Temper outbursts are common in young children (Wakschlag et al., Reference Wakschlag, Choi, Carter, Hullsiek, Burns, McCarthy and Briggs-Gowan2012); however, when they are severe and chronic, they can interfere with children's well-being and functioning in multiple domains, including family, peer, and teacher relationships, and may even precipitate psychiatric hospitalization (Brotman et al., Reference Brotman, Schmajuk, Rich, Dickstein, Guyer, Costello and Leibenluft2006; Carlson, Potegal, Margulies, Gutkovich, & Basile, Reference Carlson, Potegal, Margulies, Gutkovich and Basile2009; Leibenluft, Cohen, Gorrindo, Brook, & Pine, Reference Leibenluft, Cohen, Gorrindo, Brook and Pine2006). Severe temper outbursts (STO) are transdiagnostic, contributing to functional impairments in children with disruptive behavior disorders (Bhatia, Nigam, Bohra, & Malik, Reference Bhatia, Nigam, Bohra and Malik1991; Roy et al., Reference Roy, Klein, Angelosante, Bar-Haim, Leibenluft, Hulvershorn and Spindel2013) as well as neurodevelopmental (Dominick, Davis, Lainhart, Tager-Flusberg, & Folstein, Reference Dominick, Davis, Lainhart, Tager-Flusberg and Folstein2007; Malone, Gratz, Delaney, & Hyman, Reference Malone, Gratz, Delaney and Hyman2005) and anxiety disorders (Johnco et al., Reference Johnco, Salloum, De Nadai, McBride, Crawford, Lewin and Storch2015). In our own work, children clinically referred because of unmanageable temper outbursts most frequently presented with oppositional defiant disorder and attention-deficit/hyperactivity disorder (ADHD; Brotman et al., Reference Brotman, Schmajuk, Rich, Dickstein, Guyer, Costello and Leibenluft2006; Roy et al., Reference Roy, Klein, Angelosante, Bar-Haim, Leibenluft, Hulvershorn and Spindel2013). Several longitudinal studies of children with oppositional defiant disorder show that symptoms of irritability, including temper outbursts, are associated with elevated risk for later depressive and anxiety disorders (Burke, Hipwell, & Loeber, Reference Burke, Hipwell and Loeber2010; Rowe, Costello, Angold, Copeland, & Maughan, Reference Rowe, Costello, Angold, Copeland and Maughan2010; Stringaris, Cohen, Pine, & Leibenluft, Reference Stringaris, Cohen, Pine and Leibenluft2009; Stringaris & Goodman, Reference Stringaris and Goodman2009), highlighting their clinical importance. Little is known about the neurobiological basis of STO in young children, which could elucidate etiological trajectories of early emotion dysregulation and potentially inform preventive interventions and treatments.
Whether severe or not, temper outbursts typically occur in response to frustration. Thus, clinically significant outbursts likely reflect exaggerated frustration responses that involve crying, screaming, and yelling, as well as aggressive behaviors such as kicking, hitting, or running away (Giesbrecht, Miller, & Muller, Reference Giesbrecht, Miller and Muller2010; Potegal & Davidson, Reference Potegal and Davidson2003). Physiological studies support this dysregulated frustration response. For example, chronically irritable children with STO display significantly greater arousal in response to frustration than healthy comparisons (Rich et al., Reference Rich, Schmajuk, Perez-Edgar, Fox, Pine and Leibenluft2007). In addition to reflecting an elevated frustration response, evidence suggests that STO are associated, more generally, with poor emotional control (Gatzke-Kopp, Greenberg, & Bierman, Reference Gatzke-Kopp, Greenberg and Bierman2015). For example, when faced with frustration, children with STO exhibit deficits in negative affect regulation (Roy et al., Reference Roy, Klein, Angelosante, Bar-Haim, Leibenluft, Hulvershorn and Spindel2013) and respond relatively more slowly on an affective Posner task, implying deficits in attentional control of emotional responses (Deveney et al., Reference Deveney, Connolly, Haring, Bones, Reynolds, Kim and Leibenluft2013; Rich et al., Reference Rich, Schmajuk, Perez-Edgar, Fox, Pine and Leibenluft2007). Children rated high in temperamental anger by parents and teachers have difficulty shifting attention away from rewarding stimuli, further supporting impairments in attentional control, an aspect of emotion regulation that arises early in development (He et al., Reference He, Jin, Zhang, Huang, Shui and Shen2013).
