INTRODUCTION
To accomplish the goal of controlling uncomplicated loco-regional cancer, radiation therapy is an important treatment. Simultaneous integrated boost intensity-modulated radiotherapy (SIB IMRT) introduced by the Medical College of Virginia, USA allows treatment of the tumour in a single session at different dose levels resulting in dose escalation to treatment site.Reference Fogliata, Bolsi, Cozzi and Bernier 1 Treatment planning using SIB IMRT technique, as a single-phase planning technique, is emerging as a standard technique for treatment of head and neck (H&N) cancer. Conventional radiotherapy techniques could not offer significant tissue sparing for treatment of H&N carcinoma. SIB IMRT offers the best solution for preserving organ function by keeping dose below tolerance levels for organs at risk (OARs). The majority of H&N carcinomas are biologically similar and originate in oral cavity, lips, parotid glands, larynx and pharynx or nasal cavity. Smoking, excessive exposure to ultraviolet radiation and alcohol consumption are a few of the factors that are associated with the development of H&N cancer.Reference Caraman, Buzea, Ojica, Oprea, Zara and Iancu 2 In this study, treatment site is in the H&N region consisting of critical organs such as spinal cord, brain stem, optic chiasm, optic nerves and parotid glands. If the dose to the bilateral parotid glands, the most significant of the three main areas of salivary tissue, is exceeded above tolerance limit it may result in xerostomia.Reference Grundmann, Mitchell and Limesand 3
Radiation oncologists must have a comprehensive knowledge of tolerance dose (TD) to OARs to minimise discomfort to patients. Guidelines regarding tolerance to normal tissues, published by Rubin and CassarettReference Rubin and Casarett 4 some four decades ago, are still considered the standard practice in radiation therapy. To express the tolerances of normal tissues, dominant concept of TD 5/5 and TD 50/5 are still in practice. The probability of 5 and 50% treatment-related complications within 5 years from treatment are regarded as TD 5/5 and TD 50/5, respectively.Reference Emami, Lyman and Brown 5 OARs are allocated as serial organs (such as spinal cord and brain stem) and parallel organs (such as parotid glands). If any portion of serial organs receives radiation dose above the threshold, then there will be complete loss of organ function. For instance, a radiation dose to spinal cord beyond its threshold value may result in paralysis. In a serial structure it is necessary to ensure that the volume of organ does not receive dose above its threshold. As for parallel organs, if any portion of organ is seriously damaged by treatment, then the remaining part of the organ will continue to function. To quantify probability of complications, it is recommended to employ maximum dose for serial organs and mean dose for parallel organs.Reference Jones 6 , 7
The intensity-modulated radiotherapy (IMRT) treatment planning process can be summarised in three points. First, delineation of tumour and OARs. Second, treatment planning using system of constraints and priorities for OARs and planning target volume (PTV) to obtain the plan that may be deemed satisfactory. Lastly, there is the requirement to ensure that there is a of patient-specific quality assurance system in place to ensure accuracy of treatment plan and patient safety.Reference Gomez-Millan, Fernández and Carmona 8 For the purpose of achieving SIB IMRT plans, it is crucial that each step of treatment planning be performed on time and corrections implemented promptly. In clinical situations, it is critical to provide full dose coverage to the target volume. In the organs adjacent to the tumour, which ideally should be spared, are sacrificed and receive radiation dose that results in tissue damage and ultimately treatment side effects in the patient. Therefore, when producing an optimum treatment plan, fulfilment of dose requirements are assessed by various indices. As per the requirements of modern radiotherapy, the 95% isodose should cover PTV, so dosimetric indices are used for evaluating quality of treatment plans. Homogenous and conformal dose distribution in treatment plans lead to better dose distribution.
In order to prove the quality of treatment plans using the SIB IMRT technique; this study aims to analyse dosimetric indices, such as conformity index, homogeneity index (HI), in addition, to study the overall dose to OARs and how this dose relates to TDs, in patients treated for H&N cancer.
MATERIALS AND METHODS
In this study, 15 patients were enrolled at Shaukat Khanum Memorial Cancer Hospital and Research Centre for treatment of H&N cancer using SIB IMRT technique. Detailed dosimetric data with seven female and eight male patients in age range from 30 to 64 were analysed in this study. Patients were considered eligible, if they had pathologically confirmed pT4 pN1 carcinomas of the larynx. The selection criteria used included age >20 years. 60 Gy was delivered to gross disease and 54 Gy were delivered to PTV.
Each patient underwent computed tomography simulation with immobilisation using custom thermoplastic mask, in supine position, acquired with slice thickness of 3 mm. Treatment plans of SIB IMRT were computed on Eclipse Treatment Planning System (Varian Medical Systems, Palo Alto, CA, USA) for 6 MV beam as represented in Figure 1. Our study included two dose levels of 70 and 55·4 Gy. Doses of 2 Gy in 35 fractions and 1·68 Gy in 33 fractions were delivered for initial treatment volume known as effective PTV (PTV1) and followed by simultaneous boost dose delivered to boost PTV (PTV2), respectively. High dose of 54 Gy to PTV is considered satisfactory for elimination of disease at microscopic level. However, to eradicate disease from high risk area a boost dose is delivered for definitive elimination of disease. All SIB IMRT plans included seven fields and gantry angle was fixed at 0, 51, 102, 153, 204 and 255° delivered by DHX Clinac (Varian Medical Systems) equipped with 120-leaf Multileaf Collimator. Cumulative dose volume histogram (cDVH) generated by treatment planning system provided a wealth of information about dose delivered to PTV1 and PTV2, and normal tissues. A steep drop of isodose lines in cDVH depicts perfect homogenous distribution as discussed in the literature.Reference Pathak and Vashisht 9 Inverse planning was chosen for this study to create SIB IMRT plans which involved a trial and error process to find the best treatment plan with proper dose constraint specifications. The plan is optimised such that serial and parallel organs do not lose their functionality. In an attempt to cover target volume, the radiation oncologist must accomplish the challenging task to carefully consider plans with the objective of assuring that dose to dose limiting organ such as spinal cord and quality of life limiting organ such as parotid gland,Reference Cozzi, Fogliata, Lomax and Bolsi 10 remain well below tolerance levels.

