The principal subjects of this study, Natural History Museum London (NHMUK) specimens P7989 and P11656 (Fig. 1), are part and counterpart of a single specimen: a small nodule containing the anterior third of an early actinopterygian preserved ‘in the round'. More than a century ago, Traquair (Reference Traquair1911) observed that ‘this strange little palaeoniscid' had a peculiarly large snout, that it would probably require a new genus for its reception and that the form of its pectoral fin was unknown. Each of these topics is addressed here: a new genus is erected; the distinctive snout is shown to maximum advantage; and the skeletal anatomy of the pectoral fin is explored in unprecedented detail for a Palaeozoic ray-finned fish.

Figure 1 Trawdenia planti, part and counterpart of nodule, Trawden and Colne region, Burnley Coalfield, Lancashire, UK. (A) NHMUK P7989, natural mould of dermal bones and scales from left side, image reversed for direct comparison with NHMUK P11656, lighting from lower margin of image to create illusion of positive relief. (B) NHMUK P11656 endocranial cast in left-lateral view. Surface detail in both images enhanced with ammonium chloride.
Two factors prompted renewed interest in this species, and especially this individual fish. First, the availability and effectiveness of new imaging methods: computed tomography (CT) scans of the nodule have yielded data that deliver detailed new insights into its structure, raising questions about phylogenetic affinities and functional morphology. Second, recent studies of early actinopterygians have raised a new research agenda (Mickle et al. Reference Mickle, Lund and Grogan2009; Choo Reference Choo2011, Reference Choo2015; Giles & Friedman Reference Giles and Friedman2014; Sallan Reference Sallan2014; Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a, Reference Giles, Darras, Clément, Blieck and Friedmanb, Reference Giles, Xu, Near and Friedman2017; Mickle Reference Mickle2017). The consensus that nearly all post-Devonian Palaeozoic species were stem actinopterans or stem neopterygians (Gardiner & Schaeffer Reference Gardiner and Schaeffer1989; Coates Reference Coates1999; Gardiner et al. Reference Gardiner, Schaeffer and Masserie2005) has been overturned. Instead, most of these genera are now excluded from the actinopterygian crown clade (Giles et al. Reference Giles, Xu, Near and Friedman2017), resulting in a well-populated stem lineage (a result foreshadowed by Cloutier & Arratia (Reference Cloutier, Arratia, Arratia, Wilson and Cloutier2004) and Mickle et al. (Reference Mickle, Lund and Grogan2009)). However, phylogenetic relationships among all post-Devonian Palaeozoic actinopterygian taxa have been characterised as unstable, irrespective of specimen completeness (Giles et al. Reference Giles, Xu, Near and Friedman2017). As a result, the timescale and sequences of character and clade evolution close to the base of this major division of modern vertebrate life are yet to be resolved into a reasonable working hypothesis.
The genus Mesopoma (Traquair Reference Traquair1890a) is emblematic of this phylogenetic fog and attempts to place these fish in the actinopterygian tree raise numerous issues general to the data set as a whole. Mesopoma species are small (under 8cm long), fusiform and known from the Viséan, Serpukhovian and Westphalian of England and Scotland (but not North America; see Mickle et al. Reference Mickle, Lund and Grogan2009). Most specimens are laterally compressed (Fig. 2), and collections include numerous more-or-less complete bodies with fins and variably crushed heads (Moy-Thomas & Bradley Dyne Reference Moy-Thomas and Bradley Dyne1938; Coates Reference Coates1993, Reference Coates1999). Problematically, Mesopoma was erected as a genus intermediate to two better-known (although no better characterised) clades: Canobius Traquair and Rhadinichthys Traquair. As a result, genus diagnoses for Mesopoma (Moy-Thomas & Bradley Dyne Reference Moy-Thomas and Bradley Dyne1938; Coates Reference Coates1993, Reference Coates1999) have struggled to define this morphological hinterland. There is no unambiguous genus-specific synapomorphy for Mesopoma, and this group or grade is characterised by a combination of features widespread among other early actinopterygians. Thus, recent analyses (e.g., Giles et al. Reference Giles, Xu, Near and Friedman2017) have been careful to use the species planti alone, to avoid assumptions that specialisations present in this Westphalian form might also occur in putative congenerics from the Viséan.
The individual fish preserved in NHMUK P7989 and P11656 is valuable because it expands the range of morphological data available from post-Devonian early actinopterygians. Indications of specimen quality are already published (e.g., Coates Reference Coates1999, fig. 4, on the endocranial cast). But, for systematic purposes, rather than the generously proportioned snout that intrigued Traquair, the ornament and shape of the rostral bone emerges as more straightforwardly diagnostic. This distinguishing feature (Figs 3, 4) is clearly shared with two other Mesopoma species: carricki (Coates Reference Coates1993, pl. 1, fig. 1; Fig. 5a) and pancheni (Coates Reference Coates1993; Fig. 5b). Therefore, consistent with the aim of improving taxonomic precision and stability (Ride et al. Reference Ride, Cogger, Dupuis, Kraus, Minelli, Thompson and Tubbs1999), these three species are removed from Mesopoma to form a new genus preserved in sufficient detail to be phylogenetically instructive.
1. Materials
1.1. Image preparation
Scans of NHMUK P7989 and P11656 were completed by the X-ray CT facility at the University of Chicago, using a GE Phoenix 240/180 scanner at 160kv and 85μA, and no filter; 2024 projections with a voxel size of 10.58μm. Anatomical reconstructions were completed using Mimics v. 17 (biomedical.materialise.com/mimics; Materialise, Leuven, Belgium) for the three-dimensional (3D) modelling, including segmentation, 3D object rendering and stereolithography (STL) polygon creation. 3D Studio Max (Autodesk.com/products/3ds-max; Autodesk, San Rafael, USA) was used for further editing of the STLs (colour, texture, lighting) and mirroring for the final restoration. The photograph of NHMUK P11656 in Figure 1b was prepared using a Leica DFC490 camera attached to a Zeiss Stemi SV6 microscope. Digital image processing was performed in Image-Pro Plus 6.2, with multiple images aligned using the enhanced depth-of-field function.
1.2. Abbreviations
Explanations of anatomical abbreviations are provided in figure captions. Terminology follows Gardiner (Reference Gardiner1984) and Coates (Reference Coates1999).
Institutional abbreviations
GLAHM = Glasgow University, Hunterian Museum; MM = Manchester Museum; NHMUK = Natural History Museum London, UK; NMS = National Museums of Scotland.
1.3. Specimens and geological context
Originally described by Traquair (Reference Traquair1888, Reference Traquair1911), albeit with reservations, as Rhadinichthys planti, Coates (Reference Coates1999) redescribed the fish (specimens NHMUK P7989 and NHMUK P11656) as Mesopoma planti. The specific name planti honours John Plant who, among other notable contributions to modern biology (Smith & Walklate Reference Smith and Walklate2017), collected the first examples of this species (several laterally flattened specimens, e.g., NHMUK P8500; Fig. 2) from Collyhurst, near Bradford, UK. The collection history of the individual shown in Figure 1 is covered in more detail elsewhere (Coates Reference Coates1999). In brief, the Natural History Museum obtained NHMUK P7989 (Fig. 1a), the nodule half preserving a natural mould of the left side of the fish, from John Ward's collection in 1894. Specimen NHMUK P11656, the counterpart, exposing the cranial endocast and containing the dermal skull from the right side of the fish (Fig. 1b), was obtained from Ramsay Traquair's personal collection in 1914. Notably, Traquair's (Reference Traquair1911) rudimentary specimen sketch (Fig. 3f) shows the external morphology of the left side intact (now removed and known only from the natural mould). It seems likely that Ward and Traquair obtained their specimens from George Wild, who collected the nodule from the Soapstone Bed in the Trawden and Colne region of the Burnley Coalfield, Lancashire, UK. Notably, the similarly preserved actinopterygian Coccocephalus wildi (Poplin & Véran Reference Poplin and Véran1996) probably originates from the same locality and horizon (the species name honouring the same collector).

Figure 2 Trawdenia planti, NHMUK P8500, Collyhurst, Bradford, Yorkshire, UK. (A) Specimen. (B) Line drawing. Abbreviations: an = angular; aop = accessory opercular; br = branchiostegal rays; de = dentary; dh = dermohyal; dsp = dermosphenotic; esc = extrascapular; max = maxilla; ju = jugal; op = opercular; pa = parietal; pop = preopercular; so = suborbital; sop = subopercular; sp = surangular process; sur = surangular.
