading of the lithosphere induced a global warping of the surface (Jakosky & Phillips Reference Jakosky and Phillips2001; Phillips et al. Reference Phillips2001) and had a global influence on the distribution of valley networks (Jakosky & Phillips Reference Jakosky and Phillips2001). In addition, the outgassing derived from the volcanic activity in the Tharsis region probably released substantial quantities of water and CO2, providing input of gases to the atmosphere and possibly contributing to an early, thicker atmosphere (Jakosky & Phillips Reference Jakosky and Phillips2001). The formation of the Tharsis region probably resulted in the appearance of local geochemical environments such as fumaroles, hydrothermal vents or springs, similar to those typical of volcanic environments on Earth.
The surface of early Mars therefore would have had a relatively broad diversity of geochemical environments, not unlike the surface of early Earth. Mars also possessed a global magnetic field during the Noachian, which provided a shield against harmful Solar radiation for several hundred million years (Acuña et al. Reference Acuña1999). While we do not know the conditions in which life originated on Earth, it appears that both planets shared similar environmental conditions. The independent emergence of life on Mars therefore remains a distinct possibility.
The main threats to an early Terran and Martian biosphere were probably related to meteorite impacts and, in the case of Mars, the shutdown of the planetary dynamo and magnetic field, the increasing extreme aridity and the lowering temperatures. The effects of impacts on the early Earth and Mars largely depended on whether oceans were present or absent on the surface (Sleep & Zahnle Reference Sleep and Zahnle1998). On a planet without oceans, relatively small impacts would have heat-sterilized the surface of the planet; however, not even large-scale impacts would have affected subsurface environments (Sleep & Zahnle Reference Sleep and Zahnle1998). As mentioned earlier, there are some indications that oceans may have been present on the Martian surface early in the planet's history, when large-scale impacts were more frequent. In this scenario, the chances of survivability of a putative Martian biosphere in large-scale impacts would be greater (Sleep & Zahnle Reference Sleep and Zahnle1998). Furthermore, impact reseeding on Earth has been suggested as a possible means by which life could have survived the late heavy bombardment (Gladman et al. Reference Gladman, Dones, Levison and Burns2005), and a similar mechanism could be envisioned for Mars.
Numerical modelling of data gathered by orbital instruments indicates that early Mars possessed a dipole magnetic field with an intensity similar to that of present-day Earth (Hood & Zakharian Reference Hood and Zakharian2001). The Martian dynamo has since declined in intensity and nowadays it yields no measurable magnetic fields on the surface, and only remnant magnetizations of the original field remain preserved in crustal rocks (Acuña et al. Reference Acuña1999). The magnetosphere derived from this Martian magnetic field would have protected a putative Martian biosphere against Solar radiation for a minimum of 600 million years (from 4.4 to ca. 3.8 billion years ago). The absence of this protective shield would have had deleterious effects on any type of surface biosphere, which would have been obliged to retreat to the near subsurface, or to adapt to the increasing doses of Solar radiation.
The increasing aridity and the decrease of surface temperatures are likely to have posed the main threat to a putative Martian biosphere. Life as we know it depends on liquid water to evolve and spread. Unlike early Mars, present-day conditions on the Martian surface are extremely arid and seemingly unsuitable for terrestrial life. The transition from a relatively humid to a hyper-arid Mars occurred in a relatively short time, probably within the first 1 billion years (Jakosky & Phillips Reference Jakosky and Phillips2001). Parallel to the desiccation of the surface, the decrease in surface temperatures further limited the survivability of any putative Martian organism by sequestering the little water available in the frozen state, both at the poles and within the crust. A useful parameter to determine the habitability of Mars with respect to liquid water is water activity. The lowest known water activity value that allows microbial growth on Earth is 0.61 (Tokuoka Reference Tokuoka1993). Water activity values on the present-day Martian surface are well below 0.5. This value is used to identify ‘special regions’ on Mars, which are defined as holding potential for extant Martian life and for the forward contamination of Mars by Terran organisms brought in by spacecraft (MEPAG 2007). Thus, the transition to a colder and dryer planet was the main environmental process affecting the evolution of any putative early Martian biosphere.
The transition to a colder and dryer planet
If we were to find extant life on or near the surface of Mars today, these Martian organisms would need to be adapted to desiccation, high doses of Solar radiation, and extreme low temperatures with large daily fluctuations depending on their location. These adaptations could be physiological or ecological or a combination of both. Physiological adaptations would probably be similar to those of Terran microorganisms: increasing membrane fluidity at low temperatures, formation of spores or transitions into dormant stages, or adaptations to extremely low metabolic activities. Ecological adaptations would involve the colonization of a specific substrate with physical and chemical properties that would facilitate survival, for example the colonization of subsurface environments to avoid Solar radiation or the endolithic colonization of highly hygroscopic minerals to enable access to liquid water at low temperatures and low relative humidity. The following sections are intended to show that the survival strategies of microorganisms in extreme terrestrial environments open the possibility that Martian microorganisms could have survived and adapted to the conditions near the surface.
Life in evaporitic deposits
The hyper-arid core of the Atacama Desert is considered the dry limit for photosynthetic activity on Earth (Warren-Rhodes et al. Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gómez-Silva, Amundson, Friedmann and McKay2006). Soils in this region are practically barren of microorganisms and have trace amounts of organic matter (Navarro-González et al. Reference Navarro-González2003). With mean precipitation rates of less than 2 mm a year, this region represents a close analogue of the extreme arid conditions that dominate the Martian surface (McKay et al. Reference McKay, Friedmann, Frankel and Bazylinski2003). Yet, this hyper-arid environment hosts relatively abundant communities of endolithic photosynthetic organisms within evaporitic crusts of halite (Fig. 4) (Wierzchos et al. Reference Wierzchos, Ascaso and McKay2006). The endolithic environment provides microorganisms with mineral nutrients and more favourable moisture regimes than if exposed directly to the atmosphere, as well as protection against harmful radiation (Golubic et al. Reference Golubic, Friedmann and Schneider1981). Salt deposits have the additional advantage of mineral deliquescence, which enables the condensation of water within the pore space of salts at humidity levels that otherwise hinder the occurrence of liquid water in the surrounding environment (Davila et al. Reference Davila, Gomez-Silva, de los Rios, Ascaso, Olivares, McKay and Wierzchos2008). Halite (NaCl) has a deliquescence relative humidity of approximately 75% at 25°C (Cohen et al. Reference Cohen, Flagan and Seinfel1987; Ebert et al. Reference Ebert, Inerle-Hof and Weinbruch2002). This is of particular importance in hyper-arid environments, where liquid water is scarce, and explains the presence of endolithic microorganisms in evaporitic deposits such as halite crusts (Wierzchos et al. Reference Wierzchos, Ascaso and McKay2006; Davila et al. Reference Davila, Gomez-Silva, de los Rios, Ascaso, Olivares, McKay and Wierzchos2008).

Fig. 4. Top. Halite crusts in the hyper-arid core of the Atacama Desert (Chile), which are colonized by endolithic cyanobacteria (inset) that take advantage of the hygroscopic properties of the mineral to obtain liquid water. Bottom. Chloride-bearing deposits on Mars (bright). These deposits likely have similar properties to the crusts in the Atacama Desert and could have therefore provided a habitable niche when the conditions on the surface became increasingly arid. Top picture and inset are courtesy of Jacek Wierzchos from the University of Lleida, Spain. Bottom picture is courtesy of NASA/JPL/University of Arizona.
As Mars became drier and colder, standing bodies of water on the surface froze and sublimed or retreated into the crust. This probably resulted in the precipitation of salt deposits similar to Earth evaporites, within closed or semi-closed basins such as lakes, ponds, interdune regions or impact craters (Mancinelli et al. Reference Mancinelli, Fahlen, Landheim and Klovstad2004). Data provided by the Mars Exploration Rover (MER) Opportunity indicate that large regions of Mars such as Meridiani Planum once hosted evaporitic environments that resulted in the precipitation of salt-rich deposits (Squyres et al. Reference Squyres2004). Osterloo et al. (Reference Osterloo, Hamilton, Bandfield, Glotch, Baldridge, Christensen, Tornabene and Anderson2008) recently mapped widespread deposits in regions of the southern highlands of Mars, and interpreted them as chloride-bearing salts on the grounds of their spectral properties (Fig. 4). The chloride-bearing materials are light-toned and exhibit patterned-ground and etched-terrain morphologies. High-resolution images reveal that the deposits are highly fractured and are possibly cemented or indurated (Osterloo et al. Reference Osterloo, Hamilton, Bandfield, Glotch, Baldridge, Christensen, Tornabene and Anderson2008). The distribution of the deposits is patchy and each individual unit appears to be disconnected from other adjacent units, which is indicative of a local origin. Altogether, the geomorphology of the deposits is consistent with formation associated with precipitation from brines in an evaporitic environment (Osterloo et al. Reference Osterloo, Hamilton, Bandfield, Glotch, Baldridge, Christensen, Tornabene and Anderson2008).
As occurs in the hyper-arid region of the Atacama Desert, these deposits could have provided a last shelter to light-dependent microorganisms (Wierzchos et al. Reference Wierzchos, Ascaso and McKay2006; Davila et al. Reference Davila, Gomez-Silva, de los Rios, Ascaso, Olivares, McKay and Wierzchos2008), and may represent an important target for future life-detection missions on the planet. Active halophilic microorganisms have been found within fluid inclusions in 250 million years old, deep evaporitic deposits (Vreeland et al. Reference Vreeland, Rosenzweig and Powers2000) suggesting that similar deposits in the surface and subsurface of Mars could have sustained (or could perhaps still sustain) active microorganisms well after conditions on the surface became too harsh for life.
