Introduction
Hybodont sharks, one of the most successful chondrichthyan lineages, appeared in the Late Devonian (Ginter et al., Reference Ginter, Hairapetian and Klug2002) and became extinct at the end of the Cretaceous (Becker et al., Reference Becker, Chamberlain and Terry2004). The hybodonts attained a high diversity during the Triassic, but their abundance started decreasing from the Jurassic onwards (Cuny et al., Reference Cuny, Suteethorn, Buffetaut and Ouaja2007). These were mostly euryhaline, capable of inhabiting rivers and lakes (Cuny, Reference Cuny2012). Freshwater hybodont sharks are reported from different horizons throughout the world (Heckert, Reference Heckert2004; Fischer, Reference Fischer2008; Klug et al., Reference Klug, Tütken, Wings, Pfretzschner and Martin2010; Cappetta, Reference Cappetta2012; Johns et al., Reference Johns, Albanesi and Voldman2014; Manzanares et al., Reference Manzanares, Pla, Martínez-Pérez, Ferrón and Botella2016), although complete preservation of these forms are rare, and mostly represented by various types of teeth, cephalic spines, and scales (Fischer et al., Reference Fischer, Schneider and Ronchi2010; Klug et al., Reference Klug, Tütken, Wings, Pfretzschner and Martin2010). All hybodonts are characterized by a tooth enameloid containing single apatite crystallites (Reif, Reference Reif1973) and anaulacorhize or sponge-like pattern of root vascularization (Maisey, Reference Maisey1987; Cappetta, Reference Cappetta2012).
In India, several isolated and discrete Gondwana basins (Fig. 1.1, inset) are rich in varied vertebrate fossil assemblages (Bandyopadhyay, Reference Bandyopadhyay1999, Reference Bandyopadhyay2011), including vertebrate microfossils (e.g., Datta et al., Reference Datta, Yadagiri and Rao1978; Datta, Reference Datta1981, Reference Datta2005; Yadagiri, Reference Yadagiri1986; Prasad and Sahni, Reference Prasad and Sahni1987; Prasad and Cappetta, Reference Prasad and Cappetta1993; Patnaik, Reference Patnaik2003). Studies on fossil fish teeth collected from the Gondwana sediments of India are scarce and was initiated by Jain et al. (Reference Jain, Robinson and RoyChowdhury1964), who reported an undescribed dipnoan, subholostean, and pleuracanth fishes from the Upper Triassic Maleri Formation of the Pranhita-Godavari (PG) Basin. Later, Jain (Reference Jain1980) described Xenacanthus indicus from the same formation. The hybodont fishes, including Lonchidion indicus, were described from the Jurassic Kota Formation of the same basin by Yadagiri (Reference Yadagiri1986). Subsequently, the formation yielded different hybodont taxa (Prasad et al., Reference Prasad, Manhas and Arratia2004). Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008) described multiple hybodonts from the Upper Triassic sediments of India, whereas a diverse assemblage comprising actinopterygians, dipnoans, and indeterminate chondrichthyans was reported from the Lower Triassic Panchet Formation of the Damodar Basin by Gupta (Reference Gupta2009).

Figure 1 (1, 2), Geological maps of the (1) Rewa Gondwana Basin showing the study area. Inset: major Gondwana basins of peninsular India; (2) study area (after Mukherjee et al., Reference Mukherjee, Ray, Chandra, Pal and Bandyopadhyay2012) showing the fossil microsite; (3) litholog showing the vertebrate microfossil-bearing mudstone horizon; (4) microvertebrate-bearing mudstone unit overlying the peloidal calcirudite (after Bhat, Reference Bhat2017).
The current study focuses on a new collection of isolated hybodont teeth collected from the Upper Triassic Tiki Formation of the Rewa Gondwana Basin, India, which are described based on gross dental morphology and histology. Comparison with other existing Late Triassic hybodont taxa shows that these belong to a new genus. Multivariate analyses are performed on the hybodont teeth to determine the validity and robustness of the new taxon.
Geological setting
The Rewa Basin is relatively long in ENE–WSW direction (Fig. 1.1) with basin-fill strata gently dipping towards the North. The Upper Gondwana succession of the basin comprises a thick, conformable, and continuous Triassic succession composed of the basal Pali Formation, followed upwards successively by the sand-dominant Karki and Tiki formations, which are unconformably overlain by the Jurassic Parsora Formation (Mukherjee et al., Reference Mukherjee, Ray, Chandra, Pal and Bandyopadhyay2012). Of these formations, the Upper Triassic Tiki Formation is essentially a mud-dominated fluvial succession with subordinate amounts of coarse- to fine-grained quartzo-feldspathic sandstone units. The mudstone units have yielded a diverse array of vertebrate fossils, unionid bivalves, and petrified wood (Sahni and Tewari, Reference Sahni and Tewari1958; Datta, Reference Datta2004; Mukherjee et al., Reference Mukherjee, Ray, Chandra, Pal and Bandyopadhyay2012; Mukherjee and Ray, Reference Mukherjee and Ray2014; Ray, Reference Ray2015). Based on the overall floral and faunal content, the Tiki Formation is correlated with the lower part of the Maleri Formation of the Pranhita-Godavari Basin, the Ischigualasto Formation of Argentina, and the Hyperodapedon Assemblage Zone of the upper part of the Santa Maria Formation of Brazil, which places the Tiki fauna within the Ischigualastian Land Vertebrate Faunachron (LVF) and a proposed early Carnian age for the formation (Ray, Reference Ray2015). However, recent recovery of a mystriosuchine phytosaur from the Tiki Formation suggests that the horizon could be younger than that previously proposed (Datta et al., Reference Datta, Mukherjee and Ray2016).
A vertebrate microfossil locality or microsite (sensu Sankey, Reference Sankey2008; Fig. 1.2) has yielded the specimens described in the current work. The fossils were extracted from a mudrock-rich unit, which is ~1.5 m thick and lies above a thick unit of peloidal calcirudites (Fig. 1.3). The mudrock unit is located in an overbank deposit and shows pedogenic modifications in the form of slickensides, color mottling, and glaebules (Bhat, Reference Bhat2017). Along with the hybodonts, the mudstone unit has yielded numerous teeth of yet undescribed xenacanthids and actinopterygians, isolated teeth, skull fragments and postcrania of basal tetrapods, lepidosauromorphs, archosauriforms, and cynodonts (Bhat, Reference Bhat2015; Bhat et al., Reference Bhat, Ray and Datta2015; Ray et al., Reference Ray, Bhat, Mukherjee and Datta2016). In addition, the mudstone hosts partial and complete skeletal elements of large metoposaurid temnospondyls, phytosaurs, and rhynchosaurs.
