Introduction
Most tephritid fruit flies within the genus Rhagoletis are univoltine, specialized stenophagous frugivores that exploit groups of plants with discrete yearly phenologies (Bush, Reference Bush1966). Adults must emerge in synchrony with ripening fruit on which they meet, mate, and deposit eggs to give rise to a new generation (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom2000). Flies synchronize emergence with suitable host fruit through dormancy (Bush, Reference Bush1966; Boller and Prokopy, Reference Boller and Prokopy1976; Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom2000). This life-history strategy enables flies to exploit relatively predictable and seasonal host plants (Bush, Reference Bush1966). To cope with inter annual variability, (e.g., early frost events during flowering or fruit set, or insufficient, or extended chill periods), a proportion of the population can engage in prolonged dormancy of variable duration (2, 3, 4, or 5 years) (Boyce, Reference Boyce1931; Dean, Reference Dean1973), presumably as a bet hedging strategy (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014; Rull et al., Reference Rull, Tadeo, Lasa and Aluja2018). Dormancy regulation (induction, duration and termination) and overwintering survival in Rhagoletis have been found to be influenced by environmental factors such as winter length, temperature, relative humidity, soil moisture, photoperiod, and light intensity (Neilson, Reference Neilson1962, Reference Neilson1964; Prokopy, Reference Prokopy1968; Baker and Miller, Reference Baker and Miller1978; Feder et al., Reference Feder, Stolz, Lewis, Perry, Roethele and Rogers1997; Filchak et al., Reference Filchak, Roethele and Feder2001; Yee, Reference Yee2013).
Host plant phenology has also been found to affect the duration of dormancy among host races (morphologically similar species exploiting different closely related host plants, e.g., apple/hawthorn) and sister species (morphologically similar species exploiting different plant families, e.g., Rosaceae/Ericaceae) of Rhagoletis (Berlocher, Reference Berlocher2000) resulting in allochronic isolation among diverging taxa (Smith, Reference Smith1988; Feder and Filchak, Reference Feder and Filchak1999). Quantitative and qualitative changes in the condition of host plants can provide signals for dormancy regulation among some phytophagous insects (Denlinger, Reference Denlinger1986). For example, Hodek et al. (Reference Hodek, Bonet, Hodkova and Labeyrie1981) found that the presence of pollen induces adult diapause in the Mexican bean beetle Acanthascelides obtectus, while senescing potato leaves have been found to induce diapause in the Colorado potato beetle Leptinotarsa decemlineata (cited in Gill et al., Reference Gill, Goyal and Chahil2017). Nevertheless, the influence of host plant derived seasonal chemical cues (detectable plant derived compounds that appear or vary over the season in a predictable way) on diapause of Rhagoletis spp., or any other species of Tephritidae, has thus far not been explored.
Within the genus Rhagoletis, six described species in the Rhagoletis suavis species group exploit plants in the genus Juglans (Bush, Reference Bush1966; Rull et al., Reference Rull, Aluja, Guillen, Egan, Glover and Feder2013). Host fruit size and the presence of conspecific larvae within it have been found to influence the reproductive behaviour of some members of the R. suavis group (Papaj, Reference Papaj2005). Rhagoletis juglandis (Cresson) females exposed to host fruit (colour and shape stimuli) exhibit greater ovarian development than unexposed flies (Alonso-Pimentel et al., Reference Alonso-Pimentel, Korer, Nufio and Papaj1998). In walnut infesting Rhagoletis species, females do not avoid laying eggs in infested fruit, where usually several larvae develop without interference (Nufio et al., Reference Nufio, Papaj and Alonso-Pimentel2000). Feeding of tephritid larvae within fruit enhances the breakdown of phenolic compounds and tannins in comparison to non-infested walnuts (Oroño et al., Reference Oroño, Aluja, Ovruski, Rull, Interdonato, Prado and Hilal2019), which suggests that this behavioural trait among walnut infesting Rhagoletis may be an adaptation to the secondary plant compounds within ripening walnut fruit.
Juglone is a phenolic compound present in the walnut husk and leaves, but also released by the plant root system (Cosmulescu et al., Reference Cosmulescu, Trandafir, Achim and Baciu2011). Because juglone is poorly soluble in water, it does not move far in the soil (Dana and Lerner, Reference Dana and Lerner2001). However, juglone is more persistent in poorly drained soils than in well drained soils (Willis, Reference Willis2000). Juglone is both microbially and abiotically degraded (Von Kiparski et al., Reference Von Kiparski, Lee and Gillespie2007) and therefore its concentration in the plant and soil is both dynamic and seasonal (Coder, Reference Coder1983). Juglone concentration in the soil is highest under the tree canopy and decreases as much as 80% when measured 4.25 m away from the tree (Jose and Gillespie, Reference Jose and Gillespie1998). Juglone concentration under the tree canopy is probably influenced by the combined release of leaves, fruit and the root system, which all contain and release this compound (Cosmulescu et al., Reference Cosmulescu, Trandafir, Achim and Baciu2011). Although most detailed studies on juglone soil dynamics have been made with Juglans nigra and Juglans regia, there is evidence for non-commercial species of walnut with allelopathic effects (Willis, Reference Willis2000). Juglone concentration in the soil peaks during fall through release by decaying fruits and leaves, drops in winter, rises in spring, and decreases during the summer (de Scisciolo et al., Reference de Scisciolo, Leopold and Walton1990). These seasonal patterns are related to fruit and leaf drop but also by release by the root system. Additionally, plants in the genus Juglans are known to engage in mast seeding (the synchronous intermittent production of large seed crops) (Stapanian and Smith, Reference Stapanian and Smith1978; Kelly and Sork, Reference Kelly and Sork2002), which results in inter annual variability in fruit availability, and probably increases in juglone soil concentration. Such trait (masting), could select for prolonged dormancy among walnut infesting Rhagoletis.
Rhagoletis completa (Cresson) and Rhagoletis zoqui (Bush) are important pests of walnuts in Mexico and other parts of the world (Yee et al., Reference Yee, Hernández-Ortiz, Rull, Sinclair and Neven2014). R. completa in Mexico is typically found infesting Juglans hirsuta, J. mollis, and J. regia (Rull et al., Reference Rull, Aluja, Guillen, Egan, Glover and Feder2013), while R. zoqui has been recovered from J. mollis, J. pyriformis, and J. regia (Rull et al., Reference Rull, Aluja, Guillen, Egan, Glover and Feder2013). For both species, most of the individuals in the population are univoltine and under natural uncontrolled environmental conditions roughly take between 250 and 300 days from pupation to adult eclosion independently of walnut host species origin (Rull et al., Reference Rull, Aluja, Guillen, Egan, Glover and Feder2013). Both species become dormant as pupae to synchronize adult emergence with seasonal host fruit availability (Rull et al., Reference Rull, Tadeo, Lasa and Aluja2016, Reference Rull, Lasa, Guillén and Aluja2019a). Two parasitoid species have been found in exclusive association with walnut infesting Rhagoletis, Aganaspis alujai and Diachasmimorpha juglandis (Rull et al., Reference Rull, Aluja, Guillen, Egan, Glover and Feder2013), both parasitoids also become dormant and emerge as adults later than their fly hosts (Rull et al., Reference Rull, Lasa, Guillén and Aluja2019a). Considering the tight relationship between the phenology of Juglans fruits and adult emergence of walnut infesting Rhagoletis, here we tested the hypothesis that host plant chemicals influence adult emergence rates and emergence time of both species and their parasitoids. Given that larvae feed on the husks of walnuts, which are known to have high concentrations of this compound (Cosmulescu et al., Reference Cosmulescu, Trandafir, Achim and Baciu2011), we exposed R. zoqui and R. completa pupae in vermiculite to a warm (24°C) followed by a cold (5°C) and two consecutive warm 4-week periods. Temperature regimes were established to mimic seasonal variation in temperature (fall, winter, spring, summer) and included a chill period necessary to break diapause. Such pupae were exposed to combinations with and without walnut fruit and/or leaves. Additionally, a second experiment was set up to directly expose R. zoqui pupae to different combinations with and without a 3 μg of juglone per g of vermiculite.
