Introduction
Being the centre of rice diversity, India has a wealth of traditional landrace varieties. The landraces from the North-eastern (NE) states of the country are especially diverse morphologically and genetically and are worthy candidates for in-depth analyses (Das et al., Reference Das, Sengupta, Parida, Roy, Ghosh, Prasad and Ghosh2013; Roy et al., Reference Roy, Rathi, Misra, Bhatt and Bhandari2013). The traditional farmers of the region grow many diverse rice varieties having special cultural values and qualities. Moreover, the region is also home to many locally adapted aromatic and quality rice landraces. In the current study, we focused on examining the genetic structure of the aromatic rice cultivars grown by the traditional farmers of Manipur state of India. These rice varieties are locally called Chakhao. The literal meaning of Chakhao in Manipuri language is delicious rice (Chak= rice and ahoba or hao= delicious). In the state, the farmers describe different types of Chakhao based on grain characteristics (Amubi= black; Angoba= white). The farmers grow a number of different types of Chakhao rice landraces within a short range of altitude differences on a small scale. The variability in the grain morphology of Chakhao rice landraces is remarkable. Chakhao rice is very special to the people of Manipur, as they use it in festivals and social ceremonies to prepare various unique dishes, namely Ethe Tan (a local puri made from black Chakhao rice flour in Chandel district), Buhman Sang (a local delicacy made from Buhman landraces in Churachandpur district) and Utong Chak (a special dish prepared within bamboo sticks in Chandel district). The cultivation of Chakhao landraces is declining, as the farmers prefer growing agronomically improved high-yielding varieties. Therefore, the assessment, documentation, analysis and conservation of the level of genetic diversity are essential for exploiting these rice landraces in variety development.
Although the genetic diversity of cultivated rice germplasm on a global scale has been well characterized using molecular markers (Yu et al., Reference Yu, Xu, Vijayakumar, Ali, Fu, Xu, Jiang, Marghirang, Domingo, Aquino, Virmani and Li2003; Garris et al., Reference Garris, Tai, Coburn, Kresovich and McCouch2005; Caicedo et al., Reference Caicedo, Williamson, Hernandez, Boyko, Fledel-Alon, York, Polato, Olsen, Nielsen, McCouch, Bustamante and Purugganan2007), important issues concerning crop genetic diversity and its relationship with local cultures can only be answered by rigorously studying rice germplasm collected from geographically isolated regions (see Thomson et al., Reference Thomson, Polato, Prasetiyono, Trijatmiko, Silitonga and McCouch2009). A number of studies have been conducted in the past to characterize subsets of rice germplasm, with molecular markers being used for analysing the genetic diversity within specific countries (Prashanth et al., Reference Prashanth, Parani, Mohanty, Talame, Tuberosa and Parida2002; Jain et al., Reference Jain, Jain and McCouch2004; Gao et al., Reference Gao, Zhang, Chang, Jia, Qiu and Dong2005; Thomson et al., Reference Thomson, Septiningsih, Suwardjo, Santoso, Silitonga and McCouch2007; Reference Thomson, Polato, Prasetiyono, Trijatmiko, Silitonga and McCouch2009). Recently, an analysis of genetic diversity in the rice germplasm of NE India has been carried out using microsatellite markers (Choudhury et al., Reference Choudhury, Khan and Dayanandan2013; Das et al., Reference Das, Sengupta, Parida, Roy, Ghosh, Prasad and Ghosh2013). These studies have reported a high level of genetic diversity in the subsets of NE Indian rice landraces. However, an analysis of the genetic structure of Chakhao rice germplasm is yet to be carried out, which is essential for the systematic conservation of these landraces. The current study was carried out to determine the level of genetic diversity in a set of 37 Chakhao rice accessions collected from the state of Manipur, NE India, using 47 microsatellite markers.
Materials and methods
Seed collection and conservation
Seeds from 37 Chakhao rice cultivars were collected from the districts of Manipur, India, during a collection trip from 4 to 13 November, 2011. Seeds were collected directly from the fields in each district (Fig. 1). In a few cases, freshly harvested seeds were collected from the farmers. The farmers were interviewed to gather information on the naming, use and other characteristics of the specific cultivars. The seeds of the rice accessions used in this study are conserved in the National Genebank of National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India, and are publicly available for research purposes upon request and agreeing to an material transfer agreement (MTA).

Fig. 1 Geographical distribution of the sampled sites in Manipur. A point indicates a site where collection has been made.
