INTRODUCTION
Giardia lamblia (syn. Giardia duodenalis, Giardia intestinalis) is a common intestinal protozoan and causes diarrhoea in humans and animals. In many individuals, the infection remains asymptomatic, whereas some patients exhibit severe symptoms such as abdominal pain, nausea, as well as servere watery diarrhoea as a consequence of malabsorption (e.g. reviewed by Adam, 1991). In many cases, spontaneous resolution of the infection occurs after a few weeks but the disease may also develop to the chronic stage. The outcome of the infection is supposed to largely depend on the immunological status of the infected individual but non-immunological factors are also involved in the interaction between the host and the parasite (e.g. reviewed by Faubert, 2000; Eckmann, 2003; Müller and von Allmen, 2005).
In giardiasis, reinfections are common because acquired immunity against G. lamblia is not complete either due to insufficient immune defences or antigenic variation of the parasite. Many studies in natural and experimental rodent hosts addressed the question whether antibodies and, more specifically, local secretory immunoglobulin (Ig) A antibodies play a role in control of the parasite infection (Eckmann, 2003). Previous findings in the experimental mouse/G. lamblia GS/M-83-H7 model indicated that B-cell-deficient animals were unable to clear giardial infections (Stäger and Müller, 1997; Langford et al. 2002). Conversely, by comparing the courses of infection in IL-6-deficient and wild-type mice, no obvious correlation between intestinal IgA production and the elimination of the parasite in the duodenum was observed (Bienz et al. 2003; Zhou et al. 2003). Data from an investigation based on the use of different transgenic mouse strains indicated that an as yet unknown T-cell-dependent mechanism is essential for controlling the acute phase of a G. lamblia infection (Singer and Nash, 2000).
Although the investigations listed above could not completely elucidate the cellular network involved in anti-giardial immune defence it became evident that antibody-independent immune effector mechanisms directly, or indirectly, interfere with maintenance and growth of the intestinal parasite population particularly during the acute phase of the infection (Singer and Nash, 2000; Langford et al. 2002; Li et al. 2004, 2006). Several investigations in the past provided evidence that mast cells are substantially involved in intestinal elimination of Giardia (Mitchell et al. 1982; Erlich et al. 1983; Li et al. 2004). Li et al. (2004) found that mast cells are activated during a G. lamblia infection and that mast cell-deficient, or -depleted, C57BL/6 mice, failed to control such an infection. In this report, mast cells were considered to be a potential source for IL-6. Since in the abovementioned (Li et al. 2004) and other studies (Bienz et al. 2003; Zhou et al. 2003) murine IL-6-deficiency was associated with a much increased susceptibility to a G. lamblia clone GS/M-83-H7 infection, mast cell-derived IL-6 was suggested to be important for the control of the respective infection.
As outlined above, the immunological parameters of a G. lamblia infection have mostly been determined in experimental hosts. However, only very few of these investigations took into account that the outcome of giardiasis may be influenced by other infections that had eventually occurred prior to the G. lamblia infection. Although not necessarily clinically relevant, such infections may alter the immunological and physiological intestinal environment and thus increase, or reduce, the susceptibility of a host to a subsequent G. lamblia infection. The existence of such a phenomenon was exemplified in a former investigation that assessed various parameters of a double infection of mice with the gut-dwelling nematode Trichinella spiralis and Giardia muris (Roberts-Thompson et al. 1976). As evidenced by multiple studies, T. spiralis infection is accompanied by Th2 cell-mediated eosinophilia and IgE production (Rosenberg et al. 1971; Matossian et al. 1977; Morakote et al. 1992; Finkelmann et al. 1997; Watanabe et al. 2005). During the early-infective, intestinal phase, T. spiralis triggers recruitment of inflammatory cells in the mucosa (Friend et al. 1996), and a subsequent worm expulsion is an immune-mediated process involving activation of Th2-cells (Faulkner et al. 1997; Finkelmann et al. 1997; Urban et al. 2000) and mast cells (Grencis et al. 1993; Kamiya et al. 1985; Donaldson et al. 1996). In the co-infection experiment performed by Roberts-Thompson et al. (1976), T. spiralis-induced mucosal inflammation was associated with a strong reduction of the G. muris parasite load. This reduction in the giardial infection intensity turned out to be proportional to the mucosal inflammatory response that had emerged as a consequence of the T. spiralis infection.
Refering to these findings, we now performed a study to assess the impact of a murine T. spiralis infection on the course of a G. lamblia infection. For the infection experiments, we used the well-characterized G. lamblia clone GS/M-83-H7 (Aggarwal et al. 1989). As expected, ‘pre-infection’ of mice with T. spiralis larvae resulted in a transient mucosal infiltration of inflammatory cells (eosinophils and mast cells). However, in contrast to the T. spiralis/G. muris co-infection experiment described by Roberts-Thompson et al. (1976), intestinal inflammation during a T. spiralis/G. lamblia co-infection was accompanied by a transient massive increase of the Giardia parasite load. These results indicated that intestinal inflammatory reactions, induced by a T. spiralis infection, and more specifically T. spiralis-induced mucosal mast cell functions, did not contribute to the elimination of a G. lamblia GS/M-83-H7 infection.