Materials and methods
Material
Studied material includes 45 well-preserved, isolated hybodont teeth from the Upper Triassic Tiki Formation of India, the details of which are given in Supplemental Data 1. For comparative purpose, the Indian species of the genera Parvodus (P. tikiensis) and Lissodus (L. duffini), as described by Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008), are studied (Supplemental Data 2). The quantitative analyses include several other valid taxa in which the morphometric variables could be measured. These include Parvodus rugianus and Parvodus curvidens (Ansorge, Reference Ansorge1990; Duffin and Theis, Reference Duffin and Theis1997; Underwood and Rees, Reference Underwood and Rees2002), Lissodus minimus (Duffin, Reference Duffin1993), Jiaodontus montaltissimus, and Jiaodontus vedenemus (Klug et al., Reference Klug, Tütken, Wings, Pfretzschner and Martin2010). The details of these taxa are given in Supplemental Data 2. These hybodont teeth are quantitatively analyzed based on measured morphometric variables (Fig. 2) such as the apicobasal height (ABH), mesiodistal length (MDL), labiolingual width (LLW), and height of the principal cusp (PCH). Ratio variables such as crown profile (ABH/MDL) and crown-base proportion (BL/BW), although used for qualitative description, were not used in the statistical analyses because these overemphasize some variables and do not help in differentiating the teeth (Hendrickx et al., Reference Hendrickx, Mateus and Araújo2015).

Figure 2 Schematic representation of major morphological features and indices to the measured parameters of a hybodont shark tooth in (1) labial, (2) lingual, (3) occlusal, and (4) basal views. Abbreviations: ABH=apicobasal height; BL=basal length; BW=basal width; hor.r=horizontal ridge; lab.cr=labial crest; lab.peg=labial peg; lc=lateral cusplet; lin.cr=lingual crest; lin.peg=lingual peg; LLW=labiolingual width; MDL=mesiodistal length; oc=occlusal crest; pc=principal cusp; PCH=height of principal cusp.
Vertebrate microfossil extraction
Initial assessment of the microsite for fossil-richness was carried out by spot sampling using coning and quartering method, where the exposed area of the microsite was subdivided into four quadrants. Scree material from the surfaces of these quadrants was removed after careful examination for microfossils, and small quantities of rock samples were collected from each quadrant and examined for vertebrate microfossils. Considerable yield of vertebrate microfossils resulted in prospecting of the microsites for bulk sampling (Bhat, Reference Bhat2017). Litholog was prepared to ascertain the microfossil-bearing stratum, bulk samples of nearly three tons of red mudstone were collected, screened by wet and dry sieving methods, and residues examined under a microscope for extraction of vertebrate microfossils following the procedures outlined by Hibbard (Reference Hibbard1949), Cifelli et al. (Reference Cifelli, Madsen and Larson1996), Heckert (Reference Heckert2004), and Bhat (Reference Bhat2017). Terminology used in the present work to describe tooth morphology (Fig. 2) follows Duffin (Reference Duffin1985), Rees and Underwood (Reference Rees and Underwood2002), Shimada (Reference Shimada2002), Whitenack and Gottfried (Reference Whitenack and Gottfried2010), and Cappetta (Reference Cappetta2012).
Quantitative analyses
Statistical analyses were performed using PAST 2.07c (Hammer et al., Reference Hammer, Harper and Ryan2001). The analyses included a principal component analysis (PCA) to evaluate the differentiation of the specimens based on variance of their variables and a discriminant analysis (or canonical variate analysis, CVA) to assess the consistency of identification between qualitative and quantitative methods (Hammer and Harper, Reference Hammer and Harper2006). These analyses for taxonomic positioning of isolated teeth follow Smith (Reference Smith2005), Smith et al. (Reference Smith, Vann and Dodson2005), and Hendrickx et al. (Reference Hendrickx, Mateus and Araújo2015). Teeth with missing measurements may blur the analyses, and hence were omitted from the analyses.
Thin-section preparation
Thin-sections of the hybodont teeth were prepared by using cutting and grinding techniques as suggested by Donath (Reference Donath1995). Before sectioning, the teeth were embedded in epoxy resin (Chinsamy and Raath, Reference Chinsamy and Raath1992), and ground and polished in the required direction by carborundum (silicon carbide) powder of various grit sizes (600–1000 μm). The tooth surface was subsequently cleaned with 0.5 μm α-alumina (Al2O3), and the polished surface was mounted on glass slides, 1.5–1.75 mm in thickness, with the help of an epoxy solution. The glass slide along with the embedded specimen was then cooled for 12–16 hours and the specimen was cut down to a thickness of 45 μm with the help of a PetroThinTM (Thin Section System). The thin section obtained was again ground manually to a thickness of 30–35 μm by carborundum powder. Final polishing of the specimen was performed with a 0.5 μm α-alumina liquid. The thin sections were examined under a polarizing petrographic microscope (Leica-DMEP) for detailed histological study and photographs were taken by a Leica DFC290 camera. Terminology for describing dental histology follows Francillon-Vieillot et al. (Reference Francillon-Vieillot, Buffrénil, de, Castanet, Géraudie, Meunier, Sire, Zylberberg and Ricqlès1990).
Scanning Electron Microscopy
SEM of the specimens was carried out following the standard procedures outlined by Reed (Reference Reed2005). The specimens were first washed with distilled water followed by acetone immersion. All teeth were mounted on small aluminum metallic stubs with the help of carbon tape to reduce the charging effect. The investigation was carried out under Field Emission (FE)-SEM (ZEISS) SUPRA-TM 55, at the Indian School of Mines, Dhanbad, India, and FE-SEM (ZEISS) AURIGA COMPACT, in the Central Research Facility at the Indian Institute of Technology, Kharagpur, India.
Repositories and institutional abbreviations
The collected specimens are housed in the Vertebrate Palaeontology Laboratory of the Department of Geology and Geophysics, Indian Institute of Technology Kharagpur, India. IITKGP, Indian Institute of Technology, Kharagpur, India; VPL/JU, Vertebrate Palaeontology Laboratory, University of Jammu, India.
Tooth morphological and histological abbreviations
ABH, apicobasal height; BL, basal length; BW, basal width; hor.r, horizontal ridge; lab.cr, labial crest; lab.peg, labial peg; lc, lateral cusplet; lin.cr, lingual crest; lin.peg, lingual peg; LLW, labiolingual width; MDL, mesiodistal length; oc, occlusal crest; pc, principal cusp; PCH, height of principal cusp; en, enameloid; ord, orthodentine; osd, osteodentine.