Materials and methods
Biological material
Rhagoletis zoqui was obtained from fruit of Juglans pyriformis Liebmann collected on the old Xalapa-Coatepec road (19°29′8.15″N, 96°56′42.01″W; 1334 m a.s.l.) under the canopy of several trees during the 2nd and 3rd week of September 2017 and 2018. Rhagoletis completa was obtained from a single collection of Juglans hirsuta Manning in the locality of San Juan Bautista, Nuevo León (25°23′36.6″ N, 100°18′05.1″ W; 1385 m a. s. l.) during the 1st week of September 2017. No R. completa could be collected in 2018, the collection site is more than 1000 km away from the Xalapa laboratory, and it was difficult to predict the timing of fruit drop. Collected fruits were taken to the laboratory at the Instituto de Ecología AC and handled as described by Rull et al. (Reference Rull, Aluja, Feder and Berlocher2006) to recover pupae. Pupae were removed from trays every 3–4 days and placed in groups of 50 in transparent plastic 200 ml cups containing 5 g of vermiculite previously moistened with a sodium benzoate solution (3 g/l) applied with a hand sprayer to hinder fungal growth. All pupae were of similar age (1–4 days after pupation) when assigned to treatments. Cups were covered with an aerated mesh lid and taken to a laboratory under controlled environmental conditions at a 24 ± 1°C, a 65 ± 5% RH and a 12/12 light/dark (L/D) photoperiod for 7 days.
Host plant parts
For each Rhagoletis species, a total of 36 cups, with 50 pupae each (1800 total pupae, 450 pupae per treatment, subdivided in nine plastic cups with 50 pupae each) were gathered and transferred to 0.7 litre plastic cups with 20 g of vermiculite according to four different treatments with nine replicates each. Treatments were: (i) vermiculite alone, (ii) vermiculite with the addition of five leaves of J. pyriformis (total weight between 1.6 and 2 g), (iii) vermiculite with the addition of one walnut fruit (fresh fruit weight between 70 and 90 g) and (iv) vermiculite with the addition of five leaves and one fruit (similar weight range). Leaves and fruits were collected form J. pyriformis trees at the same time. Fruits were covered with nylon gauzes in order to avoid potential contamination of the experimental sample with larvae stemming from an infested fruit. All cups remained under a pre-winter period of 4 weeks. Thereafter, R. zoqui or R. completa pupae were transferred to a conventional refrigerator for a ‘winter’ period of 4 weeks. After the winter period, cups were returned to the lab, and kept under similar conditions as the pre-winter period to record emergence of adults after dormancy. Temperatures during periods 1, 3 and 4 were set in the lab at 24 ± 1°C; 65% RH, 12/12 L/D photoperiod, and for period 2, cups were transferred to a conventional refrigerator (Whirlpool Multi-flow WT1818A) at 5 ± 1°C; 65% RH, 0/24 L/D photoperiod.
Exposure to juglone
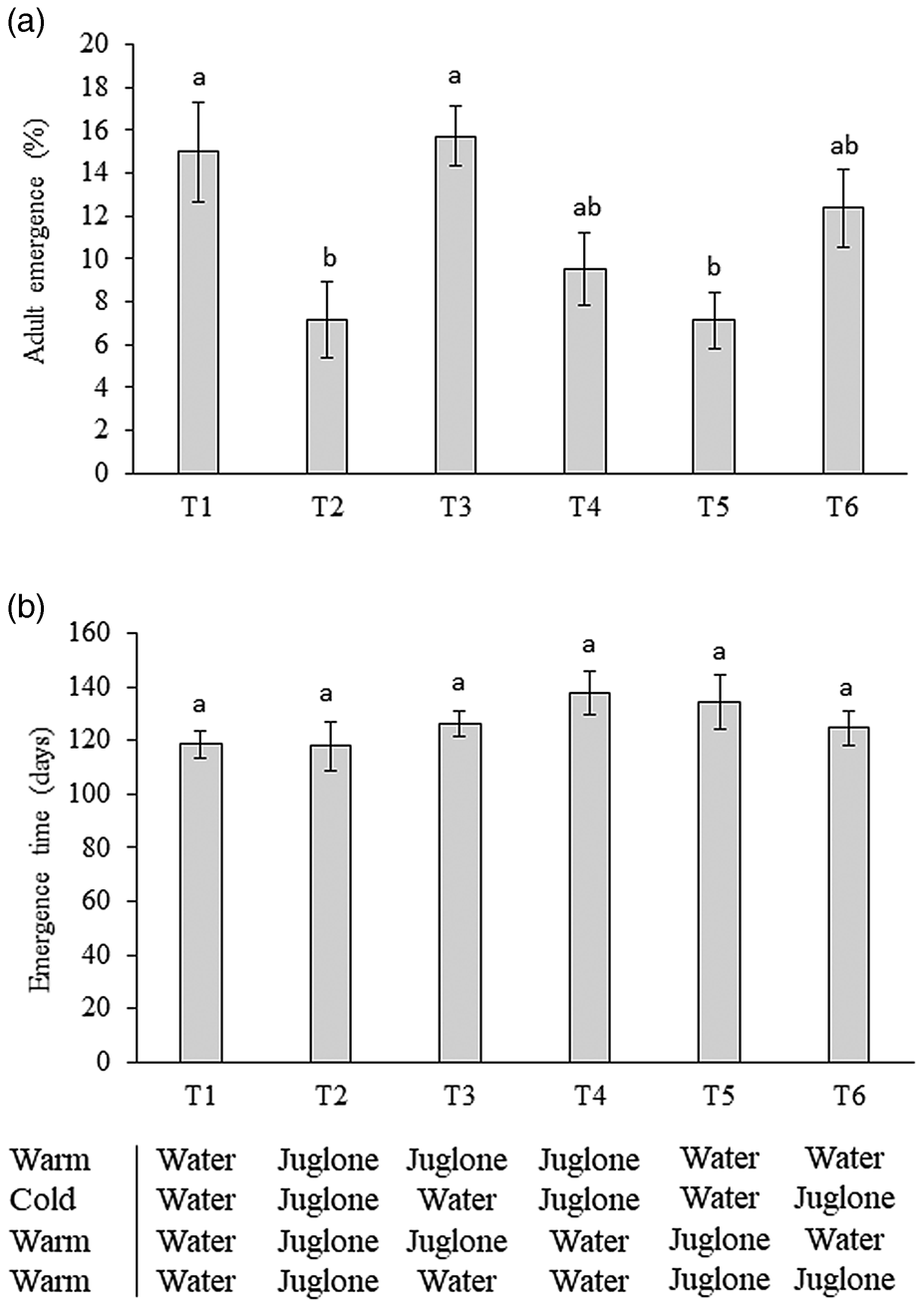
The juglone exposure experiment was only carried out with R. zoqui pupae obtained in 2018 as described above. No R. completa could be collected that season, due to the fact that the collection site is located ca. 1200 km away from Xalapa. A total of 84 cups, with 30 pupae each, were prepared and distributed according to six different treatments and 14 replicates per treatment (a total of 2520 pupae, 420 pupae per treatment). Following the 1st week after collection, all cups were exposed for a complete set of four 4-week periods under warm (24°C – period 1), followed by a cold (5°C - period 2), and two consecutive warm periods (3 and 4). The six treatments were: (i) vermiculite without juglone for the entire period (water control), (ii) vermiculite treated with juglone for the whole period, (iii) vermiculite treated with juglone during periods 1 and 3, (iv) vermiculite treated with juglone during periods 1 and 2, (v) vermiculite treated with juglone during periods 3 and 4, and (vi) vermiculite treated with juglone during periods 2 and 4 (table 1).