Figure 1 Representation of seven field treatment plan of head and neck patient with 6 MV beam measured with Eclipse Treatment Planning System.
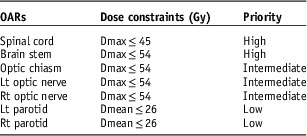
An investigation of doses delivered to normal organs was undertaken in this study. TDs given in Table 1 are based on a comprehensive review of the literature.Reference Fogliata, Bolsi, Cozzi and Bernier 1 , Reference Ang, Zhang and Rosenthal 11 – Reference Scorsetti, Fogliata and Castiglioni 18 Dose constraints and priorities of organs for treatment planning are in accordance with Danish Head and Neck Cancer Group (DAHANCA) (13–14) as represented in Table 1.
Table 1 Dose constraints and plan acceptance priority of organs at risk (OARs) for head and neck cancer

To analyse quality of treatment plans, this was done by using dose painting IMRT technique; radiation conformity index (RCI), HI and coverage were calculated. Ideal values and acceptable deviations of above mentioned indices are depicted in Table 2.
Table 2 Ideal values and acceptable deviation of commonly used indices in radiotherapy

Abbreviations: RCI, radiation conformity index; HI, homogeneity index.
The concept of the conformity index was proposed by Radiation Therapy Oncology Group (RTOG) protocol,Reference Shaw, Kline and Gillin 19 which was extended by Knoos et al.Reference Knöös, Kristensen and Nilsson 20 referred to as RCI. The dose coverage calculated in the present study is defined as the ratio of Dmin with target volume to prescribed dose.Reference Krishna, Srinivas, Ayyangar and Reddy 21 The plan is considered acceptable if target volume completely covers 90% of prescription isodose. There will be a minor deviation if 80% of prescribed dose encompass target volume. A major deviation is considered below the coverage of 80% of target volume.Reference Murphy, Chang, Gibbs, Le, Martin and Kim 22 However, most clinical practices consider±10% as an acceptable deviation.Reference Das, Cheng, Chopra, Mitra, Srivastava and Glatstein 23 The HI used in this study is referred to as the ratio of maximum dose to prescription dose.Reference Shaw, Kline and Gillin 19 It is defined as the ratio of maximum dose to target volume to prescribed dose as per RTOG protocol.Reference Kataria, Sharma, Subramani, Karrthick and Bisht 24 The treatment plan is deemed acceptable for a value of HI≤2. Plans having values between 2 and 2·5 show minor deviation and values of HI>2·5 suggest major deviation( Reference Pathak and Vashisht 9 , Reference Shaw, Kline and Gillin 19 , Reference Murphy, Chang, Gibbs, Le, Martin and Kim 22 , Reference Kataria, Sharma, Subramani, Karrthick and Bisht 24 ). Homogenous dose distribution is necessary to avoid radiation-induced toxicity.Reference Lu, Cheung, Li, Huang, Xie and Xie 25
RESULTS
For each plan, SIB IMRT technique was undertaken to qualitatively evaluate conformity and homogeneity indices and quantitatively evaluate minimum, maximum and mean doses to OARs. Statistical description of data was carried out in terms of mean±SD. Dose delivered to normal tissues for each contoured structure in terms of mean and maximum point doses is expressed in Table 3. Results of this study revealed that average dose of 15 patients to spinal cord was below tolerance level and maximum dose to this site was on average 43 Gy. For complicated H&N carcinoma PTV’s, doses to high priority series organs remained well below tolerance limits. With increase of OAR priority from low to high, decrease in dosimetric indices and doses to OARs was observed. Results demonstrate that doses to series organs spinal cord, optic nerve and chiasm were well within tolerance levels and are depicted in Figure 2.

