Core systems of the brain are established prior to birth and are foundational to infants’ emerging developmental competencies. Among the earliest to mature are the motor and sensory systems, which support the ability of the infant to interact with and receive input from the world (Fransson, Åden, Blennow, & Lagercrantz, Reference Fransson, Åden, Blennow and Lagercrantz2011; Gao et al., Reference Gao, Alcauter, Elton, Hernandez-Castillo, Smith, Ramirez and Lin2015; Grayson & Fair, Reference Grayson and Fair2017). At their start, neural systems are highly malleable (Stiles & Jernigan, Reference Stiles and Jernigan2010), enabling a learned fit between the early environment and the brain that will operate, and survive, within it. The brain is primed at birth to receive inputs from sensory organs, and the signals received play an essential role in advancing neurodevelopmental processes. Experimental disruption to normal occurrence and propagation of activity through sensorimotor systems leads to permanent loss of function in those systems and lasting atypical cortical organization. Elegant examples of this come from controlled developmental studies of rat barrel cortex (Tolner, Sheikh, Yukin, Kaila, & Kanold, Reference Tolner, Sheikh, Yukin, Kaila and Kanold2012), mouse ocular dominance columns (Xu et al., Reference Xu, Furman, Mineur, Chen, King, Zenisek and Crair2011), and cat retinogeniculate pathways (Shatz & Kliot, Reference Shatz and Kliot1982). Thus, sensorimotor systems are present at birth, and receptive to signals from the environment, which instruct further developmental patterning of neural systems. Moreover, these systems are capable of producing behavior, enabling the developing organism, or infant, to build representations of stimulus-response properties through interaction with the environment.
We expect that ontogenetic events occurring before birth set the stage for later human development in a way that varies from person to person, but direct longitudinal evidence linking the two is scarce. Recent advances in fetal functional magnetic resonance imaging (MRI) have enabled demonstration that individual differences in brain network integrity emerge in utero (Jakab et al., Reference Jakab, Schwartz, Kasprian, Gruber, Prayer, Schopf and Langs2014; Thomason et al., Reference Thomason, Dassanayake, Shen, Katkuri, Alexis, Anderson and Romero2013). One implication of these observations is that infants enter the world with unique neural assemblies that are influenced by intrauterine experiences, and it is against the background of this natural interindividual variation that experientially driven patterning of neural systems takes place. A second implication is that variation in neural development in utero may predict future development. The focus of the present study is to address this latter possibility, by examining the association between fetal sensorimotor brain system functional connectivity and infant postnatal motor ability.
Although numerous studies have linked neurological measures in high-risk newborns with subsequent infant development, direct evidence linking variation in human fetal brain networks to infant outcomes is entirely lacking. MRI studies of preterm infants at, or before, term-equivalent age have reliably identified specific mild variations in brain activity, structure, and metabolite ratios that predict behavioral outcomes (Drobyshevsky et al., Reference Drobyshevsky, Bregman, Storey, Meyer, Prasad, Derrick and Tan2007; Kendall et al., Reference Kendall, Melbourne, Johnson, Price, Bainbridge, Gunny and Robertson2014; Keunen et al., Reference Keunen, Isgum, van Kooij, Anbeek, van Haastert, Koopman-Esseboom and Benders2016; Rose et al., Reference Rose, Butler, Lamont, Barnes, Atlas and Stevenson2009; Schumacher et al., Reference Schumacher, Larsson, Sinding-Larsen, Aronsen, Lindeman, Skjeldal and Stiris2013; van Kooij et al., Reference van Kooij, de Vries, Ball, van Haastert, Benders, Groenendaal and Counsell2012; Woodward, Anderson, Austin, Howard, & Inder, Reference Woodward, Anderson, Austin, Howard and Inder2006). Related investigations have evaluated a number of other perinatal risk groups, with and without overt forms of encephalopathy, and these studies show similar associations between neural features at birth and both near and long-term neurobehavioral outcomes (Arca-Diaz et al., Reference Arca-Diaz, Re, Drottar, Fortuno, De Macedo-Rodrigues, Im and Grant2017; Johnson et al., Reference Johnson, Jenkins, Bentzley, Lambert, Hope, Rollins and Katikaneni2015; Massaro et al., Reference Massaro, Evangelou, Fatemi, Vezina, McCarter, Glass and Limperopoulos2015; Rollins et al., Reference Rollins, Asaro, Akhondi-Asl, Kussman, Rivkin, Bellinger and Soul2017). Fewer studies have attempted to bridge normal neural variation in healthy newborns with neurobehavioral outcomes. One study, by Emerson, Gao, and Lin (Reference Emerson, Gao and Lin2016), showed that, in typically developing infants, rate of transition toward interhemispheric functional asymmetry predicts language outcomes at age 4. The question that remains is whether functional integrity of core neural systems prior to birth relates to behavioral outcomes in infancy.