Life in the subsurface
If life evolved on Mars and gained a foothold, it could have evolved into efficient chemolithotrophs based on the geochemistry of Fe and S, two of the most abundant compounds on the Martian surface (e.g. Squyres et al. Reference Squyres2004; Haskin et al. Reference Haskin2005), thereby favouring the formation of sulphates and ferric iron minerals to the same extent as the first Terran communities favoured carbonate precipitation. On Earth, such ecosystems can be found in massive iron-sulphide deposits such as the subsurface ecosystem in Rio Tinto, Spain (see Fig. 5 and Amils et al. (Reference Amils2007)). Rio Tinto is located in the core of the Iberian Pyritic Belt, the largest deposit of pyrite on Earth (Barriga et al. Reference Barriga, de Carvalho, Ribeiro, Barriga and de Carvalho1997). The physico-chemical conditions and the mineralogy of the surface and subsurface of Rio Tinto are considered analogous to those in Meridiani Planum during the Noachian (Fairén et al. Reference Fairén, Fernández-Remolar, Dohm, Baker and Amils2004; Fernandez-Remolar et al. Reference Fernandez-Remolar, Morris, Gruener, Amils and Knoll2005; Amils et al. Reference Amils2007). The subsurface of Rio Tinto hosts massive iron-sulphide deposits almost 200 m thick. These pyrite deposits serve as a habitat to a community supported by chemolithotrophic microbes through oxidation of sulphur and iron, generating ferric sulphates and oxides as by-products of pyrite weathering (Fernandez-Remolar et al. Reference Fernandez-Remolar, Gómez, Prieto-Ballesteros, Schelble, Rodríguez and Amils2008).

Fig. 5. Headwaters of the Rio Tinto, showing its typical reddish colour due to the iron in solution.
A similar ecosystem can be envisioned for Mars. Pyrite was probably an abundant component of the Martian crust in the past (Burns & Fisher Reference Burns and Fisher1990, Reference Burns and Fisher1993). The Viking X-ray fluorescence spectrometer and the MER Spirit have provided direct evidence for the presence of large concentrations of sulphur in the Martian regolith (Haskin et al. Reference Haskin2005). Massive pyrite deposits could have formed on Mars through the alteration of pyrrhotite and pentlandite assemblages in mafic and ultramafic igneous rocks (Burns & Fisher Reference Burns and Fisher1990) or by volcanogenic emplacement within the crust. The jarosite deposits identified in Meridiani Planum probably formed through the aqueous oxidation of diverse iron-sulphides, including pyrite (Zolotov & Shock Reference Zolotov and Shock2005).
In a progressively drier and colder planet with a thin atmosphere, subsurface habitats offer a series of protective advantages that are lacking in surface environments. These ecosystems are protected against harmful Solar radiation and can be independent of surface conditions. Assuming that most of the surface water on Mars sublimed into the atmosphere or retreated into the crust, a small percentage of the water in the subsurface could remain liquid in the form of thin films or as brine pockets in saline soils, similar to processes in terrestrial permafrost, where liquid water makes up 3–8% of the total water volume (French Reference French1976). Terran microorganisms have devised different strategies to adapt to temperatures below the freezing point of water. The lowest established temperature for photosynthesis and growth among terrestrial organisms seems to be −20°C (Rivkina et al. Reference Rivkina, Friedmann, McKay and Gilichinsky2000). Low rates of metabolic activity have been recorded at temperatures as low as −28°C in frozen soils, both in Arctic and Antarctic permafrost. In permafrost, up to 5% of unfrozen water prevents cells from biochemical death and mechanical destruction from growing ice crystals; the viable cells present are in an overcooled state and in equilibrium with surrounding unfrozen water films (Gilichinsky et al. Reference Gilichinsky2007). Antarctic cryptoendolithic ecosystems can metabolize carbon at −10 to −8°C (Vestal Reference Vestal1988). An especially intriguing example of metabolic activity in ice was recently reported by Rohde and Price (Reference Rohde and Price2007). They found evidence of microorganisms in glacial ice metabolizing dissolved small molecules such as CO2, CO, O2, N2 and CH4 through diffusion into the ice lattice. They detected proteins associated with high concentrations of microbial organisms and determined, via modelling, that the minimum amount of metabolism that has to occur to ensure survival of the organism is 1900 molecules per cell per year. This metabolic rate is about six orders of magnitude lower than that necessary for exponential growth and mobility (Rohde & Price Reference Rohde and Price2007), but nevertheless this finding may point to a possibly feasible survival mechanism in harsh ice habitats.
Microorganisms can adapt to lowering temperatures by increasing the ‘fluidity’ of their membranes, thereby avoiding the liquid to gel-crystalline membrane transition that would result as the temperature is lowered (Finegold Reference Finegold1996). Increasing membrane fluidity is accomplished by modifying the structure or the composition of lipids that compose the membrane (Russell Reference Russell1990). Other survival strategies include the synthesis of stress proteins, reduction in cell size, dormancy or sporulation, or the use of antifreeze intracellular solutions (elaborated on below).
Microorganisms with antifreeze intracellular solutions
The high salt content of the soils and ice in permafrost lowers the freezing point, thus allowing metabolic activity to low temperatures. However, the ionic strength of the solution would be very high, making it difficult for most species to survive (Gilichinsky et al. Reference Gilichinsky2007), and requiring sophisticated microbial adaptation mechanisms to inhabit these areas. Experimental data demonstrate that halophilic bacteria remain viable at −80°C in the presence of 25% NaCl (Mancinelli & Landheim Reference Mancinelli and Landheim2002).
Houtkooper & Schulze-Makuch (Reference Houtkooper and Schulze-Makuch2007a) have recently proposed a plausible adaptation of Martian organisms to increasingly cold conditions. The authors suggested that organisms inhabiting a cold Martian surface would utilize a water–hydrogen peroxide (H2O2–H2O) mixture, rather than water, as an intracellular liquid. This adaptation would have the particular advantages in the Martian environment of providing a low freezing point, a source of oxygen, and hygroscopicity. Hygroscopicity would be an especially useful trait as it might allow the putative organisms to scavenge water molecules directly from the Martian atmosphere in an increasingly arid environment.
The surface of Mars has been intensely bathed by Solar radiation for several billion years. The intracellular space of any putative microorganisms present on or near the surface would therefore have been subjected to intense radiolysis processes. The primary oxygen radicals generated in the radiolysis of water are hydroxyl radicals (OH•) and peroxyl radicals (R-O2•) (von Sonntag Reference von Sonntag1987). Daly et al. (Reference Daly2007) recently reported that cell suspensions of the radiation-resistant species Deinococcus radiodurans released H2O2 in concentrations of 2×10−5 molar, probably produced by intracellular Mn(II,III) redox cycling, when exposed to 10 kgy of ionizing radiation. The radical and peroxide species are in turn responsible for the DNA damage observed in radiation exposure experiments (Daly et al. Reference Daly2007). Similarly, microorganisms dwelling on the Martian surface and adapted to the high doses of Solar ionizing radiation that reach the surface would naturally accumulate, and be adapted to, high concentrations of intracellular H2O2 and other radical species, thereby pointing to a possible evolutionary trajectory to support the H2O2–H2O life hypothesis suggested by Houtkooper & Schulze-Makuch (Reference Houtkooper and Schulze-Makuch2007a).
Life associated with hydrothermal systems
If life evolved on Mars, it may have left behind a fossil record in hydrothermal environments due to suitable preservation conditions combined with an abundance of long-term energy sources and water. Alternatively, extant life may still be present at moderate depths in the subsurface. Small, single-cell prokaryotes were vitally important in the evolution of life on Earth, and these microorganisms are exquisitely adapted to environmental extremes of acidic pH, high temperature, salinity, and anoxic conditions (e.g. Schulze-Makuch & Irwin Reference Schulze-Makuch and Irwin2004). Numerous authors place the origin of life on Earth at sites with hydrothermal activity (e.g. Holm Reference Holm1992; Kompanichenko Reference Kompanichenko1996; Imai et al. Reference Imai, Honda, Hatori, Brack and Matsuno1999; Leman et al. Reference Leman, Orgel and Reza-Ghadiri2004), and if fossilized and/or extant life exists on Mars, prime exploration targets would be sites of ancient and possibly active hydrothermal activity (e.g. Dohm et al. Reference Dohm, Ferris, Barlow, Baker, Mahaney, Anderson and Hare2004; Schulze-Makuch et al. Reference Schulze-Makuch, Dohm, Fan, Fairén, Rodriguez, Baker and Fink2007). A listing of top priority sites for hydrothermal activity on Mars has recently been proposed, which includes Apollinaris Patera, the Elysium rise volcanic province, and Nili Fossae (see Fig. 6 and Schulze-Makuch et al. (Reference Schulze-Makuch, Dohm, Fan, Fairén, Rodriguez, Baker and Fink2007)).

Fig. 6. Apollinaris patera is a 6 km high and 150 km diameter volcano with a large (200 km long) fan-like deposit on its southern flank. Image courtesy of E. Goldbaum from the University of Buffalo.