From a neural perspective, frustration processing has been linked to multiple brain regions involved in negative affective responses, such as the anterior insula (Abler, Walter, & Erk, Reference Abler, Walter and Erk2005; Rilling et al., Reference Rilling, Goldsmith, Glenn, Jairam, Elfenbein, Dagenais and Pagnoni2008; Yu, Mobbs, Seymour, Rowe, & Calder, Reference Yu, Mobbs, Seymour, Rowe and Calder2014), amygdala (Rilling et al., Reference Rilling, Goldsmith, Glenn, Jairam, Elfenbein, Dagenais and Pagnoni2008; Yu et al., Reference Yu, Mobbs, Seymour, Rowe and Calder2014), and anterior cingulate cortex (ACC; Spunt, Lieberman, Cohen, & Eisenberger, Reference Spunt, Lieberman, Cohen and Eisenberger2012; Yu et al., Reference Yu, Mobbs, Seymour, Rowe and Calder2014). The ACC is of particular interest because it plays an integral role in neural networks involved in emotion regulation beginning early in development (for a review, see Swingler, Perry, & Calkins, Reference Swingler, Perry and Calkins2015). Specifically, dorsal regions of the ACC have been implicated in the use of attentional strategies, such as distraction, to regulate negative affective responses to visual stimuli (e.g., negatively valenced IAPS pictures) in studies of adults (Kanske, Heissler, Schonfelder, Bongers, & Wessa, Reference Kanske, Heissler, Schonfelder, Bongers and Wessa2011; McRae et al., Reference McRae, Hughes, Chopra, Gabrieli, Gross and Ochsner2010). Consistent with this, functional magnetic resonance imaging (fMRI) studies implicate dorsal ACC regions in frustration processing. For example, adults with high trait aggression exhibit decreased activation of dorsal ACC in response to frustration (Pawliczek et al., Reference Pawliczek, Derntl, Kellermann, Gur, Schneider and Habel2013), while children (ages 6–9) with clinically significant irritability exhibit similar decreases in activation in a more rostral region of the dorsal ACC (Perlman et al., Reference Perlman, Jones, Wakschlag, Axelson, Birmaher and Phillips2015). Alternatively, decreased responding in a similar region of the ACC has been observed in a nonclinical sample of adults with high susceptibility to frustration (Siegrist et al., Reference Siegrist, Menrath, Stocker, Klein, Kellermann, Shah and Schneider2005). Finally, in a magnetoencephalography study, a similar, but older, sample (ages 8–17) than presented here (ages 5–9.9) showed increased activity of rostral ACC, along with higher ratings of agitation and sadness, in response to negative feedback, compared to healthy controls (Rich et al., Reference Rich, Carver, Holroyd, Rosen, Mendoza, Cornwell and Leibenluft2011). Thus, while task-based studies provide clear evidence supporting the role of the ACC in processes presumed to underlie STO, frustration tolerance and emotion regulation, the direction of the findings, as well as their locations within the ACC, are inconsistent.
A recent coordinate-based meta-analysis of 192 task-based studies confirms the role of the ACC both in the subjective experience of negative affect and in cognitive control (Shackman et al., Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011). A conjunction analysis revealed a single cingulate subregion, the anterior midcingulate cortex (aMCC), with activation across both domains. The anatomic connectivity of this region supports its role in cognitive control and negative affective states such as frustration. For example, the aMCC has reciprocal connections with subcortical dopaminergic pathways, suggesting that it may receive information about punishment signals such as those involved in the experience of frustration (Shackman et al., Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011). As a key node of the salience network, the aMCC is also tightly connected with the anterior insula, a region implicated in affect and cognitive control. Finally, Shackman et al. (Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011) suggest that this region is linked to multiple motor centers that permit planning of motivated instrumental responses, such as temper outbursts.