MATERIALS AND METHODS
Animals
C57BL/6 mice were obtained from Charles River GmbH (Germany). The study was performed with 4 week-old female mice that were kept under specific pathogen-free (SPF) conditions according to Swiss regulations governing animal experimentation and rules for animal protection that restrict the use of experimental animals to a minimum.
Parasite and experimental infection
The origin, axenization and cloning of G. lamblia GS/M-83-H7 has been described by Aggarwal et al. (1989). G. lamblia trophozoites were cultivated in TYI-S-33 medium with antibiotics as previously described (Keister, 1983).
Experimental G. lamblia GS/M-83-H7 infections (6 animals per experimental time-point) were done with 106 trophozoites (suspended in 200 μl of a 0·3 M NaHCO3 solution) of G. lamblia GS/M-83-H7 using a blunt-ended needle for peroral inoculation. The course of the G. lamblia infection within mice was determined according to Gottstein et al. (1993) by quantitating the parasite burden through microscopical examination of adherent trophozoites from intestinal washes.
T. spiralis (strain ISS 384) was maintained through consecutive passages in outbred CD®-1(ICR)BR mice which were purchased from Charles River GmbH (Germany). Infectious larvae (L1) were prepared by artificial digestion of muscle tissue from infected carcasses with 1% pepsin (Sigma-Aldrich, St Louis, MO) and 1% HCl and subsequent repeated washing in H2O as described by Friend et al. (1996). The released larvae were microscopically counted and 300 larvae were resuspended in 50 μl of phosphate-buffered saline (PBS), pH 7·2, and were then applied for peroral infection of mice using a blunt-ended needle. Seven weeks after T. spiralis infection (corresponding to experimental time-point week 6 p.G.i.), infectivity of the larvae was confirmed by demonstrating that the means of the total carcass larval counts within the T. spiralis-infected and T. spiralis/G. lamblia double-infected animal groups was 6413 (range: 4360–7900; S.E.: ±634) and 5046 (range: 4080–6800; S.E.: ±464) larvae per animal. The efficiency of the T. spiralis infection was determined at a time-point when muscle larvae were expected to be fully developed (6–7 weeks after T. spiralis infection).
Determination of intestinal mast cells and eosinophils
Mice were sacrificed by CO2 euthanasia. At necropsy, approximately 0·5 cm of the small intestine was fixed in formalin and embedded in paraffin wax. Serial sections of 4 μm thickness were cut from each tissue block. One section of each deparaffinized tissue sample was stained with the Luna's method (Prophet et al. 1994) to assess the number of eosinophilic granulocytes.
The mast cell numbers were determined with an enzyme-histochemical reaction for the detection of chymase activity with a commercially available detection kit (Sigma, Catalogue No. 91-C) using Naphthol-AS-D-Chloroacetate as substrate. The only modification to the kit protocol was the use of Fast Blue BB (Base) instead of Fast Red Violet LB (Base). Staining with Naphthol AS-D-Chloroacetate reveals mast cells as well as neutrophilic granulocytes and occasional macrophages. Discrimination especially between neutrophils and mast cells can be achieved by inspection of the shape and size of the nucleus. For this reason, only cells with a visible nucleus were counted in our experiments. Counting of the cells was performed by analysing the mucosa and submucosa of an entire section using a square eyepiece graticule. The number of mm2 of the total area was assessed as well as the total cell number in this area. Cell numbers are expressed as cells/mm2.
Immunohistological staining of IL-6-positive intestinal mast cells
For the detection of IL-6 produced in intestinal cells, immunohistochemical staining using a polyclonal goat anti-rat IL-6 antibody (Santa Cruz Biotechnology, Santa Cruz, Ca, catalogue no. sc 1266) was performed. The specificity of the immunohistochemical staining was demonstrated with a blocking peptide (Santa Cruz Biotechnology, Santa Cruz, Ca, catalogue no. sc 1266P). In detail, slides were blocked (0·5% BSA-PBS/TBS, 10 min at room temperature) before the specimen was incubated with the primary antibody (diluted 1[ratio ]35 in PBS) for 45 min at room temperature. This step was followed by incubation with a mouse anti-goat horseradish peroxidase conjugate (1[ratio ]40 in PBS) for 20 min at room temperature. Endogenous peroxidase was quenched by incubating the section in H2O2 (1[ratio ]10 in methanol) for 10 min at room temperature. Staining was completed by incubation with AEC Substrate-Chromogen for approximately 20 min at room temperature. Between each incubation step slides were washed twice for 5 min in PBS. Positive staining for IL-6 resulted in a red colour indicating the antigen localization of IL-6. Slides were counterstained with Ehrlich's haematoxylin and mounted in GVA mounting solution (Zymed Laboratories Inc. San Francisco CA, USA).
