Acute heart failure syndromes in children are an important source of morbidity, mortality, and resource consumption. In the United States alone, nearly 14,000 children are hospitalised annually with acute heart failure syndromes.Reference Rossano, Kim and Decker 1 Approximately 15–18 per 100,000 children are admitted each year for evaluation and management of heart failure, of which two-thirds have CHD. Infants represent a slight majority of all such admissions, whereas approximately one-quarter are aged between 1 and 12 years and about 15% are adolescents. A typical hospitalisation lasts 2–3 weeks, and overall in-hospital mortality is ∼7%.Reference Rossano, Kim and Decker 1 Approximately one-third of children hospitalised with new-onset heart failure due to heart muscle disease either die or undergo cardiac transplantation within 1 year.Reference Andrews, Fenton, Ridout and Burch 2 Children with acute heart failure syndromes present a substantial diagnostic and therapeutic challenge for clinicians. This article reviews the contemporary provision of critical care for patients with acutely decompensated heart failure and cardiogenic shock. The pathophysiology of these disease states is summarised, and the key signs and symptoms for recognition are reviewed. Diagnostic considerations are noted and management strategies are discussed in detail. Mechanical circulatory support, cardiac transplantation, and management of compensated heart failure are the focus of separate articles in this supplement of Cardiology in the Young, and thus these topics are not addressed in great detail in this manuscript. In addition, this review focusses on children with acute heart failure syndromes due to heart muscle disease. Although many principles outlined below may be applied to patients with critical CHD, intra-cardiac shunts, functionally univentricular physiology, and post-operative low cardiac output syndrome, the nuances regarding the evaluation and management of these populations of patients are not specifically discussed.
Definitions
Acute heart failure syndromes include acutely decompensated heart failure and cardiogenic shock. Acutely decompensated heart failure may be defined as the rapid onset of or change in signs and symptoms of heart failure. It is considered a life-threatening condition that requires immediate medical attention and usually leads to urgent admission to the hospital.Reference McMurray, Adamopoulos and Anker 3 Cardiogenic shock may be broadly defined as a state in which oxygen delivery to the tissues is inadequate relative to demands secondary to inadequate cardiac function. Hypotension and/or high systemic vascular resistance are typically present and ventricular end-diastolic pressures are elevated.
Physiology of acute heart failure
Understanding the physiology of systemic ventricular systolic dysfunction is a prerequisite to appreciating the manifestations of and implementing treatment for acute heart failure syndromes. In patients with systemic ventricular systolic dysfunction, the force–velocity relationship of cardiac fibres is altered such that the ability of cardiomyocytes to generate force is reduced per unit time (shortening velocity) under any given set of loading conditions. Given that ventricular systole only lasts for a finite period of time, a reduced velocity of contraction diminishes the ejection fraction. The physiology of acute heart failure syndromes may be further appreciated by reviewing the Frank–Starling law.Reference Patterson and Starling 4 In the normal heart, assuming that other variables are held constant, stroke volume may be augmented by increasing preload, but only until the excessive stretch begins to diminish force generation (Figs 1 and 2). In patients with systemic ventricular systolic dysfunction, the normal Frank–Starling curve is shifted downward and flattened, and the ventricular end-diastolic pressure increases. Inspection of ventricular pressure–volume loops in normal hearts and those with left ventricular systolic dysfunction provides additional insight. In hearts with systolic dysfunction, the slope of the end-systolic pressure volume relationship is shifted downward (Fig 3). Patients with systolic dysfunction, therefore, have elevated ventricular end-diastolic volume, diminished stroke volume, and elevated ventricular end-systolic volume. This increased end-systolic volume is added to the normal volume of pulmonary venous return, which fills the systemic ventricle, contributing to increased preload and ventricular end-diastolic pressure. This phenomenon explains the concept of “compensatory preload” seen in the Frank–Starling curve in the setting of systolic dysfunction (Fig 1). The decreased stroke volume and increased ventricular end-diastolic pressure ultimately manifest as acute heart failure. Presenting signs and symptoms are consistent with the above pathophysiological disturbances, and treatment strategies are directed at normalising them to the extent possible.

Figure 1 The Frank–Starling law. With ventricular systolic dysfunction, stroke volume falls and the end-diastolic volume increases between points A (healthy, green line) and B (dysfunction, red line).