Table 1. Four-week time periods under juglone treatment [water (white)/juglone (light grey)] applied to 50-pupae lots of Rhagoletis zoqui
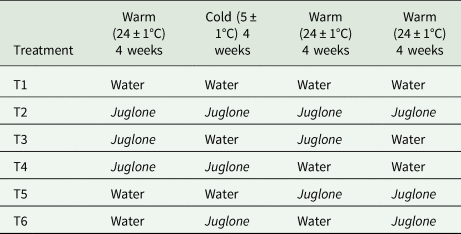
Cups were moistened once a week with 1.5 ml of a sodium benzoate solution (3 g/l) + 2% ethanol (control) or with a solution of sodium benzoate (3 g/l) that contained 10 mg/l of juglone (Sigma Aldrich, México) diluted in 2% ethanol. Ethanol occurs naturally in ripe and decaying fruit at concentrations ranging from 0.03 to 8.1% (Dudley, Reference Dudley2004). Tephritid larvae and other herbivores are therefore normally exposed to this compound without suffering mortality. However, because ethanol was used in all treatments, including the water control, differences in survival and eclosion time of exposed pupae could be attributed to different treatments. In the case of juglone treated cups, each application corresponds to approximately 3 μg of juglone per g of vermiculite, a concentration previously reported in soils under black walnut (Juglans nigra L.) trees (de Scisciolo et al., Reference de Scisciolo, Leopold and Walton1990). After the final 4-week period 4, all cups were treated with a control sodium benzoate solution (3 g/l) on a weekly basis until the end of the experiment. Temperatures during periods 1,3 and 4 were set in the lab at 24 ± 1°C; 65% RH, 12/ 12 L/D photoperiod, and for period 2, cups were transferred to a conventional refrigerator at 5 ± 1°C; 65% RH, 0/24 L/D photoperiod.
Data recording
For both experiments, emergence date and sex of flies and parasitoids were recorded twice a week (Monday and Friday) until the experiment was terminated 1 year after pupae were collected. The duration of the pre-winter period (ca. 40 days from fruit collection) was established to record proportions of non-dormant flies (Rull et al., Reference Rull, Tadeo, Lasa and Aluja2016). The emergence time was estimated as the length of time (days) from the end of the artificial winter (period 2) to adult emergence. At the end of the experiment, 1 year after pupal collection, pupae for all treatments and replicates were inspected under a dissecting microscope to establish the proportion of live pupae and the proportion of dead pupae. Time to emergence for flies and parasitoids was corrected for winter length (subtracted). Parasitoids were identified using morphological traits by one of us (LG). The rate of parasitism (percent) was calculated two ways in reference to both the total number of pupae and by dividing the number of emerged parasitoids over the sum of emerged parasitoids and emerged flies.
Statistical analyses
Cumulative percentages of adult emergence for R. completa and R. zoqui were compared among treatments and sex using a generalized linear model (GLM) with normal distribution (R. completa: Kolmogorov–Smirnov d = 0.089, P = 0.729; R. zoqui: Kolmogorov-Smirnov d = 0.062, P = 0.988) followed by Tukey's honestly significant difference (HSD) tests. The emergence time of Rhagoletis pupae was compared among treatments and sex by means of GLMs with quasi-Poisson distribution for over dispersion, followed by Bonferroni mean comparisons. Percentages of live uneclosed pupae (failing to yield adult flies) at the end of experiment were compared among treatments, for each species, through Chi-squared tests with binomial error distribution.
Cumulative percent overall adult parasitoid emergence was compared among treatments by means of a GLM with normal distribution (Kolmogorov-Smirnov: d = 0.101, P = 0.854) followed by Tukey's HSD mean comparisons. Mean length of post-winter adult dormancy was compared among treatments for each separate parasitoid species with an analysis of variance (ANOVA) in the case of parasitoids in the genus Diachasmimorpha and a non-parametric Kruskal Wallis test for Aganaspis alujai (Wharton & Ovruski). Comparison of mean length of dormancy between both parasitoid genera was subjected to a Mann–Whitney U test. Cumulative percent of adult emergence and emergence time of R. zoqui pupae exposed to juglone was compared among treatments with a two-way ANOVA with treatment and sex as factors. Statistical analyses were all performed using the R-based program Jamovi v.0.9.1.12 (Jamovi, Reference Jamovi Statistical Software2019) or with the Generalized Linear Interactive Modelling (GLIM) program (Numerical Algorithms Group, Reference Francis, Green and Payne1993).
Results
Host plant parts
Emergence rates of R. completa and R. zoqui pupae
No R. completa, R. zoqui, or parasitoid adults emerged from cups without becoming dormant during the 40-day pre-winter period in the host plant cue experiment (n = 1800 total pupae).
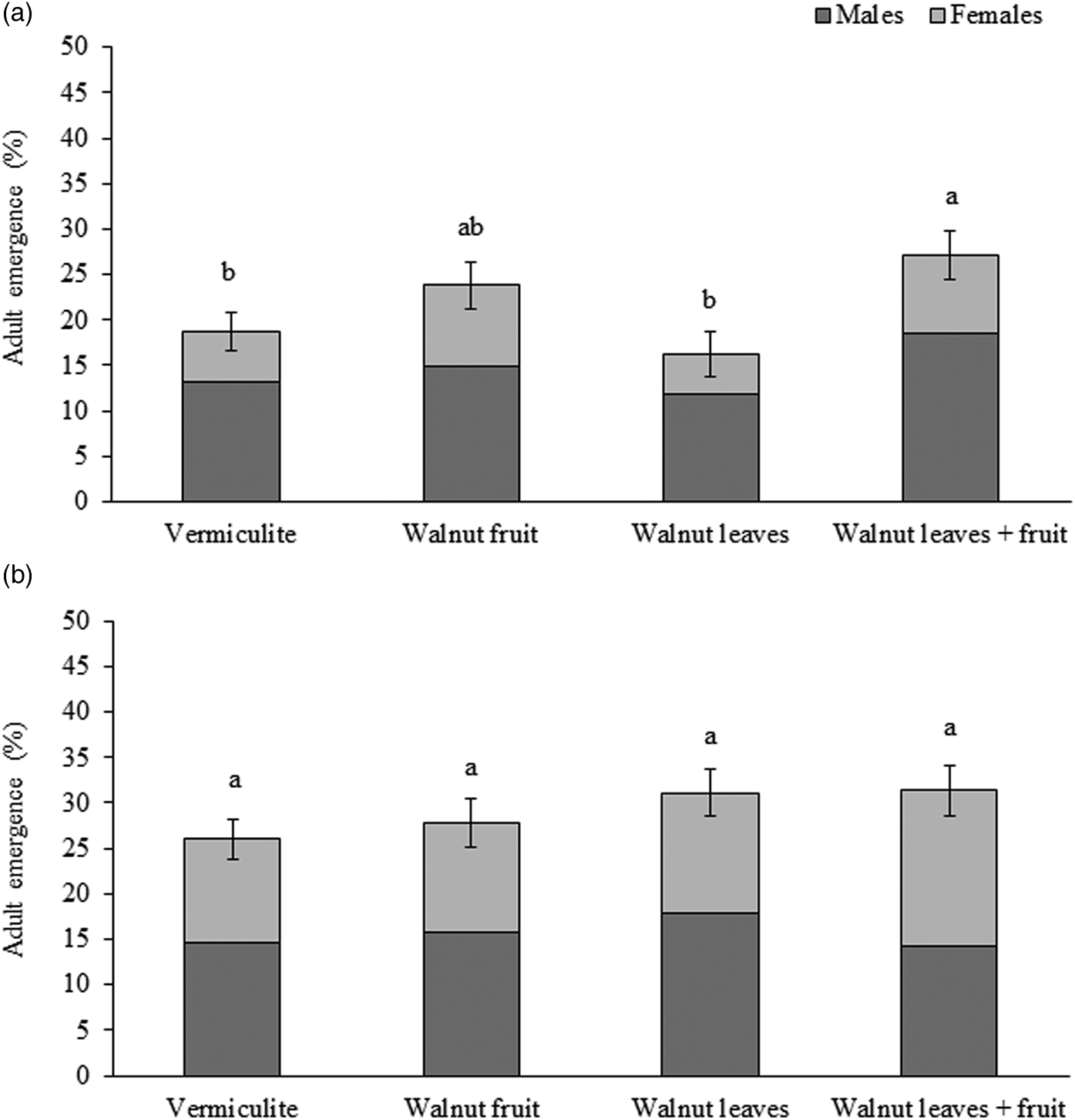
For R. completa, after the pre-winter period, a total of 385 adult flies emerged from all pupae reared in the experiment (36 cups, 50 pupae each one, overall 21.4% adult emergence), with 262 males (68%) and 124 females (32%). For males, emergence rates ranged from 11.8 ± 1.4% (mean ± SE) (walnut leaves) to 18.4 ± 2.5% (mean ± SE) (walnut leaves + fruit). For females, emergence rates ranged from 4.4 ± 1.1% (mean ± SE) (walnut leaves) to 8.9 ± 1.6% (mean ± SE) (walnut fruit).