Sex-related brain and behavioral differences are apparent across the life course, but the exact processes that guide their prenatal emergence remains a topic of vigorous scientific inquiry. There is some evidence for differences in behavior between male and female fetuses, specifically for increased leg movement in males (Almli, Ball, & Wheeler, Reference Almli, Ball and Wheeler2001), but findings are inconsistent, with many fetal behavioral studies reporting no differences, especially after controlling for wakefulness, which tends to be higher in males (DiPietro & Voegtline, Reference DiPietro and Voegtline2017; Hata et al., Reference Hata, Hanaoka, Mostafa AboEllail, Uematsu, Noguchi, Kusaka and Kurjak2016; Robles de Medina, Visser, Huizink, Buitelaar, & Mulder, Reference Robles de Medina, Visser, Huizink, Buitelaar and Mulder2003). A primary driver of sex differences in utero is exposure to hormones, such as testosterone, which begins to show varied concentrations between the sexes beginning as early as week 8 of gestation (Hines, Reference Hines2010). Testosterone influences a number of fundamental neurodevelopmental processes, including cell survival and anatomical connectivity, and does so through widespread interactions with membrane receptors, transcription factors, growth factors, and other hormones (Papenfuss & Whitacre, Reference Papenfuss and Whitacre2009). In early infancy, social processes begin to act on sex-linked biology to further influence developmental outcomes. Adults typically perceive infant females as smaller, softer, and fine-featured, and tend to vocalize and look at them more than males; in contrast, they tend to be more physical with males (Ispa, Claire Cook, Harmeyer, & Rudy, Reference Ispa, Claire Cook, Harmeyer and Rudy2015; Lewis, Reference Lewis1972).
Sex differences in brain development are apparent by infancy and persist across the life span (Caviness, Kennedy, Richelme, Rademacher, & Filipek, Reference Caviness, Kennedy, Richelme, Rademacher and Filipek1996; Choe et al., Reference Choe, Ortiz-Mantilla, Makris, Gregas, Bacic, Haehn and Grant2013; Gilmore et al., Reference Gilmore, Lin, Prastawa, Looney, Vetsa, Knickmeyer and Gerig2007; Koolschijn & Crone, Reference Koolschijn and Crone2013; Ruigrok et al., Reference Ruigrok, Salimi-Khorshidi, Lai, Baron-Cohen, Lombardo, Tait and Suckling2014; Tiemeier et al., Reference Tiemeier, Lenroot, Greenstein, Tran, Pierson and Giedd2010), and neurological differences between the sexes relate to cognitive ability (Satterthwaite et al., Reference Satterthwaite, Wolf, Roalf, Ruparel, Erus, Vandekar and Gur2015). Evidence from the antenatal period demonstrates that fetal brain structure (de Lacoste, Horvath, & Woodward, Reference de Lacoste, Horvath and Woodward1991) and neonatal brain function (Thordstein, Lofgren, Flisberg, Lindecrantz, & Kjellmer, Reference Thordstein, Lofgren, Flisberg, Lindecrantz and Kjellmer2006) develop more rapidly in females, and this relates to hormonal signaling. Further, Knickmeyer et al. (Reference Knickmeyer, Wang, Zhu, Geng, Woolson, Hamer and Gilmore2014) observed larger motor cortices in female newborns, and an inverse association between testosterone levels and both supplementary motor and paracentral lobule volumes in males. While these studies suggest marked motor differences may be present at birth, the number of studies that report sex differences in infant motor ability are surprisingly few. Many landmark publications that have produced infant motor performance age norms report nonsignificant sex differences (Bayley, Reference Bayley1965). Others either report nonsignificant female advantage (Góes, Méio, Mello, & Morsch, Reference Góes, Méio, Mello and Morsch2015; Neligan & Prudham, Reference Neligan and Prudham1969), or collapse across or remove scales with apparent sex differences (Thalagala, Reference Thalagala2015), making it possible that extant effects are underreported. The small number of studies reporting sex differences in infant motor performance highlight an important distinction, namely, that female infants excel in fine motor skill development, whereas males show gross motor advantages (Touwen, Reference Touwen1976). A study by Piek, Gasson, Barrett, and Case (Reference Piek, Gasson, Barrett and Case2002) that examined coordination of infant movements across limbs reported a complementary sex dissociation in arm versus leg coordination. They found that in 6- to 18-week-old infants, female infants show more coordinated arm movements, whereas males show a trend toward more coordinated leg movements. Overall, differences in motor ability appear to be subtle in early development, becoming more marked with maturation (Thomas & French, Reference Thomas and French1985).