Figure 2 Mechanistic depiction of the Frank–Starling law. ( a ) As the sarcomere stretches, potential force generation increases, peaks, and then diminishes with overstretch. Stylised sarcomeric units demonstrate progressive stretch from left to right, with variable degree of overlap or separation in the central M-line. Lateral movement of the sarcomere during contraction brings the Z-lines closer together in systole, whereas excessive stretch in diastole decreases maximal force generation. ( b ) A sarcomeric unit, demonstrating thick filaments – myosin heavy/light chains – thin filaments – actin complexed with their associated tropomyosin/troponin proteins. Adapted from Rogers Pediatric Intensive CareReference McBride, Costello and Epting 5 with permission.

Figure 3 Pressure–volume loops in the normal and failing ventricle. Decreased inotropy (systolic failure) shifts the pressure–volume loop along its end-systolic pressure volume relationship (ESPVR) from line A to line B, resulting in a decreased stroke volume (ray a to ray b). During compensation, the ventricle partially restores stroke volume (ray c) by increasing its end-diastolic volume (EDV), which necessitates operating at a higher filling pressure. Note the shape of the end-diastolic pressure volume relationship (EDPVR). Adapted from Rogers Pediatric Intensive CareReference McBride, Costello and Epting 5 with permission.
Delivery, consumption, and extraction of oxygen
Insight into the transition from acutely decompensated heart failure to cardiogenic shock may be gained by considering the relationship between the delivery, consumption, and extraction of oxygen in the systemic circulation (Fig 4). In patients with acutely decompensated heart failure, as delivery of oxygen declines, the oxygen extraction ratio gradually increases; however, assuming that other factors remain equal, the consumption of oxygen is unchanged. As cardiac output continues to deteriorate, a critical threshold is reached in which the demand for oxygen outstrips supply. At this point, oxygen consumption begins to decline and becomes dependent on supply. Anaerobic metabolism ensues, leading to the development of a metabolic acidosis. Clinically, patients with severe ventricular systolic dysfunction develop a progressive increase in systemic ventricular end-diastolic pressures, which results in left atrial hypertension, pulmonary oedema, and increased work of breathing. The resultant respiratory pump workload contributes substantially to increased oxygen consumption. Ultimately, the cardiac pump is insufficient to meet metabolic demands, and shock ensues. The worsening metabolic acidosis contributes to further increased work of breathing and deterioration in ventricular function. This cyclic pathway may progress to death unless timely and appropriate interventions are provided.

Figure 4 Relationship between delivery, consumption, and extraction of oxygen. Across a wide range, delivery of oxygen exceeds demand (supply-independent phase; black line) resulting in a linear extraction of oxygen (green dotted line), until a critical point (the anaerobic threshold) is reached, below which consumption of oxygen falls and is dependent on delivery (supply-dependent; red solid line), and anaerobic metabolism ensues. Oxygen extraction eventually becomes non-linear at a critically low mixed-venous oxygen saturation level (~25%) where further extraction of oxygen bound to haemoglobin is limited.
Clinical manifestations
Presenting symptoms of acute heart failure are age-dependent and non-specific, which may create diagnostic confusion.Reference Macicek, Macias, Jefferies, Kim and Price 6 Early manifestations may include fatigue, tachycardia, tachypnoea, shortness of breath, exercise intolerance, orthopnoea, cough, nausea, vomiting, abdominal pain, and failure to thrive, primarily in infants. The available cardiac output is initially shunted away from the skin and extremities, as well as from the mesenteric and renal circulations, in an effort to preserve the flow of blood to vital organs including the respiratory pump, brain, and heart. On physical examination, children may have varying degrees of distress, tachypnoea, jugular venous distension, hepatomegaly, peripheral coolness, pallor, and peripheral oedema. The cardiac impulse may be quiet if cardiomyopathy is long-standing, but the cardiac impulse may also be hyperdynamic with a third heart sound if there is volume overload; however, as shock progresses, inadequate brain perfusion may result in mental status changes including agitation, fatigue, and delirium. Blood pressure is typically normal or elevated early during the course of acute heart failure syndromes, whereas hypotension develops late.