Significant differences were observed in the percentages of R. completa emergence among treatments (F = 6.301; df = 3,64; P < 0.001) (fig. 1a). Pupae exposed to vermiculite containing walnut leaves and fruit emerged in the highest percentages, followed by pupae in vermiculite with walnut fruit with intermediate percentages and vermiculite alone or vermiculite containing walnut leaves with the lowest percentages. There was also a statistically significant effect of sex on adult percentage emergence (F = 13.147; df = 1,64; P < 0.001), but no significant interaction between sex and treatment (F = 0.137; df = 3,64; P = 0.937).
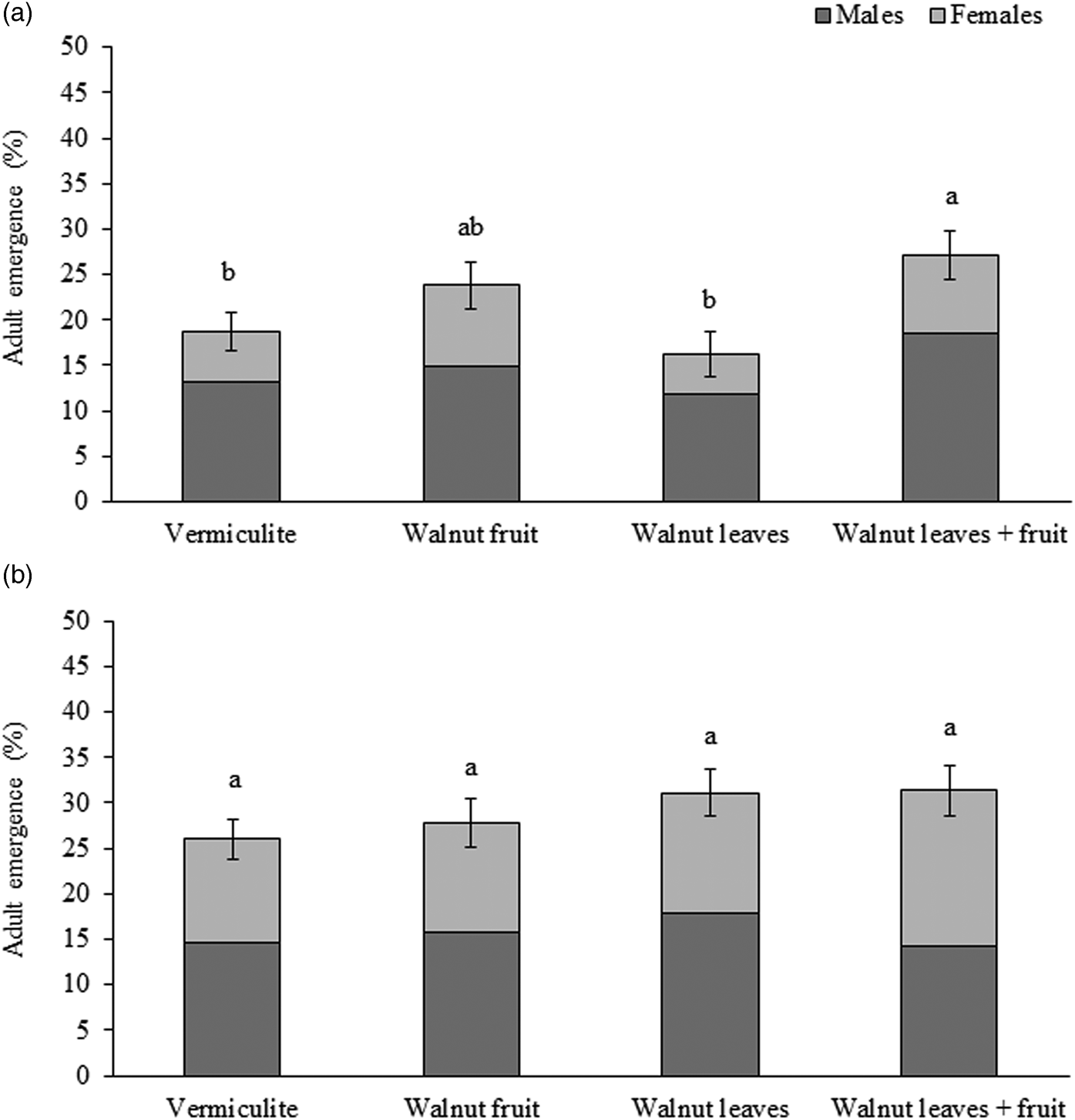
Figure 1. Mean (±S.E.) percent emerged fly adults (males in dark grey bars followed by females in clear grey bars) for (a) Rhagoletis completa (N = 262 males, 124 females) and (b) Rhagoletis zoqui (N = 281 males, 242 females) in reference to pupae exposed to vermiculite alone or vermiculite with a fruit, leaves or leaves plus a fruit. Different letters above bars indicate significant differences (P < 0.05), Tukey HSD.