Simple sequence repeat (SSR) genotyping
Five-day-old rice seedlings germinated from five well-developed seeds from a single plant of each accession were used for total genomic DNA extraction with a plant DNA extraction kit following the manufacturer's protocol (Qiagen, CA, USA). Forty-seven SSR primer pairs distributed across the rice genome were designed from the Gramene marker database (http:\\www.gramene.org\markers\microsat\). The name, chromosomal location, motif and annealing temperature of the SSR markers are given in Supplementary Table S1 (available online). Polymerase chain reactions (PCRs) were carried out using a mixture of total volume of 25 μl consisting of 50 ng of genomic DNA, 0.2 mM of dNTPs, 2.5 mM of MgCl2, 2.5 μl of 10 × PCR Buffer, 2.5 pmol of forward and reverse primers, and 0.6 U of Taq DNA polymerase on a thermocycler (Mastercycler; Eppendorf, Hamburg, Germany). The following PCR protocol was used: a denaturation period of 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at a particular annealing temperature and 30 s at 72°C, and then 7 min at 72°C for final extension.
The PCR products were resolved in ethidium bromide (10 mg/ml)-stained 2% agarose gels. The molecular sizes (in nucleotide) of the amplified alleles were determined based on their migration relative to the 50 bp DNA Step ladder (Promega, Madison, WI, USA) using the PyElph-1.4 gel image analysis software (Pavel and Vasile, Reference Pavel and Vasile2012). The band sizes of each marker were checked using the Gramene database (www.gramene.org\markers\microsat\). The band with the lowest molecular weight for each SSR marker was assigned allele number 1 and the progressively heavier bands were assigned numbers incrementally. For an individual marker, the genotypes were scored as homozygous/heterozygous for particular allele(s).
Data analysis
Genetic parameters such as observed number of alleles (A), average number of alleles per locus (N a), effective number of alleles (N e), polymorphic information content (PIC), percentage of polymorphic loci, observed heterozygosity (H o), expected heterozygosity (H e), Shannon's diversity index (I) and number of private alleles were calculated using the PopGene (version 1.31, Yeh et al., Reference Yeh, Yang and Boyle1999) and GenAlEx (version 6.5, Peakall and Smouse, Reference Peakall and Smouse2012) software packages. Pairwise genetic differentiation (F ST) values among the subpopulations were estimated and an analysis of molecular variance (AMOVA) of the rice populations was carried out to partition the levels of genetic diversity among regions (valleys and hills) and among and within Chakhao types using the GenAlEx software. For drawing the dendrogram, genetic distance was calculated using the Chord distance matrix (Cavalli-Sforza and Edwards, Reference Cavalli-Sforza and Edwards1967), followed by tree construction using neighbour-joining as implemented in the NTSYS-pc software (version 2.1, Rohlf, Reference Rohlf2000). Principal coordinate analysis (PCoA) was carried out using the genetic distance matrix calculated in the GenAlEx software.
The population structure of Chakhao rice accessions was assessed using the model-based (Bayesian clustering) method implemented in STRUCTURE version 2.3.4 (Pritchard et al., Reference Pritchard, Stephens and Donnelly2000). The number of subgroups (SGs) (K) was set from 1 to 9 based on models characterized by admixture and correlated allele frequencies. For each K, ten runs were performed separately, with 1,00,000 iterations carried out for each run after a burn-in period of 1,00,000 iterations. The number of clusters (K) was set from 1 to 9 with ten independent runs. The ‘Structure Harvester’ (version 6.0, Earl and von Holdt, Reference Earl and von Holdt2011) was used to summarize the STRUCTURE results and to find the optimum K value implementing the parameters of Evanno et al. (Reference Evanno, Regnaut and Goudet2005). The ΔK value was based on the change in the log probability of the data between successive K values.
Results
A wide range of variations were observed in the grain morphology of the collected aromatic rice landraces of Manipur (Fig. 2). All the known aromatic rice landraces such as Chakhao Poireiton, Chakhao Amubi and Chakhao Angouba (as recognized by the farmers) were collected along with other Chakhao types such as Buhman, Maklei, Chakhao Phou and Napnang Hangmei. Variations in grain shape, size and aroma are given in Table 1.

Fig. 2 Variation in the grain morphology of different Chakhao rice landraces collected from Manipur state of North-east India.