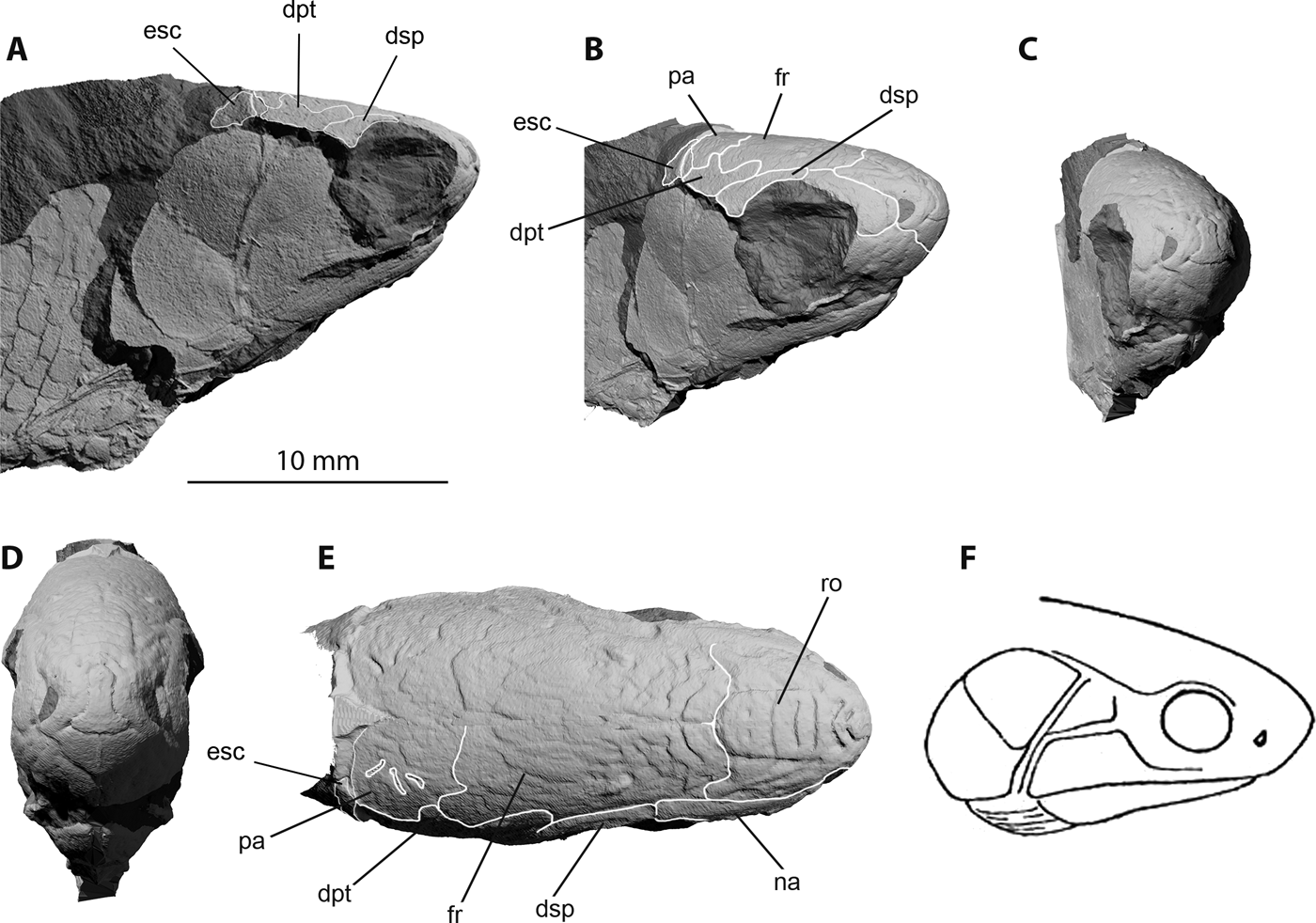
Figure 3 Trawdenia planti, NHMUK P7989, Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of natural mould of dermal bones from left side of cranium mirrored along the anatomical midline to provide estimate of complete skull shape, including the profile of the well-developed rostrum. (A) Right-lateral view. (B) Right-lateral view, slight anterior rotation. (C) Right-anterolateral view. (D) Anterior view. (E) Dorsal view. (F) Sketch of same specimen by R. H. Traquair, reproduced from Traquair (Reference Traquair1911, p. 152). Skull roof sutural boundaries highlighted in (B) and (E), with selected bones identified to avoid visual clutter. Abbreviations: dpt = dermopterotic; dsp = dermosphenotic; esc = extrascapular; fr = frontal; na = nasal; pa = parietal; ro = rostral.
The source of these nodules, Carre Heys colliery (Bolton Reference Bolton1905), has long gone, but is likely recorded by the former site of a farm, Carry Heys, near a disused shaft located at National Grid Reference 390247.439729 (M. Gill, Northern Mine Research Society, personal communication). In this context, it is worth mentioning that there is no ‘Mountain Fourfoot Mine of Trawden', contra Poplin & Véran (Reference Poplin and Véran1996). The Soapstone Bed mentioned previously is a thin band of light-grey shale including numerous small nodules consisting of ‘earthy carbonate of iron' (Bolton Reference Bolton1905). The bed lies above the Bullion coal and Mountain 1.2m coals, which lie between 135m and 206.4m beneath the Arley seam, which correlates with the Westphalian A (Langsettian) chronozone (Ramsbottom et al. Reference Ramsbottom, Calver, Eager, Hodson, Holloday, Stubblefield and Wilson1978; Williamson Reference Williamson1999). Davydov et al. (Reference Davydov, Korn, Schmitz, Gradstein, Schmitz and Ogg2012) place the base of the Langsettian at 319 mya. Thus, the age of the specimen is somewhat imprecise, but appears likely to lie within the base of the Pennsylvanian.
2. Systematic palaeontology
Class Osteichthyes Huxley, Reference Huxley1880
Subclass Actinopterygii Cope, Reference Cope1887
Incertae sedis Genus Trawdenia, gen. nov.
Type species. Trawdenia planti Traquair, Reference Traquair1888
(Fig. 4).

Figure 4 Trawdenia planti, new reconstruction, in left-lateral view. Grey lines denote sensory canal network.
Generic diagnosis. Autapomorphies: rostral with characteristic, bilaterally symmetric arrangement of broad, smooth platforms of ornament at the midline, demarcated by narrow grooves, flanked by comma-shaped margins accommodating the anterior nares; a tall, smooth, subrectangular premaxilla rising to almost mid-orbit height; preopercular dorsal limb with convex anterior margin extending anteriorly relative to the leading edge of the postorbital expanded portion of the maxilla; a semi-crescentic jugal with concave, deeply excavated, posterodorsal rim accommodating one or two simple, unornamented suborbitals. An operculum with an anterior rim notched dorsally. Pectoral fin rotated so that leading fin ray is dorsalmost.
Plesiomorphies and characters of uncertain polarity: a short, subtriangular dermohyal bordering less than half of preopercular dorsal limb length; a maxilla postorbital expansion of length equal to or slightly shorter than the suborbital portion. An operculum and suboperculum of near-equal size, with steeply inclined intervening boundary; seven or fewer branchiostegals. Surangular present; jaw articulation anteroposteriorly level with extrascapular series. Scales in 35–40 vertically oriented sigmoid rows. All fins bear fringing fulcra; dorsal fin opposite anal fin; pectoral fin rays proximally unjointed.
Etymology. From the town name, Trawden, of Lancashire, UK, close to the likely coal mine from which the exceptionally preserved individual fish was recovered.
Type species. Trawdenia planti (Traquair, Reference Traquair1888).
Synonymy. Rhadinichthys planti Traquair (Reference Traquair1888, p. 441).
Rhadinichthys planti (Traquair), Ward (Reference Ward1890, p. 177, pl. 4, fig. 6).v
Rhadinichthys planti (Traquair), Wellburn (Reference Wellburn1901, pp. 168, 174).
Rhadinichthys planti (Traquair), Traquair (1911, pp. 151, 152, text-fig. 8, pl. 33, figs 9, 10).
Mesopoma planti (Traquair), Coates (Reference Coates1999, pp. 435–462, figs 1–6).
Species diagnosis. Autapomorphies: an accessory operculum; a dermohyal of less than one third of preopercular dorsal limb length; dorsalmost of two suborbitals much larger than ventral suborbital. Scale ornament faint and limited to four or five grooves parallel to anterior edge, and around four posteriorly directed chevrons on otherwise smooth exposed surface.
Plesiomorphies and characters of uncertain polarity: lower jaw with surangular process; branchiostegal series with seven members. Pectoral radials include double propterygium enclosing canal for marginal vessels and nerves. Scales in ∼40 vertically oriented sigmoid rows; scale ornament faint and limited to four or five grooves parallel to anterior edge, and around four posteriorly directed chevrons on otherwise smooth exposed surface.
Syntypes. NHMUK P8497, NHMUK P7989 part and NHMUK P11656 counterpart of single nodule. Coates (Reference Coates1999) designated NHMUK P8497 as the lectotype after Traquair's (Reference Traquair1911, p. 151) description of this as ‘[t]he most perfect specimen I have seen'. Here, this former lectotype is ranked as of equal status with the nodule specimen part and counterpart, to present a more complete set of type material for taxon diagnosis.
Referred specimens and localities. The syntypes and the following specimens. From Collyhurst near Bradford, UK: NHMUK P8498, NHMUK P8499, NHMUK P8500, NHMUK P8501 (a box of six, small, individual fish, each on different block), NHMUK P8502, NHMUK P8503, NHMUK P8504 (a box of ten skull tables, at least eight of which are Trawdenia planti).
From Longton, Staffordshire, UK: NHMUK P7983, NHMUK P7984, NHMUK P7985, NHMUK P57019.
From Trawden and Colne region, Lancashire, UK: MM W1146 and syntypes NHMUK P7989 part and NHMUK P11656 counterpart (see discussion in Coates Reference Coates1999).
Species. Trawdenia carricki Coates, Reference Coates1993 (Fig. 5a).

Figure 5 (A) Trawdenia carricki, GLAHM V8289a, Manse Burn Formation, Bearsden, Scotland (after Coates Reference Coates1993). (B) (i) Trawdenia pancheni, NMS 1983.33.7, Manse Burn Formation, Bearsden, Scotland; (ii) Trawdenia pancheni flank scale (after Coates Reference Coates1993). Figures reproduced with permission of the Palaeontological Association. Abbreviations: an = angular; br = branchiostegal rays; cl = cleithrum; clv = clavicle; de = dentary; dh = dermohyal; dsp = dermosphenotic; esc = extrascapular; fr = frontal; ju = jugal; lpt = lepidotrichia; mx = maxilla; na = nasal; op = opercular; pa = parietal; pmx = premaxilla; po = pore; pop = preopercular; pt = post temporal; ro = rostral; sop = subopercular.
Synonymy. Mesopoma carricki Coates (Dineley & Metcalf Reference Dineley and Metcalf1999, p. 307, fig. 9.28a, d).
Diagnosis. Autapomorphies: large pores, not associated directly with sensory canal system, pierce the bulbous rostral and frontal bones. Single, large, suborbital. Scales in ∼38 vertically oriented sigmoid rows; scales lack ornament except for two or three grooves parallel to anterior edge. All median fins preceded by three basal fulcra; posterior basal fulcral scale of anal fin with narrow mid-region.