Determination of serum mast cell protease 1 and IgE levels
Serum concentrations of mouse mast cell protease 1 (MMCP-1) were determined as previously described by Li et al. (2004). Levels of serum IgE were determined by using the PharMingen IgE-capture ELISA (PharMingen, San Diego, CA; catalogue number 553413) according to the instructions of the manufacturer. IgE concentrations were extrapolated from a graph of standard OD405nm-values versus concentrations.
Determination of intestinal anti-G. lamblia IgA levels
For determination of intestinal anti-Giardia IgA concentrations, we applied the procedure described by Gottstein et al. (1993), which is suitable for the extraction of IgA from intestinal epithelium and lamina propria. Immunoreactivity of intestinal IgA antibodies was tested as previously described (Stäger et al. 1997), by using a soluble protein extract from G. lamblia clone GS/M-83-H7.
Statistical methods
The significance of the differences among the G. lamblia single-infected and the T. spiralis/G. lamblia double-infected animal groups was determined by the Student's t-test using the Microsoft Excel™ program. P values of <0·01 were considered statistically highly significant.
RESULTS
Intestinal Giardia lamblia proliferation in relation to inflammatory and local antibody reactions during primary Trichinella spiralis and secondary Giardia lamblia infection
To determine the impact of a T. spiralis infection on a subsequent G. lamblia GS/M-83-H7 infection, 4 week-old female mice either remained uninfected, or were perorally infected with 300 T. spiralis larvae (week −1, representing time-point at 1 week prior to G. lamblia infection). After 1 week (week 0, time-point at which animals were infected with G. lamblia), some of the previously uninfected, or T. spiralis-infected mice were perorally infected with 106G. lamblia trophozoites. At different time-points corresponding to weeks −1, and 0, as well as weeks 1, 2, 3, and 6 post-G. lamblia infection (p.G.i.), 6 animals per experimental group (uninfected, T. spiralis-infected, G. lamblia-infected, and T. spiralis/G. lamblia-double-infected animals) were sacrificed by CO2 euthanasia.
The efficiency of the T. spiralis infection was determined by assessing mean total carcass larval counts within the T. spiralis-infected and T. spiralis/G. lamblia double-infected animal groups at a time-point where muscle larvae were expected to be fully mature (7 weeks after T. spiralis infection). Mean larval counts did not significantly differ within the two experimental animal groups (P=0·12) and were between 6413 (range: 4368–7925; S.E.: ±634) (T. spiralis-infected) and 5046 (range: 4080–6842; S.E.: ±464) (T. spiralis/G. lamblia double-infected) per animal, respectively.
Regarding the course of the G. lamblia infection in T. spiralis-pre-infected or non-pre-infected animals, the following results were obtained: animals exclusively infected with G. lamblia, exhibited a low-intensity infection and trophozoites were only detectable at weeks 1 (mean: 4·6×104 parasites per cm duodenum) and 2 (mean: 0·2×104 parasites per cm duodenum) p.G.i. (Fig. 1A). Compared to these animals, T. spiralis-pre-infected animals exhibited an approximately 5-fold and 150-fold higher duodenal trophozoite burden at weeks 1 and 2 p.G.i., respectively. Furthermore, in double-infected animals residual trophozoites were still visible at weeks 3 (mean: 6·6×104 parasites per cm duodenum) and 6 (0·2×104 parasites per cm duodenum) p.G.i. Accordingly, pre-infection of mice with T. spiralis transiently promoted the intestinal G. lamblia trophozoite proliferation and thus up-shifted both intensity and duration of the G. lamblia infection.