Figure 2 Average of maximum doses to series organs for head and neck cancer.
Table 3 Summary of mean doses to organs at risk for simultaneous integrated boost intensity-modulated radiotherapy technique

The goal of keeping doses to bilateral parotid glands within tolerance levels was achieved in some treatment plans, except in eight cases in which dose was slightly higher than tolerance levels, shown in Figure 3.

Figure 3 Mean doses to parallel organs for 15 head and neck cancer patients.
Results of homogeneity, RCI and coverage are presented in Table 4, to demonstrate the variation in the results for the clinical treatment plan to that of an ideal pan. Conformity index for all the patients remained within limits as suggested by protocol for both PTVs. It is fair to assume that homogenous dose distribution of our treatment plans led to better treatment outcomes. For an objective evaluation of the plan’s values of RCI and HI, the results are presented and analysed for both PTV1 and PTV2 as depicted in Figures 4 and 5.

Figure 4 Graph of radiation conformity index (RCI) for effective planning target volume (PTV1) and boost planning target volume (PTV2).

Figure 5 Graph of homogeneity index (HI) for effective planning target volume (PTV1) and boost planning target volume (PTV2).
Table 4 RCI, HI and coverage of effective target volume PTV1 and boost volume PTV2 for SIB IMRT plans

Abbreviations: RCI, radiation conformity index; HI, homogeneity index; PTV1, effective planning target volume; PTV2, boost planning target volume; SIB IMRT, simultaneous integrated boost intensity-modulated radiotherapy technique.
DISCUSSION
It has been suggested by several investigators that dose delivery using SIB IMRT fractionating scheme has an ability to develop much superior dose distributions in which the radiation doses are delivered in same number of fractions for initial and boost fields.Reference Wu, Manning, Schmidt-Ullrich and Mohan 26 , Reference Ling, Burman and Chui 27 Several studies document benefits of dose shrinking technique for heterogeneous H&N tumours thus fulfilling patient-specific quality assurance requirements.Reference Sakthi, Keall and Mihaylov 28 , Reference Yang, Zeng and Qiu 29 A detailed review about the effect of TD to normal tissues was published in proceedings of 1992.Reference Cox, Stetz and Pajak 30 The classical review by Emami et al.,5 in 1991 laid the foundation of TDs for normal tissues in radiotherapy. They reported the maximum limit of dose to spinal cord around 45–50 Gy. In a study by Jun Won Kim et al.,Reference Kim, Cho and Keum 31 authors calculated the maximum tolerated dose to spinal cord, optic chiasm and brain stem as 41·1, 37·9 and 50·8 Gy, respectively. The brain stem is more prone to radiation damage than the cerebrum as expressed by BodenReference Boden 32 in his study. The results of our study clearly demonstrate that the dosimetric results of SIB IMRT are within reported tolerance limits for OARs.
Several studies reported mean dose of 24±4 Gy to bilateral parotid supporting safe sparing of this organ.Reference Deasy, Moiseenko, Marks, Chao, Nam and Eisbruch 33 – Reference Chao, Deasy and Markman 35 However, threshold dose of 26 Gy proposed by Eisbruch et al.Reference Eisbruch, Ten Haken, Kim, Marsh and Ship 36 preserved saliva flow rate thus improving quality of life. A group at the University of Michigan studied the effects of doses on parotid functioning by directly measuring stimulated and unstimulated salivary flow from each parotid gland. They concluded better conservation of parotid glands when the mean doses to these organs were kept below 24–26 Gy.Reference Eisbruch, Ten Haken, Kim, Marsh and Ship 36 – Reference Eisbruch, Ship and Dawson 38 Clinical studies suggest 50% of parotid volume should be outside the radiation field to prevent the occurrence of the xerostomia.Reference Milano, Constine and Okunieff 39 – Reference Mira, Wescott, Starcke and Shannon 40 Sparing of these glands is strongly recommended and is dependant on their complete or partial inclusion in the target volume.Reference Fogliata, Bolsi, Cozzi and Bernier 1 A study by El-Ghoneimy et al.,Reference El-Ghoneimy, Hassan, El-Bestar, Othman and Mashhour 16 reported a mean dose of 24·28 Gy to parotid glands, in their analysis of treatment plans, revealed that a mean dose to bilateral