We apply a broader network-based approach to understanding the role of the ACC in emotion dysregulation by using resting-state fMRI methods to evaluate the intrinsic functional connectivity (iFC) of an empirically derived aMCC region in young children (ages 5–9.9 years) with clinically impairing outbursts. While this approach may not directly address inconsistencies in the task-based literature, evidence of disruptions in the iFC of cingulate networks may inform comprehensive neural models that can be tested in future studies integrating activation paradigms and iFC approaches. In prior work, we found that the majority of children for whom STO are a primary concern do not exhibit chronic irritability (as defined as being in an irritable or angry mood more than 50% of the time; Roy et al., Reference Roy, Klein, Angelosante, Bar-Haim, Leibenluft, Hulvershorn and Spindel2013). Thus, the present study did not recruit children characterized by chronic irritability, as previous task-based studies of frustration responses have (Perlman et al., Reference Perlman, Jones, Wakschlag, Axelson, Birmaher and Phillips2015; Rich et al., Reference Rich, Carver, Holroyd, Rosen, Mendoza, Cornwell and Leibenluft2011), but rather focused on children with frequent, impairing outbursts. Further, our previous work suggests that a majority of young children with STO have ADHD (Roy et al., Reference Roy, Klein, Angelosante, Bar-Haim, Leibenluft, Hulvershorn and Spindel2013), which is characterized by altered function (Dickstein, Bannon, Castellanos, & Milham, Reference Dickstein, Bannon, Castellanos and Milham2006), structure (Makris et al., Reference Makris, Buka, Biederman, Papadimitriou, Hodge, Valera and Seidman2008; Seidman et al., Reference Seidman, Valera, Makris, Monuteaux, Boriel, Kelkar and Biederman2006), and connectivity (Castellanos et al., Reference Castellanos, Margulies, Kelly, Uddin, Ghaffari, Kirsch and Milham2008; Sun et al., Reference Sun, Cao, Long, Sui, Cao, Zhu and Wang2012) of the ACC. Therefore, to address the aim of the study to identify neural features associated with STO, we compare children with severe outbursts to children with ADHD who do not have severe outbursts. To distinguish from general effects of psychopathology, we included healthy comparisons, group-matched for age and sex. The emotional dysregulation of children with STO may reflect a specific dysfunction, or may be due to greater overall symptom severity. Therefore, we conducted dimensional analyses to examine emotion dysregulation and ADHD symptom severity in relation to aMCC iFC.
Methods
Participants
Boys and girls, ages 5–9.9 years (7.2±1.3; 21 female), were recruited across three groups: children with STO (85% with ADHD), children with ADHD without STO, and healthy comparisons. These children were recruited for two separate studies with identical entry criteria and imaging methods (n = 49 from Study 1, n = 24 from Study 2). Behavioral data from Study 1 (including 7 of the children in this study) have been published (Roy et al., Reference Roy, Klein, Angelosante, Bar-Haim, Leibenluft, Hulvershorn and Spindel2013). Across both studies, children with STO had to exhibit verbal rages and/or physical aggression toward people or property at least twice a week for at least 3 months. Outbursts had to (a) be characterized as out of proportion for the situation and the child's developmental level; (b) be of at least 15 min duration, during which the child was inconsolable; and (c) not occur exclusively during anxiety-provoking situations (e.g., in response to separation). Children in the ADHD group met DSM-IV criteria for ADHD without meeting criteria for STO. To ensure that the ADHD group was free of significant outbursts, children with ADHD who exhibited moderate outbursts (frequent and impairing, shorter in duration than STO) were excluded. Healthy controls (HC) were free of any major current DSM-IV diagnoses and of STO. Across all groups, participants were excluded if they displayed evidence of posttraumatic stress disorder, psychosis, or autism, IQ < 80 on the Kaufman Brief Intelligence Test (Kaufman & Kaufman, Reference Kaufman and Kaufman2004), or if there were any contraindications to MRI scanning (e.g., claustrophobia or braces). Current or past (within the past 3 months) psychotropic use was exclusionary except for stimulants, with the provision that they were withdrawn for 72 hr prior to the initial assessment and scan. Parents/legal guardians provided written informed consent as approved by the Fordham University and New York University Langone Medical Center institutional review boards and children provided written (ages 7–9) or verbal (ages 5–6) assent. A total of 73 children met criteria for study entry and were invited to complete scan procedures. Five of these failed the mock scan training session, making them ineligible for the functional scan (3 STO, 1 ADHD, 1 HC). An additional 12 failed the scan due to excessive movement (7 STO, 2 ADHD, 3 HC). Of note, 4 of these were scanned before mock scanning procedures were in place and, thus, did not have the benefit of a practice session. Overall, 56 children were included in the final sample.
Clinical assessment
Tantrum severity was determined by clinical interview across the two studies. In Study 1, parents completed a Child Emotion Dysregulation Interview that asked about frequency and duration of the child's outbursts. Parents were also asked to describe the child's most recent tantrum, a typical tantrum, and the most severe outburst that the child had experienced and to indicate how often he/she has outbursts. These responses were used to determine tantrum severity. In Study 2, we obtained the same information using a modified version of the Temper Tantrum Grid (Giesbrecht et al., Reference Giesbrecht, Miller and Muller2010) as a clinician-administered questionnaire. Parents answered the same questions about frequency and duration and were asked to describe a recent tantrum. A checklist of tantrum behaviors was provided that were rated on frequency of occurrence by the parent. For both studies, these data were then discussed during weekly diagnostic case conferences with the principal investigator (A.K.R.) and study group status was determined by consensus.