Table 1 List of the 37 Chakhao rice accessions of Manipur used in the current study

IC, indigenous collection; NA, not available.
a Determined following Cruz and Khush (Reference Cruz, Khush, Singh, Singh and Khush2000).
b Detected following Nagaraju et al. (Reference Nagaraju, Mohanty, Chaudhary and Gangadharan1991).
Genetic diversity
The whole set of 47 SSR markers detected a total of 237 alleles across 37 Chakhao rice accessions (Supplementary Table S2, available online) with an average of 5.04 alleles per locus (size ranging from 73 to 343 bp). The highest number of alleles was scored at the locus RM552 (11 alleles) and the lowest was scored at the loci RM55 and RM178 (two alleles each). The PIC values varied from 0.317 (RM178) to 0.868 (RM552) with an average of 0.63. High gene diversity (H e) was observed for several SSR loci such as RM552 (0.892), RM240 (0.874) and RM80 (0.837). Among the 237 identified alleles, 69 were private; that is, a given allele was identified in only one accession (Table 2). The highest number of private alleles was observed in Chakhao (mixed) population (29) followed by Amubi (14) and Maklei (11).
Table 2 Genetic diversity parameters of different types of Chakhao rice varietiesa
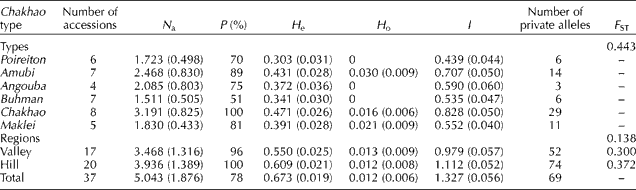
N a, average number of alleles per locus; P, percentage of polymorphic loci; H e, expected heterozygosity (Nei's gene diversity); H o, observed heterozygosity; I, Shannon's information index; F ST, genetic differentiation.
a Numbers in parentheses indicate standard deviation.
Judging from the overall genetic parameters (Table 2), considerable genetic diversity was found among the Chakhao landraces (H e= 0.673, I= 1.327). Genetic diversity was not uniformly distributed across the populations. The highest level of genetic diversity was found in Chakhao (mixed) population (H e= 0.471, I= 0.828), whereas the lowest was recorded in Poireiton (H e= 0.303, I= 0.439). Considering the regions (valley and hill) from where the rice accessions were collected, the largest genetic diversity was recorded in hilly regions (data not shown). F ST among the Chakhao types (F ST= 0.443) was higher than that among the regions (F ST= 0.138). F ST values among the populations of valleys and hills were similar to F ST values 0.300 and 0.372, respectively. The AMOVA results revealed statistically significant differentiation with 10% variation among the regions, 31% among the Chakhao types and 59% within the types (Table 3).
Table 3 Results of the analysis of molecular variance based on the 47 SSR loci of 37 Chakhao rice accessions

df, Degree of freedom; MS, mean sum of squares; CV, variance component estimates; % Total, percentage of total variation.
Genetic cluster
The genetic distance-based neighbour-joining-based phylogeny divided the Chakhao germplasm into two broad groups (Fig. 3(a)). The Poireiton and Amubi accessions having black kernel were genetically similar and grouped in Cluster 1. The Angouba and Buhman accessions possessing white/red kernels grouped in Cluster 2. During the collection trip, some ambiguities were observed in the classification of Angouba and Buhman cultivars by the farmers of Churachandpur district. Cluster 1 also included two Angouba accessions. The Maklei and Manui Maa and Napnang Hangmei, Chakhao Phou, and Kathaibuw accessions formed subclusters within Cluster 1. Similarly, the PCoA using pairwise genetic distances revealed a clear separation of the Poireiton, Amubi, Angouba and Buhman accessions from the other Chakhao cultivars (Fig. 3(b)), similar to that in the cluster analysis. The results of cluster analysis and PCoA revealed a significant population structure in the Chakhao germplasm.

Fig. 3 Genetic structure of the Chakhao rice accessions. (a) Dendrogram of 37 Chakhao accessions by neighbour-joining clustering; (b) principal coordinate analysis based on variation in 47 simple sequence repeats (SSR); and (c) six subgroups (SGs) inferred from the STRUCTURE analysis. The vertical of each SG indicates the membership coefficients (Q). The SGs (1 to 6) are indicated on the top of the bar plot.