parotid glands, for 47% cases do not fall within tolerance limits, which was due to the complex anatomy and large number of OARs in the vicinity of tumour site making planning of H&N cancer a challenging task. As per RTOG H0225 protocol,Reference Lee, Harris and Garden 41 minor deviation was observed for TD to parotid glands. This was due to tumour reformation or motion or both during treatment delivery.Reference Bjørndal, Krogdahl and Therkildsen 14 , Reference Kristensen, Nilsson and Nilsson 42 Dose limit for both glands was reached in eight patients in this present study, due to overlapping of target volume with the parotids. However, coverage of the target volume is the prime concern while keeping the dose to parotid glands as low as is achievable. Complications in the treatment of H&N cancer are due to the emergence of cold spots and hotspots, which represent minimum and maximum doses within the target volume, respectively.Reference Lu, Zhang and Li 43 , Reference Süss, Bortz, Küfer and Thieke 44
A literature survey highlights the importance of shrinking field IMRT technique over three-dimensional conformal radiotherapy and RapidArc techniques.Reference Daoud, Saleh and Habash 15 , Reference El-Ghoneimy, Hassan, El-Bestar, Othman and Mashhour 16 Based on published data it was observed that SIB not only provides a high conformal dose distribution and better coverage to the target volume but also protect susceptible organs.Reference Peszynska-Piorun, Malicki and Golusinski 45 – Reference Mohan, Wu, Manning and Schmidt-Ullrich 47 Doses to low risk PTV and boosted high risk PTV are delivered in a single plan with different doses per fraction in contrast to sequential boost (SEQ) technique. A comprehensive evaluation of previous studies suggests quality assurance results of dose painting in IMRT technique, to be better in terms of dosimetry planning then SEQ.Reference Khayaiwong, Tungboonduangjit and Suriyapee 48 – Reference Chen, Yang, Liang, Shiau and Lin 50 Improvement on the HI and RCI in our study as compared with values of dosimetric indices of a previous study, proved superiority of SIB IMRT plans over non SIB IMRT plans in H&N cancer.Reference Shamsi, Atiq, Atiq, Buzdar and Iqbal 51 Any deviation of RCI values from ideality propose over-treatment or under-treatment of target volume. Both over-treatment and under-treatment are detrimental as the former may result in acute reactions in normal cells and later increases the likelihood of tumour recurrence.Reference Lu, Cheung, Li, Huang, Xie and Xie 25
Investigation of H&N cancer radiotherapy using SIB IMRT, proves that highly conformal and homogenous dose distribution as well as better sparing of OARs is achieved, thus verifying quality assurance results to be satisfactory. Treatment of H&N carcinoma using SIB IMRT is feasible, more efficient, and dose escalation is achieved in a single plan.
CONCLUSION
This study was intended to assess quantitative dosimetric indices such as RCI, HI and coverage to PTV along with doses to critical organs for 15 H&N cancer patients. Our results confirm accuracy and efficiency of SIB IMRT to provide satisfactory target coverage and produces a highly conformal dose distribution to the target volume with significant sparing of OARs, which include nervous system and salivary glands. Minor deviation of results of dose to the parotid glands in a few cases, from defined guidelines were probably due to tumour reformation or motion or both during treatment delivery. SIB IMRT is found well tolerated and safe using doses of 70 and 55·4 Gy. In summary, good coverage to treatment site, homogeneous dose distribution within target volume and dose conformity near target volume was achieved and at the same time maintaining dose to normal tissues, well within tolerance limits. The future of IMRT using dose painting lies in exploring more patient’s treatment plans and identifying vital features for advancement in patient treatment and care.
Acknowledgement
Manuscript submitted is original work and all others participated in the work in a substantive way. All authors have seen and approved the manuscript as submitted.
Conflicts of Interest
All authors have no conflicts of interest.
Financial support
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee Shaukat Khanum Memorial Cancer Hospital and Research Centre.