Systematic paleontology
Class Chondrichthyes Huxley, Reference Huxley1880
Subclass Elasmobranchii Bonaparte, Reference Bonaparte1838
Order Hybodontiformes Maisey, Reference Maisey1975
Superfamily Hybodontoidea Owen, Reference Owen1846
Family Lonchidiidae Herman, Reference Herman1977, sensu Rees, Reference Rees2008
Genus Pristrisodus new genus
Type species
Parvodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008) collected from the Upper Triassic Tiki Formation of India.
Diagnosis
As for the type and only species.
Etymology
Generic name is derived from the Latin word ‘pristris’ meaning shark and the Greek word ‘odous’ meaning tooth.
Occurrence
Tiki Formation of the Rewa Gondwana Basin (Otischalkian, Carnian); near the village of Tiki (23°56'N, 81°22'58''E), Shahdol District, Madhya Pradesh, India.
Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008)

Figure 3 Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008). IITKGPP23, a complete anterolateral tooth (morphotype I) in (1) labial, (2) lingual, (3) occlusal, and (4) basal views. Arrows indicate lateral cusplets. Abbreviations: hor.r=horizontal ridge; lab.cr=labial crest; lab.peg=labial peg; lin.cr=lingual crest; lin.peg=lingual peg; pc=principal cusp. Scale bar represents 1 mm.

Figure 4 Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008). IITKGPP11, an anteriorly placed tooth with partially preserved root (morphotype II) in (1) labial, (2) lingual, (3) occlusal, and (4) basal views. Arrows indicate lateral cusplets. Gray shading indicates broken areas. Abbreviations: lab.cr=labial crest; lab.peg=labial peg; lin.cr=lingual crest. Scale bar represents 1 mm.

Figure 5 Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008). IITKGPP26, a posterolateral tooth with root not preserved (morphotype III) in (1) labial, (2) lingual, (3) occlusal, and (4) basal views. Arrows indicate four lateral cusplets. Gray shading indicates basal depression. Abbreviations: lab.cr=labial crest; lab.peg=labial peg; lin.for=lingual foramen; lin.peg=lingual peg; pc=principal cusp. Scale bar represents 1 mm.

Figure 6 Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008). IITKGPP13, a posterolateral tooth (morphotype IV) in (1) labial, (2) lingual, (3) occlusal, and (4) basal views. Arrows indicate four lateral cusplets. Light- and dark-gray shadings indicate broken area and basal depression, respectively. Abbreviations: lab.cr=labial crest; lab.peg=labial peg; lin.cr=lingual crest; lin.peg=lingual peg; oc.cr=occlusal crest; pc=principal cusp. Scale bar represents 1 mm.

Figure 7 Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008). IITKGPP14, an anterior tooth (morphotype V) in (1) labial, (2) lingual, (3) occlusal, and (4) basal views. Arrow indicates a lateral cusplet. Light- and dark-gray shadings indicate broken area and basal depression, respectively. Abbreviations: lab.cr=labial crest; lab.peg=labial peg; lin.cr=lingual crest. Scale bar represents 1 mm.
2008 Parvodus tikiensis Prasad et al., p. 421, fig. 3A–U.
2008 Lissodus duffini Prasad et al., p. 425, fig. 4A–R.
Holotype
VPL/JU/TF/140, a lateral tooth recovered from the Upper Triassic Tiki Formation of the Rewa Gondwana Basin (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008).
Referred specimens
IITKGPP01–IITKGPP29, IITKGPP43–IITKGPP58, 45 isolated teeth (current study), details of which are given in Supplemental Data 1; VPL/JU/TF/137–VPL/JU/TF/149, 13 isolated teeth (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008).
Revised diagnosis
Lonchidiid hybodont characterized by elongated teeth with mesiodistal length greater than or equal to twice the apicobasal height, a high principal cusp, labial horizontal ridge situated near crown-root junction, lingual ridge high up on crown, linear depression or groove along the crown-root junction, small vertical cristae as crown ornamentation. Anterior teeth are broad, triangular and robust, have subdued and blunt principal cusp, a pair of incipient lateral cusplets, and low, hanging labial peg. Anterolateral teeth have high, pyramidal principal cusp with two or three small but pointed lateral cusplets, and triangular labial and lingual protuberances. Posterolateral teeth have four incipient lateral cusplets, prominent bilobed/rounded, hanging labial and small/incipient lingual protuberances.
Remarks
Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008) reported three lonchidiid genera from the Tiki Formation, comprising Lonchidion (L. estesi, L. incumbens), Parvodus (P. tikiensis), and Lissodus (L. duffini). The Indian species of Lonchidion (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008, p. 419, fig. 2A–O) are different from Pristrisodus tikiensis in having a weakly developed principal cusp and indistinct or absence of lateral cusplets. However, the specimens assigned to Parvodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008, p. 421, fig. 3A–U) and Lissodus duffini (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008, p. 425, fig. 4A–R) are remarkably similar to each other in terms of mesiodistal length (MDL), apicobasal height (ABH), and height of principal cusp (PCH; Table 1). These specimens (see Supplemental Data 2) are much smaller than the different morphotypes of Pristrisodus n. gen., in terms of MDL, although the coronal profile (MDL/ABH=2.43) of the measured specimens of Parvodus tikiensis and Lissodus duffini falls within the ranges of the newly examined teeth in the current work. Moreover, the specimens designated as P. tikiensis and L. duffini (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008) bear morphological similarity with the new specimens (Supplemental Data 1) based on their gracile appearance, absence of strong vertical folds or ornamentation (which contrasts with other species of Parvodus [Cappetta, Reference Cappetta2012]), similar mesiodistal elongation, and well-developed lateral cusplets (in contrast to other species of Lissodus [Cappetta, Reference Cappetta2012]). In the current work, the two taxa, P. tikiensis, and L. duffini are re-assigned to the new taxon, Pristrisodus tikiensis.
Table 1 Average and standard deviation of the measured dimensions of the Indian hybodonts examined.

Index to measured parameters is given in Figure 2. Abbreviations: ABH, apicobasal height; BL, length in basal view; BW, maximum width in basal view; LLW, labiolingual width of hybodonts; MDL, mesiodistal length; N, number of specimens; PCH, height of principal cusp. Single (*) and double asterisks (**) indicate(s) after Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008) and N=3, respectively. All measurements are in mm.