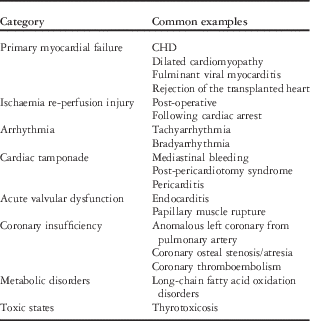
The common aetiologies of acute heart failure syndromes in children are listed in Table 1.Reference Andrews, Fenton, Ridout and Burch 2 , Reference McMurray, Adamopoulos and Anker 3 Many of these abnormalities may co-exist and may fluctuate over time in terms of relative importance. Clinicians must be astute in recognising the various prodromes, presenting signs and symptoms, and electrocardiogram and echocardiogram findings in order to arrive at a timely diagnosis.Reference Ramby, Nguyen and Costello 7 Other ancillary diagnostics that may be helpful are a chest radiograph, levels of a brain-type natriuretic peptide, and hepatic and renal function tests. Early consultation from a paediatric cardiologist, heart failure specialist, and/or electrophysiologist is often helpful in this regard.
Table 1 Common aetiologies of acutely decompensated heart failure and cardiogenic shock in children.
During the initial assessment of a child with possible acute heart failure, there are three initial questions that clinicians should consider. First, is an acute heart failure syndrome present or is there an alternative diagnosis such as sepsis, an adrenal crisis, or a pulmonary embolism?Reference Ramby, Nguyen and Costello 7 , Reference Fisher, Nelson, Beyersdorf and Satkowiak 8 Second, is the patient’s condition immediately life-threatening? Clinical judgement and pattern recognition are essential to identify patients who are at imminent risk of cardiovascular collapse – for example, our anecdotal experience suggests that most patients with long-standing myocardial dysfunction who present with an acute exacerbation of heart failure often respond well – at least initially – to the escalation of heart failure therapies. In contrast, those with fulminant viral myocarditis may progress within hours to cardiac arrest, despite the implementation of aggressive medical therapies.Reference Teele, Allan, Laussen, Newburger, Gauvreau and Thiagarajan 9 These observations underscore the highly adaptive compensatory response to chronic heart failure states, whereas a rapid decline in cardiac pump function is often poorly tolerated. Finally, is there a precipitating cause for the acute heart failure syndrome that can be immediately treated, such as re-entrant arrhythmias or pericardial tamponade?Reference McMurray, Adamopoulos and Anker 3 , Reference Berger and Dubin 10
Monitoring
The choice of a proper initial care setting is an important initial decision for children with acute heart failure syndromes. Consideration should be given to admission to an ICU for all patients until the trajectory of illness can be established.Reference Conway, Costello, Gorenfolo, Hoffman and Rossano 11 The availability of paediatric heart failure specialists, mechanical circulatory support, and a heart transplantation programme are important considerations.
The extent of monitoring used for patients with acute heart failure syndromes will vary depending on the presentation, clinical trajectory, and initial response to therapies. Members of the Pediatric Cardiac Intensive Care Society recently published consensus statements regarding the monitoring of haemodynamics and oxygen transport balance in critically ill children.Reference Domico and Checchia 12 – Reference Bronicki 18 These consensus statements provide a detailed review of the advantages, drawbacks, and nuances of the many available monitoring modalities. Published data regarding monitoring in children with acute heart failure syndromes are typically limited to single-centre case series. The evidence is very limited as to the impact of normalising key parameters on important clinical outcomes. Monitoring modalities commonly used for patients with acute heart failure syndromes can be found in Table 2. As the direct measurement of cardiac output is uncommonly performed in the clinical setting (see below), these modalities represent a variety of clinical, laboratory, and physiological variables that are useful to indirectly assess the adequacy of delivery of oxygen relative to its consumption.
Table 2 Standard haemodynamic monitoring for patients with acute heart failure syndromes.