Diagnoses were determined by semistructured clinical interviews of parents using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997) conducted by a child psychiatrist, child psychologists, or trained clinical psychology doctoral students. Only a brief clinical interview was conducted with the children, due to their young age. Final diagnoses were based on all available information, including teacher report when obtained, and determined through consensus among study clinicians and the principal investigator. A diagnosis of ADHD not otherwise specified was assigned when parents did not report ADHD-related impairments in more than one setting and teacher ratings were not available, precluding determination of cross-situational impairments.
Parents completed questionnaires about their child's emotions and behaviors including the Behavior Assessment System for Children Parent Rating Scale (BASC-2-PRS; Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004), a rating scale that assesses multiple symptom and functional domains. BASC-2 Parent Report Scales have high internal consistency with a near equivalency between clinical and general samples, and high test–retest reliability (Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004). Hyperactivity and attention problems subscale raw scores were used to compare ADHD symptoms across groups. They were also used in regression analyses to disentangle associations between ADHD symptoms and emotion dysregulation and group differences in dorsal ACC iFC. The BASC-2-PRS for one participant was missing multiple items; as a result, the attention problems subscale score could not be computed. Parents also completed the Emotion Regulation Checklist (ERC; Shields & Cicchetti, Reference Shields and Cicchetti1997), a 24-item questionnaire that yields a highly reliable (Cronbach α = 0.91) composite score, along with two subscales, emotion regulation and lability/negativity. The composite score was used in the present study to compare overall emotion regulation skills across groups and to evaluate the specific relationship between emotion regulation and group differences in aMCC iFC. ERC composite scores were not available for 6 of the participants (2 STO, 1 ADHD, 1 HC).
Scan procedures
Approximately 1 week prior to imaging, most children participated in one or more “mock” scan session(s) in which they were taught how to lay still in the scanner while watching a movie and practicing the resting-state scan. Imaging was performed using the New York University Center for Brain Imaging Siemens Allegra 3.0 T Scanner (Siemens, Iselin, NJ). Children completed a 6-min resting-state scan for which they were instructed to “stay as still as a statue” while a white cross was backprojected on a black background. The scan comprised 180 contiguous whole-brain functional volumes, acquired using a multiecho echo planar imaging (EPI) sequence: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, 33 slices, matrix = 64 × 64, voxel size = 3 × 3 × 4 mm. To minimize data loss, two EPI sequences were obtained when possible. The first EPI rest scan was used for 45 children, and the second scan was used for 11 children who moved excessively during the first scan (3 STO, 5 ADHD, 3 HC). For spatial normalization and localization, a high-resolution T1-weighted anatomical image was also acquired using a magnetization prepared gradient echo sequence (repetition time = 2500 ms, echo time = 4.35 ms, inversion time = 900 ms, flip angle = 8, 176 slices, field of view = 256 mm).
Imaging analyses
Functional image preprocessing
All brain data preprocessing and group analyses were conducted using an alpha version (0.3.9) of the Configurable Pipeline for the Analysis of Connectomes (http://fcp-indi.github.io/), which is a configurable, open-source, Nipype-based (http://nipy.org/nipype/), automated processing pipeline for resting-state fMRI data. Preprocessing consisted of slice time correction, three-dimensional motion correction (24 parameters; Friston et al., Reference Friston, Williams, Howard, Frackowiak and Turner1996), despiking (removal of extreme time series outliers), spatial smoothing (full width at half maximum = 6 mm), mean-based intensity normalization of all volumes by the same factor, and temporal bandpass filtering to isolate the low-frequency blood oxygen level dependent fluctuations of interest. Functional image registration was completed using Boundary Based Registration as implemented in FSL (Greve & Fischl, Reference Greve and Fischl2009). Structural images were registered normalized to common stereotaxic space (Montreal Neurological Institute [MNI]) using Advanced Normalization Tools (Avants et al., Reference Avants, Tustison, Song, Cook, Klein and Gee2011; http://www.picsl.upenn.edu/ANTS). Single participant nuisance regression included 24 Friston motion parameters (Friston et al., Reference Friston, Williams, Howard, Frackowiak and Turner1996) and five CompCor signals (Behzadi et al., Reference Behzadi, Restom, Liau and Liu2007). Six participants (4 STO, 1 ADHD, 1 HC) had overall mean framewise displacement values greater than 0.25 and were considered for exclusion. However, visual inspection of their time series showed movements greater than 3 mm only occurred within the last 100 s of their scans. Thus, rather than exclude them, we truncated their time series by removing the volume with motion >3 mm and all subsequent volumes.