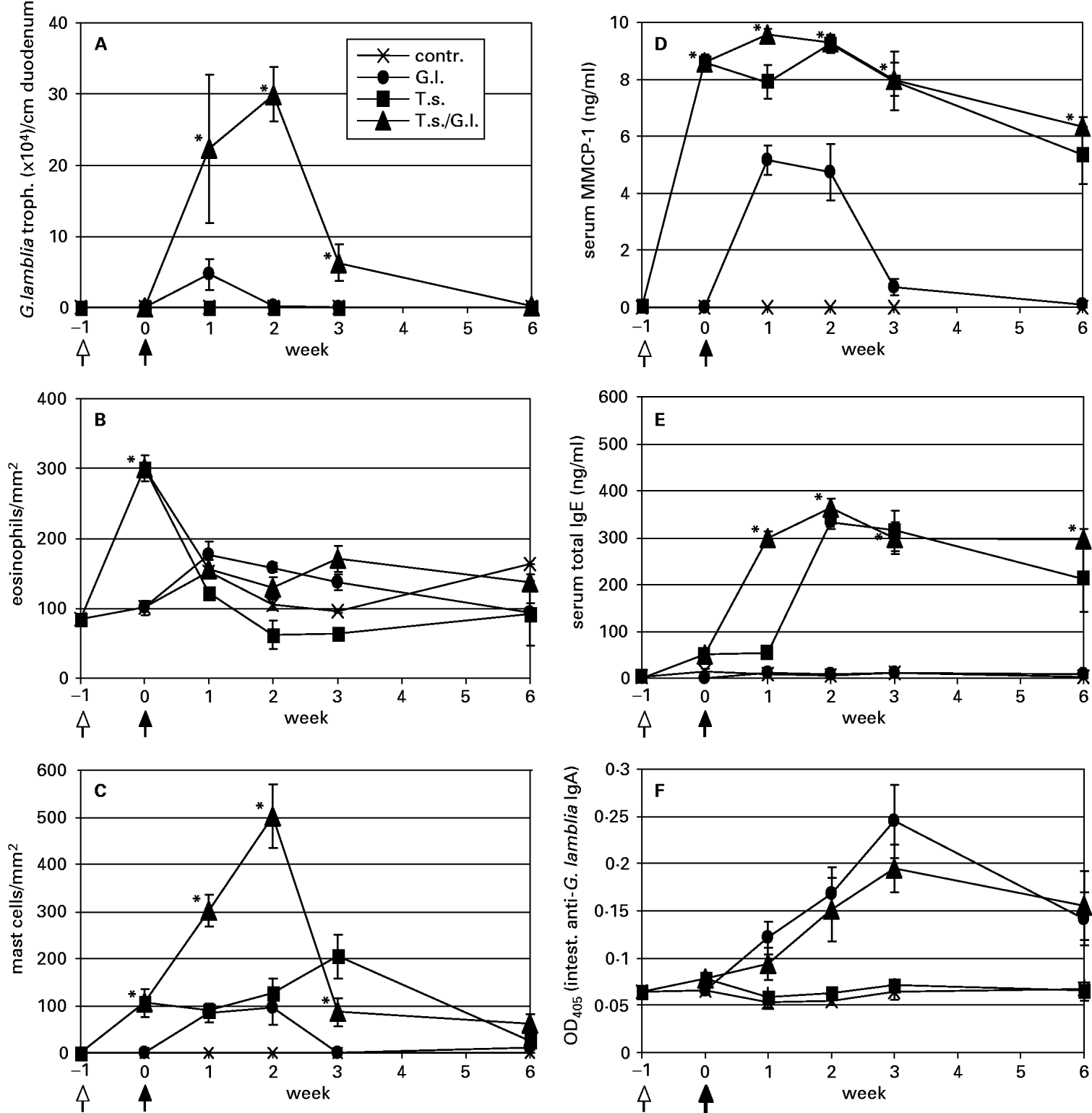
Fig. 1. Determination of intestinal Giardia lamblia trophozoite (A), intestinal eosinophil (B), and intestinal mast cell numbers (C), and serum MMCP-1 (D), serum total IgE (E), and intestinal anti-G. lamblia IgA levels in uninfected control (contr.), G. lamblia – (G.l.), T. spiralis – (T.s.), and T. spiralis/G. lamblia – (T.s./G.l.) infected mice. Samples were taken at week −1 (time-point of T. spiralis infection as indicated by an arrow with an open head), week 0 (time-point of G. lamblia infection as indicated by an arrow with a closed head), and at weeks 1, 2, 3, and 6 post G. lamblia infection. Values are given as means (±S.E.) and highly significant differences between the values representing G. lamblia- and G. lamblia/T. spiralis-infected animal groups were assessed by using the Student's t-test (*P<0·01).
In addition, we analysed various parameters linked to the intestinal inflammatory status of the different experimental animal groups in relation to the course of the G. lamblia infection in single- and double-infected mice. In particular, we found that the T. spiralis infection resulted in a transient accumulation of eosinophils in the intestinal mucosa, which already reached its maximum around the time-point where the G. lamblia infection was initiated (week 0 p.G.i.) (Fig. 1B). Conversely, the G. lamblia infection alone did not lead to a significant eosinophil infiltration.
In T. spiralis-infected mice, mucosal mast cells started to accumulate around week 0 p.G.i. and reached a maximal density around week 3 p.G.i. (Fig. 1C). At week 6 p.G.i., only relatively small numbers of mast cells resided in the intestinal mucosa of these animals. In G. lamblia-single-infected mice, mucosal mast cell numbers were somewhat lower and peak levels were observed at week 2 p.G.i. before respective cell numbers dropped to basal levels during week 3 p.G.i. Interestingly, T. spiralis/G. lamblia double-infected mice exhibited mucosal mast cell numbers that at weeks 1 and 2 p.G.i. were significantly higher than those observed in T. spiralis or G. lamblia single-infected animals. Accordingly, consecutive infection of mice with T. spiralis and G. lamblia apparently had a synergistic effect on mast cell infiltration into the mucosa.