The primary goal of this investigation was to evaluate evidence of a prospective, longitudinal association between fetal sensorimotor brain system functional connectivity and infant postnatal motor ability. We measured fetal brain resting-state functional connectivity (FC) during MRI scans that were performed in late pregnancy, and followed up infants and their mothers at 7 months postpartum (N = 96), at which time we assessed infant motor ability. We tested the hypothesis that increased FC within the fetal sensorimotor brain network would predict more mature individual motor ability at age 7 months. We also explored sex differences, examining whether this brain–behavior relationship differs between the sexes. Analyses accounted for other salient sociodemographic factors that could influence our hypothesized relationships, including maternal education, income, fetal MRI motion, and maternal prenatal mood symptomology.
Method
Participants
One hundred forty-nine pregnant women were recruited into a longitudinal study of pediatric brain development during routine obstetrical appointments. Selective inclusionary criteria included no contraindications for MRI, maternal age >18 years, and healthy singleton pregnancy. Individual cases were subsequently excluded due to high motion during fetal scan (n = 22) or missing outcome data at child age 7 months (n = 31), resulting in a final sample of 96 fetal cases (37 female; 59 male), maternal mean age of 25.9 years (SD = 4.6), for examination of longitudinal associations. The mean age of fetuses at the time of MRI was 33.1 weeks of gestation (SD = 4.1), and mean age at birth was 39 weeks of gestation (SD = 1.4). Sample descriptive statistics are provided in Table 1. All study procedures were approved by the Wayne State University Human Investigation Committee, and all women provided written informed consent prior to participation.
Table 1. Summary of data and participant characteristics

Note: Chi-square tests compared race/ethnicity, education, and income between groups and two-sample t tests compared all other variables. All comparisons were nonsignificant, using two-tailed, p < .05.
Prenatal fetal MRI
Fetal magnetic resonance exams were performed with a Siemens Verio 70-cm open-bore 3-Tesla scanner with a light-weight abdominal Siemens Flex Coil. The MRI examination lasted 45 min. Images were collected with the following parameters and system-derived estimates of specific absorption rate: echo planar imaging blood oxygen level dependent, response time/echo time 2000/30 ms, minimum 180 frames, axial 4-mm slice, specific absorption rate = 0.22 (SD = 0.08). For the final sample of 96 cases included in all analyses, frame count, after excluding high motion frames, was range = 90 to 330, M = 155, SD = 48. Fetal MRI motion was included as a covariate in all subsequent analyses for included participants.
Study measures
Infant motor development
At 7 months postpartum (M = 7.4 months, SD = 0.5) infants’ developmental skills were assessed with the Bayley Scales of Infant Development (3rd ed.; Bayley-III; Bayley, Reference Bayley2006). In the current study, fine and gross motor subtests, as well as a scaled composite motor score, were evaluated. For these measures, scale scores on the fine and gross motor subtest range from 1 to 19; scores between 7 and 10 are considered to be typical development and scores below 4 indicate a developmental delay at –2 SD. The motor composite ranges from 45 to 145. Scores ranging from 85 to 115 are considered to be typical motor development, while scores at or below 70 are indicative of a developmental delay at –2 SD. In the present sample, motor development scores ranged from 58 to 130 with a mean and SD of 97.01 and 13.7.
Maternal psychosocial adaptation
During the prenatal period maternal anxiety symptoms were measured using the State Trait Anxiety Inventory (Spielberger, Reference Spielberger1984). The State Trait Anxiety Inventory is an anxiety inventory with a trait subscale, used here, on which participants rate their anxiety on a Likert scale ranging from 1 (almost never) to 4 (almost always). Scores range from 20 to 80 with higher scores reflecting greater trait anxiety. Mothers also completed a demographic survey that included questions about education and income.