Description
Within the taxon Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008), five tooth morphotypes (I–V) can be distinguished based on crown proportions, number of cusplets, height of the principal cusps, forms of the labial and lingual pegs, and ornamentation (Table 2). Because the cusps are straight and upright, the different morphotypes of P. tikiensis represent teeth along different positions of the lower jaw, as suggested for the genus Lissodus (Rees and Underwood, Reference Rees and Underwood2002) and for hybodonts in general (Cappetta, Reference Cappetta2012), and show a gradual monognathic heterodonty. Morphotypes I–II were positioned more anteriorly in comparison to the more posterior positioning of morphotypes III–IV, as is suggested by the sharper, high, and pointed principal cusps of the former in comparison to the relatively rounded principal cusps of the latter (sensu Cappetta, Reference Cappetta2012). Morphotype V is considered to be anteriorly placed because of its broad and triangular shape, as suggested by Heckert et al. (Reference Heckert, Ivanov and Lucas2007) for Lonchidion humblei. In Parvodus tikiensis and Lissodus duffini, the crown proportions in longitudinal (MDL/ABH) and occlusal (MDL/LLW) views are equal (=2.4) to each other, and nearly equal to that of morphotypes IV and V of Pristrisodus n. gen. In the current study, all the morphotypes of Parvodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008, p. 421, fig. 3A–U) and Lissodus duffini (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008, p. 425, fig. 4A–R) are re-assigned as lateral and anterior teeth of morphotypes IV and V of Pristrisodus n. gen., respectively, based on their mesiodistal elongation, number of lateral cusplets, and triangular shape of the anterior teeth (Patterson, Reference Patterson1966; Maisey, Reference Maisey1983; Hodnett et al., Reference Hodnett, Elliot, Olson and Wittke2013).
Table 2 Characteristic features distinguishing the five morphotypes of Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008).

Index to the abbreviations is given in Table 1.
Single (*) asterisk indicates that total number of specimens examined for the morphotypes IV and V incorporate specimens of Parvodus tikiensis and Lissodus duffini, respectively.
Morphotype I
Morphotype I comprises three well-preserved anterolateral teeth. The anterolateral tooth IITKGPP23 (Fig. 3.1–3.4) shows a well-developed principal cusp and two pairs of cusplets. Average mesiodistal length (MDL) of morphotype I is 3.05±0.6 mm (N=3, Table 1) and average labiolingual width (LLW) is 0.8±0.06 (N=3, Table 1). The crown ratio (MDL/LLW=3.8) in occlusal view is much greater than that in longitudinal (MDL/ABH=2.65) view, suggesting that the teeth are robust. The principal cusp is pointed and strongly developed in all the specimens. The occlusal crest is sharp and blade-like (Fig. 3.1, 3.2). The labial and lingual crest regions show bulges, whereas the rest of the lateral region of the crown is compressed (Fig. 3.3). There are straight horizontal ridges on the labial and lingual faces of the crown, although the former is near the crown-root junction and the latter is situated higher up on the crown (Fig. 3.1, 3.2). Small vertical apical cristae are seen on the principal cusp and lateral cusplets. Both labial and lingual protuberances are triangular in shape, although the former is much smaller than the latter (IITKGPP23). There is a constricted, slightly concave groove at the crown-root junction on all sides. The roots are either partially or completely preserved in all specimens of morphotype I, and display an anaulacorhize vascularization pattern (Fig. 3.1). A set of regular foramina is arranged linearly along the dorsal end of the labial face of the root, whereas ventrally, the foramina are randomly arranged. The crown-base is slightly curved in outline, concave towards the labial face and has multiple circular pits or foramina (Fig. 3.4).
Morphotype II
There are three isolated teeth which show overall similarity with morphotype I in terms of a pointed, robust principal cusp, but have three lateral cusplets on one side of the principal cusp and two on the other (Fig. 4.1). These teeth have crown proportions similar to morphotype I, where MDL/LLW > MDL/ABH (Table 2). In most of the specimens, the height of the principal cusp is twice the height of the three lateral cusplets (e.g., IITKGPP11, Fig. 4.1, 4.2). A similar high principal cusp is also seen in IITKGPP20. Small vertical ridges (or cristae) extending along the crest are seen in the specimens (Fig. 4.1). The labial and lingual crests are sharp and prominent. The labial peg is large, triangular, and displaced towards the root, whereas on the lingual side, the peg is absent though the region has a prominent and large subcircular bulge (Fig. 4.1, 4.2). The ridge near the crown-root junction is curved and crenulated in labial view (Fig. 4.1), whereas it is smooth, curved, and situated midway on the crown in lingual view (Fig. 4.2). In occlusal view, the labial and lingual sides show a distinct difference in outline, especially at the labial and lingual pegs (Fig. 4.3). The root is partially preserved, showing an anaulacorhize vascularization pattern, and contains several large and irregular foramina. In basal view, the crown-base is distinctly sub-triangular in outline (Fig. 4.4).
Morphotype III
Morphotype III is based on six well-preserved teeth (Supplemental Data 1), where the crown proportions in longitudinal (MDL/ABH) and occlusal (MDL/LLW) views are equal, unlike that in the morphotypes I and II (Table 2). All the specimens of morphotype III (Supplemental Data 1) are characterized by a bilobed labial peg, a slightly rounded principal cusp, and three or four pairs of lateral cusplets on either side of it (Fig. 5.1). Vertical ridges are present on the principal cusp and cusplets (Fig. 5.1). The curved ridges on the labial and lingual faces of the crown are similar to those seen in morphotype II. Although the lingual face is smooth, it contains an incipient lingual peg and a curved horizontal ridge. There is a foramen just below this lingual peg (Fig. 5.2). In occluso-lingual view, the crest is prominent (Fig. 5.3). The tooth has a centrally depressed base, which has a triangular outline that is slightly concave towards the lingual side (Fig. 5.4).
Morphotype IV
Twenty-three isolated teeth were examined and comprise sixteen newly collected specimens (Supplemental Data 1) and the specimens originally assigned to Parvodus tikiensis (VPL/JU/TF/137–VPL/JU/TF/143, Supplemental Data 2). The labiolingual width (LLW) equals the apicobasal height (ABH) in morphotype IV (Table 1), whereas the crown proportions (MDL/ABH and MDL/LLW) are nearly equal to each other, as in morphotype III (Table 2). The principal cusp is broadly V-shaped with a rounded apex and has four pairs of cusplets on each side of the principal cusp (Fig. 6.1). The large triangular labial peg is positioned towards the base of the crown (Fig. 6.1), which contrasts with a small, subcircular lingual peg (Fig. 6.2). The latter is shifted towards the crown in comparison to the labial peg. The occlusal crest is sharp and slightly curved (Fig. 6.3). The ridges on the labial and lingual faces of the crown are curved and crenulated. There are small but distinct cristae along the lateral cusplets in labial and lingual views. The base of the crown is subtriangular but narrow and highly elongated (avg. BL/BW=4.2 [N=22]), centrally depressed and concave towards the lingual side (Fig. 6.4).