aMCC region of interest analysis
Given our interest in a cingulate region involved in both frustration responses and cognitive control, we selected a region of interest (ROI) based on the coordinate-based meta-analysis described earlier (Shackman et al., Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011). Using the coordinates for the center of intensity of the conjunction analysis (Talairach coordinates: 0, 12, 42, which were converted to MNI space), we created a 4-mm radius sphere as our aMCC ROI (see Figure 1). The mean time series of this ROI was calculated by averaging the time series of all contained voxels. For each participant, aMCC connectivity strength with other regions of the brain was assessed using a whole-brain analysis that involved correlations between the aMCC ROI time series and all other voxels in the brain. This resulted in individual participant-level maps of all voxels exhibiting significant iFC with the aMCC (Gaussian random field correction: p < .05, Z > 2.3). Group-level analyses were conducted using a random-effects, ordinary least-squares model, including two group mean predictors (STO vs. ADHD) and three nuisance covariates (sex, age, and mean framewise displacement); all were Gaussian random field corrected at p < .05, Z > 2.3. We opted not to conduct a one-way analysis of covariance to compare all groups at once, as this would emphasize findings related to overall psychopathology more than associations with STO.
Figure 1. (Color online) Anterior midcingulate cortex region of interest based on coordinates from Shackman et al. (Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011).
Additional analyses
Normative comparison
To clarify differences in the aMCC iFC between the STO and ADHD groups, we compared each group with the matched healthy comparisons, using independent samples t tests in SPSS 19.0 (IBM SPSS Statistics, Version 19.0 IBM Corp: Armonk, NY).
Dimensional analyses
The increased emotion dysregulation in the STO group might reflect greater overall severity of symptoms. To assess the unique contributions of emotion dysregulation and ADHD symptoms to aMCC iFC, we conducted multiple linear regression analyses for each of the regions showing significant group iFC differences, using SPSS 19.0. Each model included the ERC composite score as a measure of emotion regulation and BASC-2-PRS hyperactivity and attention problems raw scores as measures of ADHD symptoms. Raw scores were used rather than T scores to allow for greater equivalence with the ERC composite, which does not have standardized norms. To account for putative effects of age and sex, these were included as covariates in the regression models. These regressions were conducted across STO and ADHD groups to increase power to detect significant effects.
Results
Demographic and clinical characteristics
Group characteristics of the 56 children who successfully completed fMRI scans are presented in Table 1. The three groups did not differ in age, F (2, 55) = 0.28, p = .76, sex, χ2 (2) = 2.64, p = .27, IQ, F (2, 54) = 2.4, p = .10, or movement as measured by mean framewise displacement, F (2, 55) = 1.58, p = .22. Regarding diagnosis, the STO group exhibited significantly greater psychopathology, with 85% meeting diagnostic criteria for two or more comorbid diagnoses. In contrast, only 16.6% (n = 3) of the children in the ADHD group met criteria for at least one other disorder, χ2 (2, 38) = 18.64, p < .001. Seven children (4 ADHD, 3 STO) were receiving pharmacological treatment at the time of the study; all stimulants were withdrawn at least 3 days prior to the MRI scan as per study entry criteria. One child had taken melatonin the night before the scan; otherwise, the children were medication free.
Table 1. Demographic and clinical characteristics of the study sample

Note: STO, severe temper outburst group; ADHD, attention-deficit/hyperactivity disorder group; HC, healthy comparisons group; BASC-2 PRS, Behavioral Assessment Scale for Children, Second Edition, Parent Report Scales; ERC, Emotion Regulation Checklist.
Emotional and behavioral regulation
As shown in Table 1, group differences were observed for all emotion regulation and behavioral variables. Overall group differences were observed in emotion regulation as measured by the ERC composite score, F (2, 55) = 31.88, p < .001. As predicted, children in the STO group had poorer emotion regulation, evidenced by significantly lower ERC composite scores than children in both comparison groups, ADHD (p = .002) and HC (p < .001). The ADHD group also had significantly poorer emotion regulation scores than the HC group (p < .001). The groups also differed significantly on BASC-2-PRS hyperactivity raw scores, F (2, 55) = 29.97, p < .001. Post hoc tests showed greater scores in the STO group than the ADHD group (p = .001) and the HC group (p < .01). Children with ADHD were rated higher on BASC-2-PRS hyperactivity than HC children (p = .001). Finally, group differences were also observed for BASC-2-PRS attention problems raw scores, F (2, 54) = 16.69, p < .001. Compared to the HC group, these scores were significantly higher in the STO (p < .001) and ADHD (p = .001) groups with no significant difference between the latter two groups.