Analytic plan
Functional data preprocessing
Time frames corresponding to periods of minimal fetal head motion were identified using FSL image viewer (http://www.fmrib.ox.ac.uk/fsl/). Brainsuite (Shattuck & Leahy, Reference Shattuck and Leahy2002) was used to manually generate three-dimensional masks for single reference images drawn from time periods, or segments, of fetal movement quiescence. Masks were binarized and applied only to frames corresponding to their select segment, and only those data were retained for further analyses, resulting in, on average, 31% of data censored, or eliminated, due to movement artifacts. It should be noted that this value underestimates total data motion censoring, given that excessive motion was an exclusionary criterion and resulted in exclusion of 22 cases. As a point of reference, prior fetal functional MRI (fMRI) studies have reported censoring 39%–41% of total number of acquired fMRI frames (Thomason et al., Reference Thomason, Dassanayake, Shen, Katkuri, Alexis, Anderson and Romero2013, Reference Thomason, Brown, Dassanayake, Shastri, Marusak, Hernandez-Andrade and Romero2014, Reference Thomason, Scheinost, Manning, Grove, Hect, Marshall and Romero2017). Each segment was manually reoriented, realigned, resliced, and normalized to a 32-week fetal brain template (Serag et al., Reference Serag, Aljabar, Ball, Counsell, Boardman, Rutherford and Rueckert2012) using Statistical Parametric Mapping (SPM8) software implemented in Matlab (http://icatb.sourceforge.net). To correct for variation in normalization across segments within-participant, normalized images were then concatenated into one run, realigned, and smoothed with a 4-mm Gaussian kernel.
Independent component analyses (ICA)
Following preprocessing and quality assurance steps, fetal resting-state time series data were concatenated across all fetuses and submitted to group ICA implemented in Matlab using the Group ICA/IVA of fMRI Toolbox (GIFT; Calhoun, Adali, Pearlson, & Pekar, Reference Calhoun, Adali, Pearlson and Pekar2001). The Infomax (MST) ICA algorithm was used to estimate 30 components, after which visual inspection of spatial and temporal/spectral features was used to separate noise components (n = 13) from networks of interest (n = 17) following priors (Griffanti et al., Reference Griffanti, Douaud, Bijsterbosch, Evangelisti, Alfaro-Almagro, Glasser and Smith2017; Robinson et al., Reference Robinson, Basso, Soldati, Sailer, Jovicich, Bruzzone and Moser2009). Resultant networks of interest met criteria of dynamic range >0.06 and power frequency ratio <2.0 (cf. Allen et al., Reference Allen, Erhardt, Damaraju, Gruner, Segall, Silva and Calhoun2011). GIFT was used to implement back reconstruction of single-subject spatial maps for the component corresponding to the sensorimotor system (Figure 1).
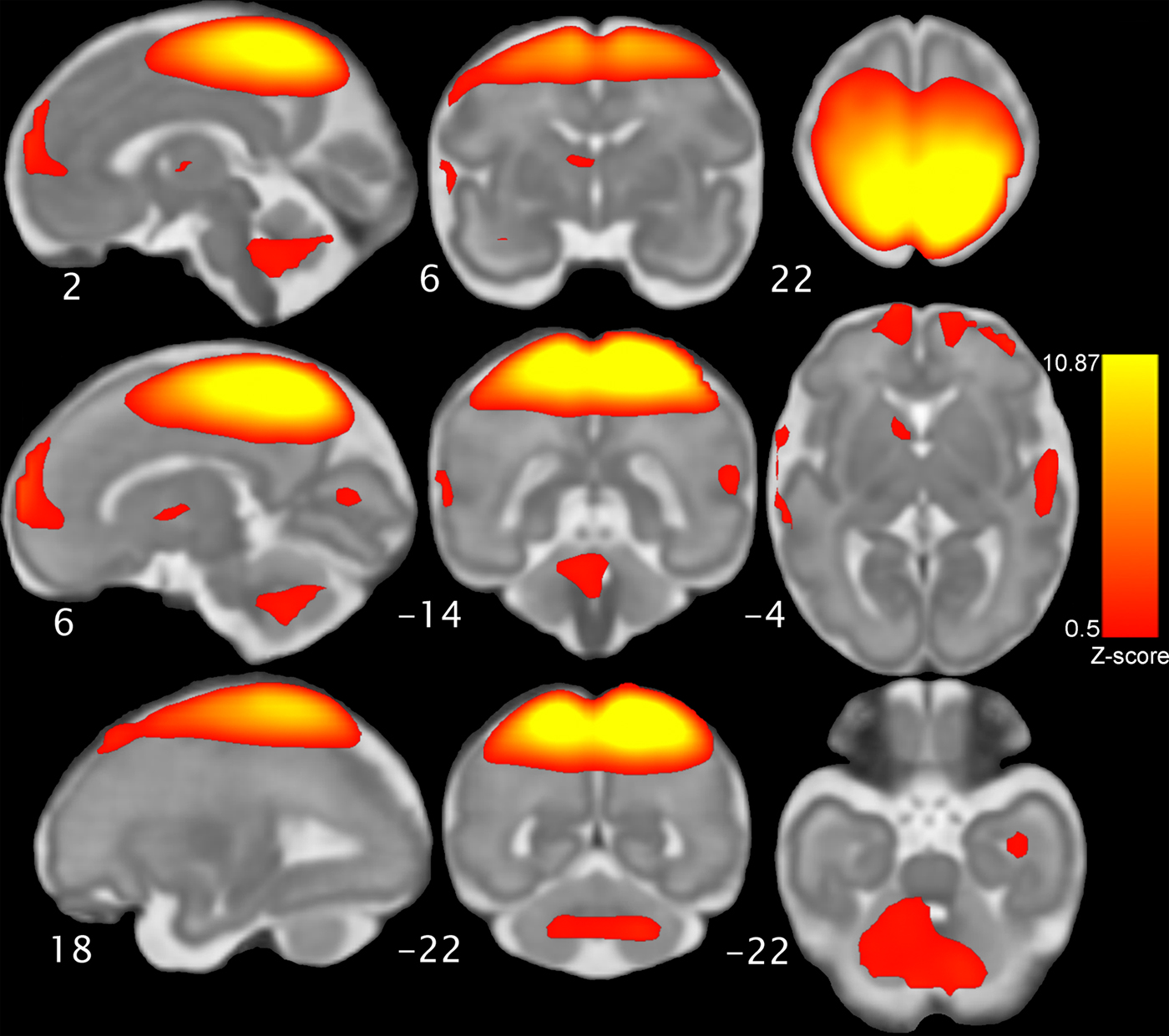