Morphotype V
This morphotype is based on twenty-four isolated teeth comprising seventeen newly collected specimens (Supplemental Data 1) and the specimens originally assigned to Lissodus duffini (VPL/JU/TF/143–VPL/JU/TF/149, Supplemental Data 2). Morphotype V is characterized by robust teeth with a rounded principal cusp (Fig. 7.1, 7.2) and one incipient lateral cusplet on each side. The teeth have distinct triangular outlines in occlusal and basal views where MDL/LLW (=2.3) and BL/BW (=3.1) are low in comparison to the other morphotypes (Table 2). However, their crown proportions are similar to those seen in morphotypes III and IV (MDL/LLW=MDL/ABH, Table 2). The crown has an enlarged and robust labial peg that extends beyond the crown-root junction (Fig. 7.1), whereas the lingual peg is absent (Fig. 7.2). The horizontal ridge is not visible in labial view, but it is prominent, wide, and crenulated in lingual view. The occlusal crest is broad and almost flat (Fig. 7.3). The base of the crown is distinctly triangular and has a central depression (Fig. 7.4).
Discussion
The low-crowned teeth and presence of anaulacorhize root vascularization suggest that Pristrisodus n. gen., conforms to the hybodontiform tooth morphology (Ginter et al., Reference Ginter, Hampe and Duffin2010). Pristrisodus n. gen., fits the familial diagnosis of the Lonchidiidae, which includes small teeth with mesiodistal expansion, well-developed labial protuberances, and presence of irregularly arranged foramina on the root (Rees and Underwood, Reference Rees and Underwood2002). The family Lonchidiidae, established for the genus Lonchidion (Herman, Reference Herman1977), was synonymized with Polyacrodontidae (Cappetta, Reference Cappetta1987), although recent works show that the former is valid (Rees and Underwood, Reference Rees and Underwood2002; Cappetta, Reference Cappetta2012). The family comprises eleven genera characterized by the shape of the crown in labial and occlusal views, height of the principal cusp, number and nature of lateral cusplets, ornamentation, and nature of the root (Wang et al., Reference Wang, Zhang, Zhu and Zhao2009; Klug et al., Reference Klug, Tütken, Wings, Pfretzschner and Martin2010; Cappetta, Reference Cappetta2012). Of these, Dabasacanthus and Gansuselache are essentially Paleozoic forms (Ginter et al., Reference Ginter, Hampe and Duffin2010). Both forms bear similarity with Lissodus, although these possess several distinguishing features. Dabasacanthus is represented by an articulated juvenile with very small teeth, which have prominent labial pegs but lack lateral cusplets and ornamentations (Ginter et al., Reference Ginter, Hampe and Duffin2010). On the other hand, teeth of Gansuselache have a high crown-root junction labially, widely separated lateral cusplets from the principal cusps, and a strongly developed, flared occlusal crest (Wang et al., Reference Wang, Zhang, Zhu and Zhao2009; Ginter et al., Reference Ginter, Hampe and Duffin2010). The tooth morphology of five Mesozoic lonchidiid genera is distinctly different from the teeth of Pristrisodus n. gen., and includes Bahariyodon (Fig. 8.1, 8.2), Hylaeobatis (Fig. 8.3–8.5), Isanodus (Fig. 8.6, 8.7), Vectiselachos (Fig. 8.8–8.10), and Diplolonchidion (Fig. 8.11, 8.12). In contrast to Pristrisodus n. gen., Bahariyodon is characterized by a high cusp with wide base, prominent cristae, and a lingual crown face that is produced into a distinct shelf (Cappetta, Reference Cappetta2012). The shapes of teeth of Hylaeobatis are rectangular (Fig. 8.5), whereas Isanodus teeth are deeper than long and have a stout crown with rounded apex (Fig. 8.6, 8.7; Cuny et al., Reference Cuny, Suteethorn, Kamha, Buffetaut and Philippe2006). The teeth of Vectiselachos, conversely, are small, have a well-demarcated principal cusp, no lateral cusplets, and a root that is much thinner than the crown (Fig. 8.8–8.10). Diplolonchidion differs from other lonchidiid genera, including Pristrisodus n. gen., in possessing two distinct and robust labial pegs slightly offset mesiodistally from the central crown (Fig. 8.11, 8.12). Diplolonchidion was included in Polyacrodontidae by Heckert and Lucas (Reference Heckert and Lucas2006), and Lissodus was placed in an unknown family by Rees (Reference Rees2008), although Cappetta (Reference Cappetta2012) placed them in the family Lonchidiidae with a scope for future revision of the family.

Figure 8 Representative lonchidiid hybodonts, (1–2) Bahariyodon: (1) anterior tooth in lingual view, (2) lateral tooth in labial view; (3–5) Hylaeobatis: (3–4), symphyseal tooth in (3) occlusal, (4) lingual views, (5) lateral tooth in labial view; (6–7) Isanodus: (6) anterolateral tooth in lingual view, (7) posterior tooth in labial view; lateral tooth of (8–10) Vectiselachos: (8) occlusal, (9) labial, and (10) lingual views; (11–12), Diplolonchidion in (11) occlusal and (12, labial views; (13–15) Lissodus in (13) occlusal, (14) labial, and (15) lingual views; (16–18), Lonchidion in (16) occlusal, (17) labial, and (18) lingual views; (19–20), Parvodus in (19) labial and (20) lingual views; (21–24) Jiaodontus: (21–22) J. montaltissimus in (21) labial and (22) lingual views; (23–24) J. vedenemus in (23) labial and (24) lingual views; (25), Pristrisodus n. gen., (morphotype I) in labial view. Sources of information: Klug et al. (Reference Klug, Tütken, Wings, Pfretzschner and Martin2010), Cappetta, (Reference Cappetta2012), and current study. Scale bars represent 1 mm, except for 5 mm (3–5), 500 µm (21−24).