An extremely acidic endolithic microbial community has been described by Walker et al. (Reference Walker, Spear and Pace2005) inhabiting the pore space of rocks in the geothermal area of Yellowstone National Park, USA, which is an analogue site of possibly active hydrothermal sites on Mars. Subjected to silica mineralization, the endolithic communities can become fossilized and potentially preserved in the geological record. If similar processes occurred on Mars, ancient hydrothermal sites will provide important clues about early life associated with Martian geothermal environments.
Endogenic activity on Mars has been manifested as large-scale, tectono-magmatic complexes, Tharsis and Elysium (Komatsu et al. Reference Komatsu, Dohm and Hare2004), and to a lesser extent in volcanic provinces that have formed along impact-induced basement structures. Impact craters of varying sizes, degrees of preservation (from pristine to highly degraded), and morphologic characteristics mark an active exogenic-induced history at the Martian surface. The presence of significant amounts of ground ice or water would cause impact-induced hydrothermal alteration at Martian impact sites and nearby surroundings (Schulze-Makuch et al. Reference Schulze-Makuch, Dohm, Fan, Fairén, Rodriguez, Baker and Fink2007). The primary heat source of a hydrothermal system associated with a complex impact crater is likely to be shock-emplaced heat. The estimated lifetime of impact-induced hydrothermal systems on early Mars ranges from 67 000 years for a 30 km crater, 380 000 years for a 180 km crater, to nearly 10 million years for a Hellas-sized basin, depending strongly on the assumed permeability of the subsurface (Abramov & Kring Reference Abramov and Kring2005). Both geological evidence and modelling suggest that the flow of hydrothermal fluids in large craters will be concentrated at the margins of the melt sheets in zones of higher permeability, focused along the crater rims and central uplifts. Hydrothermal modelling conducted by Rathbun & Squyres (Reference Rathbun and Squyres2002) indicated that a lake could form in a large, complex impact crater from the associated heat of the impact, even under current Martian atmospheric conditions.
Thus, Martian environmental conditions are certainly challenging. However, habitable conditions certainly existed in the Martian past and may exist in certain environmental niches even today. Another alternative would be the use of specialized adaptation mechanisms by putative Martian life.
Evidence for past martian life?
The Martian meteorite ALH84001
McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) made a case for ancient life on Mars based on detailed analyses of the ALH84001 meteorite. The authors suggested several lines of evidence for relic biological activity present in the meteorite (Fig. 7). While each of the observations had reasonable alternative non-biological explanations, the totality of their observations, if considered collectively, was claimed to constitute evidence for microbial activity on early Mars.

Fig. 7. Top left: The ALH84001 Martian meteorite. Scale bar is approximately 1 cm. Top right: Carbonate globules in a small piece of the meteorite. Scale bar is approximately 1 mm. Bottom left: Putative bacteria nanofossils within the meteorite. Scale bar is approximately 100 nm. Photo courtesy of NASA/JSC. Bottom right: Two suggested lines of evidence for fossil magnetosomes within the meteorite. The three transmission electron microscopy images show individual magnetite particles with sizes and shapes that match those of bacterial magnetosomes (from Thomas-Keprta et al. Reference Thomas-Keprta, Clemett, Bazylinski, Kirschvink, McKay, Wentworth, Vali, Gibson, McKay and Romanek2001). The arrow in the scanning electron microscopy-backscattered image shows a putative chain of magnetite particles with shape and dimensions similar to those of magnetotactic bacteria (from Friedmann et al. Reference Friedmann, Wierzchos, Ascaso and Winklhofer2001). The scale bar for the single magnetosomes is 20 nm and the scale bar for the SEM picture from Friedmann et al. (Reference Friedmann, Wierzchos, Ascaso and Winklhofer2001) is 500 nm.
Carbonate globules
ALH84001 contains secondary carbonate minerals in the form of globules from 1 to 250 μm across that formed between 1.3 and 3.6 billion years ago on Mars (see Fig. 7 and Knott et al. (Reference Knott, Ash and Turner1995)). The conditions in which the carbonate globules formed are central to the discussion regarding relic evidence of life in ALH84001. Petrographic and electron microprobe results (Mittlefehldt Reference Mittlefehldt1994; Scott et al. Reference Scott, Yamaguchi and Krot1997) indicate that the carbonates formed at relatively high temperatures (>500°C). However, the stable oxygen isotope data indicate that the carbonates formed between 0 and 80°C (Romanek et al. Reference Romanek, Grady, Wright, Mittlefehldt, Socki, Pillinger and Gibson1994), a range of temperatures compatible with life. The magnetic properties of pyroxene grains within the ALH84001 seemed to reflect primary features of the original martian magnetic field, again supporting a low-temperature origin for the carbonate globules (Kirschvink et al. Reference Kirschvink, Maine and Vali1997), but thermochronometry analyses indicated that the meteorite may have been shocked multiple times, reaching peak temperatures of 400°C (Min & Reiners Reference Min and Reiners2007). None of the studies on the origin of the carbonate globules published since 1996 has provided conclusive evidence for the conditions in which the globules formed. Currently there seems to be a consensus that the carbonates did form at temperatures below 300°C. This temperature range is not conclusive, but being an upper-end value it cannot be used to rule out the biogenic hypothesis.
Complex organic compounds
Polycyclic aromatic hydrocarbons (PAHs) are complex organic compounds with two or more fused aromatic rings. PAHs were detected in the interior of ALH84001 at a concentration in the parts per million range (McKay et al. Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996). The restricted types of PAHs identified in the meteorite and their apparent association with the carbonate globules was taken as indicative of diagenetic alteration of microorganisms accumulated within ALH84001. The controversy around this finding centred on whether these organic compounds formed on Mars, or represent terrestrial contamination from the Antarctic ice where ALH84001 was found. McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) noted that the content of PAHs increased towards the centre of the meteorite, with a tendency to accumulate around the carbonate globules, while the meteorite is depleted in PAHs near its fusion crust (Clemett et al. Reference Clemett, Dulay, Seb Gillette, Chillier, Mahajan and Zare1998). This suggests that the PAHs were already present in the meteorite when it landed on Earth. On the other hand, positive Carbon-14 analyses suggest that a large portion of the organic carbon present in the meteorite is terrestrial contamination, despite a small percentage (~8%) which has no Carbon-14 signature and is too old to be terrestrial. This carbon component remains to be characterized, and could be an inorganic carbonate phase or a high molecular weight organic component (Jull et al. Reference Jull, Courtney, Jeffrey and Beck1998; Becker et al. Reference Becker, Popp, Rust and Bada1999). Other meteorites with no indigenous PAHs also found in the Allan Hills region are clean of terrestrial PAH contamination (Clemett et al. Reference Clemett, Dulay, Seb Gillette, Chillier, Mahajan and Zare1998), and the heterogeneous distribution of PAHs and their relatively large concentrations within the rock compared with the Allan Hills ice are hard to reconcile with contamination processes.
Coexistence of iron-oxides, iron-sulphides and carbonates
McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) reported the coexistence of nanophase magnetite (Fe3O4) and pyrrhotite (Fe1-xS, x=0 to 0.2) crystals in rims surrounding the carbonate globules. The authors argued that while both minerals can be formed through biotic and abiotic processes, their coexistence was difficult to explain by simple inorganic precipitation models. Alternatively, some bacteria co-precipitate intracellular iron-oxide and iron-sulphide particles, and the biologically controlled mineralization of iron-sulphides and magnetite can take place under anaerobic conditions (Blakemore Reference Blakemore1982; Petersen et al. Reference Petersen, von Dobeneck and Vali1986; Bazylinski & Frankel Reference Bazylinski and Frankel2003; Frankel & Bazylinski Reference Bazylinski and Frankel2003). However, Anders (Reference Anders1996) pointed out that some C-chondrites, a different type of meteorite, also have a similar suite of mineralogies, but clearly formed under abiotic conditions.
Fossilized ancient microbes
McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) reported ovoid and elongated forms ranging in size from 20 to 100 nm in their longest dimension (Fig. 7). These forms were similar to terrestrial nanobacteria and fossilized filamentous bacteria found in calcite concretions, travertine and limestone (Folk Reference Folk1993), a suggestive argument in favour of their biotic origin. The authors ruled out any possible artefacts associated with sample preparation, and the same analyses conducted on other meteorites recovered from Allan Hills did not show any of the structures, which ruled out a possible terrestrial origin. However, Bradley et al. (Reference Bradley, Harvey and McSween1997) found that similar biomorphs were lamellar growth steps on pyroxene and carbonate crystals, and their segmented surface merely artefacts due to the gold coating process followed during sample preparation for electron microscopy. Accepting that argument, McKay et al. (Reference McKay, Gibson, Thomas-Keprta and Vali1997) replied that it did not apply to the entire suite of biomorphs in ALH84001, and that some of them could still be remnants of Martian microorganisms. Folk & Taylor (Reference Folk and Taylor2002) reported 30–70 nm spheres and ovoids in uncoated samples, thus suggesting that sample preparation was not an issue. In the same study, the authors reported abundant nanobacteria-like structures on pyroxene/olivine crystals of ALH84001, similar to those reported by McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) associated with the carbonate globules, although they could not rule out terrestrial contamination.