Figure 1. The fetal sensorimotor resting state network, which was derived from group independent components analysis of resting state scans obtained in 96 fetuses. The network component map pictured here is threshold at Z = 0.5 and displayed on a 32-week gestational age template for anatomical reference. The network includes motor and sensory cortices, cerebellum, striatum, thalamus, and bilateral insula. Neurological convention is used.
Group-level neuroimaging analyses
Individual fetal sensorimotor maps were submitted to a full factorial model in SPM8. Bayley infant motor ability at 7 months (median-split) and sex were treated as separate factors, and gestational age at scan was included as a covariate. Conjunction analyses were used to isolate brain regions with both significant main effect of Bayley performance and sex interaction effects from constituent maps thresholded at p < .05, resulting in four regions reaching p < .05 criteria for both main and interaction effects: anterior cingulate cortex (ACC; 8,29,6), prefrontal cortex (PFC; 8,40,0), cerebellum (2,-16,-26), and inferior parietal lobule (IPL; -24,-32,8). FC values from these regions were then exported from reconstructed single-subject spatial maps and exported into Mplus version 7.2 (Muthén & Muthén, Reference Muthén and Muthén2014).
Structural equation modeling (SEM) was implemented in Mplus to test whether the brain–behavior associations were impacted by salient demographic variables: maternal education, income, fetal MRI motion, and maternal prenatal anxiety symptomology. SEM was implemented separately for the ACC, PFC, cerebellum, and IPL, where each model included the main effect of FC to motor ability, gender to motor ability, and the interaction of FC × Gender predicting motor ability, with gross motor ability included as a continuous variable. In addition, we accounted for the associations between maternal education, income, fetal MRI motion, and maternal prenatal anxiety symptoms to motor ability in each model. Finally, to stringently rule out the impact of these variables on any significant brain–behavior associations, we also included pathways from maternal education, income, fetal MRI motion, and maternal prenatal anxiety symptoms to FC for each region and FC × Gender interaction term. Models were tested in Mplus using maximum likelihood procedures with robust standard errors. Further, we corrected for multiple comparisons by applying a Bonferroni correction of p < .0125 to each model, determined by dividing .05 by 4, which reflects the total number of models tested. Significant interactions between motor ability and sex were probed by comparing the simple slopes for boys and girls separately. We established regions of significance for significant interactions using an online tool, www.quantpsy.org/interact/ (Preacher, Curran, & Bauer, Reference Preacher, Curran and Bauer2006).