Quantitative monitoring of cardiac output
Several monitoring systems may be used for quantitative measurement of cardiac output and other haemodynamic variables. Examples include the Fick principle, indicator dilution techniques, pulse contour analysis, Doppler ultrasound, and thoracic bio-impedance.Reference Gazit and Cooper 13 , Reference Perkin and Anas 16 A nuanced understanding of each monitoring modality is essential to ensure that the potential benefits outweigh the risks, and that the limitations and accuracy are appreciated to minimise erroneous interpretation of haemodynamic data and subsequent decision-making.Reference Tibby and Murdoch 19 , Reference Gnaegi, Feihl and Perret 20 Furthermore, the need for special equipment and catheters, the limited utility in patients with intra-cardiac shunts, and the invasive nature and requirement for frequent re-calibration of some of the systems are all important considerations when using these modalities outside of investigational research protocols. In light of the above issues, the recent guidelines published by representatives of the International Society of Heart and Lung Transplantation do not recommend these advanced monitoring techniques for routine use in children with acute heart failure syndromes.Reference Conway, Costello, Gorenfolo, Hoffman and Rossano 11
Monitoring with predictive modelling and streaming analytics
The increasing availability of technological products that allow acquisition of complex clinical data for storage and analysis create opportunities for more accurate characterisation of patient state and rate of change.Reference Goldstein, McNames and McDonald 21 High-resolution data may allow for more discriminating characterisation of physiological phenotypes and pathological states, which in turn may provide clinically actionable information about potentially important differences between patients that are an important adjunct in the evolution and development of personalised medicine.Reference Murdoch and Detsky 22 For example, the calculation and monitoring of derived physiological variables such as heart rate variability, pulse waveforms, shock index, and ratio of dead space to total ventilation have been shown to correlate with outcomes in critically ill patients.Reference Vender, Betancourt, Lehman, Harrell, Galvan and Frankenfield 23 – Reference Winchell and Hoyt 25 Data transformation by predictive modelling and streaming analytics offers the potential for insights gained from research to be transformed into automated, real-time clinical decision support tools that may revolutionise how patients with acute heart failure syndromes are monitored as their disease processes evolve.Reference Herasevich, Pickering, Dong, Peters and Gajic 26
Treatment of acute heart failure syndromes
Although the aetiology of an acute heart failure syndrome is apparent in some patients at the time of admission, for others, treatment must be initiated while the diagnostic evaluation is ongoing. The two most important critical-care strategies used to treat acute heart failure syndromes include optimising the systemic delivery of oxygen and decreasing the consumption of oxygen (Fig 5). These goals are achieved by the thoughtful manipulation of the many factors that influence the delivery and consumption of oxygen. Although we will discuss supply-side factors first, the ability to augment cardiac output in primary pump failure may be very limited, whereas decreasing the metabolic demand for oxygen may have a relatively greater clinical impact.

Figure 5 Factors contributing to the delivery and consumption of oxygen in acute heart failure syndromes. MAP=mean arterial pressure; CVP=central venous pressure; SVR=systemic vascular resistance.
Interventions to augment systemic delivery of oxygen
The systemic delivery of oxygen is determined by the product of cardiac output and arterial oxygen content. The latter is largely influenced by haemoglobin concentration and systemic arterial oxygen saturation. Fortunately, both these variables can be easily measured and manipulated. In patients with acute heart failure syndromes with relative anaemia, oxygen-carrying capacity may be enhanced by the transfusion of packed red blood cells, with due consideration given to the usual hypervolemic state. Patients with arterial hypoxaemia that is secondary to lung disease with pulmonary venous desaturation should be provided supplemental oxygen and additional respiratory support as needed – for example, positive pressure ventilation – while the underlying pulmonary condition is treated.
Cardiac output is the product of heart rate and stroke volume. Stroke volume is influenced by pre-load, afterload, and contractility. These variables are inter-related, and in the clinical setting modulation of one factor may have an important impact on others – for example, a tachyarrhythmia or sinus tachycardia will limit diastolic filling time, thereby compromising ventricular filling and coronary perfusion, increasing myocardial oxygen consumption, and diminishing cardiac output. It is critical to recognise that a sinus tachycardia is an expected compensatory response in heart failure, and thus the degree of heart rate elevation must be placed into clinical context. Sinus bradycardia and other bradyarrhythmias may also compromise cardiac output. In an intensive care environment, the presence of an arrhythmia is commonly suspected by inspection of the electrogram on the bedside monitor. Occasionally, tachyarrhythmias may be insidious – for example, ectopic atrial tachycardia or atrial flutter with 2:1 atrioventricular conduction – but revealed by a review of heart rate trends on a telemetry system. A 12-lead electrocardiogram is usually diagnostic. Prompt treatment of important arrhythmias should be initiated, which typically leads to an improvement in ventricular preload and cardiac output.
Preload
Preload represents passive ventricular wall stress at the end of diastole. Based on the Law of Laplace, ventricular preload is influenced by the radius of the ventricle at end diastole, end-diastolic transmural wall pressure, and thickness of the ventricular wall. Preload effects on ventricular stroke volume are demonstrated by the Frank–Starling mechanism, where increased venous return augments ventricular filling, end-diastolic volume, thus increasing stretched state of the cardiac myocytes before contraction.Reference Patterson and Starling 4 To a point, myocyte stretching increases sarcomere length, which causes an increase in force generation and enables the heart to eject the augmented venous return, thereby increasing stroke volume (Fig 1).