However, it was the size of these biomorphs, 100 times smaller than the smallest known organism on Earth, that made the whole argument unconvincing for many. The main critics of the biogenic hypothesis argued that something that small could not contain all of the molecules necessary for basic cellular activity. The images presented by McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) stirred a debate that resulted in the meeting of a panel of experts in microbiology to discuss the size limits of life. Before the panel of experts released their conclusions, a number of publications related to nanobacteria had already come forward. Uwins et al. (Reference Uwins, Webb and Taylor1998) reported the detection of living colonies of nano-organisms on Triassic and Jurassic sandstones and other substrates. These nanobes had cellular structures similar to Actinomycetes and fungi, but their diameters ranged from 20 to 100 nm. Ultrathin sections revealed membrane-like structures and different staining techniques indicated the presence of DNA (Uwins et al. Reference Uwins, Webb and Taylor1998). Kajander et al. (Reference Kajander, Kuronen, Akerman, Pelttari and Ciftcioglu1998) reported nanobacteria isolated from blood and blood products. The authors observed growth in culture plates seeded with samples that had been filtered through 0.07 μm pores and estimated a lower size limit for the nanobacteria of 80 nm in diameter. Kajander & Ciftcioglu (Reference Kajander and Ciftcioglu1998) also reported the culture of nanobacteria and the partial characterization of a nanobacterial ribosomal RNA.
These results were later debated by Cisar et al. (Reference Cisar, Xu, Thompson, Swaim, Hu and Kopecko2000), who argued that the observed bacterial growth was, in fact, inorganic precipitates and that the isolated RNA was likely to be a contaminant from the reagents used in the experiments. Taking into account these precedents, and considering theoretical constrains about the minimum amount of biomolecules required for the basic living processes, the consensus of the panel of experts dictated that a sphere of 200 nm in diameter was the minimum volume required for a single-cell organism (Steering group for the Workshop on Size Limits of Very Small Microorganisms, National Research Council 1999). With this threshold the putative biomorphs in ALH84001, and some of the reported terrestrial nanobacteria, did not seem to make the cut. However, new findings seem to call for a redefinition of the theoretical size limits of life. Baker et al. (Reference Baker, Tyson, Webb, Flanagan, Hugenholtz, Allen and Banfield2006) discovered nano-archea by genomic analysis ranging in length from 193 to 299 nm in biofilms of an acidic environment. Some of the reported organism's sizes fall below the theoretical limit of 200 nm.
Biogenic magnetite particles
Perhaps the most contentious discussions erupted about the magnetite grains in ALH84001 (Fig. 7). They were claimed by McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) to represent a biomarker for life on Mars. The original argument was based on the single-domain crystals, purity, and lack of structural defects of the magnetite grains. This idea was extended by Thomas-Keprta et al. (Reference Thomas-Keprta, Bazylinski, Kirschvink, Clemett, McKay, Wentworth, Vali, Gibson and Romanek2000), who argued that 25% of the magnetite crystals in ALH84001 conformed to six properties, which constitute a robust magnetite assay for biogenicity (MAB). The MAB cannot be accounted for by any known abiotic process; however, magnetotactic bacteria, a widespread type of aquatic prokaryote on Earth, synthesize intracellular magnetite crystals called magnetosomes, which typically meet all properties in the MAB (Fig. 7). The magnetite crystals in the magnetotactic bacteria appear aligned in chains, a highly unstable configuration that is achieved by means of cytoskeletal microfilaments and proteins (Komeili et al. Reference Komeili, Li, Newmann and Jensen2006; Scheffel et al. Reference Scheffel, Gruska, Faivre, Linaroudis, Plitzko and Schüler2006). Comparative studies of morphological and structural defects between magnetite crystals from magnetotactic bacteria and from ALH84001 also seemed to support the biogenic origin of the latter (Taylor et al. Reference Taylor, Barry and Webb2001).
Barber & Scott (Reference Barber and Scott2002) noted that solid-state diffusion as a result of carbonate decomposition during impact heating could result in magnetite nanocrystals similar to those found associated with the carbonate globules. Thomas-Keprta et al. (Reference Thomas-Keprta, Clemett, Bazylinski, Kirschvink, McKay, Wentworth, Vali, Gibson and Romanek2002) responded by pointing out that the heat necessary to decompose iron carbonates and form magnetite was not present and would require a homogenization of all magnetic dipoles. Instead, they observed considerable heterogeneity in the ALH84001 carbonates inconsistent with significant heating. Golden et al. (Reference Golden, Ming, Morris, Brearley, Lauer, Treiman, Zolensxy, Schwandt, Lofgren and McKay2004) presented detailed electron microscopy work on magnetite crystals extracted from ALH84001, and compared them with magnetite crystals from the magnetotactic bacteria strain MV-1. The authors concluded that the shape of the [111]-elongated magnetite crystals in ALH84001 is not identical to that from the bacterium MV-1, an argument that somewhat undermines the biogenic hypothesis. Bell (Reference Bell2007) conducted shock-recovery experiments with naturally occurring siderite, and obtained magnetite crystals with a similar composition, size and morphology as those found in ALH84001. The shock temperatures required for siderite composition were above 470°C, somewhat in excess of the 300°C upper limit mentioned above for the formation of the carbonate globules. The author argued that local thermal excursions within the meteorite could account for the high temperatures required by this process, without substantially altering the bulk temperature of the rock.
While several authors have concluded that the magnetite crystals in ALH84001 cannot be taken as evidence for biological activity, it is important to note that some results supporting the biogenic hypothesis remain undisputed. Particularly intriguing is the claim by Friedmann et al. (Reference Friedmann, Wierzchos, Ascaso and Winklhofer2001) that chains of magnetite particles are present in ALH84001 in close association with the carbonate globules. The authors used backscattered scanning electron microscopy to study undisturbed, carbonate-rich fragments of the meteorite, and imaged chains of single-domain magnetite crystals, the trademark characteristic for magnetotactic bacteria. The chain arrangement of magnetic particles is highly unstable and represents a powerful tool to assess biogenicity. Barber & Scott (Reference Barber and Scott2002) argued that magnetite crystals growing on ledges and kink sites on microfractures could align in chains; however, they failed to support their claim with any form of evidence.
The seminal paper by McKay et al. (Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996) inspired a large number of studies aiming to prove or disprove the evidence for the hypothesis of past life on Mars. While some lines of evidence favouring the biogenic hypothesis have been put into question, the presence of chains of magnetic single-domain particles remains plausible, albeit controversial. Irrespectively of their origin in ALH84001, these structures ought to be considered as important biomarkers and should therefore be searched for in samples returned from Mars in the future.
New signs of ancient life in other Martian meteorites?
Gibson et al. (Reference Gibson, McKay, Thomas-Keprta, Wentworth, Westall, Steele, Romanek, Bell and Toporski2001) reported possible biogenic features in two additional Martian meteorites, Nakhla and Shergotty, which indicated a strong resemblance to very similar structures that were almost surely biogenic in origin, discovered in Earth rocks. More recently, Fisk et al. (Reference Fisk, Popa, Mason, Storrie-Lombardi and Vicenzi2006) reported tunnel and borehole-like structures in basalts from Earth, and also in the Martian meteorites Nakhla and Lafayette. They reported that these tunnel structures tested positive for the presence of cellular material in the terrestrial samples, and that the tunnel structures in Nakhla were indistinguishable in size, shape, and distribution from the Earth samples. Tunnel structures had previously been discovered in 3.5 billion-year-old rocks in the Barberton Greenstone Belt in South Africa (Furnes et al. Reference Furnes, Banerjee, Muehlenbachs, Staudigel and de Wit2004), providing evidence of submarine microbial activity during the very early history of Earth. These new indications of possible life in other Martian meteorites are tenuous, however, mostly because these meteorites were not well protected from possible organic contamination that could have derived from Earth microbes since their collection. For example, organic ingredients were used in the preparation and preservation of samples from Nakhla. Nevertheless, the observations of tunnel structures made in the other Martian meteorites are consistent with the hypothesis of ancient life on Mars, and indications for the presence of fossil life on Mars are accumulating.
Revisiting the Viking mission
The Viking programme, which included two unmanned space missions to Mars, Viking 1 and Viking 2, was the most ambitious mission ever sent to Mars. Despite the remaining and on-going controversy about the Viking life-detection experiments it was highly successful, providing an enormous amount of information about the environmental conditions on Mars. Detailed descriptions of the biology payload onboard the Viking landers can be found elsewhere in the literature (Oyama Reference Oyama1972; Horowitz et al. Reference Horowitz, Hobby and Hubbard1977; Klein Reference Klein1978; Levin Reference Levin1997). Here we focus on the mission results and their interpretation with regards to possible life on Mars.
The initial results from both landers were very exciting, but also very confusing. All three experiments observed chemical changes that indicated the possible presence of life, although the signals were not always as large as expected and eventually tapered off, starting to cast doubt on a biological explanation.
First, the Gas Exchange (GEx) experiment was conducted in the humid mode. The nutrient medium was added in such a way that the soil did not come into contact with the nutrient, but was exposed to water vapour in the atmosphere. Obtained data indicated that some carbon dioxide and nitrogen gas was desorbed from the soil, and there was a surprising and rapid accumulation of oxygen after humidification each of the times the experiment was carried out. The release of oxygen upon humidification had not been previously observed with surface samples either from Earth or the Moon, and is still puzzling today. After the initial rapid gas release, later addition of water to the soil caused no further release of oxygen. In another experiment that was conducted in the humid mode at a sterilizing temperature of 145°C, oxygen was again released from the sample, suggesting a chemical explanation for the observed phenomenon.