For R. zoqui, after the pre-winter period, a total of 523 adult flies emerged from all pupae reared in the experiment (36 cups, 50 pupae each one, representing an overall 29.1% adult emergence), with 281 males (54%) and 242 females (46%). For males, emergence rates ranged from 14.2 ± 1.6% (mean ± SE) (walnut leaves + fruit) to 17.8 ± 2.8% (mean ± SE) (walnut leaves). For females, emergence rates ranged from 12.0 ± 1.5% (mean ± SE) (walnut fruit) to 17.8 ± 2.8% (mean ± SE) (walnut leaves + fruit). No significant differences were observed in percentage R. zoqui adult emergence for pupae exposed to a control treatment or different host stimuli (F = 0.988; df = 3,64; P = 0.404) (fig. 1b). There was no statistically significant effect of sex (F = 2.594; df = 1,64; P = 0.112) or for the interaction between sex and treatment (F = 0.1.502; df = 3,64; P = 0.222).
Emergence time of R. completa and R. zoqui pupae
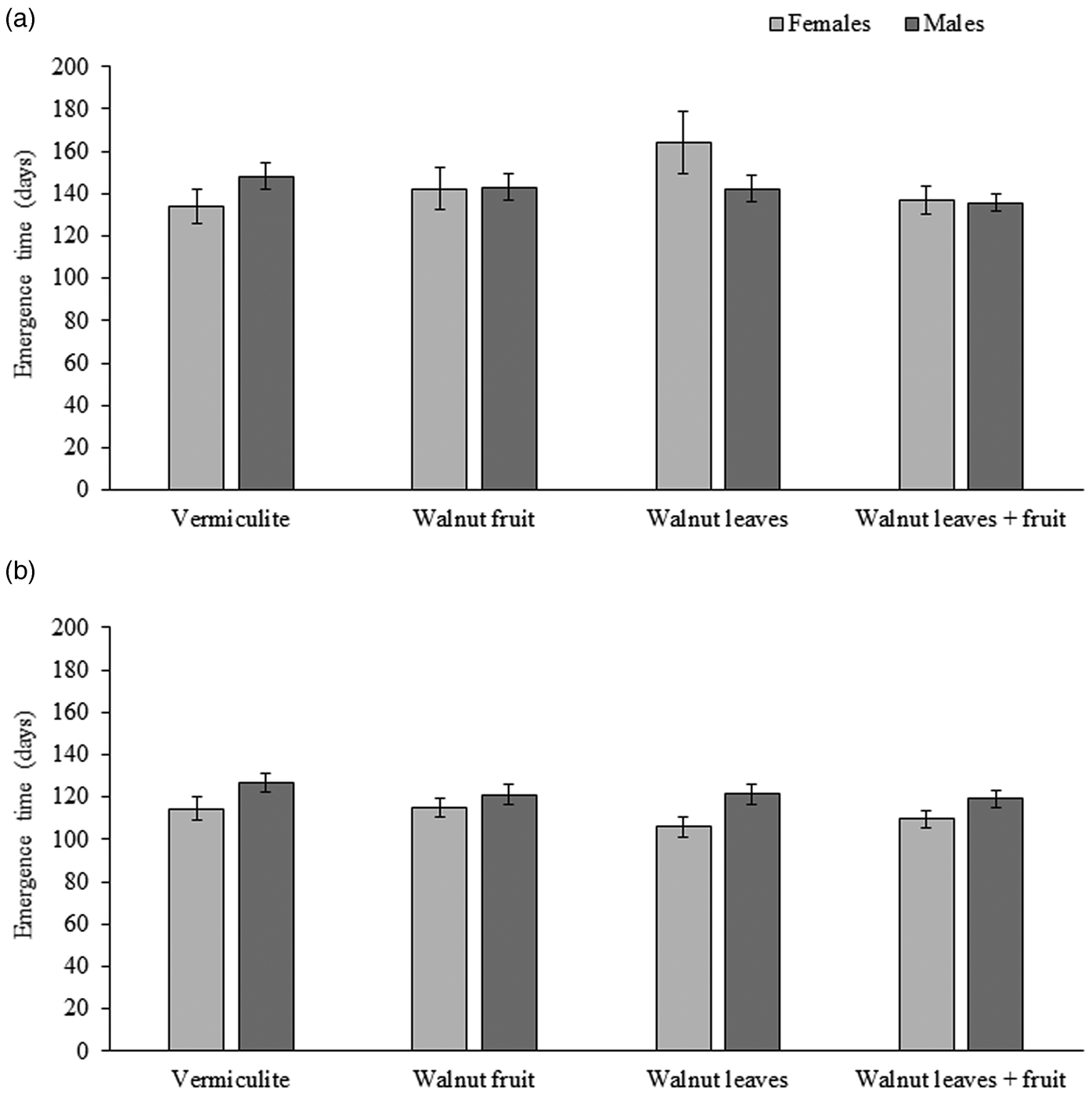
For R. completa, the mean emergence time for males ranged from 136 to 148 days, and for females it ranged from 134 to 164 days. The mean emergence time for R. completa adults was not statistically different among treatments (χ2 = 4.75; df = 3; P = 0.191). No statistical difference was observed between sexes (χ2 = 0.071; df = 1; P = 0.790) or the interaction between sex and treatment (χ2 = 1.2; df = 3; P = 0.240) (fig. 2a).
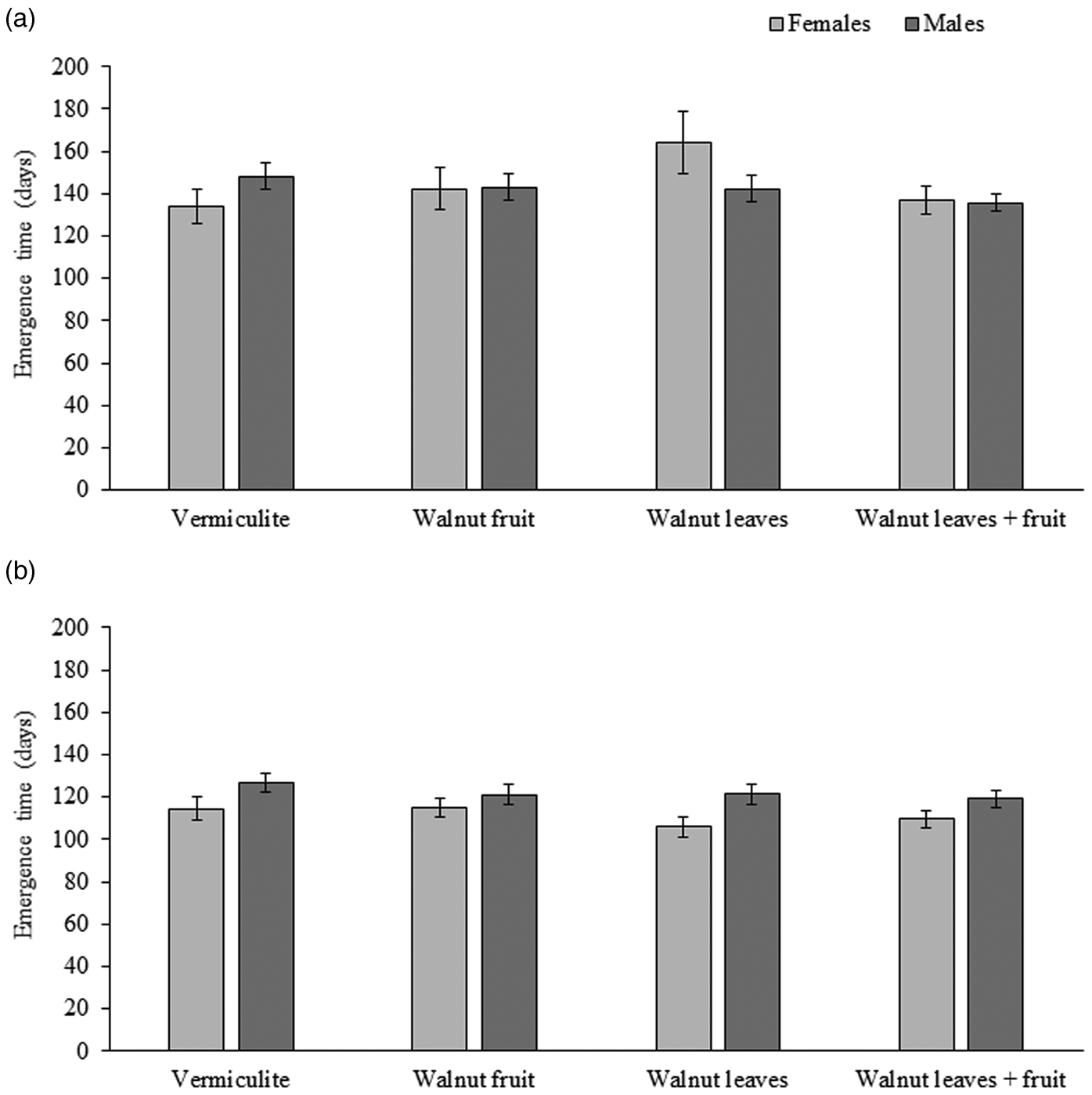
Figure 2. Mean days (± SE) to adult emergence of (a) Rhagoletis completa (N = 262 males, 124 females) (b) R. zoqui (N = 281 males, 242 females) in reference to pupae exposed to vermiculite alone or vermiculite with a fruit, leaves or leaves plus a fruit.