Population structure
An analysis of the population structure of the 37 Chakhao landraces identified the most appropriate grouping with six SGs. The number of SGs (K) was identified based on the ΔK versus K plot, which showed an evident knee at K= 6 (Supplementary Fig. S1, available online). Using a membership probability threshold of 0.60, eight accessions (Chakhao Phou, Napnang Hangmei, Chakhao and Maklei) were assigned to SG1, three accessions (Angouba and Pungdol Amubi) to SG2, nine accessions (Buhman, Langphou Angouba and Chakhao) to SG3, eight accessions of Poireiton and Amubi to SG4, four accessions of Amubi to SG5, and four accessions (three Maklei and one Manui Maa) to SG6 (Fig. 3(c)). The STRUCTURE results indicated that the inferred population structure mostly correlated with the farmers' classification of the Chakhao landraces. The assignments of the 37 Chakhao accessions to six SGs derived from STRUCTURE were consistent with their grouping in the neighbour-joining tree.
Discussion
The current study estimated the genetic relatedness among 37 Chakhao rice landraces from Manipur state of NE India using 47 SSR markers and analysed the population structure among the landraces. The PCR amplification results with all the markers are consistent with those reported previously (Garris et al., Reference Garris, Tai, Coburn, Kresovich and McCouch2005). The N a value obtained in the current study was 5.04. This value is lower when compared with a value of 7.8 across a set of 52 aromatic rice cultivars from India (Jain et al., Reference Jain, Jain and McCouch2004). The N a value obtained in the current study was also lower than that reported across diverse sets of global rice accessions (11.9, Xu et al., Reference Xu, Beachell and McCouch2004; 13.0, Thomson et al., Reference Thomson, Septiningsih, Suwardjo, Santoso, Silitonga and McCouch2007; and 12.4, Borba et al., Reference Borba, Brondani, Rangei and Brondani2009). Recently, Choudhury et al. (Reference Choudhury, Khan and Dayanandan2013) and Das et al. (Reference Das, Sengupta, Parida, Roy, Ghosh, Prasad and Ghosh2013) have reported higher N a values (13.57 and 7.9, respectively) across sets of indigenous rice cultivars of eastern and NE Indian states. However, the N a value obtained in the current study is comparable to that (5.5) recorded in a set of 183 rice accessions collected from 18 villages on the island of Borneo, Indonesia (Thomson et al., Reference Thomson, Polato, Prasetiyono, Trijatmiko, Silitonga and McCouch2009). The average PIC value (0.63 per marker) was similar to the value (0.66) reported within a set of traditional and improved rice varieties of Indonesia (Thomson et al., Reference Thomson, Septiningsih, Suwardjo, Santoso, Silitonga and McCouch2007). A similar PIC value (0.6) was also recorded by Jain et al. (Reference Jain, Jain and McCouch2004) across a set of aromatic rice cultivars distributed throughout India using 30 SSR markers. Das et al. (Reference Das, Sengupta, Parida, Roy, Ghosh, Prasad and Ghosh2013) recorded an average PIC value of 0.57 within the rice accessions collected from different NE Indian states using 23 trait-linked SSR markers.
In the current study, the highest gene diversity (H e) value was recorded in the subpopulation of Chakhao (mixed) landraces (0.47) and the lowest value was observed in that of Poireiton landraces (0.303). It is obvious that gene diversity in the Chakhao (mixed) subpopulation was due to the inclusion of different Chakhao landraces within this group. Higher H e values were also recorded in both Amubi and Maklei subpopulations. The Chakhao landraces had a lower gene diversity value when compared with the value of 0.78 across a set of indigenous rice landraces of NE India. However, the value was higher than that recorded in agronomically improved varieties (0.46) of the region (Choudhury et al., Reference Choudhury, Khan and Dayanandan2013). Although the Chakhao landraces represent a subset of total genetic diversity in NE India, the average gene diversity recorded in the current study was at par with that recorded across diverse sets of rice germplasm of Indonesia (0.68, Thomson et al., Reference Thomson, Septiningsih, Suwardjo, Santoso, Silitonga and McCouch2007) and China (0.71, Tu et al., Reference Tu, Lu, Zhu and Wang2007) and across sets of global rice collections (0.55, Garris et al., Reference Garris, Tai, Coburn, Kresovich and McCouch2005; 0.64, Borba et al., Reference Borba, Brondani, Rangei and Brondani2009). The gene diversity value obtained in the current study was higher than that recorded in a set 183 rice landraces (0.49) from the island of Borneo, Indonesia (Thomson et al., Reference Thomson, Polato, Prasetiyono, Trijatmiko, Silitonga and McCouch2009). Generally, the cultivars sampled from a small geographical region would be inherently less diverse as they are grown in a similar set of environmental conditions, whereas global collections of germplasm being sampled from a wider range of geographically and ecologically distinct regions will exhibit greater genetic diversity due to divergent selection pressures. The high genetic diversity in the current set of 37 Chakhao accessions may be a reflection of the prevalent diverse agro-climatic conditions and diverse cultural practices followed by the farmers of the region. However, further characterization of more landraces from this region would help in establishing this association. In general, the genetic diversity maintained in a plant species is considered a function of its ecological and evolutionary history (Hamrick and Godt, Reference Hamrick, Godt, Avise and Hamrick1996). The conservation of high gene diversity within the Chakhao landraces could be the outcome of traditional farming systems and varied human preferences in Manipur.