Anterior midcingulate iFC analyses
Group analyses revealed significant differences, as shown in Figure 2. The STO group exhibited reduced positive aMCC iFC with a cluster in the ACC extending ventrally and rostrally from the aMCC seed (max = 3.83; MNI: 2, 22, 26) compared to the ADHD group. Conversely, the STO group exhibited significantly greater positive aMCC iFC with the precuneus (max = 3.58; MNI: –8, –68, 20) than the ADHD group.
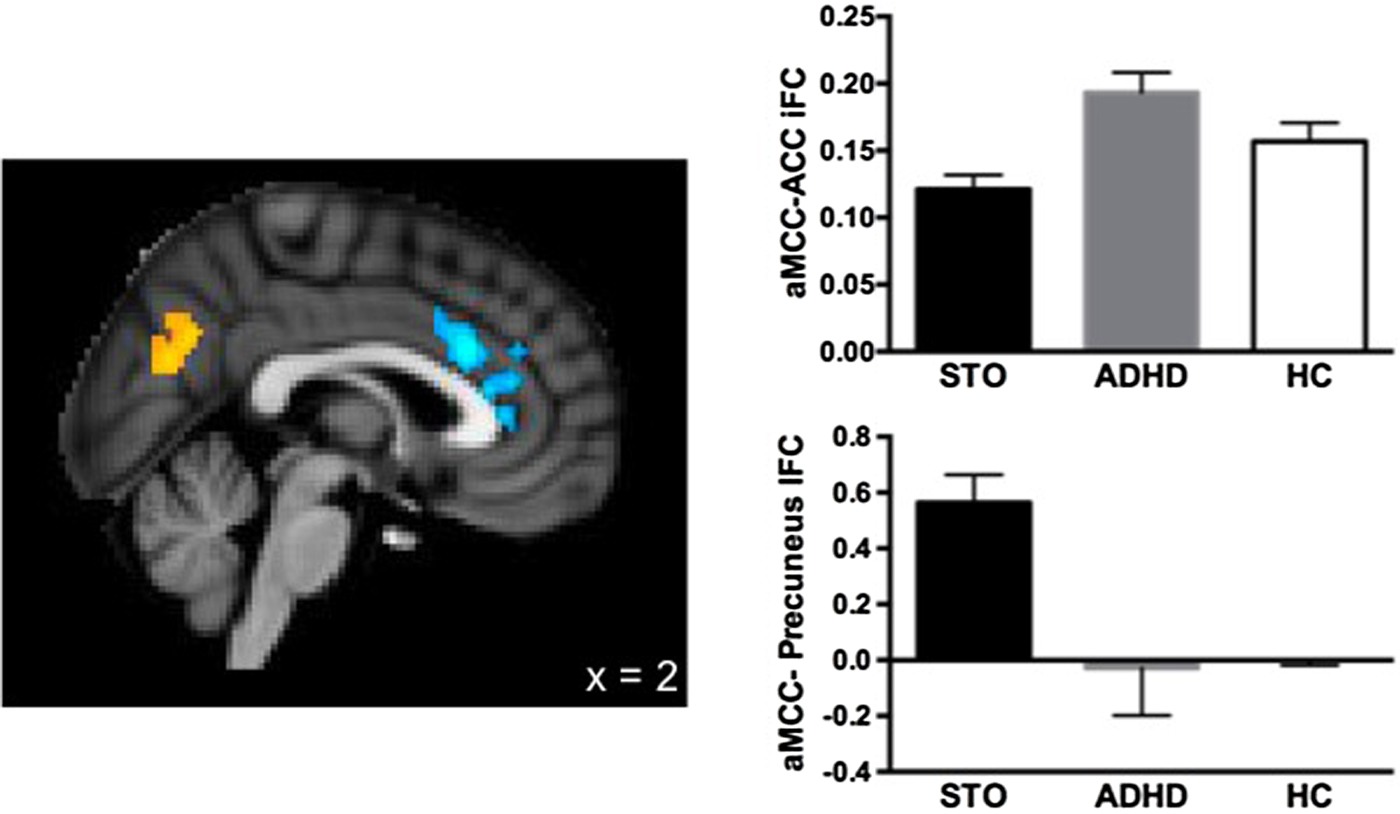
Figure 2. (Color online) Clusters showing significant differences in anterior midcingulate cortex (aMCC) intrinsic functional connectivity between the severe temper outburst (STO) and attention-deficit/hyperactivity disorder (ADHD) groups.
Normative comparisons
We then compared the iFC values extracted from these clusters (ACC and precuneus) for the STO and ADHD groups to those from the HC group. The STO group showed weaker positive iFC between the aMCC and local ACC regions, t (36) = –2.661, p = .013, and stronger positive aMCC–precuneus iFC, t (36) = 2.062, p = .047, than the HC group. No significant differences were found between the ADHD and HC groups on these measures (see Figure 2).