For R. zoqui, the mean emergence time for males ranged from 119 to 126 days, and for females it ranged from 105 to 114 days. The mean emergence time for R. zoqui adults was not statistically different among pupae subjected to a control treatment or different host stimuli (χ2 = 2.84; df = 3; P = 0.418) (fig. 2b). However, the mean emergence time for males ~122 days was statistically different than for females ~111 days (χ2 = 10.54; df = 1; P < 0.001). No significant interaction between sex and treatment was observed (χ2 = 1.17; df = 3; P = 0.167) (fig. 2b).
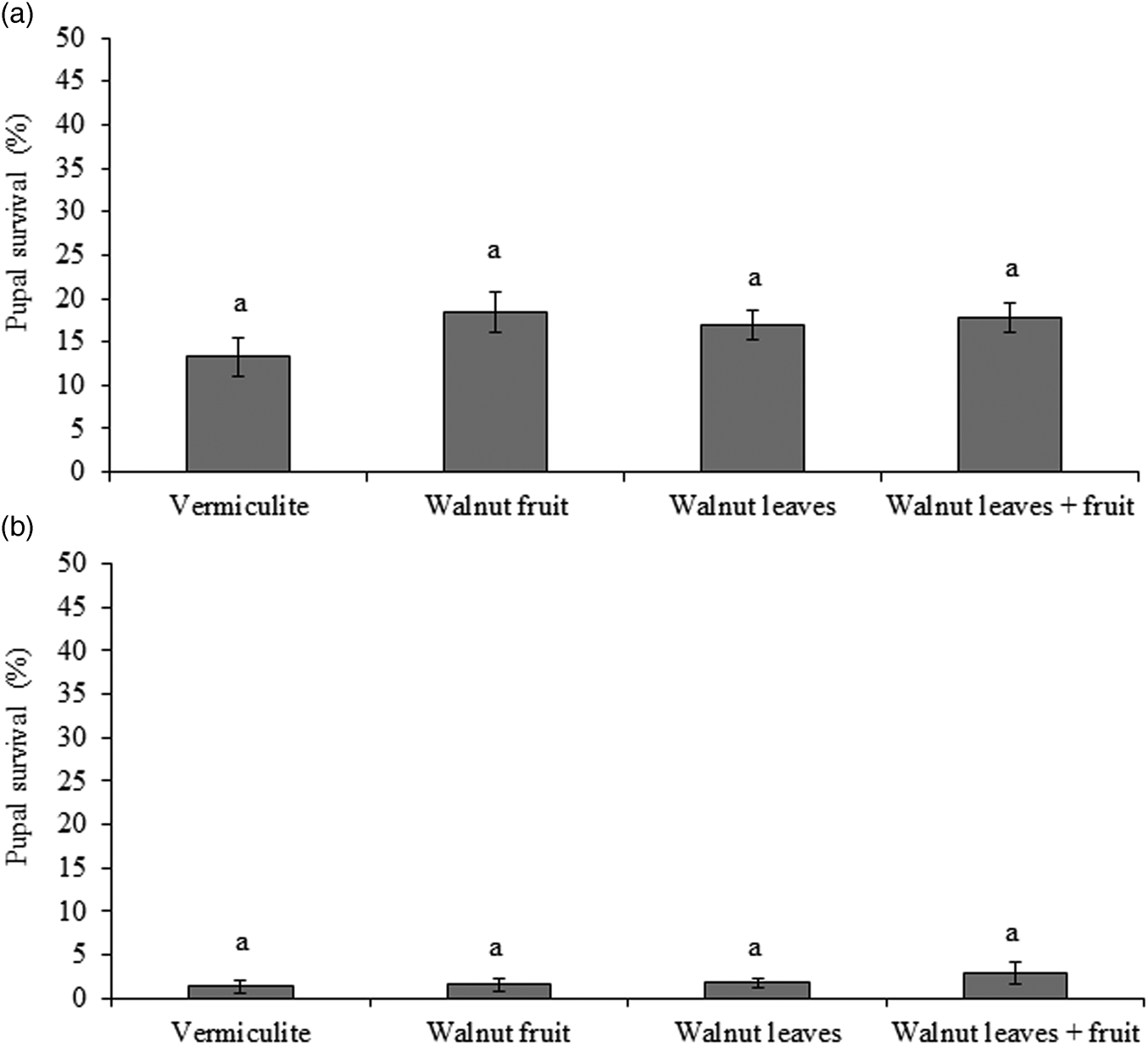
The percentages of R. completa, pupae that remained alive at the end of experiment ranged from 13.3% to 18.4% among treatments. However, the variation among treatments was not statistically significant (χ2 = 5.22; df = 3; P = 0.16) (fig. 3a).