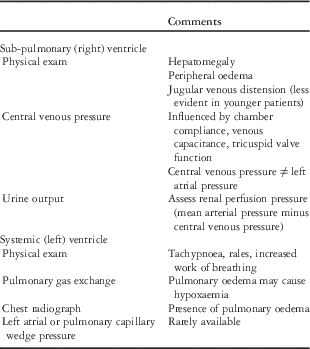
Practical methods to assess right (sub-pulmonary) ventricle and the left (systemic) ventricular preload can be found in Table 3. Note that preload is dependent on intra-vascular volume, which may be depleted in states of increased endothelial permeability. The goal is to identify ventricular filling pressures that optimise stroke volume without producing systemic or pulmonary oedema. Note that patients with poor ventricular compliance may benefit from additional preload, but in such cases volume needs to be administered judiciously and with careful monitoring. Excessive tachycardia, via loss of diastolic filling time, loss of atrioventricular synchrony, and pericardial tamponade may all compromise preload. Patients with elevated filling pressures may be treated with diuretics and fluid restriction, and those with advanced cases may benefit from vasodilators or inotropic infusions.
Table 3 Pre-load assessment.
Afterload
Afterload is the force opposing ventricular fibre shortening during ejection of the ventricle – ventricular wall stress during systole. The Law of Laplace specifies that systemic ventricular afterload is influenced by ventricular transmural wall pressure, the radius of the ventricle at end systole, and the thickness of the ventricular wall. Ventricular transmural wall pressure is influenced by outflow tract resistance – for example, stenosis of the aortic valve – and systemic arterial pressure (Fig 6).

Figure 6 Factors influencing afterload. Adapted with permission from Norton.Reference Norton 27
Manipulation of afterload is an important cornerstone in the management of acute heart failure syndromes; however, it is impractical to measure the systemic ventricular wall stress, and thus afterload at the bedside. Blood pressure is influenced by cardiac output, and thus does not strongly correlate with systemic vascular resistance, and quantitative data on the latter are rarely available. The assessment of peripheral perfusion, including capillary re-fill and strength of pulses, is somewhat subjective, but useful in extreme situations.
Provided that adequate blood pressure exists, systemic vascular resistance may be reduced by the administration of vasodilators. Nitroglycerin or nitroprusside may be used for this purpose, but in contrast to adults there is very little experience with the use of pure vasodilators in children with acutely decompensated heart failure. Milrinone is often used with the dual goals of lowering systemic vascular resistance and improving contractility (see below). Nesiritide – a brain-type natriuretic peptide – has been shown to improve haemodynamics and symptoms in adults with acutely decompensated heart failure. On the other hand, in a multi-centre randomised double-blinded, placebo-controlled trial that enrolled over 7000 patients, patients assigned to receive nesiritide did not have improved clinical outcomes including mortality.Reference O'Connor, Starling and Hernandez 28 In children, published experience with nesiritide in acute heart failure patients is limited to single-centre retrospective case series, which suggested that the drug was reasonably well-tolerated and may have improved symptoms.Reference Mahle, Cuadrado, Kirshborn, Kanter and Simsic 29 , Reference Jefferies, Price and Denfield 30
When initiating therapy with vasodilators or diuretics in patients with acute heart failure syndromes, caution is needed to maintain coronary perfusion pressure. The difference between diastolic blood pressure and the left ventricular end-diastolic pressure, which is often markedly elevated in heart failure states, estimates coronary artery perfusion pressure of the systemic ventricle. An adequate diastolic pressure must be maintained to prevent sub-endocardial ischaemia.
Elevated pulmonary artery pressure may occasionally complicate the management of children with acute heart failure syndromes.Reference Gazit and Canter 31 Long-standing systemic ventricular dysfunction may result in pulmonary arterial hypertension, which may be exacerbated in acute heart failure by the hypoxia and carbon dioxide retention that may occur with pulmonary oedema. Worsening pulmonary hypertension may lead to right ventricular dilation and dysfunction, which may cause adverse effects on inter-ventricular septum position and ventricular–ventricular interactions, thereby compromising left ventricular filling and systolic function. Treatment strategies are focussed on normalising pH, provision of supplemental oxygen to treat hypoxaemia, and judicious use of inotropes to support right ventricular function. Atelectasis, pleural effusions, parenchymal lung infections, and alveolar over-distension all may increase pulmonary vascular resistance, and these conditions should be avoided if possible and promptly treated when present. Caution is warranted regarding the use of inhaled nitric oxide in patients in whom the aetiology of pulmonary hypertension is severe left ventricular dysfunction, given the propensity for pulmonary vasodilators to cause acute worsening of left atrial hypertension and pulmonary oedema in such patients.