Dimensional analyses
As noted, psychopathology in the STO group might be more “severe” than in the ADHD group, and this difference, rather than differences in emotion dysregulation per se, may account for STO versus ADHD differences in the aMCC iFC. To address this issue, we performed multiple linear regression analyses across the STO and ADHD groups. The dependent variable was aMCC iFC, and the independent variables were ERC composite scores and BASC-2-PRS hyperactivity and attention problems raw scores. As shown in Table 2, analyses revealed that ERC composite scores were not a significant predictor of aMCC iFC with surrounding cingulate regions (p = .96). However, BASC-2-PRS hyperactivity scores significantly contributed to variability in this local ACC iFC (p = .01). There was also a trend for a relationship with BASC-2-PRS attention problems scores (p = .08). Conversely, ERC composite scores contributed significantly to the variance of aMCC– precuneus iFC (p = .04), while BASC-2-PRS hyperactivity and attention problems scores did not (ps = .10 and .16, respectively).
Table 2. Independent contributions of attention-deficit/hyperactivity disorder symptoms and emotion dysregulation to aMCC intrinsic functional connectivity

Note: aMCC, anterior midcingulate cortex; ACC, anterior cingulate cortex; iFC, intrinsic functional connectivity; BASC-2-PRS, Behavior Assessment System for Children Parent Rating Scale; ERC, Emotion Regulation Checklist.
Discussion
The present study provides preliminary information about neural mechanisms underlying STO in children. Analyses focused on cingulate circuitry given the known involvement of this region in frustration and cognitive control of emotion. We predicted that children with STO would demonstrate altered iFC of aMCC circuits, as compared to children with ADHD free of STO, as well as healthy comparisons, with no differences expected between the two comparison groups. Compared to children with ADHD without outbursts, children with STO exhibited weaker positive aMCC iFC with local ACC regions and stronger positive iFC with the precuneus. These differences were also found between the STO group and healthy comparisons; in contrast, the ADHD and HC groups did not differ. Further, multiple regression analyses suggest unique associations between local ACC iFC and hyperactivity symptoms, and between aMCC–precuneus iFC and emotion regulation capacity. Thus, our findings suggest that disruptions in cingulate iFC may underlie both the behavioral and emotional dysregulation observed in children with STO.
Consistent with previous work (Kelly et al., Reference Kelly, Di Martino, Uddin, Shehzad, Gee, Reiss and Milham2008), all three groups showed positive iFC between the aMCC ROI and local ACC regions extending rostrally. However, children with STO exhibited a significant reduction in this iFC, compared to children with ADHD without outbursts and healthy comparisons. Multiple regression analyses suggest that the group difference in local aMCC iFC reflects greater severity of ADHD symptoms in the STO group, rather than differences in emotion dysregulation. Previous work implicates the cingulate cortex in the overall pathophysiology of ADHD (Bush, Valera, & Seidman, Reference Bush, Valera and Seidman2005; Cortese et al., Reference Cortese, Kelly, Chabernaud, Proal, Di Martino, Milham and Castellanos2012). Compared to healthy youth, children with ADHD typically exhibit reduced task-related activation in the ACC (Dickstein et al., Reference Dickstein, Bannon, Castellanos and Milham2006) and reduced gray matter in the rostral ACC, which was identified in the current iFC analyses (Bonath, Tegelbeckers, Wilke, Flechtner, & Krauel, Reference Bonath, Tegelbeckers, Wilke, Flechtner and Krauel2016). Disruption in local ACC iFC may also have implications for differential developmental outcomes between STO and ADHD youth. A recent study examined the associations between iFC of the executive function network in late adolescence (~age 17) and changes in hyperactivity/impulsivity symptoms since age 11 (Francx et al., Reference Francx, Oldehinkel, Oosterlaan, Heslenfeld, Hartman, Hoekstra and Mennes2015). Greater symptom reduction was associated with stronger iFC of the ACC, suggesting that integration of this region into the executive control network may underlie symptom improvement. Similarly, the STO group in the present study evidenced a cluster of decreased local aMCC iFC that includes the same ACC region; this may reflect less integration of these regions into the executive control network, reflected behaviorally by increased severity of hyperactivity and impulsivity symptoms. As such, these children may be resistant to improvement in these symptoms with age. Longitudinal follow-up is needed to test this hypothesis further.