The results based on both F ST and AMOVA confirmed that a major genetic variation exists within different types of Chakhao rice landraces. A moderately high estimate of F ST (0.443) considering the Chakhao types indicated less admixed ancestry among the cultivars. However, when analysing F ST for the regions, a value of 0.138 indicated the presence of admixed ancestry within the regions. Similarly, Thomson et al. (Reference Thomson, Polato, Prasetiyono, Trijatmiko, Silitonga and McCouch2009) reported a lack of geographical differences within rice landraces collected from three different regions within the island of Borneo, Indonesia. It was also proposed that the lack of seed exchange among the traditional farmers has prevented any significant genetic differences occurring over time. The F ST results are also supported by the AMOVA results, which indicated that 31% of the total variation was due to differentiation among the Chakhao types. It is to be noted that 59% of the total variation is explained by differentiation within the types, i.e. among the different Chakhao landraces. Similarly, Choudhury et al. (Reference Choudhury, Khan and Dayanandan2013) also reported that differentiation among rice varieties explained 66% of the total genetic variation in a set of diverse rice genotypes comprising indigenous cultivars, agronomically improved varieties and wild relatives from NE India.
The cluster analysis revealed obvious grouping of the Chakhao accessions corresponding to different cultivar types. The black-kernelled landraces such as Chakhao Poireiton and Chakhao Amubi had a close genetic relationship, whereas white-kernelled Angouba landraces were genetically similar to the Buhman accessions having white or red kernels. Both cluster analysis and PCoA showed a clear grouping of Poireiton, Amubi, Angouba and Buhman. The other Chakhao cultivars such as Maklei, Napnang Hangmei, Chakhao Phou and Chakhao were found to be genetically diverse from the major Chakhao cultivars such as Poireiton, Amubi, Buhman and Angouba. Population structure is an important component of association analyses between molecular markers and traits. All the three population structure approaches, neighbour-joining phylogeny, PCoA and STRUCTURE analysis, revealed a significant genetic structure within the Chakhao accessions.
During the collection trip, attempts were made to collect all the available Chakhao cultivars from the state. Multiple accessions of each cultivar with the same name were collected with the idea that each accession is inherently valuable for the conservation of rice germplasm. It was found that the landraces with the same name collected from adjacent areas were often genetically similar and could be considered members of the same cultivar, while this was not true for cultivars with the same name collected from distant areas (Thomson et al., Reference Thomson, Polato, Prasetiyono, Trijatmiko, Silitonga and McCouch2009). The current study showed that the farmers, who are the custodians of the traditional landraces, are very efficient at conserving different cultivars within a small geographical region.
Conclusions
In conclusion, the high gene diversity detected among various Chakhao cultivars is comparable to that detected in rice populations in various parts of the world. There is also a pronounced genetic structure among different types of Chakhao, which could be associated with limited gene flow and introgression with different rice varieties, mainly due to geographical isolation of the state of Manipur. The current study demonstrated that the traditional farmers of Manipur are efficiently preserving several types of aromatic rice varieties within a small geographical region over time. The genetic characterization of these landraces, which are a set of dynamic gene pool of traditional varieties adapted to the local environment and in close association with human cultural preferences, offers an important foundation for conserving these valuable genetic resources and exploiting genetic diversity for improving rice varieties.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262113000580
Acknowledgements
This study was funded by the Indian Council of Agricultural Research (ICAR). The authors are grateful to the farmers of Manipur for providing necessary information and seed samples used in this study. They also thank the scientific staff of ICAR Research Complex for NEH Region, Manipur, particularly N Prakash, G Singh and LT Monsang.