Results
Neonatal parameters, MRI data quality, demographic variables, and prenatal maternal self-report anxiety symptoms did not differ between male and female infants included in this study (see Table 1). In line with prior research, significant associations were observed among maternal age, income, and education, all ps < .015. In addition, as would be expected, infant gestational age at birth was related to birth weight, r = .40, p < .001.
Fetal sensorimotor network development
A robust fetal sensorimotor neural network emerged from the group-level ICA. The network included regions of the motor and sensory cortices, cerebellum, thalamus, posterior cingulate cortex, and striatum (see Figure 1). Greater FC, operationally defined as fMRI signal coherence between a given region and the mean signal for the fetal motor network, was positively related to infant 7-month Bayley-III composite motor scores (M = 97.01, SD = 13.7) in regions of the posterior cingulate cortex, supplementary motor cortex, medial temporal lobe, and superior frontal gyrus, and negatively related in regions of the ACC, insula, lateral cerebellum, and right inferior motor cortex, controlling for gestational age at scan. See Table 2 for a full list of regions in which fetal motor network connectivity was linked to age 7-month infant Bayley motor performance.
Table 2. Brain areas in which fetal motor network functional connectivity positively or negatively predicts infant Bayley performance at 7 months

Note: Significant clusters are reported for regions in which fetal functional connectivity positively or negatively predicted motor performance at 7 months postnatal age, given at p < .05, and k-min ≥ 20.
Associations between brain and motor outcomes in boys and girls
Conjunction analyses identified four brain regions in which FC values predicted motor outcomes differently in males and females. These were areas of the PFC, somatomotor cerebellum, IPL, and ACC. FC associations for each of these regions are plotted in Figure 2. SEM performed for each region confirmed that brain–behavior associations remained significant even after controlling for prenatal maternal anxiety and psychosocial variables and accounting for multiple comparisons (Bonferroni p < .0125). Interactions between sex and FC values in those models were significant for the cerebellum and PFC, but not for the ACC and IPL (see Table 3). The region of significance analysis conducted for the cerebellum and PFC established that brain–behavior relationships were significant for high FC values only (see Figure 3). That is, there were no significant differences between males and females in how fetal cerebellum and PFC motor network connectivity related to infant motor ability at approximately the bottom half of the FC values. Estimates of simple slopes between fetal FC and Bayley motor performance, separated by sex, demonstrated significant slopes were present in females, ps < .01, but not in males, ps > .3 (see Table 4).

Figure 2. Scatterplots depicting significant associations between fetal functional connectivity and Bayley performance at infant age 7 months. Scatterplots depict mean signal extracted for each subject from 3-mm radius spheres positioned at the center of mass in regions associated with both motor outcomes and sex differences, shown in left panel in orange and projected onto 32-week fetal reference anatomical brain images. We observe that for the cerebellum, prefrontal cortex, and inferior parietal lobule, increases in functional connectivity to the motor system predicts better Bayley scores. The reverse is true for the anterior cingulate cortex (ACC), suggesting decreased signal coupling between the fetal motor system and ACC is indicative of better future motor outcomes.

Figure 3. Sex differences were observed in four brain regions exhibiting associations between fetal functional connectivity and Bayley motor performance at 7 months postnatal age. Mean functional connectivity values are plotted by median Bayley performance and by sex, and given for (a) the cerebellum, (b) the anterior cingulate (ACC), (c) the prefrontal cortex (PFC), and (d) the inferior parietal lobule (IPL). Structural equation models controlling for maternal education, income, anxiety, and motion during magnetic resonance imaging demonstrate significant sex interactions in the cerebellum, p = .03, and the PFC, p = .002. An examination of the region of significance in sex interactions showed these effects to be significant at higher fetal functional connectivity values (see shaded regions in [a] and [c]). When groups were tested individually, brain–behavior associations were significant among girls (ps < .01), but not boys (ps ≥ .3), across all four areas (see Table 4). Brain images to the left of plots highlight areas from which functional connectivity is plotted, corresponding to main effects of Bayley motor outcome (dark blue), interaction of Motor × Sex (cyan), and the conjunction of these (fuscia) at p < .05.