Contractility
Inotropic agents may be administered to augment myocardial contractility in patients with advanced acute heart failure syndromes. The goal of inotropic therapy in this setting is to augment stroke volume at the same or lower systemic ventricular end-diastolic pressure. As an accurate bedside measure of contractility does not exist, the response to inotropic infusions must be assessed by indirect measures. Data from animal models indicate that inotropes increase myocyte injury and apoptosis.Reference Caspi, Coles and Benson 32 , Reference Caspi, Coles and Benson 33 The use of inotropes in adults with acutely decompensated heart failure has been associated with increased mortality in multiple clinical trials and registry reports.Reference Abraham, Adams and Fonarow 34 , Reference Cuffe, Califf and Adams 35 Nevertheless, in children, inotropes remain a cornerstone of critical-care management for acute heart failure. In one multi-centre study, inotropic infusions were administered to approximately one-half of children admitted with acute heart failure due to cardiac muscle disease.Reference McMurray, Adamopoulos and Anker 3 Inotropic agents should be used cautiously and at the lowest dose and for the shortest duration possible, given that all increase myocardial oxygen consumption, except levosimendan (see below), and are pro-arrhythmic.Reference Pflugfelder, O'Neill and Ogilvie 36 , Reference Smith, Owen, Borgman, Fish and Kannankeril 37 Selective characteristics of commonly used inotropes are summarised in Table 4.
Table 4 Characteristics of selected inotropic agents.
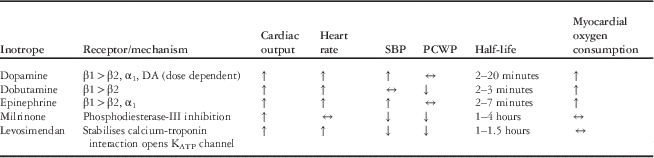
α1,=alpha-1 adrenergic receptor; β1=beta-1 adrenergic receptor; β2=beta-2 adrenergic receptor; DA=dopamine receptor 1; KATP=adenosine tri-phosphate sensitive potassium; PCWP=pulmonary capillary wedge pressure; SBP=systolic blood pressure
Milrinone, an inodilator, is commonly administered to children with acutely decompensated heart failure with the goals of reducing systemic vascular resistance and augmenting myocardial systolic and diastolic function. Milrinone has been shown to improve and, when administered empirically, prevent low cardiac output syndrome following complex paediatric cardiac surgery, but little published data exist in non-surgical children.Reference Chang, Atz, Wernovsky, Burke and Wessel 38 , Reference Hoffman, Wernovsky and Atz 39
Levosimendan is a newer inodilator that stabilises the calcium–troponin interaction, thereby prolonging crossed bridging of actin and myosin, which augments myocardial contractility. Levosimendan also opens ATP-sensitive potassium channels in the systemic arterial circulation, thereby causing direct vasodilation. Levosimendan has been shown to have favourable effects on haemodynamics, neurohormonal balance, and patient symptoms in multiple prospective adult studies. Importantly, this drug does not increase myocardial oxygen consumption nor does it impair diastolic function, and patients do not seem to develop tolerance to the drug. Despite these favourable effects, when compared with dobutamine or placebo in large clinical trials, levosimendan has not been shown to reduce mortality.Reference Mebazaa, Nieminen and Packer 40 , Reference Packer, Colucci and Fisher 41 Published experience in children is limited to pilot studies in post-operative patients, the results of which have been mixed.Reference Ebade, Khalil and Mohamed 42 – Reference Momeni, Rubay and Matta 44 Although levosimendan is approved for use in adults with acutely decompensated heart failure in ∼55 countries, it has not been approved by the United States Food and Drug Administration. A phase III trial designed to determine whether levosimendan will prevent low cardiac output syndrome in adults undergoing cardiac surgery is enrolling patients at present (ClinicalTrials.gov Identifier: NCT02025621).
Interventions to decrease metabolic demands
Interventions to decrease metabolic demands play a critical role in the management of patients with acute heart failure syndromes, particularly those with cardiogenic shock. Primary treatment options include the use of positive pressure ventilation, sedation, and temperature control.