In the second mode of analysis, nutrient was in contact with the samples (heterotrophic mode). Soon after the nutrient solution came into contact with the samples, about 30% of carbon dioxide gas went into solution. Also noted was an uptake of oxygen that previously had been released in the humid mode. With continued incubation of these samples, carbon dioxide was slowly and continuously produced, so that eventually the total carbon dioxide returned to the original levels and thereafter continued to increase with time. No other gas changes were noted, even in experiments that involved incubation periods of several months. The absorption of carbon dioxide in this manner was also observed in samples that had been sterilized at 145°C, and similar reactions had been seen in sterile terrestrial samples in pre-mission testing (Oyama & Berdahl Reference Oyama and Berdahl1977; Oyama et al. Reference Oyama, Berdahl and Carle1977).
In the Labelled Release (LR) experiment the addition of an aqueous solution of dilute organic compounds with radioactive 14C to Martian samples resulted in a rapid release of labelled gas (Fig. 8). The process was virtually eliminated by prior heating of the samples at a sterilizing temperature of about 160°C for three hours, and was substantially reduced by heating to only 45–50°C (Levin & Straat Reference Levin and Straat1981). Upon prolonged incubations there was a slow continued release of labelled gas after the initial reactions were over (Levin & Straat Reference Levin and Straat1976, Reference Levin and Straat1977), comparable to the observations in the GEx experiment. Also, each time additional liquid was added, about 30% of the labelled gas in the test cell went into solution. In contrast to the results obtained in the GEx experiment, however, storage of the samples for two to four months essentially eliminated the agent responsible for the rapid decomposition of the nutrient in the LR experiment (Klein Reference Klein1978).

Fig. 8. Radioactivity evolved in the Labelled Release experiment following the first injection of radioactive nutrient to each analysis cycle in the Viking 2 Lander. A fresh sample was used for each cycle except cycle 5 which used a sample stored approximately 84 Sols (Martian days) at 7°C prior to injection. The sample used in cycle 3 was obtained from under a rock. Cycles 1, 3 and 5 were active sequences, whereas cycles 2 and 4 were control sequences in which the samples were heated for three hours at approximately 51.5 and 46°C, respectively, prior to nutrient injection. All data have been corrected for background counts observed prior to injection (figure courtesy of Gilbert Levin, Spherix, Inc.).
In the Pyrolytic Release (PR) experiment assimilation of 14C into organic constituents of putative microorganisms was measured. The organic carbon was derived from 14CO2 and/or 14CO using samples of surface soil incubated under a close simulation of ambient conditions, performed under light or dark conditions, with or without added water vapour (Hubbard Reference Hubbard1976). In the PR experiments, positive reactions were found at both Viking landing sites (see Table 1 and Horowitz et al. (Reference Horowitz, Hobby and Hubbard1976)). Prior heating of a sample at a sterilizing temperature of 175°C for three hours drastically cut down the reaction, while heating at 90°C appeared to have no effects (Klein Reference Klein1978). The collected data indicated that the observed reaction was proceeding better in light, but this conclusion was based on comparing reactions under ‘light’ conditions on one lander site with ‘dark’ conditions on the other (Horowitz et al. Reference Horowitz, Hobby and Hubbard1977). Storage did not reduce the capacity of the Martian samples to yield statistically positive results. During one experiment the soil sample was first humidified for several hours, after which the test cell was heated and vented to dry out the sample. This treatment was conducted to remove, or at least greatly decrease, any major postulated oxidants such as superoxides and hydrogen peroxide. However, even after this treatment the Martian soil sample yielded a positive result.
Table 1. Data from the Pyrolytic Release experiment (Horowitz et al. Reference Horowitz, Hobby and Hubbard1976). The ‘Conditions’ column indicates whether the lamp was on or off, whether or not water vapour was injected, and whether the soil sample was heat-sterilized (control is 175°C for three hours). The radioactivity of the ‘Peak 2’ column represents organic matter synthesized from the labelled gases. Note that the lowest peak (even smaller than the control) was observed in the soil sample from Utopia 2 under wet conditions, providing a hint to some problems that putative Martian organisms might have had with excess water

The gas chromatography-mass spectrometry (GC-MS) instrument on board the Viking landers did not detect conclusively any indigenous organics in any of the samples tested at levels down to what was thought to be the parts per billion range (Biemann et al. Reference Biemann, Oro, Toulmin, Orgel, Nier, Anderson, Flory, Diaz, Rushneck and Simmonds1977; Biemann Reference Biemann1979). Much later analyses indicated that the Viking GC-MS was much less sensitive than originally thought (Benner et al. Reference Benner, Devine, Matveeva and Powell2000; Navarro-González et al. Reference Navarro-González2006), although this view remains controversial (Biemann Reference Biemann2007). The Viking experiments were designed on the assumption of a widespread distribution of Martian biota (Klein et al. Reference Klein1976; Klein Reference Klein1978). If, however, life were to exist on Mars in highly localized habitats, and perhaps constrained by unfamiliar metabolic limitations, then ambiguous results could well have been obtained in the Viking experiments, with inconclusive evidence for the presence of life on Mars (Klein Reference Klein1999).
After several months of receiving data from the Viking landers, most participating scientists believed that the results from the experiments, taken all together, could best be explained by non-biological chemical reactions. With the apparent lack of detection of any organic molecules by the GC-MS, a case for biology was difficult to make, although the GC-MS experiment would not have detected organics present in Antarctica either. Klein (Reference Klein1978) compared the merits of various chemical and biological explanations for the detailed results of each experiment. He concluded that while some of the results were consistent with a biological interpretation, most were not. The experiment that he deemed to be closest to a biological explanation was the PR experiment (Table 1).
However, Levin & Straat (Reference Levin and Straat1977, Reference Levin and Straat1981) argued that the LR experiment results were entirely consistent with a biological interpretation, based on the remarkably uniform production of gas from the LR nutrient when it was placed on Martian soil at both lander sites and, even more importantly, on the unique responses from heat-treated control samples that favoured a biological explanation over a chemical one. They showed that the Viking biology control established for all three life-detection experiments – exposing a duplicate sample to the one that produced a positive response to 160°C for three hours – eliminated the otherwise positive result. This satisfied the pre-mission criterion for confirming that the response was biological, because it was likely that chemical reagents would have survived the heating to produce another positive LR response. Moreover, improved tests showed that the active agent in the soil was destroyed at 51°C, reduced by 70% at 46°C, and eliminated after standing for three months in the dark inside the sample container held at 7–10°C. However, the samples kept their activity for up to several Martian days in the sample test chamber held at approximately 10°C before testing. This kind of thermal sensitivity profile was thought to be more consistent with microorganisms rather than chemical reactions.
Numerous authors proceeded to advance the idea that inorganic compounds were responsible for the observed behaviour in the Viking experiments (Ballou et al. Reference Ballou, Wood, Wydeven, Lehwalt and Mack1978; Banin & Rishpon Reference Banin and Rishpon1979; Oyama & Berdahl Reference Oyama and Berdahl1979; Mancinelli Reference Mancinelli1989; Quinn & Zent Reference Quinn and Zent1999; Benner et al. Reference Benner, Devine, Matveeva and Powell2000; Yen et al. Reference Yen, Kim, Hecht, Frant and Murray2000), most of them suggesting some kind of very strong oxidant that would react with the added water to produce oxygen and hydrogen, and with the nutrients to produce carbon dioxide. However, no suitable oxidant has been detected to date by any of the subsequent Mars missions. The oxidant would have to be a chemical with which we are unfamiliar on Earth, because the presence of humidity in Earth's atmosphere would immediately destroy any oxidant strong enough to react with water.
Zent & McKay (Reference Zent and McKay1994) pointed out the problems with oxidant reaction explanations under the environmental conditions on Mars. Their analysis of the Viking mission concluded that none of the hypotheses in the literature is free from serious objections, many having to do with the instability of putative oxidants in the presence of heat, light, or atmospheric carbon dioxide. Or, alternatively, the suggested hypotheses would require elaborate formation mechanisms for which there is no evidence. However, Zent & McKay (Reference Zent and McKay1994) rejected a biological explanation, believing rather that the results obtained by Viking could be best explained by some kind of heterogeneous surface chemistry, yielding one or more types of oxidizing surfaces on the Martian regolith particles.
A biological explanation for the Viking results was recently advanced by Houtkooper & Schulze-Makuch (Reference Houtkooper and Schulze-Makuch2007a). They suggested that the Viking lander findings could be explained if putative Martian organisms were able to utilize a water–hydrogen peroxide (H2O–H2O2) mixture rather than water as an intracellular liquid. This adaptation would have the particular advantages in the Martian environment of providing a low freezing point, a source of oxygen, and hygroscopicity (Table 2). Hygroscopicity would be an especially useful trait as it might allow the suggested organisms to scavenge water molecules directly from the Martian atmosphere. However, at the same time it would make them extremely vulnerable to abundant liquid water (conditions used in some of the Viking experiments) against which they would have little defence.