The teeth of Pristrisodus n. gen., show superficial similarities to the genera Lissodus (Fig. 8.13–8.15), Lonchidion (Fig. 8.16–8.18), Parvodus (Fig. 8.19, 8.20), and Jiaodontus (Fig. 8.21–8.24) based on symmetry and shape and size of the principal cusp and lateral cusplets. However, the lateral teeth of Pristrisodus n. gen., (Figs. 3–7, 8.21) are mesiodistally at least three times more elongated than those of Lissodus africanus (Broom, Reference Broom1909; Duffin, Reference Duffin1985), and the lateral cusplets are well developed in contrast to the weakly developed ones or their near absence in all species of Lissodus (Duffin, Reference Duffin1985; Rees and Underwood, Reference Rees and Underwood2002; Duncan, Reference Duncan2004; Fischer, Reference Fischer2008; Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008), such as L. cassangensis (Teixeira, Reference Teixeira1956). In addition, the crown of Lissodus possesses weakly developed folds (Underwood and Rees, Reference Underwood and Rees2002), in contrast to Pristrisodus n. gen., Lonchidion (Fig. 8.16–8.18) differs from Pristrisodus n. gen., (Fig. 8.25) in having a weakly developed principal cusp, multiple cusplets, a prominent and narrow labial peg, and a broad root. The root of Pristrisodus n. gen., has a single row of foramina proximal to the crown-root junction, and distally, the foramina are randomly oriented (Fig. 8.25). Such a pattern contrasts with that of Hybodus parvidens (Patterson, Reference Patterson1966), where the foramina are irregularly distributed throughout the root without any specialized pattern. The lateral teeth of Parvodus (Fig. 8.19, 8.20; Rees and Underwood, Reference Rees and Underwood2002) are different from those of Pristrisodus n. gen., in having a very high crown profile and strong vertical folds. The two known species of Jiaodontus (J. montaltissimus, [Fig. 8.21, 8.22] and J. vedenemus [Fig. 8.23, 8.24]) are distinctly different from Pristrisodus tikiensis in having teeth with a high coronal profile, protruding and bulging labial peg, strong ornamentation in the form of prominent ridges on their labial and lingual faces, and a strongly convex labial face (Klug et al., Reference Klug, Tütken, Wings, Pfretzschner and Martin2010). Hence, Pristrisodus constitutes a new genus belonging to the family Lonchidiidae, which is distinct from all other valid lonchidiid genera.
Quantitative assessments
Results
To overcome discrepancy arising due to positional differences, only lateral teeth of the genera examined are considered (Supplemental Data 2). In the case of Pristrisodus tikiensis, the anterior and anteriorly placed teeth are omitted from the analyses, whereas those teeth that are definitely lateral in position are used in the analyses. This includes twenty-two lateral teeth, which are placed posteriorly (morphotypes III–IV) with respect to morphotypes I–II (Supplemental Data 1). The examined teeth of Lissodus duffini were identified as lateral in position by Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008), although these are found to be morphologically similar to morphotype V (anterior) teeth of Pristrisodus tikiensis.
PCA was applied to the variance-covariance matrix of the four variables, ABH, MDL, LLW, and PCH, which characterize the crown elongation, width, thickness, and height of the principal cusp. Because all of the variables were measured in the same unit (mm), the variance-covariance matrix is used for analyses (Hammer et al., Reference Hammer, Harper and Ryan2001). As the first three principal components account for 99.29% of the total variance (Table 3), PC 4 is discarded. PC 1 is mainly a size axis, as shown by the high and positive coefficients of the four variables. On PC 2 or the first shape axis, high positive value is seen for crown heights (ABH and PCH), which load opposite to the crown length and width (MDL and LLW). On the other hand, LLW shows high positive value and loads opposite to other crown-proportion variables on PC 3.
Table 3 Principal component coefficient of the first four axes.

Principal component scores are plotted on PC 1 and PC 2 (Fig. 9.1), and PC 2 and PC 3 (Fig. 9.2) to show the scatter of the hybodont teeth examined. Although distinct clustering of the taxa is seen, Lissodus duffini and Parvodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008) show close occupation of morphospaces with that of Pristrisodus tikiensis (Fig. 9.1). However, Parvodus rugianus shows considerable overlapping of convex hull polygon with that of Parvodus tikiensis and Pristrisodus n. gen., (Fig. 9.1), suggesting that all these taxa have similar size ranges. The other available lonchidiids examined (Parvodus curvidens, Lissodus minimus, and Jiaodontus [J. montaltissimus and J. vedenemus]) show distinctly separate occupation of zones in the morphospaces (Fig. 9.1, 9.2). Principal component scores plotted on PC 2 and PC 3 (Fig. 9.2) show that the Indian taxa have overlapping of morphospaces with each other and with that of Lissodus mimimus. Other taxa occupy separate and distinct morphospaces.

Figure 9 Graphical result of principal component scores of hybodont teeth plotted on (1) first two principal components, PC 1 and PC 2, and (2) second and third principal components, PC 2 and PC 3. Variable loadings and component eigenvalues are given in Table 3.
CVA shows that 78% of the total teeth analyzed (N=45) have been correctly assigned (Table 4), whereas all the European forms show 100% correct assignment. The anterior teeth of L. duffini are 100% correctly assigned. Species of Jiaodontus show 67%–75% correct assignment of lateral teeth, whereas Parvodus tikiensis, as originally described by Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008), show only 33% correctly assigned lateral teeth. Such a low percentage accounts for considerable overlap of the convex hull polygon of Parvodus tikiensis with that of Pristrisodus tikiensis (Fig. 10). On the other hand, the morphospace of Lissodus duffini, although distinct, is close to the morphospace of Pristrisodus tikiensis. Such close clustering of the morphospaces of the Indian genera (Fig. 10) contrasts with that of other genera examined, in which the latter are distributed in distinct and separate morphospaces.

Figure 10 Graphical result of the canonical variate analysis of hybodont teeth along the first two canonical axes of maximum discrimination in the dataset (Eigenvalue of Axis 1=8.086, which accounted for 74.46% of the variation; Eigenvalue of Axis 2=1.45, which accounted for 13.35% of the variation).
Table 4 Number of correctly assigned teeth as determined by canonical variate analysis (CVA); (Eigenvalue of Axis 1=8.086, which accounted for 74.46% of the variation; Eigenvalue of Axis 2=1.45, which accounted for 13.35% of the variation).

Discussion
Fossil chondrichthyans are mostly described based on tooth morphology (e.g., Duffin, Reference Duffin1985; Shimada, Reference Shimada2002; Fischer et al., Reference Fischer, Voigt, Schneider, Buchwitz and Voigt2011; Johns et al., Reference Johns, Albanesi and Voldman2014), which has inherent problems because most of the teeth are found isolated and exhibit various forms of heterodonty (Shimada, Reference Shimada2005; Whitenack and Gottfried, Reference Whitenack and Gottfried2010; Cappetta, Reference Cappetta2012). In the current work, application of PCA and CVA for hybodont teeth tests the validity of Pristrisodus n. gen., in comparison to other Indian, European, and Chinese taxa, and is represented graphically (Figs. 9, 10) by the occupation of different zones of morphospaces (convex hull polygons). Although the sample size is small, in both the analyses, the specimens of Parvodus tikiensis and Lissodus duffini (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008) show either overlapping of zones or close clustering with the morphospace of Pristrisodus tikiensis (Figs. 9, 10). These multivariate analyses thus corroborate the qualitative findings of the current study, where the specimens designated as P. tikiensis and L. duffini by Prasad et al. (Reference Prasad, Singh, Parmar, Goswami and Sudan2008) are found to be similar to the newly collected Tiki specimens, and are re-assigned to Pristrisodus n. gen. Moreover, loadings on different measured parameters in PCA (Table 3) show that crown height (ABH and PCH) loads opposite to crown length and width (MDL and LLW), and these crown proportions are key identification features for the different hybodont taxa analyzed.