Holotype. GLAHM V8289a-b.
Referred specimens and locality. The holotype, and NMS 1981.63.44, NMS 1981.63.46, NMS 1981.63.47, NMS.63.53, NMS 1981.63.54a-b, NMS 1981.63.55a-b, NMS 1987.7.131, GLAHM V8254, NHMUK P62370, NHMUK P62372a-e. All specimens from Bearsden, Glasgow, UK.
Species. Trawdenia pancheni Coates, Reference Coates1993 (Fig. 5b).
Synonymy. Mesopoma pancheni Coates (Dineley & Metcalf Reference Dineley and Metcalf1999, p. 307).
Diagnosis. Autapomorphies: rostral bone with three, broad posteriorly directed chevrons on posterodorsal surface. Scales arranged in 35+ vertically oriented sigmoid rows. Scales directly behind post-temporals have convex posterior denticulated edge; scale ornament includes around four grooves parallel to anterior edge; distinct posteriorly directed chevrons, most prominent on scales close to dorsal midline.
Holotype. NMS 1983.33.7.
Referred specimens and locality. The holotype and GLAHM V8283a-b. Both specimens from Bearsden, Glasgow, UK.
3. Description of Trawdenia planti dermal skull, pectoral girdle and fin
3.1. Skull table
The external features of NHM P7989 (Figs 1a, 3) bear a striking resemblance to Trawdenia carricki (Fig. 5a) and, to a less certain extent, T. pancheni (Coates Reference Coates1993; Fig. 5b). The total head length of NHM P7989 measures 16mm from rostral apex to posteriormost edge of the suboperculum. The skull table consists of paired frontals, parietals, medial and lateral extrascapulars and post-temporals, flanked by a dermopterotic and dermosphenotic, as previously reported (Coates Reference Coates1999). The dermal ornament of broad, flattened tubercles and ridges (Fig. 3e), as described by Traquair (Reference Traquair1888), closely resembles that of T. carricki (Coates Reference Coates1993), and markedly differs from the patterns of vermiform ridges commonly encountered among early actinopterygians. The ornament material resembles ganoine, but a histological examination has not been completed. Surfaces, complete and broken, bear none of the characteristics of cosmine. The frontals are broad posteriorly and taper anteriorly. The posterolateral edge of each frontal is embayed where it sutures with the anterior limb of the dermopterotic. The parietal is approximately equilateral, less than half the anteroposterior length of the frontals, with a small projection into the posterior edge of the frontal, which probably marks the passage of the supraorbital sensory canal. The three pit-lines on the parietal are clearly marked. The medial extrascapular is most clearly preserved in NHMUK P8500 (Fig. 2) and the lateral extrascapular in NHMUK P7989 (Fig. 3). The medial extrascapular is smaller than the lateral, and both are seated on a broad flange extending from the posterior margin of the parietal and dermopterotic (Fig. 6a). The relative proportions of these bones are indicated by the space for the missing medial extrascapular in NHMUK P7989 (Fig. 3a, b, e), adjacent to the larger and laterally flared lateral extrascapular. The lateral extrascapular reaches the edge of the skull table.
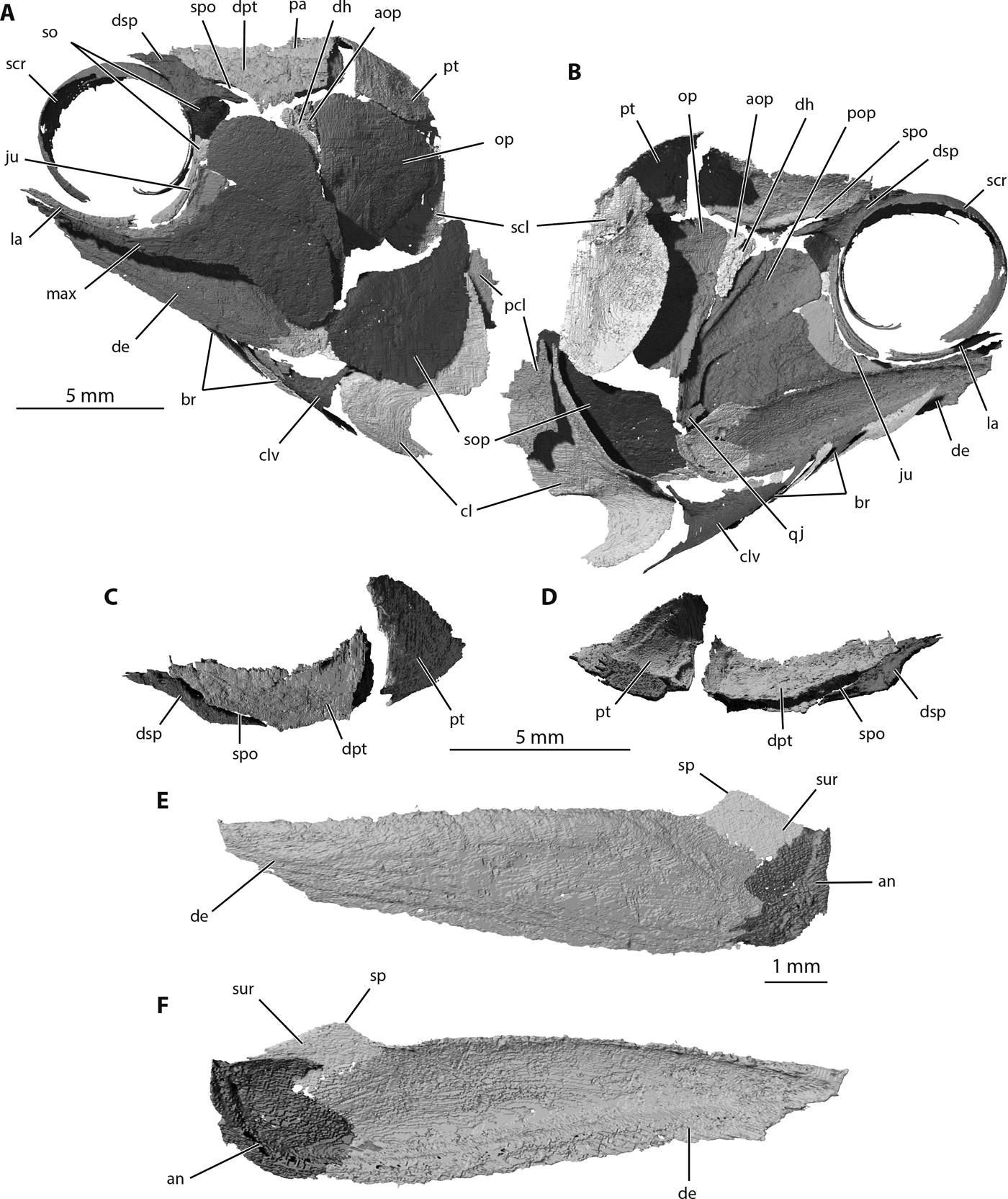
Figure 6 Trawdenia planti, NHMUK P11656, Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of cranial dermal bones from nodule-enclosed right side of cranium (reversed). (A) Left-lateral view. (B) Left-medial view. (C) Temporal series dorsal view. (D) Temporal series ventral view. (E) Lower-jaw left-lateral view. (F) Lower-jaw left-medial view. Abbreviations: an = angular; aop = accessory opercular; br = branchiostegal rays; cl = cleithrum; clv = clavicle; de = dentary; dh = dermohyal; dpt = dermopterotic; dsp = dermosphenotic; ju = jugal; la = lachrymal; max = maxilla; op = opercular; pa = parietal; pcl = postcleithrum; pop = preopercular; pt = post temporal; qj = quadratojugal; scl = supracleithrum; scr = sclerotic ring; so = suborbital; sop = subopercular; sp = surangular process; spo = spiracular opening; sur = surangular.
The dermopterotic is slightly broader than described previously (Coates Reference Coates1999), with a notched anterolateral edge for the spiracular opening (Fig. 6a, b). Note that this notch is not readily visible in the natural mould shown in Figures 1a, 3. The irregular medial margin with a broad tongue projecting anteromedially, between the dermosphenotic and the frontal, is strikingly similar to the dermopterotic (supratemporo-intertemporal) of Pteronisculus (Nielsen Reference Nielsen1942). The path of the otic portion of the main lateral line canal is well preserved on the mesial surface (Fig. 6b, d), passing dorsal to the spiracular opening before turning laterally and ventrally to enter the dermosphenotic. The dermosphenotic is noteworthy for its externally slender posterior ramus, which forms the lateral boundary of the spiracular opening (thus, the spiracle is mostly enclosed by otic- and infraorbital-canal-bearing bones). The internal, mesial view of this ramus shows it to be triangular in cross section, forming a robust lateral rim and internal lip for the spiracular duct. The short, narrow ventral ramus of the dermosphenotic that projects towards the dorsal extremity of the jugal (posterior infraorbital) shows no clear sign of a sensory canal enclosure. Thus, the general canal pattern, including a union with the infraorbital canal, is not obviously present.
3.2. Snout
Traquair (Reference Traquair1888, 1911) recognised the ‘peculiarly large development of the snout', of which the most prominent part is the bulbous rostral (Fig. 3b–d). Unfortunately, the ethmoid complex is known only from its external morphology (NHM P7989), in which the rostral is flanked by nasals and bordered ventrally by premaxillae. The apex of the snout has a bilaterally symmetrical ornament of broad, comma-shaped platforms flanking a broad inverted T-shaped area at the midline. This arrangement is also present in Trawdenia carricki and T. pancheni (Coates Reference Coates1993; Fig. 5). Dorsal and posterior to this, the rostral midline bears a wide-margined rectangular shield (Fig. 3e), bearing, in T. planti, a set of four deeply incised grooves: one chevron and three transverse slots. This suite of features closely resembles the incompletely preserved rostral of T. pancheni (Fig. 5b), but less so the equivalent area of T. carricki (Fig. 5a). The ventral extremity of the rostral bone is remote from the gape. The nasal bone is simple and smooth, with no deep notches for accommodating the anterior and/or posterior nostrils: the notch for the anterior nostril lies within the lateral rim of the rostral bone and not on the mesial margin of the nasal. The dentigerous premaxillae, left and right, are simple, broad and tall, with a long median suture, and held at a shallow anterodorsal angle relative to the gape margin. The premaxillae form a smooth wall containing the ventral surface of the projecting rostrum, and the posterolateral lateral edge of each contributes to the anteroventral margin of the orbit. As for the nasal bone, there is no evidence of the notch contributing to part of the posterior nostril rim.