The implementation of mechanical ventilation may have a number of important benefits in this population of patients – for example, under resting conditions in a healthy patient, the muscles used to support the respiratory pump only consume ∼5% of the total body consumption of oxygen; however, in patients with substantial increased work of breathing, as may be seen in patients with pulmonary oedema or metabolic acidosis, the respiratory pump may account for up to 50% of the total body consumption of oxygen. In such patients, the use of positive pressure ventilation will substantially unload the diaphragm and intercostal muscles, thereby decreasing systemic oxygen consumption. Acute heart failure patients with pulmonary oedema often have impaired gas exchange, and in such patients the provision of supplemental oxygen and positive pressure ventilation may be beneficial. Positive pressure ventilation increases intra-thoracic pressure, which effectively decreases the transmural pressure of the systemic ventricle, thereby reducing wall stress and thus afterload (Fig 7). Positive pressure ventilation may be provided non-invasively – for example, continuous or bilevel positive airway pressure via mask – or via endotracheal intubation.Reference Conway, Costello, Gorenfolo, Hoffman and Rossano 11 , Reference Gray, Goodacre, Newby, Masson, Sampson and Nicholl 46

Figure 7 Effect of positive pressure ventilation on transmural wall stress. Adapted with permission from Bronicki.Reference Bronicki 45
The use of mechanical ventilation allows for the provision of sedation and neuromuscular blockade, if needed, in an intubated patient, both of which will further reduce the systemic consumption of oxygen. Sedation and paralysis also facilitate the safe performance of diagnostic tests – for example, cardiac catheterisation – and invasive procedures – for example, central and arterial line placement.
The performance of endotracheal intubation and the initiation of positive pressure ventilation carry substantial risk in patients with acutely decompensated heart failure, particularly those in cardiogenic shock. The provision of sedation and analgesia may blunt endogenous catecholamines, increase systemic venous capacitance, thus decreasing preload, cause peripheral vasodilation, and have direct myocardial depressant effects. Right ventricular preload may be further reduced due to the increase in intra-thoracic pressure that occurs with conversion to positive pressure ventilation. Laryngoscopy may cause a catecholamine surge leading to arrhythmias or acute bradycardia due to an exaggerated vagal response. In patients with pre-existing significant pulmonary oedema, frothy oedema may emerge through the vocal chords, which may compromise visualisation for endotracheal tube placement. Patients with long-standing heart failure with secondarily elevated pulmonary vascular resistance are at risk for developing a pulmonary hypertensive crisis. Given the above factors, patients with acute heart failure syndromes are at substantial risk of cardiac arrest around the time of intubation. To mitigate this risk, the following precautions should be considered. An experienced team of clinicians is needed to manage the patient around the time of intubation. Ideally, at least one experienced clinician should manage the airway, while another is available to provide general oversight for the procedure, and serve as a co-ordinator for other clinicians in the room. Pre-medication with a catecholamine infusion or small boluses of epinephrine may be beneficial to maintain coronary perfusion pressure and offset the potential haemodynamic effects of sedation and analgesia. Although paradoxical in the setting of acute heart failure syndromes, volume may be judiciously administered to maintain preload during the transition from negative pressure to positive pressure ventilation. Judicious selection of induction agents is essential, and those that are most commonly associated with vasodilation or myocardial depression should generally be avoided. The cardiac surgical team should be notified that the procedure is taking place, as emergent extracorporeal membrane oxygenation may be needed for patients who experience a refractory cardiac arrest.
Control of temperature
Meticulous management of body temperature is an under-appreciated and under-utilised treatment strategy to decrease total oxygen consumption in patients with acute heart failure syndromes. Total body oxygen consumption increases approximately by 10% for every degree Celsius increase in body temperature. Although it is apparent to most clinicians that fevers are poorly tolerated in patients with marginal cardiac output, it is less well-appreciated that temperatures between 37 and 38°C may be detrimental. The use of a cooling blanket is quite effective for temperature management in this population of patients.
Anti-coagulation
Patients with acute heart failure syndromes are at risk for developing thromboses.Reference McMurray, Adamopoulos and Anker 3 , Reference Law, Sharma, Feingold, Fuller, Devine and Webber 47 The low flow state and the frequent use of arterial and central venous catheters are often the most important risk factors. Consideration should, thus, be given to the use of systemic anti-coagulation;Reference Conway, Costello, Gorenfolo, Hoffman and Rossano 11 , Reference Law, Sharma, Feingold, Fuller, Devine and Webber 47 however, there are no published data regarding the optimal anti-coagulant drug dose or duration in children with acute heart failure syndromes.