Tooth histology
Description
Two lateral teeth (morphotype IV, IITKGPP50 and morphotype III, IITKGPP29) were longitudinally and transversely sectioned, respectively, to reveal their tooth micro-morphology (Fig. 11). In both specimens, the crown has a thin (47 μm) outer enameloid layer overlying a thick dentine (Fig. 11.1). The latter is composed of an outer thick orthodentine (sensu Sire et al., Reference Sire, Donoghue and Vickaryous2009) and an inner narrow osteodentine (sensu Carlson, Reference Carlson1989). Although orthodentine usually comprises an outer dense pallial dentine surrounding an inner layer of circumpulpar (Carlson, Reference Carlson1989), in Pristrisodus n. gen., the pallial dentine is restricted to the enameloid-dentine boundary and is hard to differentiate as in other hybodonts (Johnson, Reference Johnson2003). Profuse dentine tubules of two distinct types are seen in longitudinal view. The first type is coarse and situated towards the crown base (Fig. 11.2), whereas the second type is slender, feather-like, and found in the apical region of the crown (Fig. 11.3). In occlusal view (Fig. 11.4–11.6), a thick zone of orthodentine (285 µm along the vertical axis) overlies a narrow osteodentine (89 µm along the same vertical axis). The latter surrounds a small, central pulp cavity, which has a circular outline (diameter=113 µm). The osteodentine is characterized by multiple, elliptical, and circular denteons that form a radiating pattern surrounding the pulp cavity (Fig. 11.6).
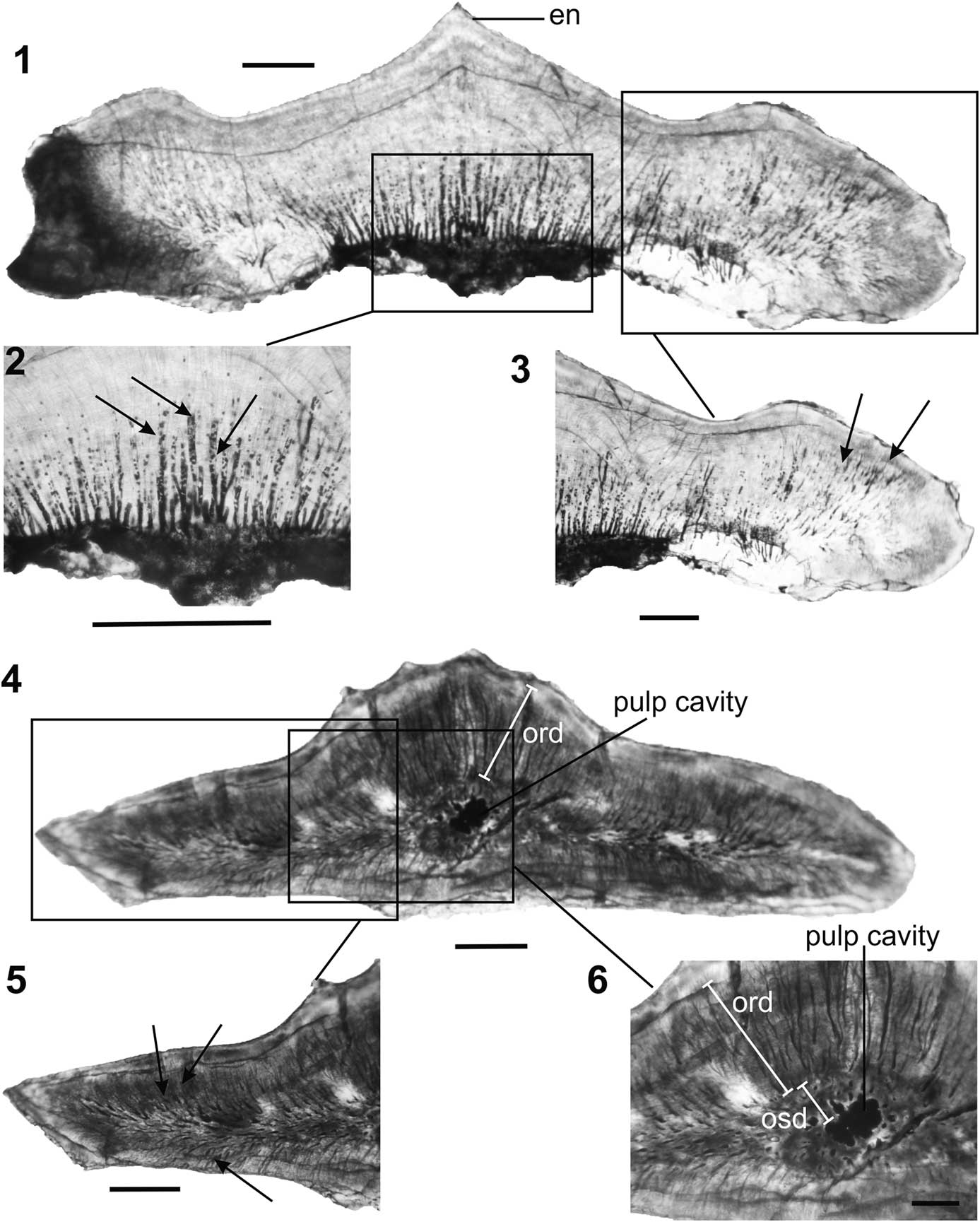
Figure 11 Pristrisodus tikiensis (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008). (1–3), Longitudinal section of a lateral tooth (IITKGPP50, morphotype IV) in lingual view showing (1) a thin capping of enameloid on the principal cusp, (2) vascular canals (arrows), and (3) dentinal tubules (arrows) at higher magnification; (4–6), transverse section of a lateral tooth (IITKGPP29, morphotype III) in occlusal view showing (4) a small, centrally located pulp cavity surrounded by a thick zone of orthodentine, (5) profuse dentinal tubules (arrows), and (6) a distinct pulp cavity surrounded by a narrow zone of osteodentine, which is overlain by a thick zone of orthodentine. Abbreviations: en=enameloid, ord=orthdentine, osd=osteodentine. Scale bars represent 0.2 mm (1, 2, 4, 5), 0.1 mm (3, 6).