Table 3. Fetal intrauterine functional connectivity relates to higher infant motor function in structural equation models accounting for sex, movement during the scan, and salient maternal characteristics

Note: Connectivity of the ACC negatively predicts motor outcome; all other regions are positively related to infant motor ability at 7 months.
Table 4. Estimated simple slopes for associations between fetal functional connectivity and Bayley performance in male and female infants

Note: Simple slopes estimated for male and female fetal functional connectivity predicting Bayley motor scores at 7 months. ACC, anterior cingulate cortex. PFC, prefrontal cortex. IPL, inferior parietal lobule. *p < .05. **p < .001.
With regard to sex differences in postnatal motor ability, we observed a trend for better motor performance in female compared to male infants, t = 1.85, p = .069. Examination of sex differences separately for fine and gross motor performance revealed fine, t = 2.14, p = .037, but not gross, p = .258, motor functioning was significantly more mature in females than in males at 7 months postnatal age.
In post hoc analyses, we examined potential distinctions between neural FC and outcomes when stratefied by fine and gross motor performance. We observed that the relationship between fetal IPL FC was uniquely related to gross but not fine motor skills when these were considered as correlated dependent variables. In contrast, for the other three areas exhibiting both gender and composite motor effects (ACC, PFC, and cerebellum), the association between FC and fine and gross motor outcomes were not distinct. In other words, with the exception of the IPL, fetal FC is correlated with both fine and gross motor ability in a similar way to the composite score; for the IPL, FC strongly predicts gross but not fine motor outcomes in infancy.
Discussion
Neural processing in the newborn brain depends on global connectional systems present at birth. The ordered steps by which the brain becomes organized into a collection of interactive highways for information transfer across gestational development are presently not well understood. To our knowledge, this study is the first report on fetal brain–function and infant–behavior associations. Specifically, we address the extent to which strength of FC within the fetal sensorimotor neural system predicts variation in infant motor ability at age 7 months, and examine infant sex as potentially influencing this effect.
We observed that several regions in the fetal brain have connectional patterns that relate to subsequent infant motor ability (Table 2). Observed effects support a model of more rapid infant motor development relating to earlier specialization of neural systems that support sensorimotor function. Specifically, we observed that regions that support motor function, particularly prefrontal regions, the posterior cingulate, and supplementary motor regions, show increased connectivity with the motor network in fetuses that will develop motor abilities more rapidly in infancy. The idea that more rapid specialization of fetal neural systems predicts more advanced motor ability in infancy, over and above variation introduced by age of the fetus at the time of MRI, fits with previous developmental literature showing that infants that “start out ahead” tend to go on to be individuals that “stay ahead.” For example, earlier attainment of motor milestones in early childhood is related to better cognitive performance and completion of higher educational levels later in life (Murray, Jones, Kuh, & Richards, Reference Murray, Jones, Kuh and Richards2007; van Batenburg-Eddes et al., Reference van Batenburg-Eddes, Henrichs, Schenk, Sincer, de Groot, Hofman and Tiemeier2013).
In addition to observing brain regions in which fetal FC and child motor ability were positively related, we identified a separable set of regions that showed the reverse effect. These were regions of the anterior cingulate, insula, and lateral cerebellar regions, where diminished FC was associated with more advanced infant motor ability. Thus, it is not just enhanced signal coherence within neural systems that relates to individual motor outcomes, but also reduced connectivity to potentially superfluous regions. In this context, significant negative correlations could result from more active reorganization or pruning in individuals that will develop infant motor abilities more quickly. Another possibility is that these are extraneous connections that are more often present in individuals that reach motor milestones more gradually. An example is in the cerebellum where advanced motor outcomes were associated with decreased lateral cerebellar FC and increased midline anterior cerebellar FC. Prior studies in large samples of adults demonstrate that the cerebellum possesses multiple representations of the cerebral cortex (Buckner, Krienen, Castellanos, Diaz, & Yeo, Reference Buckner, Krienen, Castellanos, Diaz and Yeo2011; Guell, Schmahmann, Gabrieli, & Ghosh, Reference Guell, Schmahmann, Gabrieli and Ghosh2018). Of note, regions of the cerebellum that Buckner, Guell, and others showed to be mapped to sensorimotor cortical regions encompass the region in which we observed a positive association with future motor ability. On the contrary, the region of the cerebellum in which we observed reduced connectivity predicting future motor ability fell within regions of the cerebellum they identified as linked to cognitive and association cortices. One can imagine that in assembling neural systems, increased functional integrity within the system is balanced with reductions in unnecessary connections. Our observations in the cerebellum dissociation noted here may be an example of this.