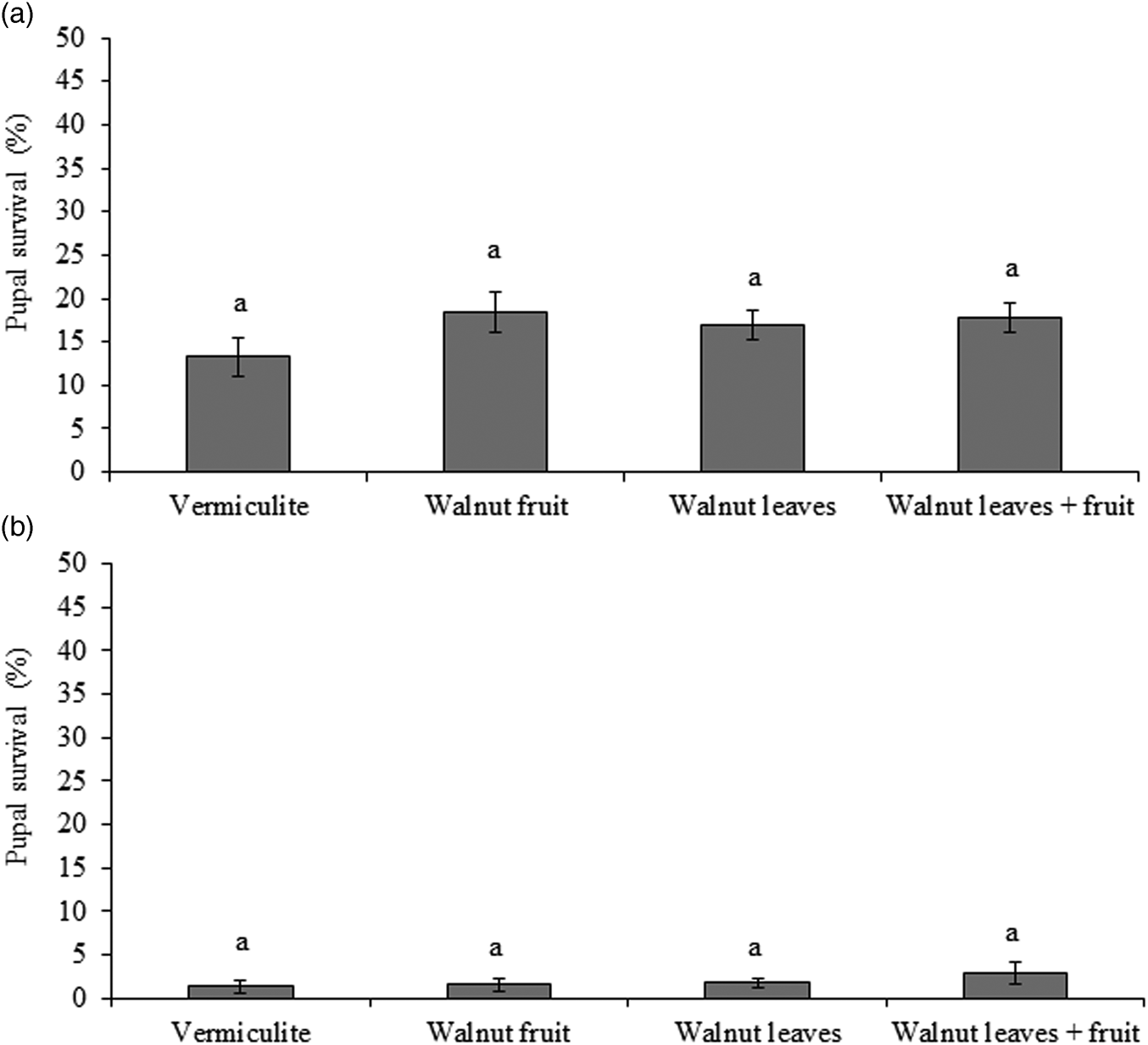
Figure 3. Mean pupae (± SE) survival of (a) Rhagoletis completa (N = 1800) (b) R. zoqui (N = 1800) in reference to pupae exposed to vermiculite alone or vermiculite with a fruit, leaves or leaves plus a fruit. Similar letters above bars indicate no-significant differences (P < 0.05).
The mean percentage of R. zoqui pupae that remained alive at the end of experiment among treatment of the effects of host plant parts varied between 1.3% and 2.8%, and there was no significant difference among treatments (χ2 = 3.25; df = 3; P = 0.34) (fig. 3b).
Parasitoids
A total of 104 parasitoids emerged from cups with R. completa pupae. Of these 104 parasitoids, 51 were identified as Anagaspis alujai (Wharton & Ovruski) and 53 were classified as Diachasmimorpha spp. All specimens of A. alujai parasitoids were females whereas Diachasmimorpha parasitoids were composed of Diachasmimorpha juglandis (Muesebeck) (27 adults) and Diachasmimorpha mellea (Gahan) (24 adults × 12 females and × 12 males). In the case of D. juglandis, the sample was damaged so the sex of individuals could not be accurately determined, however, a sub-sample not included in the experiment yielded a male biased sex ratio of 2.57 males per female. Diachasmimorpha species were identified at the end of the experiment and therefore emergence and mean duration of dormancy were evaluated together for both species. Mean percentage parasitism on total pupae varied between 4% and 8% and there were no significant differences among treatments (F = 1.74; df = 3,32; P = 0.179). When parasitism was considered, in reference to the total emerged adult flies, it reached 21% (104 parasitoids form 490 R. completa flies). Parasitoid species found on R. completa entered a dormancy of longer duration than that of their fly host.
Among 45 cups of fly pupae, parasitoids in the genus Diachasmimorpha emerged form 27 cups whereas A. alujai only emerged from eight cups. No emergence of A. alujai was observed in cups of the treatment that contained vermiculite + walnut leaves which was excluded from analyses. No significant differences were observed in the meantime to adult emergence of A. alujai or Diachasmimorpha for pupae subjected to different fly host plant parts (χ2 = 2.15; df = 2; P = 0.340) (F = 0.183; df = 3,49; P = 0.907) respectively. The mean time to adult emergence for A. alujai, was 260 ± 54 days (mean ± SD), and was significantly longer than that recorded for Diachasmimorpha parasitoids (216 ± 39 days (mean ± SD)), (U = 365, P < 0.001). As expected, both parasitoid species emerged significantly later than their fly host.
Only one parasitoid was reared from all R. zoqui pupae in the experiment and it was identified as female of A. alujai.
Exposure to juglone
Adult emergence of R. zoqui pupae
Out of 2520 pupae used for the juglone experiment, only 17 R. zoqui adults (0.67%) emerged from cups during the pre-winter period (35 days) without becoming dormant. No parasitoids emerged from any R. zoqui pupae recovered in 2018.
After the pre-winter period, a total of 272 R. zoqui adult flies emerged from all pupae reared in the experiment (84 cups, 30 pupae each one), with 131 males (48%) and 141 females (52%). Significant differences were observed in percent R. zoqui adult emergence for pupae exposed to juglone treatments or a non-treated vermiculite control (F = 5.78; df = 5146; P < 0.001) (fig. 4a). There was no statistically significant effect of sex (F = 0.273; df = 1146; P = 0.602) or of the interaction between sex and treatment (F = 0.379; df = 5146; P = 0.862). Exposure to two consecutive time periods under juglone treatment resulted in lower percent emergence of flies (fig. 4a).

Figure 4. (a) Mean (± SE) percent emerged fly adults (males in dark grey bars followed by females in clear grey bars) (N = 131 males, 141 females), (b) mean days (± SE) to adult emergence of R. zoqui in reference to pupae exposed to vermiculite alone (water) or vermiculite treated with juglone over four 4-week time periods (warm 24°C and cold 5°C), following treatments indicated in table 1. Different letters above bars indicate significant differences (P < 0.05), Tukey HSD.
Emergence time of R. zoqui pupae
The mean emergence time of R. zoqui adults (excluding non-dormant flies) was not statistically different among pupae subjected to different juglone treatments (F = 1.278; df = 5,242; P = 0.274) (fig. 4b). The mean emergence time for males (mean ± SE) (122.1 ± 4.7 days) was not statistically different than that of females (117.1 ± 4.6 days) (F = 0.941; df = 1242; P = 0.333). No significant interaction between sex and treatment was observed (F = 0.644; df = 5242; P = 0.666) (fig. 4b).
Discussion
No, or very small proportions of R. completa and R. zoqui emerged as adults without becoming dormant. By contrast, a proportion of R. completa and a smaller proportion of R. zoqui pupae engaged in prolonged dormancy. Exposure to host plant parts (leaves and/or fruit) or juglone affected overwintering survival but not the duration of dormancy of R. completa and R. zoqui. During the host plant part exposure experiment, only overwintering pupae of R. completa exposed to fruit in vermiculite survived (emerged as adults) in greater proportion than R. completa or R. zoqui exposed to leaves and the control. In the case of exposure to juglone, at the tested concentration, regimes under prolonged exposure (two consecutive 4-week periods or more) caused mortality of overwintering R. zoqui. Overall, we failed to gather conclusive evidence indicating that host plant derived cues or juglone are used as a signal (token stimulus) by walnut infesting Rhagoletis for regulation or fine tuning of dormancy.