As assessed by Li et al. (2004), the serum MMCP-1 concentration is a suitable marker to monitor the degranulation status of intestinal mast cells activated during a G. lamblia infection. In the present study, infection of animals with T. spiralis resulted in an immediate and strong induction of serum MMCP-1 already detectable at week 0 p.G.i. (Fig. 1D). In both, T. spiralis single-infected and T. spiralis/G. lamblia double-infected mice, the serum MMCP-1 levels remained relatively high until week 3 p.G.i., and only slighty declined between weeks 3 and 6 p.G.i. Compared to these two experimental animal groups, G. lamblia single-infected animals only produced approximately 50% of serum MMCP-1 between weeks 1 and 2 p.G.i., and MMCP-1 production essentially did not extend beyond week 3 p.G.i.
As previously described, murine trichinellosis is associated with IgE production that is involved in mast cell homeostasis, and that partially triggers mast cell degranulation including the release of mediators such as MMCP-1 (Gurish et al. 2004). An assay monitoring serum total IgE levels revealed that serum from T. spiralis single-infected and T. spiralis/G. lamblia double-infected mice, but not serum from G. lamblia-infected and non-infected mice, contained detectable amounts of IgE (Fig. 1E). In the 2 positive groups, IgE production started around week 0 p.G.i. and extended the final time-point (week 6 p.G.i.) of the infection experiment. Interestingly, double-infected animals exhibited a marked rise in total IgE levels between weeks 0 and 1 p.G.i. whereas in T. spiralis-single-infected animals high-level serum IgE production started between weeks 1 and 2 p.G.i.
Since in the current literature (e.g. reviewed by Eckmann, 2003) intestinal IgA is discussed as a possible immunological effector involved in the control of giardiasis, we decided to examine the different experimental animal groups in view of their intestinal anti-G. lamblia IgA-production. For this purpose, intestinal IgA was extracted from intestinal tissue samples and IgA-antibody reactivities to soluble G. lamblia GS/M-83-H7 trophozoite antigen were determined by ELISA as described in the Materials and Methods section (Fig. 1F). In this analysis, both G. lamblia single- and T. spiralis/G. lamblia double-infected animals elicited a significant intestinal IgA response to G. lamblia. Respective antibody reactions were already detectable between 1 and 2 weeks p.G.i.
Intestinal Giardia lamblia proliferation in relation to inflammatory and local antibody reactions during primary Giardia lamblia and secondary Trichinella spiralis infection
To determine the impact of a T. spiralis infection on the course of a previous G. lamblia GS/M-83-H7 infection, 5-week-old female mice were perorally infected with 106G. lamblia trophozoites (week 0). Some of these animals were sacrificed at time-points corresponding to weeks 1 (time-point at which T. spiralis infection had been carried out) and 3 p.G.i. As shown in Fig. 2A, during the acute-phase of G. lamblia infection around week 1 p.G.i., exposure of the animals to a subsequent T. spiralis infection resulted in (re-)activation of the intestinal G. lamblia trophozoite growth within a following 2-week period. However, when the secondary T. spiralis infection was performed at later stages, no relapsing of an eventual residual G. lamblia population was observed (data not shown). The efficiency of the T. spiralis infection was determined by assessing mean total carcass larval counts within the T. spiralis-infected and T. spiralis/G. lamblia double-infected animal groups at a time-point where muscle larvae were expected to be fully mature (6 weeks after T. spiralis infection, not shown). Mean larval counts did not significantly differ within the two experimental animal groups (P=0·15) and were between 6976 (range: 4865–7810; S.E.: ±462) (T. spiralis-infected) and 5703 (range: 4025–7980; S.E.: ±662) (T. spiralis/G. lamblia double-infected) per animal, respectively.