Discussion
In comparison to the numerous valid hybodont genera known (Cappetta, Reference Cappetta2012), histologies of only a few have been examined. Pioneering work on hybodont tooth histology was carried out by various workers, such as Stensiö (Reference Stensiö1921), Patterson (Reference Patterson1966), Reif (Reference Reif1973), Johnson (Reference Johnson1981), and Maisey (Reference Maisey1987). Presence of orthodentine and absence of osteodentine is considered a basal/primitive feature of hybodonts (Maisey, Reference Maisey1987). In general, dental histology of the hybodonts may be subdivided into orthodont and osteodont types. In the first case, the whole crown beneath the enameloid is formed by the orthodentine with a pulp cavity present, whereas in the second type, a central thick osteodentine is present with the orthodentine occurring as a thin layer between the enameloid and the osteodentine. Polyacrodus, Lonchidion, and Paleobatus have teeth with an outer enameloid and pallial dentine overlying a thick orthodentine, thereby forming the orthodont taxa (Maisey, Reference Maisey1987). In contrast, Hybodus and Acrodus have a central core of osteodentine underlying an outer enameloid and pallial dentine, and are known as the osteodont type. However, in Lissodus (L. angulatus), both types are found (Błażejowski, Reference Błażejowski2004). The osteodentine type is usually found in lateral and posterior teeth of L. angulatus, whereas its anterior teeth are orthodentine type. Subsequently, Rees and Underwood (Reference Rees and Underwood2002) and Heckert et al. (Reference Heckert, Ivanov and Lucas2007) corroborated that Lissodus (L. minimus) and Lonchidion (L. humblei), respectively, are of orthodentine type and lack osteodentine in the crown.
Histology of the lateral teeth of Pristrisodus n. gen., is distinctly different from that of Polyacrodus, Lonchidion, Paleobatus, Hybodus, and Acrodus, which are either orthodont or osteodont (Maisey, Reference Maisey1987), but similar to the lateral tooth of Lissodus (L. angulatus Stensiö, Reference Stensiö1921) in having a thin outer layer of enameloid overlying an outer orthodentine and an inner osteodentine. In contrast to Lissodus, the lateral teeth of Pristrisodus n. gen., are characterized by a thick orthodentine surrounding a thin osteodentine, which in turn surrounds the pulp cavity (Fig. 11.6). Hence, co-existence of the two types (orthodont and osteodont) within a single taxon is evident in Pristrisodus n. gen., as seen in Lissodus, which contrasts with that of other hybodont genera studied.
Concluding remarks
The Late Triassic Tiki Formation of the Rewa Gondwana Basin, India has yielded a new lonchidiid shark, Pristrisodus n. gen., based on numerous well-preserved, isolated teeth. Five distinct morphotypes are identified within Pristrisodus n. gen., based on crown proportions, number of cusplets, height of the principal cusps, form of the labial and lingual pegs, and ornamentation, which suggest a gradual monognathic heterodonty. Based on their overall shape and robustness, morphotype V is considered an anterior tooth, morphotypes I–II are positioned relatively anteriorly with respect to morphotypes III–IV. Parvodus tikiensis and Lissodus duffini are synonymized and reassigned to Pristrisodus n. gen., as Pristrisodus tikiensis, which is corroborated by multivariate analyses. Dental histology of Pristrisodus n. gen., is extremely distinctive and does not pertain to either the orthodont or osteodont type, and is of a mixed type, where a thick covering of orthodentine surrounds a relatively thin osteodentine and a central pulp cavity.
The Tiki Formation has yielded a rich assemblage of freshwater hybodonts along with various yet undescribed ceratodontiform dipnoans, xenacanthids, and actinopterygians (Bhat, Reference Bhat2015). Such high abundance of Late Triassic freshwater fishes is similar to that found in the Chinle Group of USA (Heckert, Reference Heckert2004). Additionally, the Tiki dromatheriid Rewaconodon (Datta et al., Reference Datta, Das and Luo2004) and archosauriform teeth as found in the Tiki Formation are present in the lower Tecovas Formation of the Chinle Group, USA (Heckert, Reference Heckert2004; Bhat et al., Reference Bhat, Ray and Datta2017). Because the latter is Adamanian in age (Tanner et al., Reference Tanner, Spielmann and Lucas2013), such similarity in the vertebrate assemblages corroborates the findings of Ray et al. (Reference Ray, Bhat, Mukherjee and Datta2016) and Datta et al. (Reference Datta, Mukherjee and Ray2016) that the Tiki Formation may be younger than previously suggested.
India is one of the three widely separated regions, including Argentina (Johns et al., Reference Johns, Albanesi and Voldman2014) and Madagascar (Burmeister et al., Reference Burmeister, Flynn, Parrish and Wyss2006), from where Late Triassic freshwater hybodonts are reported. Three hybodont genera Lonchidion, Polyacrodus (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008), and Pristrisodus n. gen., (current study) are known from India, of which the latter genus is endemic. Lonchidion is known from North America (Murry, Reference Murry1981; Cappetta, Reference Cappetta2012), India (Prasad et al., Reference Prasad, Singh, Parmar, Goswami and Sudan2008), and Spain (Manzaneras et al., 2016), whereas Polyacrodus is known from Germany and eastern France (Cappetta, Reference Cappetta2012), although there are doubts regarding its validity (Rees, Reference Rees2008; Cappetta, Reference Cappetta2012). Hence, the Indian hybodont fauna shows resemblance to the European and North American forms that are known from coeval horizons. Most of these genera were euryhaline in nature (Maisey, Reference Maisey1989; Cuny et al., Reference Cuny, Suteethorn, Kamha, Buffetaut and Philippe2006), which may have resulted in their adaptation to freshwater systems in India during the Carnian. Further study is required to explain their migration along the coastlines prior to Pangaean rifting.
Acknowledgments
We thank D. Mukherjee of the Geological Studies Unit, Indian Statistical Institute, Kolkata, India for active participation during fieldwork, and critical comments on an earlier draft of the manuscript. We are grateful to C. Duffin of the Earth Science Department, Natural History Museum UK for valuable suggestions and insightful discussions during preparation of the revised draft of the manuscript. The constructive criticism and comments of two anonymous reviewers, D. J. Ward, and J. Kriwet are gratefully acknowledged. We are indebted to A. Bhaumik of the Department of Applied Geology, Indian School of Mines, Dhanbad, India, N. Bhowmik, and K. Datta for facilitating Scanning Electron Microscopy. C. Desarkar is acknowledged for thin-section preparations. Financial assistance and infrastructural facilities were provided by the Department of Science and Technology, India and the Indian Institute of Technology, Kharagpur, India, respectively.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.mq282

