3.3. Cheek region
The cheek region was previously reconstructed from the natural mould preserving the left side of the head in NHMUK P7989 (Figs 1a, 3a, b). Here, the buried right side of the cranium is revealed for the first time, exposing a slightly more elaborate and taxonomically distinctive suite of dermal bones (Fig. 6). Most unusually, the anterodorsal limb of the preopercular has a convex anterior margin and the ventral rim projects anteriorly beyond the sharply descending anterior margin of the expanded posterior portion of the maxilla (Figs 4, 6a, b). After identifying this feature in Trawdenia planti, it now appears that the same condition is probably also present in T. carricki, although the preopercular is damaged (Fig. 5a). Furthermore, in T. planti the preopercular sensory canal is not positioned parallel to the posterior edge of the bone, but takes a more anteriorly angled trajectory, terminating close to the mid-point of dorsal rim of the anterodorsal limb (Fig. 6b). Two ovoid suborbitals are present: one large (dorsal) and one small (ventral).
The dermohyal is not long and slender (contra Coates Reference Coates1999), but is, instead, short and attached loosely to the head of the hyomandibula. In external view, the dermohyal is wedged between a similarly shaped but smaller accessory opercular, and the preopercular. Both bones (dermohyal and accessory opercular) are visible not only in NHMUK P11656 (Fig. 6a, b), but also the Collyhurst flattened cranium of NHMUK P.8500 (Fig. 2). The jugal is preserved on the right side of the skull in NHMUK P11656, but here, it is split along the course of the infraorbital canal so that a crescent-shaped sliver forming the orbit rim is bent medially and appears to be a separate bone. Reference to the jugal of Collyhurst specimens (e.g., NHMUK P.8497, P.8500; Fig. 2) confirms the interpretation of the NHMUK P11656 jugal as broken, and the complete shape is very similar to that of Trawdenia carricki (Coates Reference Coates1993). The jugal section of the infraorbital canal is simple and lacks accessory branches. A small quadratojugal is present, and, thus far, only identified in NHMUK P11656 (Fig. 6b). No pit line is evident. In lateral view, the quadratojugal is largely overlapped by the posterior of the maxilla. The anterior infraorbital or lachrymal is a simple, slender tube. The maxilla is mostly as described previously (Coates Reference Coates1993, 1999). No pit-lines are present; the anterior slender ramus is about the same length as the posterior expanded portion. The marginal dentition is small, simple and arranged as a single tooth row. The presence or absence of acrodin caps is uncertain.
3.4. Lower jaw (external surface)
The external surface of the lower jaw (Fig. 6e) consists mostly of the dentary, through which the mandibular canal passes. This is most clearly seen in mesial view (Fig. 6f) hugging the ventral margin and passing forwards to the anterior extremity. The angular is simple, pierced by at least two large sensory canal pores, and anteroposteriorly broader than previously reconstructed. A surangular is present, projecting dorsally to form a distinct process. Similar processes are reported in a range of genera, with contrasting examples in Pteronisculus (Nielsen Reference Nielsen1942), Birgeria (Nielsen Reference Nielsen1949), Aesopichthys (Poplin & Lund Reference Poplin and Lund2000) and Coccocephalichthys (Poplin & Véran Reference Poplin and Véran1996). Confusingly, these processes tend to be tagged ‘coronoid' (e.g., Poplin & Véran Reference Poplin and Véran1996; Poplin & Lund Reference Poplin and Lund2000, but see Nielsen Reference Nielsen1949), although they lack any coronoid contribution whatsoever. Here, the simpler label ‘surangular' is used for the process, and examples are visible in NHMUK P11656 as well as the figured Collyhurst cranium NHMUK P.8500 (Fig. 2). The marginal dentition consists of uniformly small, conical teeth arranged in a single row, more clearly observed in NHMUK P.8500 than in the CT renderings of NHMUK P11656. Once again, the presence or absence of acrodin caps is uncertain.
3.5. Operculogular series
The operculogular series includes a small accessory opercular situated within a concave embayment in the anterodorsal rim of the opercular bone (Figs 2, 4, 6a, b). A similar embayment is present in the opercular of Trawdenia carricki (Fig. 5a). The opercular and subopercular of T. planti are anteroposteriorly broad and of approximately equal size. The opercular is barely four-sided because the dorsal and posterior edges run into a nearly continuous convex margin. The ventral edge of the opercular is slightly convex and angled steeply from anteroventral to posterodorsal. The subopercular is more distinctly four-sided, with a strongly convex posterior rim and a gently concave dorsal margin. Posterodorsally, these edges meet as a laterally flattened process overlapped in life by the opercular, but clearly visible in NHMUK P11656 (Fig. 6a). As noted by Traquair (Reference Traquair1888), the opercular and subopercular are smooth except for a few, concentric striae parallel to the posterior rim.
Seven branchiostegal rays are present, which diminish in size, slightly, anteriorly. The dorsalmost of the series is much the same size as its ventral neighbour. The subtriangular lateral gular is larger than the leaf-shaped branchiostegals, but extends for less than a third of total jaw length. The presence or absence of a median gular is unknown.
3.6. Pectoral girdle dermal bones
Few details of the pectoral skeleton were provided in the previous description (Coates Reference Coates1999): ‘[t]he pectoral girdle resembles that of M. carricki, with a short cleithrum, dorsoventrally deep supracleithrum, and post-cleithrum. The leading edges of all fins appear to have fringing fulcra, except the dorsal edge of the caudal fin. The pectoral fin includes numerous, proximally unjointed lepidotrichia'. These notes can now be augmented significantly (Figs 7–11).
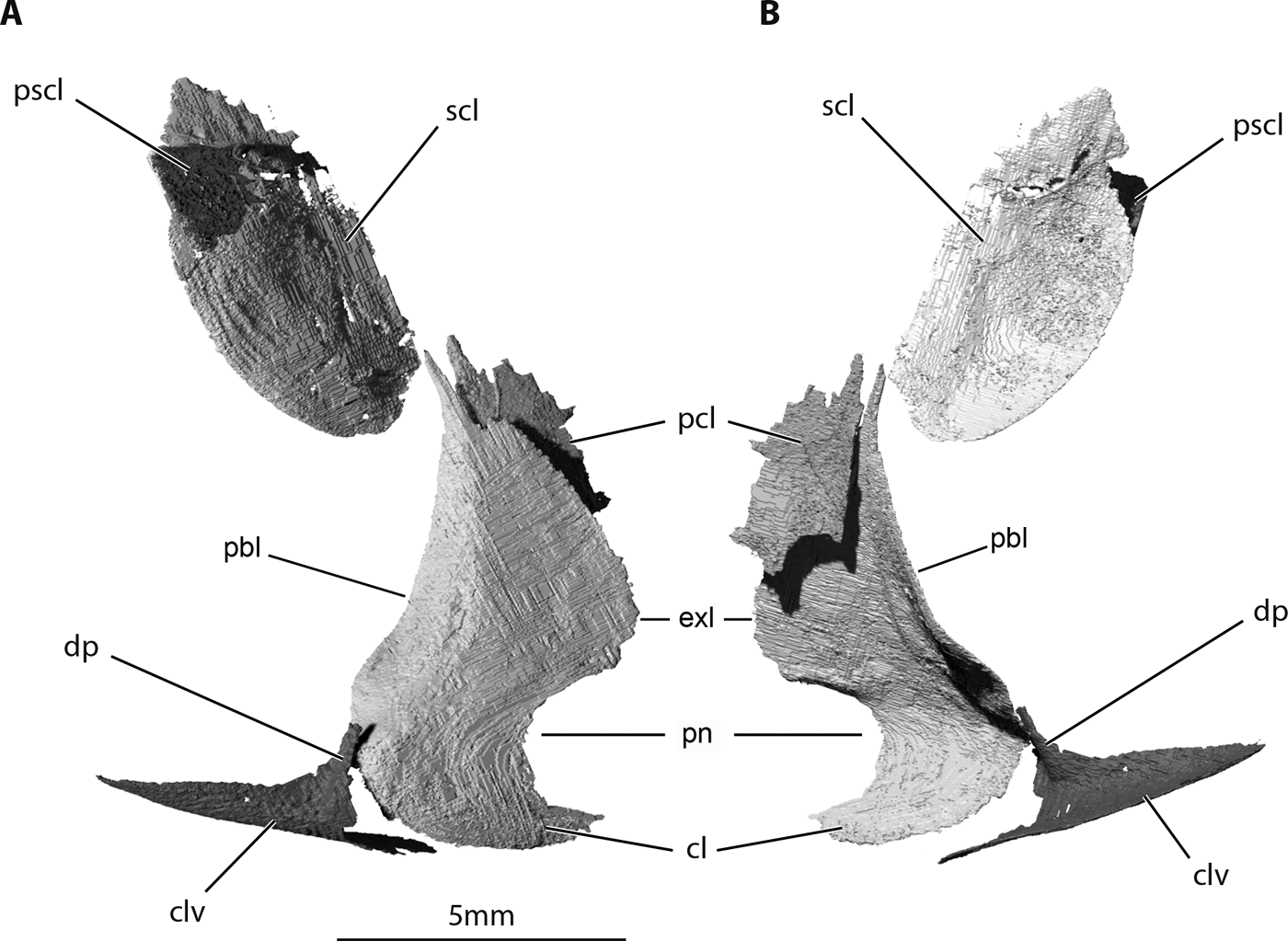
Figure 7 Trawdenia planti, NHMUK P11656, Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of pectoral girdle dermal bones from nodule-enclosed right side of cranium (reversed). (A) Left-lateral view. (B) Left-medial view. Abbreviations: cl = cleithrum; clv = clavicle; dp = dorsal process of clavicle; exl = external lamina; pbl = postbranchial lamina; pcl = postcleithrum; pn = pectoral notch; pscl = presupracleithrum; scl = supracleithrum.