Table 2. Comparison of chemical and biological H2O2–H2O hypotheses based on Viking observations (modified from Houtkooper & Schulze-Makuch 2007a)

Hydrogen peroxide has many roles in biology; all complex organisms produce it, mainly in their mitochondria, and it is used for many purposes such as cell signalling, modulation of growth, and activation of transcription factors. Some microbial organisms produce hydrogen peroxide (e.g. certain Streptococcus and Lactobacillus sp., see Eschenbach et al. (Reference Eschenbach, Davick, Williams, Klebanoff, Young–Smith, Critchlow and Holmes1989)), while other microbes utilize H2O2 (e.g. Neisseria sicca, Haemophilus segnis; see Ryan & Kleinberg (Reference Ryan and Kleinberg1995)), and some others such as the microbe Acetobacter peroxidans use H2O2 in their metabolism (overall reaction H2O2(aq)+H2(aq)↔2H2O; see Tanenbaum (Reference Tanenbaum1956)).
The hydrogen peroxide–water hypothesis, though highly speculative, provides a logically consistent explanation for the Viking results and has the advantage that it is testable with future missions. For example, a testing procedure using the Thermal and Evolved-Gas Analyzer instrument on board the Mars Phoenix mission was recently suggested by Schulze-Makuch et al. (Reference Schulze-Makuch, Turse, Houtkooper and McKay2008).
Methane in the Martian atmosphere
The detection of CH4 in the Martian atmosphere was reported by three different research groups (Mumma et al. Reference Mumma, Novak, DiSanti and Bonev2003; Formisano et al. Reference Formisano, Atreya, Encrenaz, Ignatiev and Giuranna2004; Krasnopolsky et al. Reference Krasnopolsky, Maillard and Owen2004). In addition, Mumma et al. (Reference Mumma, DiSanti, Novak, Bonev, Dello Russo, Hewagama and Smith2005) detected the oxidation products of methane H2CO, CH3OH, and C2H6. Methane concentrations were observed at a level of about 10 parts per billion, which seem to be about the maximum levels of methane present, since it has not been detected by the cryogenic echelle spectrograph at the NASA Infrared Telescope Facility on Mauna Kea, Hawaii, at a concentration of 14 parts per billion despite perfect observation conditions (Krasnopolsky Reference Krasnopolsky2007). The remarkable importance of the methane discovery is related to the fact that methane is produced on Earth most commonly by the microbial metabolism of methanogens (Kotelnikova Reference Kotelnikova2002; Sassen et al. Reference Sassen, Milkov, Ozgul, Roberts, Hunt, Beeunas, Chanton, DeFreitas and Sweet2003). Virtually all of the methane on Earth is produced by these microbes, which have been present on the planet for more than 3.46 billion years (Ueno et al. Reference Ueno, Yamada, Yoshida, Maruyama and Isozaki2006). Secondly, methane in the Martian atmosphere is extremely unstable due to the strong ultraviolet flux on Mars and the oxidative conditions in the atmosphere and the near-surface. The photochemical lifetime of methane in the atmosphere of Mars is only about 430 years, which means that the methane must be currently produced. The spectrometer on board Mars Express was also able to measure longitudinal variations in the methane detections that showed the highest values over certain areas, such as Arabia Terra, Elysium Planitia, and Arcadia-Memnonia, suggesting localized sources for the methane (Formisano et al. Reference Formisano, Atreya, Encrenaz, Ignatiev and Giuranna2004; Kerr Reference Kerr2004).
Abiogenic ideas about possible origins of methane were advanced, starting with volcanism (Max & Clifford Reference Max and Clifford2000; Pellenbarg et al. Reference Pellenbarg, Max and Clifford2003). However, the first direct measurement of the volcanogenic production of methane, in the Mauna Loa volcano of Hawaii, revealed that it was not a measurable source of methane, producing less than 9 metric tons per year (Ryan et al. Reference Ryan, Dlugokencky, Tans and Trudeau2006). Thus, if Mauna Loa is a valid Martian analogue, these results suggest that volcanic activity is not a significant source of methane in the Martian atmosphere. Other alternative abiogenic sources proposed were (1) cometary and meteoritic impacts (Krasnopolsky et al. Reference Krasnopolsky, Maillard and Owen2004); (2) geothermal synthesis via serpentinization (Oze & Sharma Reference Oze and Sharma2005), a process that involves the breakdown of minerals in liquid water; (3) the alteration of the basaltic crust by carbon-loaded hydrothermal fluids (Lyons et al. Reference Lyons, Manning and Nimmo2005); and (4) sublimation of methane from the near-surface, hydrate-rich ice (clathrates) at low latitudes, where most of the methane appears to come from (Prieto-Ballesteros et al. Reference Prieto-Ballesteros, Kargel, Fairén, Fernández-Remolar, Dohm and Amils2006). However, the net production of cometary and meteoritic impacts combined accounts for less than 7% of the total CH4 measured (Krasnopolsky et al. Reference Krasnopolsky, Maillard and Owen2004), and the breakdown of minerals in liquid water seems to require high water temperatures, near 400 K. Clathrate hydrates are non-stoichiometric crystalline compounds in which a water-ice lattice forms cages that contain apolar gas molecules, such as methane (CH4 · nH2O) and carbon dioxide (CO2 · nH2O). Max & Clifford (Reference Max and Clifford2000) previously pointed out the probable presence of large amounts of carbon compounds trapped in water-ice-rich structures on Mars. Interestingly, both serpentinization and clathrate formation require the presence of liquid water, as does life, and they are not mutually exclusive.
As none of the abiogenic hypotheses revealed satisfactory results to explain the origin of methane, the biogenic option has gained importance. The methane in the Martian atmosphere could point to a currently restricted biosphere in suitable environmental niches on Mars. Alternatively, it could be explained as the result of an extensive and planet-wide community of microorganisms running a complete biological cycle of methane hidden underground, in which the methanogenic microorganisms are producing methane and methanotrophic microorganisms are degrading it (Hanson & Hanson Reference Hanson and Hanson1996; Roslev et al. Reference Roslev, Iversen and Henriksen1997).
Onstott et al. (Reference Onstott, McGown, Kessler, Lollar, Lehmann and Clifford2006) compared the Martian conditions with the environmental conditions in the deep rocks of the Witwatersrand Basin in South Africa. In these rocks, microbial methanogenesis and methane oxidation are both prevalent, as was confirmed by 16S DNA sequencing of the microbial communities inhabiting the rocks. The major nutrient source for methanogenic microbes was hydrogen gas, which can either be produced by abiogenic water–rock interactions or the radioactive decomposition of water in the crust. The existence of such an ecosystem was previously demonstrated in a deep basalt rock at the Hanford nuclear site in Washington State (e.g. Stevens & McKinley Reference Stevens and McKinley1995). The model developed by Onstott et al. (Reference Onstott, McGown, Kessler, Lollar, Lehmann and Clifford2006) indicated that radiolytically produced hydrogen would suffice to support the observed methane flux on Mars, if the hydrogen was microbially converted to methane. Onstott et al. (Reference Onstott, McGown, Kessler, Lollar, Lehmann and Clifford2006) also suggested measuring the abundance of methane, helium and hydrogen, and determining the carbon and hydrogen isotopic composition of methane and higher hydrocarbons such as ethane from an instrument that could be attached to a rover. Either metabolic pathway used by microorganisms would produce large isotopic fractionation shifts in heavy carbon of the residual methane. A final proof, however, would require direct detection of the cellular organic matter in a sample that exhibited a methanogenic metabolism.
Alternatively, methane could have formed by biological processes occurring in the distant Martian past, when conditions were more suitable for life. Prieto-Ballesteros et al. (Reference Prieto-Ballesteros, Kargel, Fairén, Fernández-Remolar, Dohm and Amils2006) showed that methane may be released by the destabilization of methane clathrate hydrates. Martian geology suggests that some icy crust has persisted since the Noachian Period or even from the crust's origin (Kargel Reference Kargel2004). The contemporary loss of shallow ground ice eliminates confining pressure, initiating the destabilization of the clathrate hydrates below and the release of trapped methane to the atmosphere. The release could be triggered by the cyclic movement of water/ice deposits towards the poles or the equator, induced by orbital modulations of the Martian climate for the past 0.4 million years. In fact, in some of the areas from where methane emissions appear to have originated, retreating glaciers have been detected (Head et al. Reference Head2005). A related version of this idea is that the hydrate dissociation and CH4 release is triggered by small changes in salinity (Elwood Madden et al. Reference Elwood Madden, Ulrich, Onstott and Phelps2007). The Planetary Fourier Spectrometer experiment on board Mars Express has shown that methane correlates well with the water vapour in the atmosphere and with the near-surface ice-enriched areas at middle and low latitudes identified by Mars Odyssey (Formisano Reference Formisano2005). This alternative process does not restrict the methane's age to 430 years, because clathrate hydrates can preserve methane of ancient origin for long time periods. It means that methane production did not have to be active during the past hundreds of years of Martian history, but instead it could have been originated at the bottom of the Noachian oceans from communities of methanogenic microorganisms. There, the CH4 might have been encaged into clathrates, and what we see now in the atmosphere is the record of past biological activity. Nevertheless, this explanation would still invoke biology, in fact a substantial microbial biosphere in the distant past of Mars. If so, some of these organisms would still be expected to be present in some environmental niches of Mars today.
Panspermia between Earth and Mars?