The STO group also exhibited significant positive iFC between the aMCC ROI and the precuneus that was not observed in either of the other two groups. Previous studies show no significant iFC between this cingulate region and precuneus in healthy children (Kelly et al., Reference Kelly, Di Martino, Uddin, Shehzad, Gee, Reiss and Milham2008) or adults (Margulies et al., Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007). Multiple regression analyses suggest that this hyperconnectivity is associated specifically with the emotion dysregulation demonstrated by children with STO. Similarly, greater global connectivity of a similar precuneus region has been shown in children and adults with bipolar disorder, a condition characterized by dysregulated emotion (Stoddard et al., Reference Stoddard, Gotts, Brotman, Lever, Hsu, Zarate and Leibenluft2016). The precuneus is a core region of the canonical default network, which plays a role in social emotion regulation (Xie et al., Reference Xie, Mulej Bratec, Schmid, Meng, Doll, Wohlschlager and Sorg2016). Thus, the increased iFC between the aMCC and this region may reflect a greater reliance on others (i.e., parents or teachers) to help regulate strong emotions because children with STO do not possess sufficient skills to adequately self-regulate. Alternatively, hyperconnectivity with the precuneus may prevent the aMCC from effectively regulating negative affect. As discussed earlier, the aMCC and surrounding ACC regions are typically considered part of the executive control network, and significant shifts occur in the iFC between and within this network and the default network across early development (Fair et al., Reference Fair, Dosenbach, Church, Cohen, Brahmbhatt, Miezin and Schlaggar2007; Sato et al., Reference Sato, Salum, Gadelha, Picon, Pan, Vieira and Jackowski2014). Thus, the present finding may represent a deviation or delay in typical development that is mirrored by delayed acquisition of emotion regulation in children with STO. Of note, we failed to find differences in aMCC–precuneus iFC between the ADHD group without outbursts and healthy comparisons, which contrasts with previous findings in adults (Castellanos et al., Reference Castellanos, Margulies, Kelly, Uddin, Ghaffari, Kirsch and Milham2008) and pediatric ADHD samples (Sun et al., Reference Sun, Cao, Long, Sui, Cao, Zhu and Wang2012). It is possible that the abnormalities found in these earlier studies may have been due to the presence of emotion regulation impairments in ADHD, rather than to ADHD itself. This is an important hypothesis to consider; however, symptoms of emotion dysregulation were not included in these investigations, so this cannot be directly tested.
The present findings need to be considered in the context of several study limitations. First, sample sizes were limited, reducing power to detect significant effects. It is challenging to obtain usable fMRI data from children as young as 5, particularly those with an ADHD diagnosis. Our overall success rate was good, allowing us to analyze more than 75% of our starting sample, but larger samples are needed to test complex interactions among ADHD and emotion regulation symptoms. A larger sample would also permit a more data-driven approach to examine emotion dysregulation and the functional connectivity of multiple regions of the ACC. Second, assessments of temper outbursts, ADHD symptoms, and emotion regulation all relied on parent reports. Thus, shared method variance may have contributed to elevated scores for both behavioral and emotional dysregulation in the STO group. However, inconsistent with this possibility, parents of children in the STO group did not endorse greater symptoms of inattention than parents of children in the ADHD group. Teacher reports were not available for all participants and, thus, could not be used as an additional measure of ADHD symptoms in this study. Inclusion of objective experimental or observational measures would further mitigate this concern. Third, study inclusion criteria did not allow examination of temper outbursts as a continuous measure. All children in the STO group had severe outbursts (limiting variability in severity), and to study clearly differentiated groups, children with ADHD with moderate tantrums were excluded (also providing little variance in outburst severity in the ADHD group). Such dimensional analyses might have provided greater power and increased the clinical relevance of the findings to a broader group of children with ADHD. Fourth and finally, to truly investigate the neural circuitry of STO without other confounding symptoms of dysregulation (i.e., hyperactivity and impulsivity), a 2 × 2 balanced design would be needed. While we have been able to examine three of these four groups in this study and previously, it has not been feasible to recruit a sufficient sample of children with STO without ADHD. Inclusion of a comparison group of children with ADHD without STO represents our best, albeit imperfect, effort to disentangle the associations of behavioral and emotional dysregulation with iFC measures. Thus, while we cannot definitively state that findings are specific to emotion dysregulation (STO), and not related to other symptoms of dysregulation (i.e., hyperactivity), they provide initial observations that such differentiation may exist, warranting further study.
In summary, findings provide initial evidence of disruptions in a core cingulate network in young children with STO. This represents a first attempt at disentangling iFC alterations associated with symptoms of hyperactivity/impulsivity from those of emotion dysregulation in a heterogeneous clinical group of children with STO. Replication of these results will be key to our understanding of neurobiological models of impairing STO in children and, ultimately, of the importance of investigating their longitudinal outcome.