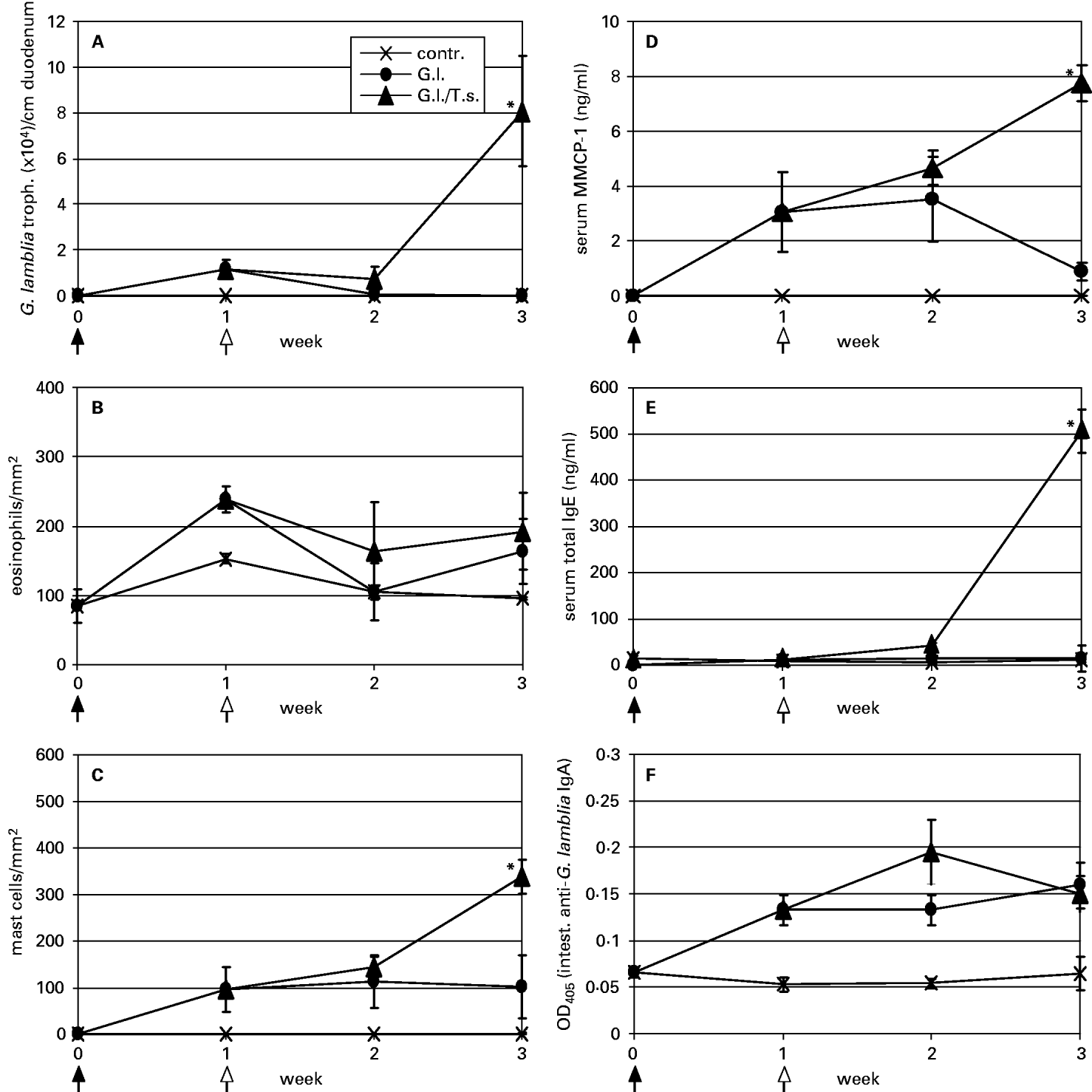
Fig. 2. Determination of intestinal Giardia lamblia trophozoite (A), intestinal eosinophil (B), and intestinal mast cell numbers (C), and serum MMCP-1 (D), serum total IgE (E), and intestinal anti-G. lamblia IgA levels in uninfected control (contr.), G. lamblia – (G.l.), and G. lamblia/T. spiralis – (G.l./T.s.) infected mice. Samples were taken at week 0 (time-point of G. lamblia infection as indicated by an arrow with a closed head), week 1 (time-point of T. spiralis infection as indicated by an arrow with an open head), and at weeks 2 and 3 post-G. lamblia infection. Values are given as means (±S.E.) and highly significant differences between the values representing G. lamblia- and G. lamblia/T. spiralis-infected animal groups were assessed by using the Student's t-test (*P<0·01).
Comparative analyses of intestinal inflammation and local antibody production during T. spiralis-induced re-activation of a G. lamblia GS/M-83-H7 infection provided the following findings: while no massive intestinal accumulation of eosonophils was detected in any of the experimental animal groups (Fig. 2B), G. lamblia single- and G. lamblia/T. spiralis double-infected mice exhibited a significant increase of intestinal mast cells (Fig. 2C). As shown in Fig. 2D, this increase coincided with a mast cell degranulation pattern. Thus, at week 3 p.G.i., G. lamblia/T. spiralis double-infected animals contained approximately 9-fold higher MMCP-1 serum levels than G. lamblia-single infected animals. Furthermore, elevated serum total IgE production was restricted to double-infected animals and was only observed at week 3 of the co-infection experiment (Fig. 2E). Conversely, anti-G. lamblia IgA was detected in both G. lamblia-single and G. lamblia/T. spiralis-double-infected mice (Fig. 2F). In both animal groups, antibody concentrations reached similar levels and started around week 1 p.G.i.