Mars and Earth are neighbouring planets and panspermia as a means to travel between planets has been advocated for quite some time (e.g. Arrhenius Reference Arrhenius1903). The possibility of panspermia is intrinsic to the question of whether life exists on Mars. There are two possibilities for the presence of life on Mars: (1) life originating on Mars under habitable conducive conditions, and (2) transfer of microorganisms from Earth to Mars. Based on our previous discussion of environmental conditions on early Mars, scenario (1) seems to be a promising option but due to our lack of knowledge about required conditions for an origin of life (for either Earth or Mars), we cannot arrive at a prudent assessment at this time. Option (2) is easier to assess, because we know that life has been prevalent on our home planet for about 3.5 billion years (Schopf Reference Schopf1993; Schopf & Packer Reference Schopf and Packer1987; Rosing Reference Rosing1999; Furnes et al. Reference Furnes, Banerjee, Muehlenbachs, Staudigel and de Wit2004; Schulze-Makuch et al. Reference Schulze-Makuch, Irwin, Lipps, LeMone, Dohm and Fairén2005) or perhaps longer. Thus, the remaining problem that we need to discuss for option (2) is whether microorganisms can spread from Earth to Mars.
Any organism taking this type of journey would have to survive a series of hazards, including survival of the meteorite impact that ejects the organism into space from the planet of its origin; maintenance of viability for long durations of time inside the meteoritic material; intense ultraviolet and cosmic radiation, cold, and vacuum; and the shock and heat of impact on the planetary body to which the organism is transferred (Schulze-Makuch & Irwin Reference Schulze-Makuch and Irwin2004).
It is necessary to consider the huge hypervelocity impact of a large object, such as a meteorite or a comet, required to extract a rock fragment from the planetary surface of Earth and eject it into space. This will produce an intense heating of the ejected materials, as well as a great acceleration. Any cells included in the rock will be subjected to acceleration, jerk, shock pressure, and heating, applied singly or in combination. Nicholson et al. (Reference Nicholson, Fajardo-Cavazos, Langenhorst and Melosh2006), Horneck (Reference Horneck2006) and Stöffler et al. (Reference Stöffler, Horneck, Ott, Hornemann, Cockell, Moeller, Meyer, de Vera, Fritz and Artemieva2007) have simulated the impact process bombarding granite, quartz and gabbro targets, respectively, embedded with bacterial endospores of Bacillus subtilis. After heating the rock fragments up to 80°C and subjecting them to a shock pressure between 5 and 50 GPa, followed by incubation, all three studies separately reported a very similar survival rate of up to 0.01% bacteria (a total of about 10 000 bacteria), confirming the viability of the initial stage of the panspermia process.
The next challenge is the space environment, which is hostile to life. The damaging potential of ultraviolet and particle radiation, the extremely low temperature and non-existent vapour pressures exclude any forms of active life. However, there is increasing evidence that microbes, especially when in the dormant spore form, can survive space conditions fairly well (Horneck Reference Horneck1981; Koike et al. Reference Koike, Oshima, Koike, Taguchi, Tanaka, Nishimura and Miyaji1991; Nicholson et al. Reference Nicholson, Munakata, Horneck, Melosh and Setlow2000). This is especially the case if the microbe is surrounded by a thin layer of solid material that would shield it from cosmic and ultraviolet radiation. The effect of the vacuum of space is another constraint. Some space experiments have shown that up to 70% of bacterial and fungal spores survive 10 days exposure to space vacuum, even with no protection (Horneck Reference Horneck1993). Survival rates increased when Bacillus subtilis spores were embedded in salt crystals, or if they were exposed in thick layers (Horneck et al. Reference Horneck, Bücker and Reitz1994). Other studies showed that bacterial survival rates decreased by two to four orders of magnitude when exposed to space vacuum and short-wavelength ultraviolet radiation (Saffary et al. Reference Saffary, Nandakumar, Spencer, Robb, Davila, Swartz, Ofman, Thomas and DiRuggiero2002), but confirmed the protection provided by salt crystals (Mancinelli et al. Reference Mancinelli, White and Rothschild1998).
Finally, the organisms would have to face landing on the new world. This would be more favourable on a planet with lower gravity than the Earth, and easier if it occurred during a time period when Mars had a thicker atmosphere. The thicker the atmosphere, the more likely is that the impactor breaks, which would reduce the speed of the resulting fragments in the lower atmospheric layers. This would decrease the impact with the surface to subsonic velocities (Fajardo-Cavazos et al. Reference Fajardo-Cavazos, Link, Melosh and Nicholson2005), and thus raise the chances of survival for any microorganisms travelling within the meteorite. The transfer of microorganisms from Earth to Mars is no longer merely a hypothesis, but an exciting possibility, scientifically tested in many ways.
Mileikowsky et al. (Reference Mileikowsky, Cucinotta, Wilson, Gladman, Horneck, Lindegren, Melosh, Rickman, Valtonen and Zheng2000) and Clark (Reference Clark2001) provided estimates on the likelihood of microbial survival for the different steps. Davies (Reference Davies1996) analysed this possibility for the Mars–Earth case and concluded that it is a plausible scenario. A critical parameter is travel time, which can be as little as two months for microscopic particles from Mars to Earth (Moreno Reference Moreno1988). Boulder-sized rocks, however, have been estimated to need a mean travel time of several hundred thousands to millions of years for the same distance (Melosh Reference Melosh1988). The Mars to Earth scenario is the more favoured case on physical grounds due to travel in the direction of the Sun's gravitational well. However, travel from Earth to Mars is also possible. Either way, the end result is the same: that both worlds would be inhabited by (micro)organisms and are most likely related to each other.
A transfer of organisms from Earth to Mars would also require habitable conditions for Terran organisms. While this may be a challenge today (although most organisms would probably be transferred in the form of spores and be able to wait for more habitable conditions), more conducive conditions are likely to have existed during earlier periods in the history of Mars (e.g. when oceans were present) (Fairén et al. Reference Fairén, Dohm, Baker, de Pablo, Ruiz, Ferris and Anderson2003; Perron et al. Reference Perron, Mitrovica, Manga, Matsuyama and Richards2007) and also during the purported warmer and wetter intermittent climate periods on Mars.
Discussion
We have reviewed and analysed the most pertinent developments to date with regards to whether life on Mars exists or not. There is no definitive response, and the answer to the question of whether life has already been discovered on Mars depends on the viewpoint of the observer. Some argue that it has (McKay et al. Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996; Levin Reference Levin1997, Reference Levin2007), but doubts and uncertainties remain. If a proof requires the observation under the microscope of organisms that move, metabolize and are composed of known life's biochemistry, then the answer is no. However, if the observer assumes that it is relatively likely that life either has originated on Mars under conditions that were very similar to the conditions on early Earth and/or that microbial life can easily be transferred from Mars to Earth and vice versa, then the hurdle to overcome should not be set that high. In fact, the likely event of panspermia between the terrestrial planets and the accumulation of reports of past and (limited) present habitability of Mars would seem to make it a surprising outcome if Mars was and is lifeless.
The analogy of Occam's razor is usually used to argue against life on Mars. However, if we factor in the knowledge of the adaptability of extremophilic life (e.g. Rothschild & Mancinelli Reference Rothschild and Mancinelli2001), evidence from the Martian meteorites, the Viking experiments and the detection of methane, these points in combination strongly favour the existence of Martian life. If ALH84001 were a rock from Earth from nearly any location, there would have been very little controversy that it indicated evidence of life. The same could be said for the LR results from the Viking mission, but with a little less confidence. The main arguments against a biological interpretation of the Viking mission results were points that were largely shown to be flawed, such as the lack of organics. Thus, applying the analogy of Occam's razor to what we know today, the more extraordinary claim appears to be that Mars is, and has been, lifeless.
If we accept the hypothesis for life on Mars, then the question is: where is it, and of what type is it? Obviously, this takes us into the realm of speculation, but we will attempt to make some inferences. First, there has to be some kind of ecosystem on Mars, otherwise resources would be quickly utilized and only a very scant amount of biomass could be supported. Recycling of nutrients and energy would allow the possibility of a reasonably diverse and abundant biosphere (although much less so than that on Earth).
If a biological explanation of the Viking results is entertained, the Viking observations would indicate that any biomass was low, possibly comparable with the microbial abundance in Antarctica or the Atacama Desert on Earth. Observations would also indicate that Martian organisms would be quick to adapt and capable of utilizing organic food sources. The suggested presence of heterotrophic organisms would point to a microbial ecosystem, which would have to be sustained by chemoautotrophs or photoautotrophic organisms, or both, as the basis of the food chain. Given the nutrient-poor and hostile conditions in the near-surface environment on Mars, any organisms present at the Viking Lander sites would either have to be organisms revived from their dormant state, or organisms exhibiting special adaptation mechanisms to the dry Martian environment (Houtkooper & Schulze-Makuch Reference Houtkooper and Schulze-Makuch2007a).
Further, under the nutrient-poor conditions microbial sizes should be small, probably in the nanobacteria size range. This falls in line with the putative fossilized nanobacteria in ALH84001 (McKay et al. Reference McKay, Gibson, Thomas-Keprta, Vali, Romanek, Clemett, Chillier, Maechling and Zare1996). The existence of nanobacteria has been and still is controversial, but despite the fact that viable nanoorganisms have not yet been discovered, the recent finding of an acidophilic Archaea with recovered DNA and RNA and a cell volume of below 0.006 μm3 (Baker et al. Reference Baker, Tyson, Webb, Flanagan, Hugenholtz, Allen and Banfield2006) – well below the suggested size limit by the National Research Council (1999) – warrants further examination.