Figure 8 Trawdenia planti, NHMUK P11656, Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of pectoral girdle and fin from nodule-enclosed right side of individual, slightly restored with fin rays excluded. (A) Right-lateral view, fin radials abducted. (B) Right-medial view, fin radials adducted. (C) Anterior view, fin radials abducted. (D) Posterior view, fin radials abducted. Abbreviations; apr = anterior process; cl = cleithrum; clv = clavicle; dmc = dorsal muscle canal; dp = dorsal process of clavicle; endg = endoskeletal pectoral girdle; exl = external lamina; mca = mesocoracoid arch; mcp = mesocoracoid process; pbl = postbranchial lamina; vmc = ventral muscle canal.
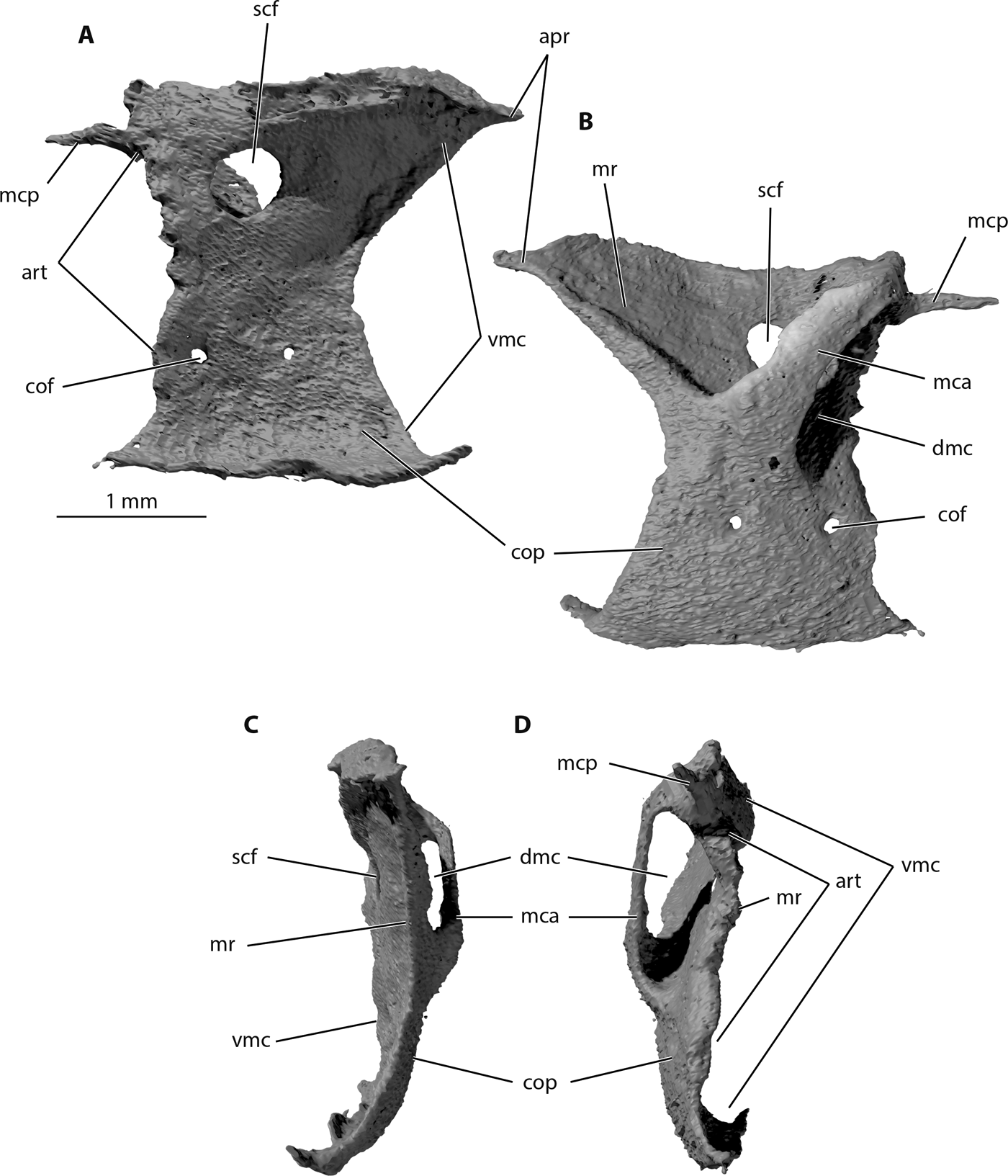
Figure 9 Trawdenia planti, NHMUK P11656 Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of pectoral endoskeletal girdle from nodule-enclosed right side of individual. (A) Right-lateral view, abductor surface. (B) Right-medial view, adductor surface. (C) Anterior view. (D) Posterior view. Abbreviations: apr = anterior process; art = articular surface; cof = coracoid foramen; cop = coracoid plate; dmc = dorsal muscle canal; mca = mesocoracoid arch; mcp; mesocoracoid process; mr = middle region; scf = supracoracoid foramen; vmc = ventral muscle canal.

Figure 10 Trawdenia planti, NHMUK P11656 Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of pectoral radials from nodule-enclosed right side of individual. (A) Right-lateral view, abductor surface. (B) Proximal view. (C) Distal view. (D) Marginal and submarginal propterygium in right-lateral proximal view. (E) Marginal and submarginal propterygium in distal view. (F) Marginal and submarginal propterygium separated in right-lateral view. (G) Marginal and submarginal propterygium separated in proximal view. (H) Proximal radial 3, adductor surface to left of image. Abbreviations: dr = distal radials; mpt = metapterygium; pc = propterygial canal; ppt i = marginal propteygium; ppt ii = submarginal propterygium; pr = process from proximal surface; r1–3 = radials 1–3.
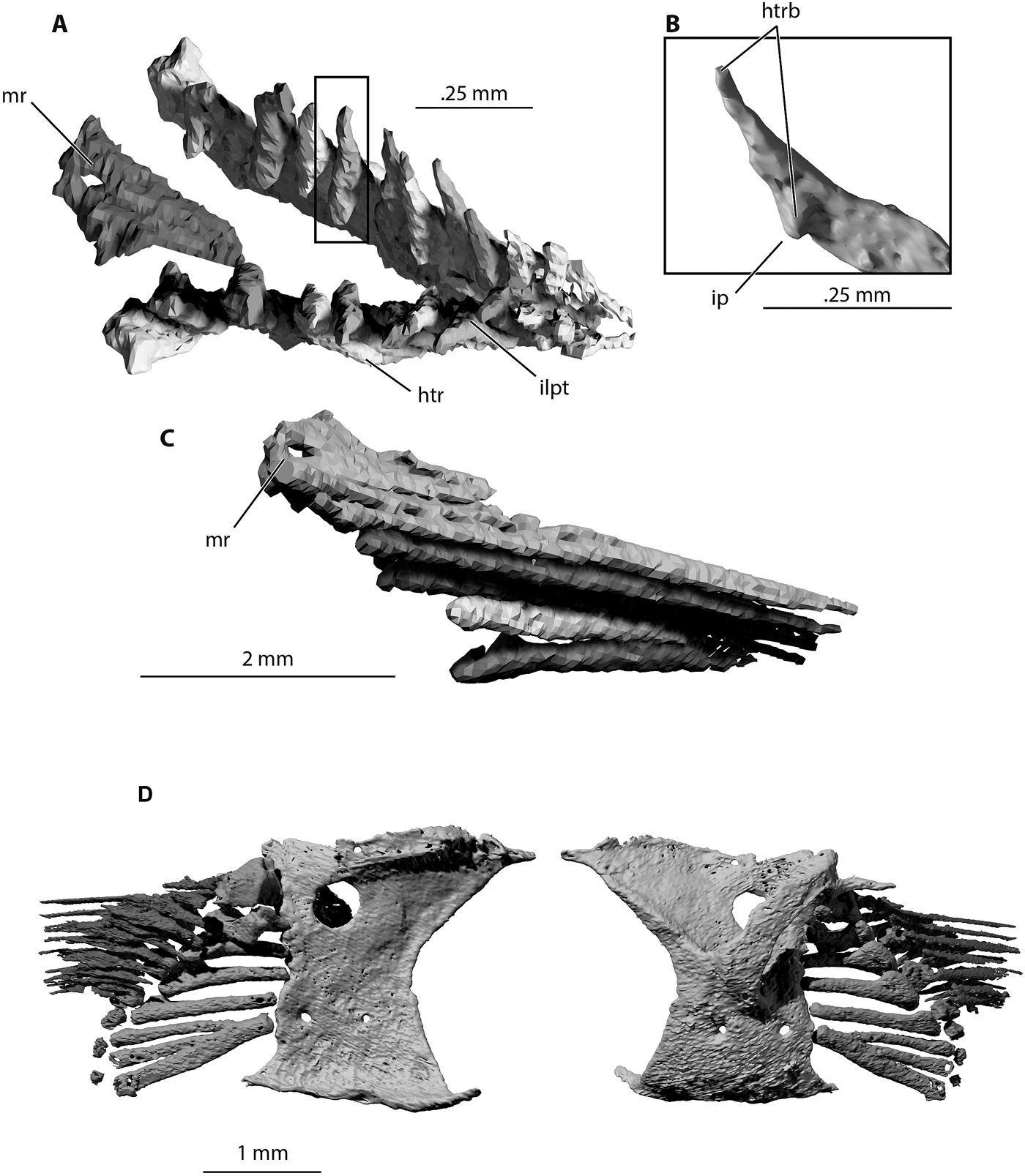
Figure 11 Trawdenia planti, NHMUK P11656 Trawden and Colne region, Burnley Coalfield, Lancashire, UK. μCT model of pectoral lepidotrichia from nodule-enclosed right side of individual. (A) Proximal view. (B) Detail of hemiray base. (C) Right medial view. (D) Rearticulated lepidotrichia and pectoral endoskeleton in lateral (left) and medial (right) views. Abbreviations: htr = hemitrich; htrb = hemitrich or hemiray base; ip = internal process; ilpt = interlepidotrichial space; mr = marginal ray.