Mechanical circulatory support
For patients with acute heart failure syndromes refractory to medical management, mechanical circulatory support should be considered. Both extracorporeal membrane oxygenation and ventricular assist devices are useful for supporting patients in specific clinical scenarios. Mechanical circulatory support is discussed in great detail in a separate article in this supplement of Cardiology in the Young, and thus only selected issues are reviewed below.
In paediatric cardiac patients, extracorporeal membrane oxygenation was initially used to support those who were unable to separate from cardiopulmonary bypass or those who developed low cardiac output syndrome following cardiac surgery. The utility of extracorporeal membrane oxygenation to support other patients, including those with acute fulminant myocarditis, refractory arrhythmias, and rejection of the transplanted heart, was quickly realised.Reference Rajagopal, Almond, Laussen, Rycus, Wypij and Thiagarajan 48 Extracorporeal membrane oxygenation is most beneficial for cardiac patients who emergently need circulatory support, those with concurrent pulmonary failure, and those who are expected to have myocardial recovery within a few days.
Extracorporeal support initiated during cardiopulmonary resuscitation may be life-saving for paediatric cardiac patients who are in refractory cardiac arrest.Reference Kane, Thiagarajan and Wypij 49 , Reference Thiagarajan, Laussen, Rycus, Bartlett and Bratton 50 In many cardiac ICUs, saline-primed extracorporeal membrane oxygenation circuits are readily available and protocols exist for mobilisation of the personnel and resources essential for rapid cannulation. Congenital and acquired abnormalities of the central blood vessels, such as an interrupted inferior caval vein, occluded vessels, or a surgically constructed superior caval pulmonary connection, are not uncommon in children with congenital and acquired heart disease. Therefore, when feasible, plans should be made in advance with the cardiac surgeons regarding the preferred site for extracorporeal membrane oxygen cannulation – that is, neck, groin, or chest. Review of previous imaging and operative and cardiac catheterisation reports is essential in this regard. At Ann & Robert H. Lurie Children’s Hospital of Chicago, we routinely obtain arterial and venous Doppler ultrasounds of the femoral artery and vein, as well as the right carotid artery and internal jugular vein, in all patients who are considered to be at above-average risk for cardiac arrest and emergent initiation of extracorporeal membrane oxygenation support. In addition, we obtain written informed consent for possible emergent extracorporeal membrane oxygen cannulation from the parents/guardians of all patients admitted with acute heart failure syndromes who were treated with inotropic support.
Although of tremendous value in the acute setting, support with extracorporeal membrane oxygenation for more than 2–4 weeks is often not feasible due to the development of complications including bleeding, infection, and development of thromboses. For patients who are unable to be decannulated within this time frame, and for those expected to benefit from ongoing mechanical circulatory support, transition to a ventricular assist device should be considered.
The use of ventricular assist devices to support children with refractory acute heart failure syndromes is increasing.Reference Almond, Morales and Blackstone 51 Most commonly, ventricular assist devices are used as a bridge to heart transplantation, although myocardial recovery is occasionally seen. Rarely, ventricular assist devices are used for destination therapy in carefully selected children. Given the risks including bleeding, stroke, infection, the decision to place a ventricular assist device is difficult and practice varies widely across centres. At present, outcomes are sub-optimal for patients weighing ∼5 kg and those with functionally univentricular physiology.
Conclusions and future directions
A nuanced understanding of the pathophysiology is essential for the recognition and management of acute heart failure syndromes. The cornerstones of critical-care management focus on manipulation of the many variables that influence systemic delivery and consumption of oxygen. Multidisciplinary collaboration is critically important to optimising outcomes in this population of patients. Enhanced monitoring in the form of predictive modelling and streaming analytics holds promise for the development of clinical decision support tools. The successful completion of clinical trials in children with acute heart failure syndromes is fraught with feasibility issues given the rarity of the disease, the heterogeneous population of patients, and the unpredictability of timing of hospital admission. Going forward, it is likely that multi-centre observational data may best inform clinical practice. Registries such as the Pediatric Cardiac Critical Care Consortium, the Society for Thoracic Surgeons Congenital Heart Surgery Database, and the Pediatric Heart Transplant Study Group are a few examples of existing databases that may be of substantial utility.
Acknowledgements
None.
Financial Support
Dr Costello’s contribution to this manuscript was supported in part by an endowment given by Mr Warren Batts to the Division of Cardiology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Conflicts of Interest
Dr Epting is an unpaid consultant of Vivacelle Bio. Inc. Dr Costello has recently served on a Scientific Advisory Board for Ikaria Inc.