The Martian meteorite evidence suggests the presence of magnetotactic bacteria on Mars. Mars had an ancient magnetic field and abundant liquid water for at least the first 600 million years, and therefore development of the ability of putative microorganisms to orient themselves to a magnetic field would make sense. Furthermore, the magnetite could serve another function. If hydrogen peroxide is extensively used in the intercellular liquid, as suggested by the H2O2–H2O hypothesis for Martian life, then magnetite could catalyze its breakdown in an energy-yielding way to produce water and oxygen. Thus, we propose here that the notion of magnetotactic bacteria and the use of hydrogen peroxide would be complementary. Could it be that these ingredients are part of a basic biochemistry in near-surface Martian organisms? Also, one line of evidence has often been overlooked in ALH84001: the close spatial association of oxidizing and reducing mineral assemblages in the carbon globules deriving from the same time (H. Vali, personal communication). These kinds of spatially linked microenvironments are usually considered a strong biomarker for life processes when examining ancient Earth rocks.
The emanation of methane suggests the presence of methanogenic bacteria on Mars, which are also common on Earth, especially in the deep biosphere. Most of them utilize hydrogen and carbon dioxide to produce methane and water. Given that volcanic activity was and probably still is a presently active mechanism on Mars (Schulze-Makuch et al. Reference Schulze-Makuch, Dohm, Fan, Fairén, Rodriguez, Baker and Fink2007), the same chemicals are likely to be present on Mars, allowing for the same methanogenic metabolic reactions as known from Earth. Another option for methane production was suggested from a photosynthetic pathway applying the hydrogen peroxide–water hypothesis (Houtkooper & Schulze-Makuch Reference Houtkooper, Schulze-Makuch, Hoover, Levin, Rozanov and Davies2007b):
This pathway would produce H2O2 biochemically through energy obtained from sunlight. The metabolism is constrained by the necessity to produce an excess of H2O2 from the environment and the release of possible metabolites. The production of H2O2 can be realized from the available constituents in the atmosphere, CO2 and H2O, when coupled to a photosynthetic reaction. In turn, the produced H2O2 could be used in an energy-yielding reaction by decomposing it to oxygen and water. Some other variations of this type of photosynthetic reaction pathway were suggested by Houtkooper & Schulze-Makuch (Reference Houtkooper, Schulze-Makuch, Hoover, Levin, Rozanov and Davies2007b); some of which would release formaldehyde or carbon monoxide as metabolites.
The most reasonable interpretation of the origin of methane is biogenic, either from past or present life, from the deep subsurface or the near-surface environment. Future experiments based on carbon isotope fractionation ratios should be performed to confirm this notion. Living organisms prefer to use the lighter 12C rather than 13C, and thus a spectroscopic analysis of the Martian CH4 could render an initial answer. The problem is that such an analysis can only be performed directly over the surface of Mars, as it is viable neither from Earth-based instruments nor from orbiters. Even from the surface, the analysis would be difficult because of the low methane concentrations. In addition, isotopic analyses do not always offer conclusive results. For example, in some hydrothermal smokers of the Atlantic Ridge, the isotopic signal is far from conclusive, as the gas is not clearly fractionated (Kelley et al. Reference Kelley, Karson, Blackman, Früh-Green, Gee, Butterfield, Lilley, Olson, Schrenk and Roe2001, Reference Kelley2005). Thus, the analysis must be extended to hydrogen isotopes, as the organisms would also prefer hydrogen to deuterium, and should perhaps be extended to heavy hydrocarbons related to methane, such as ethane or propane. It is fortunate that some near-future missions to Mars will include spectrometers for isotope measurement both in the soil (Phoenix Lander 2008) and in the atmosphere (Mars Science Laboratory, 2009–2011; Beagle 3, after 2009). It is important to note that the production of methane on a planetary scale during the Noachian time period would have resulted in a substantial increase of the greenhouse effect (Kasting Reference Kasting1997). This thermal heating would have enhanced the biomass production of methanogens in a positive feedback that would have contributed to the stability of the early Martian atmosphere and oceans.
What kind of picture can be drawn as of today? Perhaps a planet that is dominated in dry and cold periods (like those of today) by a microbial community that lives deep in the subsurface, is small in size and thrives on inorganic food sources. These chemolithotrophs could be based on iron and sulphur geochemistry, favouring the formation of sulphates and ferric iron minerals to the same extent as the primeval Earth communities favoured carbonate precipitation, and as the carbonate aqueous system on Earth is mediated by photosynthetic microbes and is a key buffer that maintains pH values favourable for life in the oceans. On Earth, microorganisms have evolved to accommodate a variety of extreme conditions, including desiccation, extreme cold, salinity and acidic conditions. Active communities of psychrophilic bacteria that thrive within liquid inclusions in frozen environments at temperatures as low as 250 K in Siberia, Greenland, the Arctic (Rivkina et al. Reference Rivkina, Friedmann, McKay and Gilichinsky2000; Bakermans et al. Reference Bakermans, Tsapin, Souza-Egipsy, Gilichinsky and Nealson2003; Junge et al. Reference Junge, Eicken and Deming2004; Tung et al. Reference Tung, Price, Bramall and Vrdoljak2006) and in Antarctica (Priscu et al. Reference Priscu, Fritsen, Adams, Giovannoni, Paerl, McKay, Doran, Gordon, Lanoil and Pinckney1998; Mahaney et al. Reference Mahaney, Dohm, Baker, Newsom, Malloch, Hancock, Campbell, Sheppard and Milner2001; de Angelis et al. Reference de Angelis, Morel-Fourcade, Barnola, Susini and Duval2005), and that derive energy from iron and sulphur compounds in areas covered by glaciers and thick permafrost in or near the Arctic (Grasby et al. Reference Grasby, Allen, Longazo, Lisle, Griffin and Beauchamp2003; Tung et al. Reference Tung, Price, Bramall and Vrdoljak2006) and the Antarctic (Mikucki & Priscu Reference Mikucki and Priscu2004), could serve as analogues of iron–sulphur-based ecosystems living at extremely low temperatures within tiny brine pockets in crustal ice on Mars. Subsurface microbial metabolism coupled to sulphate reduction can produce large amounts of CH4 (Winfrey & Zeikus Reference Winfrey and Zeikus1977; Mountfort et al. Reference Mountfort, Kaspar, Asher and Sutherland2003) at very cold temperatures and in anoxic conditions, and some groups of methanogenic Archaea are acidophilic (Bräuer et al. Reference Bräuer, Cadillo-Quiroz, Yashiro, Yavitt and Zinder2006). These types of microorganisms may be the most likely candidates as the source of the Martian methane. They could form communities of acidophilic methanogenic psychrophiles, existing contemporarily and/or in the wetter past.
Any surface dwellers would be dormant during the currently dry Martian time period, perhaps aside from some suitable niche environments such as in lava caves (Boston et al. Reference Boston, Ivanov and McKay1992). However, during the hypothesized wetter periods, for example due to periodic obliquity changes and extending for considerable time (Head et al. Reference Head, Mustard, Kreslavsky, Milliken and Marchant2003), dormant organisms in certain areas near the surface can come into action, and feed on the nutrients and autotrophic organisms. After each of these episodes, Mars will cool, the near-surface microbial dwellers become dormant, and the biosphere is once again dominated by organism in the deep subsurface thriving on inorganic energy sources. Most of them will live in favoured local areas, probably close to an area that still releases remnant heat associated with volcanism (Schulze-Makuch et al. Reference Schulze-Makuch, Dohm, Fan, Fairén, Rodriguez, Baker and Fink2007). This scenario would be consistent with the methane-detection peaks that seem to be concentrated around volcanic areas such as Arabia Terra and Elysium Planitia. Rather than a global rich biosphere as on Earth, a contemporary biosphere on Mars is likely to be much lower in numbers, very segmented into specific suitable niches, and adapted to generally nutrient-poor conditions. Alternatively, life on Mars could be widespread on the surface if microorganisms were able to adapt through natural section to the hostile surface and near-surface conditions (e.g. Houtkooper & Schulze-Makuch Reference Houtkooper and Schulze-Makuch2007a).
What can be done next? Missions should be designed that not only look aggressively for biogenic signs on Mars, but also try to hone in on suitable environments that can be tested for a diverse group of microorganisms, both fossil life and extant life. Water ice is plentiful on Mars, even if liquid-water availability on the surface is severely limited. Perhaps we ought to look for nitrogen in the Martian soil, as suggested by Mancinelli & Banin (Reference Mancinelli and Banin2003). Nitrogen is a key element for life as we know it on Earth, and it has not yet been detected on Mars other than in the atmosphere.
Conclusions
Current lines of evidence, cumulatively, strongly suggest the past and present existence of microbial life on Mars (Table 3). Considering the adaptability of extremophilic life on Earth, the results from the Viking biological experiments, the proposed evidence for biological activity from various Martian meteorites, the detection of methane in the atmosphere of Mars, the likelihood that life originated on Mars under conditions that were very similar to the conditions on early Earth, and that microbial life can be transferred from Mars to Earth and vice versa, it would be surprising if Mars has always been lifeless. Future life-seeking strategies must focus on where extant Martian life can be best detected (highest accumulation of biomass), what type of life can be expected and where remains of extinct Martian organisms may be preserved.
Table 3. Major arguments for and against life on Mars