Dermal bones of the pectoral girdle include a presupracleithrum, supracleithrum, cleithrum, postcleithrum and clavicle (Fig. 7). The presence of an interclavicle is uncertain. The presupracleithrum (Fig. 7a) was discovered in NHMUK P11656, sandwiched between the supracleithrum, opercular and post-temporal. The outline is vaguely scale-like, with an anterodorsal angle, but with convex ventral margin curving into a convex posterior margin bearing four deep serrations. The supracleithrum is broad, with a strongly convex anterior margin projecting beneath the opercular. The course of the lateral line canal through the supracleithrum is short, curves dorsally and enters the bone about one quarter of the way down its posterior margin. As in Trawdenia carricki, the ornament consists of well-marked concentric grooves.
The cleithrum and clavicle resemble those of other early actinopterygians, but marked differences from well-preserved examples, such as Mimipiscis and Moythomasia (Gardiner Reference Gardiner1984; Choo Reference Choo2011, Reference Choo2015), Gogosardina (Choo et al. Reference Choo, Long and Trinajstic2009) and Raynerius (Giles et al. Reference Giles, Darras, Clément, Blieck and Friedman2015b), highlight characteristics not yet used in phylogenetic analyses. The cleithrum of Trawdenia planti (Figs 7, 8) is high, with a pointed dorsal margin, a strongly concave anterior margin and postbranchial lamina, which forms the anteriorly facing posterior wall of the gill chamber. The posterior margin of the external lamina is strongly convex. The breadth-to-height ratio of the external lamina is notably greater than in Mimipiscis and Moythomasia (Gardiner Reference Gardiner1984). The ventral part of the convex posterior rim is deeply notched at the insertion of the pectoral fin. Notably, this semicircular notch is completely visible in lateral aspect, unlike Mimipiscis and especially Moythomasia (Gardiner Reference Gardiner1984; Choo Reference Choo2015) in which the notch faces lateroventrally, or Gogosardina (Choo et al. Reference Choo, Long and Trinajstic2009) in which the notch is shielded laterally by a stubby, pre-pectoral spine-like outgrowth. A similar, although less prominent, outgrowth is present in Moythomasia. The ventral surface of the T. planti cleithrum curves gradually in a medial direction where it is overlapped by the clavicle. In contrast, the curvature in Moythomasia is acute (Choo Reference Choo2015) and the ventral surface of the clavicle almost flat.
The clavicle of Trawdenia planti, like the cleithrum, is strongly but gradually curved medially to encompass the rounded anteroventral projection of the cleithrum. The ‘flat ventral expanse' reported in Mimipiscis and Moythomasia (Gardiner Reference Gardiner1984) is absent. The dorsal process of the clavicle in T. planti is short and terminates below the level of the upper rim of the pectoral notch in the cleithrum. In contrast, the dorsal process of the clavicle in Moythomasia and Gogosardina extends to half the total height of the cleithrum and appears similarly extended in Raynerius. The proportions of the clavicle dorsal process of Mimipiscis are less extreme but extend to at least the full height of the pectoral notch.
The postcleithrum of Trawdenia planti is incomplete; the preserved portion resembles a simple, enlarged flank scale.
3.7. Endoskeletal girdle
The endoskeletal pectoral girdle of left and right sides is preserved intact in NHMUK P11656 (Figs 8, 9, 11d). Each consists of a single ossification, and, in general, resembles other early actinopterygian examples. The structure is more or less tripartite with a well-developed middle region, and ventral, coracoid region. The dorsal extremities and margin of the middle region suture with the mesial surface of the cleithrum, as does the ventral rim of the coracoid region (Fig. 8b). Thus, the attachment to the dermal girdle is bipartite, as in Mimipiscis, Moythomasia (Gardiner Reference Gardiner1984) and many other fossil and Recent actinopterygians (Nielsen Reference Nielsen1942; Jessen Reference Jessen1972). A mesocoracoid arch, enclosing a dorsal muscle canal, is directed dorsally and posteriorly (Fig. 9b), also as in Mimipiscis and Moythomasia (Gardiner Reference Gardiner1984) and many other fossil actinopterygian pectoral girdles, with the possible exception of the earliest known example: that of Cheirolepis trailli (Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a). The ventral muscle canal (in Trawdenia) is contained medially by the coracoid region, and laterally by the cleithrum.
Where the mesocoracoid arch reaches the posterior edge of the endoskeletal unit, a spur-like mesocoracoid process projects posteriorly (Fig. 9a, b). The leading edge of the mesocoracoid arch aligns with the leading edge of the coracoid region, as it does in Mimipiscis and Moythomasia (Gardiner Reference Gardiner1984). However, unlike Mimipiscis and Moythomasia, the descending posterior margin of the mesocoracoid arch is not curved to join the posterior of the coracoid plate, aligned with the edge, but joins the dorsal rim of the coracoid some distance anterior to the level of the coracoid foramen. Furthermore, the ‘horizontal' (Gardiner Reference Gardiner1984) middle region of the girdle is rotated so that the conventional ‘dorsal' surface faces medially (Fig. 9b). Therefore, the dorsal margin of the endoskeletal girdle is the lateral rim of the so-called middle region, and this is confluent with the dorsal extremity of the mesocoracoid arch. The scapular region identified in Moythomasia and Mimipiscus (Gardiner Reference Gardiner1984) is completely absent in Trawdenia. As a result, the articular surface for the pectoral fin radials is oriented almost vertically (inclined at approximately 70° relative to the horizontal) (Figs 8d, 9d). The middle region is produced far anteriorly, and, in mesial view, the dorsal surface appears as a right-angled triangle, with the hypotenuse running along the ventral edge.
The supracoracoid foramen (posterior canal of the middle region; Jessen Reference Jessen1972) is large and circular (Fig. 9a), unlike the tiny openings present in Mimipiscis and Moythomasia (Gardiner Reference Gardiner1984), and apparent absence of openings in Cheirolepis (Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a). The ventral surface (effectively, the lateral surface) of the middle region in Trawdenia is smooth and lacks any obvious sign of a ventral ridge dividing separate spaces for abductor muscles and a ventral marginal/arrector muscle. The articular surface is narrow, divided into an irregularly spaced series of facets, but broadens dorsally for the articulation with the propterygium.
The coracoid region is a broad, trapezoid plate. Mesially and laterally, the plate is mostly featureless, except for two foramina. The posterior of these openings, the coracoid foramen, lies anterior to the articulation for the metapterygial radials (Fig. 11d), and directly ventral to the much larger scapular foramen, which lies anterior to the propterygial articulation. These locations are consistent with Jessen (Reference Jessen1972) dissections and wax-plate tomographic reconstructions of modern actinopterygian pectoral fin complexes, in which these openings transmit the marginal blood vessels and nerves to the leading and trailing edges of the fin. The identity of the anterior foramen in the coracoid is less certain.
3.8. Pectoral fin endoskeleton
The pectoral fin endoskeleton consists of proximal and distal radials preserved in articulation, barely disturbed from in-life positions. This is probably the most completely visualised paired-fin skeleton known from any Palaeozoic actinopterygian. The proximal radials include, from posterior to anterior (or ventral to dorsal, given fin rotation), a metapterygium, three radials and a two-part propterygium (Fig. 10a, b). The distal radials grade from small to large, from posterior to anterior (or ventral to dorsal) across the tips of the proximal radials (Figs 8, 10a, c).
The metapterygium (Fig. 10a) branches twice, and thus articulates with two elongate preaxial radials, as in Acipenser (Jessen Reference Jessen1972) and Boreosomus (Nielsen Reference Nielsen1942). Whether the preaxial radials are entirely separate from the metapterygium is unclear. Each of these radials is slender throughout its length: a slim cylindrical rod. The three preceding radials share a similarly sized, slender base (Fig. 10b), but each flares distally to form a spatulate head (Fig. 10h). These three radials are successively shorter, from the metapterygial to propterygial side of the series. Once again, the number and arrangement of these radials resembles conditions in Acipenser and Boreosomus. The contrast with possibly plesiomorphic conditions exhibited by Cheirolepis (Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a) is marked: five incompletely ossified radials flanked by a blocky propterygium and squat metapterygium.
The propterygium of Trawdenia (Fig. 10d–g) is unusual: it consists of two, short, broad radials. The maximum width of the proximal surface of each propterygial radial is at least three times that of other radials in the fin. When the complete fin skeleton is viewed from proximal or distal perspectives (Fig. 10b, c), the ‘extra' portions of these bulky, propterygial radials are seen to project laterally, on the abductor surface of the fin skeleton. The opposing, inter-radial surfaces of these propterygia each bear a broad groove, and combine to encompass a wide, propterygial canal (Fig. 10b, c, e). Both of the propterygial radials (termed, for convenience, the marginal and submarginal) bear processes on the proximolateral surface (Fig. 10d, f, g). These processes probably mark the insertion of parts of the musculature. The proximal articular surfaces of the two parts of the propterygium saddle the dorsal portion of the scapulocoracoid articulation surface (Fig. 8d).
Six distal radials are preserved, barely moved from in-life position, fringing the distal extremities of the proximal radials (Figs 8, 10a, c). The polygonal shapes of the distal radials sit between the distal ends of the proximal radials, and the largest three distal radials bear facets, providing evidence of inter-articulation across the distal radial series.