Characterization of IL-6-producing intestinal cells in Trichinella spiralis/Giardia lamblia single- and double-infected mice
Intestinal mast cell-derived IL-6 may be important for control of a G. lamblia clone GS/M-83-H7 infection in mice (Li et al. 2004). In order to find out if a previous T. spiralis infection increases murine susceptibility to infection with G. lamblia clone GS/M-83-H7 and if this is associated with a reduced IL-6-positivity of intestinal mast cells, a combined histological/immunohistological investigation was carried out on formalin-fixed, paraffin-embedded intestinal tissue, representing the different experimental animal groups from week 2 p.G.i. In these histological sections, mast cells were visualized by chymase staining. In corresponding sections, IL-6-producing cells were detected with an IL-6-specific antibody and the specificity of the immunostaining was confirmed by demonstrating neutralization of the antibody reactivity with a blocking peptide (not shown).
Typical features from the different (immuno-)histological stainings as outlined above are shown in Fig. 3. Interestingly, the vast majority of intestinal mast cells in G. lamblia single-infected mice (Fig. 3A, panel a) were located in the crypt area whereas mast cells in T. spiralis single-infected (Fig. 3B, panel a) and T. spiralis/G. lamblia double-infected (Fig. 3C, panel a) animals were located in the crypt area as well as in the villi. In uninfected control animals (Fig. 3D, panel a), no intestinal mast cells were detected. In intestinal tissue from G. lamblia-single-infected (Fig. 3A, panel b), T. spiralis-single-infected (Fig. 3B, panel b), T. spiralis/G. lamblia-double-infected (Fig. 3C, panel b), and uninfected (Fig. 3D, panel b) mice no anti-IL-6 antibody staining patterns were observed that may have reflected the distribution of intestinal mast cell infiltrates. In contrast, epithelial cells exhibited a specific and consistent reactivity with the anti-IL-6-antibodies, and in all experimental animal groups epithelial immunostaining was visible at about the same intensity. Conclusively, these results indicated that neither in single- and double-infected mice, nor in uninfected control mice, intestinal mast cells (and other intestinal cellular infiltrates) synthesized detectable amounts of IL-6. Conversely, intestinal epithelial cells seemed to produce IL-6 irrespective of the infection status of the animals investigated.

Fig. 3. Detection of intestinal mast cells and IL-6-producing cells in mice at week 2 post-Giardia lamblia infection. Enzyme-histochemical staining for mast cell chymase demonstrated different numbers of mast cells in G. lamblia – (A, panel a), T. spiralis – (B, panel a), and T. spiralis/G. lamblia – (C, panel a) infected mice but apparently no mast cells in the controls (D, panel a). Tissue areas with mast cell accumulations are indicated by arrows. Immunohistochemical staining using an anti-murine IL-6 antibody revealed a mild to moderate IL-6 expression in the epithelial cells of infected (A, B, and C, panel b) and control (D, panel b) mice. No IL-6 expression was detected in the areas of mast cell accumulation. Areas representing epithelial cell layers are indicated by arrows (magnification×200).
DISCUSSION
As recently reported, G. lamblia (clone GS/M-83-H7) infections in mice were associated with intestinal mast cell infiltration and this process seemed to be involved in resolution of infection (Li et al. 2004). The aim of the present study was to find out if intestinal mast cells – stimulated during a concurrent T. spiralis infection – are also able to modulate the course of such a G. lamblia infection. Our investigation was performed in analogy to a former T. spiralis/G. muris co-infection experiment that demonstrated a suppression of G. muris trophozoite proliferation as a consequence of a preceding T. spiralis infection (Roberts-Thompson et al. 1976). However, in contrast to the findings from this former study, our results revealed that a T. spiralis infection did not suppress, but rather stimulated, intestinal growth of a respective G. lamblia trophozoite population. The divergent outcome of these two analogous co-infection experiments may reflect differences in either the (immuno-)biology of the G. lamblia and the G. muris species or the genetic background and/or the age of the infected mice. However, the particular reasons for these differential giardial growth patterns under conditions of a concomittant T. spiralis infection still remain to be elucidated.
In our study, C57BL/6 mice pre-infected with T. spiralis demonstrated a transiently increased susceptibility to a G. lamblia (clone GS/M-83-H7) infection. Furthermore, the intensity of a primary G. lamblia infection was enhanced by secondary infection of animals with T. spiralis. In concordance with previous data (Gurish et al. 2004), the present co-infection experiment in mice revealed that early phase T. spiralis infection coincided with transient intestinal inflammation and was particularly associated with a massive eosinophil and mast cell infiltration into the mucosa, mast cell degranulation, and IgE production. Intestinal eosinophil infiltration was visible only during a short period (approximately 1 week) during the initial stage of the T. spiralis infection and thus most likely did not have a substantial influence on the course of the secondary G. lamblia infection. Conversely, in our co-infection experiments, intestinal accumulation of mast cells was observed during a much longer period (approximately 3 weeks) and this phenomenon strikingly coincided with a transient increase of the intestinal G. lamblia trophozoite burden. Thus, it became evident that mast cells emerging within the intestinal inflammatory environment during an acute T. spiralis infection were not able to control a concurrent G. lamblia infection. Rather, our findings suggested that these mast cells might have contributed to the enhanced growth of the intestinal G. lamblia population. However, this hypothesis is challenged by data from a recent study demonstrating that mast cells induced by G. lamblia display an inhibitory effect on giardial proliferation in mice (Li et al. 2004). In combination, the previous and present results outlined above may indicate that murine mast cells induced by either T. spiralis or G. lamblia were involved in functionally distinct immunological processes that either promoted, or suppressed, intestinal growth of G. lamblia trophozoites. Since both of these putatively distinct mast cell populations turned out to release MMCP-1, we concluded that the differential effect on giardial growth was probably not related to differential degranulation characteristics of these particular cells.