Proportions of non-dormant flies and prolonged dormancy
While most species of Rhagoletis are considered to be univoltine (Bush, Reference Bush1966; Boller and Prokopy, Reference Boller and Prokopy1976), there is a proportion of the population that emerges as adults without becoming dormant. The proportion of non-dormant individuals among Rhagoletis can range from 53% to less than 1%, (Boyce, Reference Boyce1931; Rull et al., Reference Rull, Tadeo, Lasa and Aluja2016; Neven and Yee, Reference Neven and Yee2017). For R. pomonella, total forgoing of dormancy can be induced by manipulating pre-winter environmental conditions (Prokopy, Reference Prokopy1968). There is a latitudinal cline in the proportion of non-dormant individuals among North-American R. pomonella, with populations at northern latitudes being more prone to produce second generation individuals than those at lower latitudes (Dambroski and Feder, Reference Dambroski and Feder2007). The latitudinal cline holds true for Mexican populations at the southern extreme of the distributional range of this species (Rull et al., Reference Rull, Tadeo, Lasa and Aluja2016), and appears to include R. cingulata (Rull et al., Reference Rull, Tadeo, Lasa and Aluja2018) which forgo dormancy in proportions under 1%. In the case of R. completa, AliNiazee and Boyce (Reference Boyce1931) reported up to 75% and 53% of non-dormant individuals while Rull et al. (Reference Rull, Lasa, Guillén and Aluja2019a) and our current results failed to detect any non-dormant individuals for the Mexican population in this species. Similarly, very few R. zoqui individuals forgo dormancy (Rull et al., Reference Rull, Tadeo, Lasa and Aluja2016). Overall, it seems that ecological conditions at the southern extreme of the genus do not favour persistence of this trait. This finding has negative implications from an applied perspective because it complicates selection of non-dormant strains necessary for development of continuous rearing.
As found for other members of the genus Rhagoletis (Boyce, Reference Boyce1931; Dean, Reference Dean1973; Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014; Rull et al., Reference Rull, Tadeo, Lasa and Aluja2018) a proportion of Mexican populations of R. completa and R. zoqui, engaged in prolonged dormancy. It has been proposed that prolonged dormancy in R. cerasi, evolved as a bet edging mechanism to cope with unpredictable environmental variability (Moraiti et al., Reference Moraiti, Nakas and Papadopoulos2014). Plants in the genus Juglans are known to engage in mast seeding events which result in seasons with abundant fruit followed by seasons with scarce fruit (Stapanian and Smith, Reference Stapanian and Smith1978; Kelly and Sork, Reference Kelly and Sork2002). Such recurrent inter annual variation could explain the existence of prolonged diapause in walnut infesting Rhagoletis.
Host plant part effects on survival and duration of dormancy
Exposure to host plant fruit and/or leaves or juglone had little effect on the duration of dormancy of R. completa and R. zoqui. Only R. zoqui pupae continuously exposed to juglone (four consecutive 4-week periods), emerged as adults sooner than pupae exposed to other regimes. However, duration of dormancy for such pupae was not different from that recorded for the untreated control, and therefore we cannot conclude that host plant derived chemicals are used by walnut flies as environmental cues for fine tuning of dormancy.
When examining adult emergence, the presence of fruit, with and without leaves, enhanced emergence of R. completa in comparison with pupae exposed to walnut tree leaves only and the control (vermiculite). By contrast, fruit presence had no effect of adult R. zoqui emergence. Humidity has been found to be important for survival of overwintering Rhagoletis pupae, especially during the final stages of dormancy (Rull et al., Reference Rull, Lasa and Aluja2019b). The positive effect of fruit presence could have been due to release of plant produced chemicals. However, it is also possible that fruit presence enhanced survival by increasing humidity in the pupation media through water release during fruit breakdown. Thus, it is possible that R. completa may be more susceptible to desiccation than R. zoqui, which could account for the difference in response when fruit was present in treatments between the two species.
Exposure to juglone
Toxic effects of juglone on phytophagous insects have been widely reported (Islam and Widhalm, Reference Islam and Widhalm2020). Nevertheless, some phytophagous species have been shown to be tolerant to this compound, yet at high concentrations it can still produce toxic effects (Thiboldeaux et al., Reference Thiboldeaux, Lindroth and Tracy1994). When exposing R. zoqui pupae to juglone, continuous application during two consecutive 4-week periods resulted in a reduction of adult emergence in comparison to other treatments and the control. Juglone concentration in our study was established on records found in soil under the canopy of J. nigra trees (de Scisciolo et al., Reference de Scisciolo, Leopold and Walton1990), which is known to have the highest juglone concentration among studied species of Juglans (Willis, Reference Willis2000) and is a natural host of R. completa (Yee et al., Reference Yee, Hernández-Ortiz, Rull, Sinclair and Neven2014). Additionally, our methodology involved moistening vermiculite containing pupae on a weekly basis. Because we did not estimate the rate at which juglone degraded in cups, it could be that the compound accumulated at higher concentrations than 3 μg per g of vermiculite over the course of the experiment. In any case, it appears that rather than functioning as an environmental cue, at the tested concentration, juglone had a toxic effect on R. zoqui. Perhaps, juglone concentration under J. pyriformis trees, the native host of R. zoqui (Rull et al., Reference Rull, Aluja, Guillen, Egan, Glover and Feder2013), is normally lower than that under other species of Juglans trees and therefore the potential effect of juglone on regulation of dormancy of walnut infesting Rhagoletis should be tested at different lower concentrations than the one evaluated in this study.
Parasitism
As found in other studies involving Mexican populations of R. completa (Rull et al., Reference Rull, Lasa, Guillén and Aluja2019a), we recorded 21% parasitism by three hymenopteran species (A. alujai, D. mellea, and D. juglandis). By contrast, parasitism on R. zoqui was almost non-existent, a surprising finding considering the fact that this fly species was collected within its native range and from its natural host plant. Because A. alujai did not emerge from fly pupae exposed to walnut leaves. it was not possible to reach conclusions on susceptibility of this parasitoid to host plant derived compounds such as juglone.
Conclusion
While our study yielded information on dormancy of Mexican populations of walnut infesting Rhagoletis and associated parasitoids, we failed to produce evidence indicating that juglone or host plant parts are used as a cue for fine tuning of dormancy in R. completa or R. zoqui. At the tested concentrations, juglone appeared to be toxic to exposed pupae. Lower concentrations of juglone should be evaluated to conclusively discard a potential effect, such tests should include R. completa. However, at present, juglone applications to the soil to disrupt synchrony of walnut infesting Rhagoletis and susceptible fruit are not supported by our data.
Acknowledgements
We thank Larissa Guillen for identification of parasitoids, Eduardo Tadeo for technical assistance, and Emilio Acosta for collection of fruit. Funding was provided by the International Atomic Energy Agency, (IAEA Research Contract No. 18331/R0), Dormancy Management to Enable Mass-rearing and Increase Efficacy of Sterile Insects and Natural Enemies) to JR and RL and the Mexican Campaña Nacional contra Moscas de la Fruta, Mexican Ministry of Agriculture (SADER) via the National Consultative Phytosanitary Council (CONACOFI) through projects 41011-2017, 41012-2018, and 41013-2019 awarded to MA. We also acknowledge funding by the Instituto de Ecología AC.