3.9. Pectoral fin lepidotrichia
NHM P7989 preserves a natural mould of the left-side pectoral fin lepidotrichia (Fig. 1a). The previous reconstruction (Coates Reference Coates1999) co-opted the reconstructed fin from Mesopoma carricki (Coates Reference Coates1993), in which at least eight fin rays were thought to be present. Close inspection of NHMUK P7989 suggests that the fin ray count exceeds 11. The fin is preserved fully adducted against the flank with the leading edge dorsalmost, exposing the flexor (abductor) surface. The primary (leading edge) rays project furthest laterally; thus, the exposed surface of the fin is concave, from leading (dorsal) to trailing (ventral) edges.
CT scans of NHMUK P11656 reveal the proximal portions of the fin rays (Fig. 11), showing the hemitrichs in detail. The fin web includes a marginal ray, and the ‘dorsal' (adductor surface) and ‘ventral' (abductor surface) hemirays remain in register (Fig. 11a, c). The base of each proximal hemitrich diverges strongly from its counterpart, and each base bears a distinct internal process (Jessen Reference Jessen1972; Fig. 11b). The primary lepidotrichia, including the marginal ray and at least the first two paired rays, clasp the propterygium (Fig. 11d), but there is no evidence of fusion between these structures. Comparison of Figures 10a, 11d illustrates the extent to which fin rays overlap the distal radials, seated between hemiray bases. As noted in previous descriptions, the proximal segments of the fin rays are elongate, with segmentation and bifurcation confined to the distal and posteromedial parts of the fin.
4. Discussion
Until recently, Trawdenia planti (as Mesopoma) was thought to branch from within the actinopterygian crown group, from a node close to the divergence of chondrostean and neopterygian stems (Coates Reference Coates1999). However, recent analyses of much larger assemblages of early actinopterygian taxa and characters have excluded T. planti from the crown and placed it deep within a transformed, and now well-populated actinopterygian stem lineage (Giles et al. Reference Giles, Xu, Near and Friedman2017). Notable features excluding T. planti from its former, derived position include the presence of fulcral scales preceding midline fins, and the absence of a supraorbital bone. Reference to the new restoration in Figure 4 suggests that despite the influx of new material from the present study, T. planti seems likely to remain a stem member, at least in the context of the most recent data sets. Nevertheless, the level of detail from this study alongside others (Pradel et al. Reference Pradel, Maisey, Mapes and Kruta2016; Giles et al. Reference Giles, Xu, Near and Friedman2017; Friedman et al. Reference Friedman, Pierce, Coates and Giles2019) exemplifies the impact of CT as a tool for accessing additional data, not only revealing morphology at different scales, but also for visualising the 3D connectedness of parts; especially small parts such as the radials of paired fins.
4.1. Comparative morphology and pectoral characters
The character set compiled by Giles et al. (Reference Giles, Xu, Near and Friedman2017) includes 16 characters (out of a total 265) describing pectoral girdle and fin conditions. Here (referenced with C-numbers from Giles et al. Reference Giles, Xu, Near and Friedman2017), these are summarised with Trawdenia planti scores in parenthesis and, where they differ, with Giles et al. scores for Mesopoma planti shown in square brackets: C-226, the presence of a presupracleithrum (present: Fig. 7a) [?]; C-227, the number of presupracleithra (single: Fig. 7a) [?]; C-228, the shape of the dorsal margin of the cleithrum (pointed: Fig. 7a); C-229, a medial wing on the cleithrum (absent: Fig. 8c) [?]; C-230, presence of an anocleithrum (absent: Fig. 7a); C-231, the condition of the clavicle (large: Fig. 8b); C-232, presence of a serrated organ (absent: Fig. 8) [?]; C-233, presence of an interclavicle (unknown); C-234, a triradiate endoskeletal girdle (present: Fig. 9) [?]; C-235, a perforated propterygium (absent: Fig. 10) [?]; C-236, the propterygium is embraced by the anterior rays of the fin (present) [?]; C-237, the propterygium is fused to the first fin ray (absent: Figs 10, 11) [?]; C-238, the pectoral endoskeleton extends far beyond the body wall (absent: Fig. 8) [?]; C-239, the pectoral radials are jointed (absent: Fig. 10) [?]; C-240, the fin articulation is monobasal versus polybasal (polybasal) [?]; C-241, the pectoral fin ray segments are elongate proximally and segmented distally (present: Fig. 11); and C-242, the fin outline is leaf-shaped (absent: Figs 1a, 4).
The high proportion of scores differing from former entries, or new scores where data were formerly unknown or uncertain, is clear, but the CT renderings also reveal new detail suggesting a series of revised and/or entirely new character statements.
The description of the cleithrum includes new observations on the location and orientation of the notch for the pectoral fin, especially the differences in notch position evident in early genera. Functionally, the notch creates space to accommodate the bulk of fin abductor muscle below, or, as in Trawdenia planti, lateral to the middle region of the endoskeletal girdle (Fig. 8a). Reconstruction drawings of early actinopterygians conventionally display the notch as visible in lateral aspect, although, in many early genera it would have been hidden from view: Moythomasia, Gogosardina, and, perhaps, Mimipiscis (Gardiner Reference Gardiner1984; Choo et al. Reference Choo, Long and Trinajstic2009; Choo Reference Choo2015). In existing character lists, this likely plesiomorphic condition, with the notch directed ventrally, is not distinguished from the derived condition: notch visible laterally.
The height of the clavicle dorsal process is similarly varied. In outgroups (e.g., Onychodus; Andrews et al. Reference Andrews, Long, Ahlberg, Barwick and Campbell2006) and several Devonian actinopterygians (Gardiner Reference Gardiner1984; Choo et al. Reference Choo, Long and Trinajstic2009; Choo Reference Choo2015), the dorsal process is remarkably tall, extending dorsally to overlap a large stretch of the anterior surface of the cleithrum. Process height is much reduced in many, and probably most, Carboniferous actinopterygians, as exemplified by the diminutive stump in Trawdenia (Fig. 8a); especially visible in anterior view (Fig. 8c). Such reduction might be an early expression of the widespread phylogenetic trend in actinopterygians of clavicle reduction and loss.
The endoskeletal portion of the pectoral girdle, widely referred to as a scapulocoracoid, is coded by Giles et al. (Reference Giles, Xu, Near and Friedman2017) as ‘triradiate' (C-234) after Friedman (Reference Friedman2007). Here, Gardiner's (Reference Gardiner1984) term ‘tripartite' is preferred. Janvier (Reference Janvier and Panchen1980) recognised that triradiate girdles with three distally separate buttresses are characteristic of sarcopterygians, whereas early actinopterygian girdles consist of dorsal and ventral portions divided by a shelf-like middle region or ‘bar' (Mabee & Noordsy Reference Mabee and Noordsy2004). A further distinction might be drawn between the bipartite girdle-to-cleithrum attachment of actinopterygians (whether or not the girdle is tripartite, contra Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a), and the tripartite attachment of some sarcopterygians. Among neopterygians and polypterids the girdle is variously modified, but how this relates to the presence or absence of the triradiate character state, as coded by Giles et al. (Reference Giles, Xu, Near and Friedman2017), and especially the use of absence of this character as a crown group actinopterygian synapomorphy, is unclear. No actinopterygian has yet been shown to have a sarcopterygian-like triradiate condition.
The anterior extremity or process of the pectoral girdle middle region (Figs 8b, 9a, b) represents a further, distinguishing actinopterygian characteristic (Jessen Reference Jessen1972). Although incomplete, the girdle condition in Cheirolepis (Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a) lends further support to this generally accepted hypothesis. The anteroposterior length of the middle region provides a measure of pectoral marginal muscle length, because the arrector dorsalis muscle, or the levator muscle of teleost fins (Wilhelm et al. Reference Wilhelm, Du, Standen and Larsson2015), originates at the apex of this shallow tray. The broader, posterior part is perforated by one or more openings for nerves and blood vessels supplying the leading edge of the fin. The large, circular foramen in the middle region of the Trawdenia planti girdle (Fig. 9a) resembles strikingly similar examples in neopterygian girdles (Jessen Reference Jessen1972), and probably represents a further derived condition. In Mimipiscis and Moythomasia durgaringa, this region is perforated by a scatter of small foramina (Gardiner Reference Gardiner1984). Jessen (Reference Jessen1972) named the single, large opening the ‘posterior canal', but Janvier (Reference Janvier and Panchen1980), noting positional similarity, suggested homology with the supracoracoid foramen of early tetrapods.
The dorsal part of the girdle is often identified as the scapular, with lateral, and, in some texts, mesial processes (e.g., Dillman & Hilton Reference Dillman and Hilton2015), enclosing a dorsal muscle canal. The ventral part of the girdle is identified as the coracoid and forms the mesial wall of a ventral muscle canal (the cleithrum forms the lateral wall). Rotation of the girdle middle region in Trawdenia planti and other actinopterygians (Jessen Reference Jessen1972) obliterates the lateral process of the scapular region. Consequently, unless the scapular region bears a dorsal prolongation, as expressed in near-chondrichthyan proportions in crown group chondrosteans (Jessen Reference Jessen1972; Dillman & Hilton Reference Dillman and Hilton2015), the scapular portion of the girdle is very nearly absent, and consists of little more than the slender, mesial process bridging the dorsal muscle canal. However, note that Gardiner (Reference Gardiner1984, source of present labelling conventions) identifies this bridge as part of the coracoid.
Presence of a large coracoid plate, such as that of Trawdenia, appears to be a straightforward primitive retention. There is no indication of reduction or emargination of the caudal side of the coracoid, and the anteroventral extremity displays only a slight extension to allow it to contact the anterior margin of the cleithrum.