We observed that female infants demonstrated higher motor development scores than males, especially in the fine motor domain. While investigators commonly report language performance differences between male and female infants (Neligan & Prudham, Reference Neligan and Prudham1969), few studies report sex differences in motor skills during infancy, and in the limited studies we found, sex differences occur in fine motor skills or upper limb coordination, rather than in gross motor performance (Piek, Gasson, Barrett, & Case, Reference Piek, Gasson, Barrett and Case2002; Touwen, Reference Touwen1976). Thus, the current observations replicate prior developmental sex differences, and add greater context, establishing that fetal neural system development may set the stage for later variation in motor ability.
We also discovered that associations between fetal neural sensorimotor system connectivity and infant motor behavior were particularly strong in females. This is illustrated in slope values provided in Table 4, and in plots of FC separated by participant sex in Figure 3. Prior studies report sex differences in developmental trajectories, and report that female mental abilities stabilize at an earlier age (Bayley, Reference Bayley1966), and that early speech ability predicts subsequent verbal ability in females only (Bayley, Reference Bayley1968). Here, we also find that females show a behavioral advantage and that longitudinal associations between brain and behavior are stronger in females. Nancy Bayley remarked, “The girls appeared to be more resilient in returning to their own characteristic inherent response tendencies. Boys, on the other hand, were more permanently affected by the emotional climate in infancy” (Reference Bayley1968, pp. 14–15). This sentiment inclines that female infants may have a less malleable disposition than their male counterparts, and it appears that our neuroimaging findings may fall in line with this position. We may be observing that the female fetal brain may be more “hard-wired” and less susceptible to early environmental programming.
The idea that females may be less sensitive to environmental programming dovetails nicely with prior observations that insults and injuries sustained during the perinatal period have larger negative consequences in males than in females. Evidence for enhanced male susceptibility has been observed across numerous conditions, including stress exposure (Bale & Epperson, Reference Bale and Epperson2015), prematurity (Ingemarsson, Reference Ingemarsson2003; Skiöld et al., Reference Skiöld, Alexandrou, Padilla, Blennow, Vollmer and Ådén2014; Whitfield, Grunau, & Holsti, Reference Whitfield, Grunau and Holsti1997), and negative caregiving (Barnett & Scaramella, Reference Barnett and Scaramella2013; Góes et al., Reference Góes, Méio, Mello and Morsch2015; Mileva-Seitz et al., Reference Mileva-Seitz, Ghassabian, Bakermans-Kranenburg, van den Brink, Linting, Jaddoe and van Ijzendoorn2015). Recognition that maladaptive outcomes are more frequent in males across exposure types suggests that a general mechanism, or common pathway, may contribute to overall greater male vulnerability in early life. Findings from this study provide a candidate pathway in neural system functional integrity.
If males are more sensitive to environmental programming than females, the implications of this warrant consideration. It is notable that increased sensitivity to environmental programming may be disadvantageous under conditions of adversity, but may be advantageous when conditions are more favorable. In a review on prenatal origins of behavioral differences, Coe and Lubach (Reference Coe and Lubach2005) address the adaptive nature of flexibility, stating “fetal vulnerability to adverse events experienced by the mother may be a price paid for the adaptive benefits and flexibility gained by allowing developmental processes to be responsive to the environment. As long as the extrinsic conditions remain within a tolerable norm, then the reactions of the fetus and young infant serve a useful function and benevolently guide development” (p. 48). Within this framework, flexibility is adaptive under more favorable conditions.
In conclusion, the present study demonstrates for the first time that variation in the human fetal neural connectome correlates with infant motor ability. This relationship was significant even after controlling for salient sociodemographic factors that could influence our hypothesized relationships, including socioeconomic disadvantage and maternal prenatal anxiety. In addition, brain–behavior relationships were influenced by the sex of the child such that slope of effects was strongest in female participants. These observations extend prior longitudinal research back into prenatal brain development, and raise exciting new ideas about the advent of risk, and the ontogeny of early sex differences.