One possible mechanism for mast cell activation is dependent on IgE and Fcε-receptor I although other factors such as IL-10 are involved in the degranulation process as well (Ghildyal et al. 1992). Activation of mast cells by IgE is supposed to elicit the release of an array of cytokines and chemokines that are involved in regulation of the acute inflammatory response (Gurish et al. 2004). Interestingly, in our investigation the G. lamblia infection was found to trigger MMCP-1 release in the absence of IgE, whereas in T. spiralis-infected mice this process occurred in the presence of IgE. This finding certainly needs further attention and future investigations e.g. based on the use of an IgE-deficient mouse model will have to explore whether production of IgE has a negative effect on anti-giardial immunity and increases susceptibility of the animals to the parasite infection. Respective results will be particularly interesting because a correlation between increased IgE production and susceptibility to symptomatic giardiasis has already been observed in an allergic versus non-allergic group of Venezuelan children (Di Prisco et al. 1993).
As reported formerly, epithelial cells (McGee et al. 1993) but also mast cells (e.g. reviewed by Galli et al. 2005) have the capacity to express IL-6. Furthermore, previous experimental G. lamblia infections in IL-6-deficient mice clearly evidenced a central role of IL-6 in antigiardial immunity (Bienz et al. 2003; Zhou et al. 2003). These observations are in agreement with recent data from Li et al. (2004) suggesting that IL-6-producing mast cells may be key players within the intestinal immunological network that mediates immunity against a G. lamblia infection in mice. However, this assumption is challenged by our present immunohistological data indicating that murine mast cells, induced by a G. lamblia infection, seemed not to produce IL-6 in a quantity above the level of IL-6 production of intestinal epithelial cells from uninfected, or G. lamblia-infected, mice. Our conclusion was also confirmed by results from a quantitative reverse transcription (RT-)-PCR approach, which did not demonstrate increased intestinal IL-6 expression upon infection of mice with G. lamblia (data not shown). The conflicting results listed above make it evident that further extensive experimental work will be needed to elucidate the IL-6- and mast cell-dependent processes that contribute to murine anti-giardial immunity.
Intestinal mucosal inflammation has already previously been evaluated regarding its importance in resistance to murine giardiasis (e.g. reviewed by Müller and von Allmen, 2005). For example, a study on G. muris infections in a resistant versus susceptible mouse model did not evidence a correlation between resistance against infection and mucosal inflammation (Venkatesan et al. 1997). Conversely, Li et al. (2004) demonstrated that accumulation of inflammatory mast cells contributed to the elimination of a G. lamblia infection in the experimental murine host. Finally, our present data indicated that a T. spiralis infection, and perhaps particularly the intestinal inflammation induced by this infection, favours establishment and maintenance of a G. lamblia population in mice. It is obvious that in natural hosts, a G. lamblia infection often occurs in a situation where the intestinal tissue is inflamed e.g. as a consequence of a ‘pre-existing’ nematode infection. Nematode infections are evidently widespread in most endemic areas of giardiasis (Gendrel et al. 2003; Owen, 2005) and the phenomenon of a ‘pre-existing’ nematode infection prior to a G. lamblia infection may therefore have a direct influence on the epidemiological relevance of the disease.
As for example demonstrated for nematode infections, the inflammatory process involved in intestinal (immuno-)pathogenesis can change both the immunological and physiological conditions inside the intestinal environment (Garside et al. 2000; Lawrence, 2003; Khan and Collins, 2004; Maizels et al. 2004). Inflammation-associated modulation of the Th1/Th2 balance, alterations in intestinal nitric oxide, mucin and defensin production, and alterations in the composition of the intestinal flora may influence proliferation and persistence of G. lamblia trophozoites in the gut (e.g. reviewed by Müller and von Allmen, 2005). Accordingly, approaches investigating giardial growth in relation to an eventual intestinal inflammatory reaction will provide novel information on the immunological and physiological functions that promote either resistance or susceptibility to a G. lamblia infection.
We acknowledge U. Brönnimann, H. Sager (Institute of Parasitology), M. Bozzo, E. Garchi, and E. Rohner for technical support, and T. E. Nash (NIH, Bethesda, Maryland, USA) for his gift of G. lamblia clone GS/M-83-H7. This work was financed through a grant obtained from the Swiss National Science Foundation (No. 31-066795.01).