Within the fin endoskeleton, the simple characterisation of a propterygium as either perforate or imperforate (C-235) is no longer sufficient to capture the newly expanded range of known conditions (Fig. 10). Giles et al. (Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a) show a classic, block-like, imperforate propterygium in Cheirolepis, but Trawdenia planti demonstrates that pectoral fin marginal nerves and vessels can be enclosed (Jessen Reference Jessen1972; Patterson Reference Patterson1982), irrespective of whether a propterygium is perforated. A two-part or double propterygium enclosing a propterygial canal might be unique to T. planti, but in the present context the observation that sturgeon propterygia develop from a pair of cartilaginous precursors rather than a single cartilage (Dillman & Hilton Reference Dillman and Hilton2015) is of possible systematic interest. Paddlefish pectorals appear to be similarly equipped, with a propterygium that expands laterally late in development (Mabee & Noordsy Reference Mabee and Noordsy2004). In contrast, teleost propterygia (e.g., Danio; Grandel & Schulte-Merker Reference Grandel and Schulte-Merker1998) are not known to show any of these characteristics and holostean examples are, as yet, insufficiently described.
Processes on the Trawdenia propterygia probably represent muscle insertion areas comparable to dorsal and ventral marginal muscle insertions on the proximal face of the Acipenser propterygium (Jessen Reference Jessen1972, pl. 5, fig. 6 and pl. 13, fig. 4). These are probably plesiomorphic conditions, comparable to fin musculatures in polypterids and outgroups, where pectoral fin muscles insert on the distal parts of all radials (Wilhelm et al. Reference Wilhelm, Du, Standen and Larsson2015). The general, and probably derived, condition of actinopterygian fins is for the adductor and abductor muscles to bypass the fin endoskeleton altogether and insert directly on fin-ray bases.
The hemirays (Flammang et al. Reference Flammang, Alben, Madden and Lauder2013) of Trawdenia enclose an adbasal, interlepidotrichial space between their divergent bases (Fig. 11). Jessen (Reference Jessen1972) proposed this space, which in living species houses the outer marginal nerves and vessels, as a defining characteristic of the Actinopterygii. However, no such space exists in the fins of Polypterus and outgroups. In these taxa, the base of each proximal hemitrich is a simple prong that overlaps an extremity of the fin endoskeleton. The flared ‘boot' at the base of the proximal hemitrichs of Trawdenia likely provided an insertion for adductor or abductor fin muscles, and connection with the endoskeleton is achieved via an internal process, which articulates with a distal radial (Fig. 11d). These three characters – divergent fin ray base, adbasal interlepidotrichial space and proximal hemitrichs with an internal process – are so closely correlated that they might be treated as a single condition. Taxon descriptions rarely communicate these details, but examples are visible in the literature. Disarticulated proximal hemitrichs show these features to be absent in Cheirolepis (Giles et al. Reference Giles, Coates, Garwood, Brazeau, Atwood, Johanson and Friedman2015a), Mimipiscis (Choo Reference Choo2011, fig. 15), Moythomasia nitida (Choo Reference Choo2015, fig. 12a) and Woodichthys (Coates Reference Coates1998, fig. 7). Examples displaying the likely ‘present' condition include Chondrosteus, Palaeoniscus, Perleidus and Caturus (Jessen Reference Jessen1972, pls 20–24).
4.2. Fin morphology and functional implications
Well-preserved paired fins of Palaeozoic actinopterygian are rare. Early sarcopterygian and chondrichthyan examples are far better represented in collections and descriptive literature (e.g., Coates Reference Coates2003). The most completely described, illustrated and, thus, standard early actinopterygian fins to compare with are those of Mimipiscis (Gardiner Reference Gardiner1984) and Pteronisculus (Nielsen Reference Nielsen1942). But Pteronisculus is Triassic, thereby highlighting the remarkable lack of Palaeozoic examples to compare with, especially so given the abundance of post-Devonian ray-finned fish material (fossil specimens and taxa). Against this background, the significance of the morphological disparity provided by the Trawdenia pectoral skeleton is readily apparent. Not only does this glimpse prompt a series of new character statements, the preservation is of sufficient quality to allow a discussion of its likely functional and palaeoecological significance.
Much of the groundwork of what is now understood about fin function stems from McNeill Alexander (Reference McNeill Alexander1967) seminal text on fish functional morphology. This work characterised ‘primitive palaeoniscoid' fins as structurally and functionally indistinguishable from those of modern sturgeons. In both, the rays are closely packed, the muscles arranged as in elasmobranch chondrichthyans and the fin movements limited. These conditions contrast markedly with the more derived fins of neopterygians in which a flexible web interconnects fewer, more widely spaced rays, with much greater mobility of all parts of the appendicular (fin) skeleton. In all of these fins, the halves of each ray are bound together tightly and attached to a distal radial by ligaments. Joints are located between each ray and distal radial, and between distal and proximal radials. In paired fins, adductor and abductor muscles insert on the fin rays, on dorsal and ventral surfaces respectively, assuming that the fin is held horizontally. These muscles may be subdivided into superficial and deep portions, and an arrector dorsalis muscle extends along the leading edge of the fin base, between adductors and abductors, to insert on the base of the first fin ray (Wilhelm et al. Reference Wilhelm, Du, Standen and Larsson2015).
However, unlike sturgeons and modern chondrosteans in general, the pectoral fin of Trawdenia planti is rotated so that the adductor muscle is medial and abductor lateral. Like many non-teleostean neopterygians, and, once again, unlike modern chondrosteans, the fin ray to radial ratio is between 2:1 and 4:1, and the strongly divergent bases of the proximal hemitrichs support the hypothesis that these fin rays functioned like neopterygian examples. Importantly, extant neopterygian fishes can control individual ray curvature, and thereby govern the curvature and stiffness of the entire fin surface. Each fin ray half can slide relative to the other half, its counterpart (Fig. 11), and if the base of one half-ray is pulled past the other, the ray will curve and/or resist hydrodynamic loading (Alben et al. Reference Alben, Madden and Lauder2007). Significantly, in neopterygians such fin mobility and control is functionally linked to the presence of an effective swim bladder and the ability to seemingly rest almost motionless in water (McNeill Alexander Reference McNeill Alexander1967). Swim bladder volume determines the depth at which a fish and its surrounding water share the same density, but this equilibrium is unstable and vertical displacements are continually corrected by fin movements. Similarly, all fins correct for instability arising from swim bladder location relative to the centre of gravity, and pectoral fins, in particular, work to counteract the slight jet propulsion effect caused by the opercular pump. Neither polypterids nor chondrosteans achieve this degree of motion control. Polypterids are demersal and hold station by propping themselves against available surfaces; chondrosteans, like many chondrichthyans, swim continuously unless resting on a substrate.
Trawdenia planti pectoral fins are oriented to provide a rowing action. Figure 8a, c, d show the radials moderately abducted, with the fin blade swung anteriorly. The relationship to the space occupied by the abductor muscle is abundantly clear (Fig. 8a). However, the caudal fin is fully heterocercal: a combination is unknown in extant fishes. The classic functional interpretation of a heterocercal tail is that it generates forward thrust and lift but pitches the rostrum downwards, an effect counteracted by the lift-providing surfaces of near-horizontal pectoral fins, and/or an appropriately shaped rostrum (Wilga & Lauder Reference Wilga and Lauder2002). Trawdenia planti pectoral fins are evidently neither horizontal nor, primarily, lift-generating. This implies that this ‘palaeoniscid', at least, had an effective swim bladder to compensate for the functional burden of its heterocercal tail and extensive dermal skeleton. These data further suggest that T. planti, like modern forms, was able to hold position in mid-water. If correct, this represents a significant functional advance: the fact that this swimming behaviour and this degree of motion control is commonplace among living neopterygians, especially in low-energy environments, attests to its functional importance.
Among the abundant species of small fusiform actinopterygians of the mid to late Palaeozoic (Sallan Reference Sallan2014), Trawdenia planti is the earliest to reveal clear and detailed structural evidence of locomotor specialisation in the paired fins. Earlier instances of rotated fins are known, but these are poorly preserved with scant evidence of their endoskeletal supports. However, such fins are consistently linked with deep body forms. Examples include eurynotiform (Sallan & Coates Reference Sallan and Coates2013) species such as Cheirodopis (Moy-Thomas & Bradley Dyne 1938) and Amphicentrum (Young Reference Young1866; Traquair Reference Traquair1879, 1890b; Bradley Dyne Reference Bradley Dyne1939), but appear to be absent in less derived members of the clade, e.g., Eurynotus (Traquair Reference Traquair1879; Coates Reference Coates1994; Friedman et al. Reference Friedman, Pierce, Coates and Giles2019, although the postcranium is not addressed). Further examples of rotated pectorals occur in Aesopichthys (Poplin & Lund Reference Poplin and Lund2000), Discoserra and Guildayichthys (Lund Reference Lund2000). Importantly, these rotated pectorals are very likely independently derived, and the link with laterally compressed, circular or rhombic body shapes probably relates to the degree of control demanded by the consequent hydrodynamic instability. There appears to be no pressing, functional demand for such mobile fins in early fusiform fishes, unless predicated on, or perhaps released by, increased buoyancy. Some ‘primitive palaeoniscoids', at least, were not swimming like sturgeons (contra McNeill Alexander Reference McNeill Alexander1967), although the heterocercal tail persisted. An implication is that several groups of post-Devonian fishes achieved this functional advance independently. Pectoral fins like those of T. planti become far more common in fusiform non-teleostean neopterygian ray-finned fishes of the early Mesozoic, such as Gracilignathichthys or Platysiagum (Bürgin 1992), but in these genera, a fully heterocercal tail is absent. As for the vast array of seemingly similar ‘trash fish' of the Carboniferous, such detailed anatomy of the fins signals cryptic palaeoecological diversity.
5. Acknowledgements
This paper is presented in celebration of Professor Jennifer Clack's career and numerous contributions to studies of early vertebrates, with many thanks for being such a supportive colleague and research partner. N. Fraser and S. Walsh, of the National Museums of Scotland, are thanked for providing access to fossil specimens, and Mike Gill, Recorder of the Northern Mine Research Society, is thanked for his advice on the history of the Burnley Coalfield. Valuable reviews were received from Sam Giles and another anonymous source. This project was supported by National Science Foundation grants DEB-0917922 and DEB-1541491.













