Introduction
Patient expectations about treatment are of substantial importance to clinicians and researchers given their potential impact on health-care utilization, adherence and clinical outcomes (Martin et al. Reference Martin, Guhr, Hunter and Acree1977a, Reference Martin, Moore, Sterne and Lindseyb; Sotsky et al. Reference Sotsky, Glass, Shea, Pilkonis, Collins, Elkin, Watkins, Imber, Leber, Moyer and Oliveri1991; Adams & Scott, Reference Adams and Scott2000; Mondloch et al. Reference Mondloch, Cole and Frank2001; Krell et al. Reference Krell, Leuchter, Morgan, Cook and Abrams2004; Gaudiano & Miller, Reference Gaudiano and Miller2006; Anglin et al. Reference Anglin, Alberti, Link and Phelan2008). Expectations are of particular interest because they are modifiable and related to behavior change (Andersen, Reference Andersen1995).
Positive expectations about recovery were related to better health outcomes in 15 of 16 studies spanning myocardial infarction, cardiac surgery, chronic pain, alcoholism, and other disorders and surgeries (Mondloch et al. Reference Mondloch, Cole and Frank2001). Positive expectations of treatment effectiveness also predicted improved outcomes in depression (Sotsky et al. Reference Sotsky, Glass, Shea, Pilkonis, Collins, Elkin, Watkins, Imber, Leber, Moyer and Oliveri1991; Krell et al. Reference Krell, Leuchter, Morgan, Cook and Abrams2004), bipolar disorder (Gaudiano & Miller, Reference Gaudiano and Miller2006) and schizophrenia (Martin et al. Reference Martin, Guhr, Hunter and Acree1977a, Reference Martin, Moore, Sterne and Lindseyb). Studies of the placebo effect, a positive expectancy about improvement, have provided intriguing support for theories that highly active neurobiological processes, including brain reward circuitry, are mediated by psychological expectations (Enck et al. Reference Enck, Benedetti and Schedlowski2008; Howland, Reference Howland2008). Patient expectations about improvement are therefore a promising area for outcome research.
The importance of health-related attitudes and beliefs has also been highlighted in several models of health services use. The Behavioral Model (Andersen & Aday, Reference Andersen and Aday1978; Andersen, Reference Andersen1995) proposes that the use of health services for health improvement is associated with: (1) predisposing characteristics such as demographic, social structure and health beliefs; (2) personal, family and community enabling resources; and (3) perceived and objective need. The Health Belief Model (Rosenstock, Reference Rosenstock1966, Reference Rosenstock1974; Janz & Becker, Reference Janz and Becker1984) similarly proposes that individuals evaluate a health behavior's feasibility and efficaciousness through an estimate of perceived benefits in reducing their susceptibility to or severity of illness, weighed against the psychological, physical, financial or other costs or barriers associated with the behavior. The balance of benefits and costs influences the likelihood of taking action regarding health care (Becker et al. Reference Becker, Haefner, Kasl, Kirscht, Maiman and Rosenstock1977). Predictors of health-care utilization, however, have focused on barriers to service such as availability, accessibility or insurance status; psychological barriers; exposure to mental health services; and patients' attitudes toward mental illness (Alvidrez, Reference Alvidrez1999) rather than on expectations about treatment.
There are assessments available that evaluate beliefs and attitudes about health, illness, treatment and medication (Weinman et al. Reference Weinman, Petrie, Moss-Morris and Horne1996; Horne & Weinman, Reference Horne and Weinman1999; Petrie et al. Reference Petrie, Jago and Devcich2007), or general expectations about improvement (Devilly & Borkovec, Reference Devilly and Borkovec2000). There are also patient-rated questionnaires that evaluate the perceived benefits of specific interventions, such as mammography (Beaulieu et al. Reference Beaulieu, Beland, Roy, Falardeau and Hebert1996; Finney & Iannotti, Reference Finney and Iannotti2001; Petro-Nustas, Reference Petro-Nustas2001), Pap smear screening (McFarland, Reference McFarland2003) and breast cancer screening (Meana et al. Reference Meana, Bunston, George, Wells and Rosser2001). However, patient expectations specifically about the potential for improved functioning associated with treatment is an important factor in understanding both treatment seeking and treatment outcome and this area has been minimally investigated.
Health-related quality of life, including patient perceptions about daily functioning, is increasingly understood to be a crucial health outcome (Trivedi et al. Reference Trivedi, Rush, Wisniewski, Warden, McKinney, Downing, Berman, Farabaugh, Luther, Nierenberg, Callan and Sackeim2006), but currently no validated tool is available for use with psychiatric or general medical disorders that assesses expectations about the impact of treatment on domains of everyday functioning. An assessment identifying these perceived benefits of treatment would be a useful contribution to both health services and outcomes research.
The Anticipated Benefits of Care (ABC) was developed for use in research or clinical settings for populations with medical or psychological conditions, and was first used (previously called the Patient Perception of Benefits) in the Texas Medication Algorithm Project (TMAP; Gilbert et al. Reference Gilbert, Altshuler, Rago, Shon, Crismon, Toprac and Rush1998; Rush et al. Reference Rush, Rago, Crismon, Toprac, Shon, Suppes, Miller, Trivedi, Swann, Biggs, Shores-Wilson, Kashner, Pigott, Chiles, Gilbert and Altshuler1999, Reference Rush, Crismon, Kashner, Toprac, Carmody, Trivedi, Suppes, Miller, Biggs, Shores-Wilson, Witte, Shon, Rago and Altshuler2003). This report uses data from TMAP for patients with major depressive disorder (MDD), bipolar disorder and schizophrenia to evaluate the psychometric properties of the ABC, including its ability to predict outcome in these samples. The following questions were asked:
(1) What is the factor structure of the ABC?
(2) What is the reliability and validity of the ABC?
(3) Does the ABC have adequate psychometric properties for use in clinical and research ettings?
(4) Is the ABC associated with symptom response?
Method
TMAP compared the clinical and economic impact of algorithm-based medication treatment combined with clinical support and a patient/family education program to treatment as usual for patients with psychotic and non-psychotic MDD, bipolar disorder and schizophrenia treated in 19 public sector mental health clinics in Texas. The rationale and design of the study and description of the samples and treatment assignments are reported elsewhere (Rush et al. Reference Rush, Crismon, Kashner, Toprac, Carmody, Trivedi, Suppes, Miller, Biggs, Shores-Wilson, Witte, Shon, Rago and Altshuler2003). Participants were enrolled from March 1998 to April 1999 and treated for up to 2 years. Algorithm-based care for the three disorders was provided in four separate clinics each and the seven remaining clinics provided treatment as usual only. Each clinic providing algorithm-based care for one disorder also provided treatment as usual for a second disorder.
Institutional Review Boards at the University of Texas Southwestern Medical Center and the University of Texas at Austin approved and monitored the study. All participants provided written informed consent prior to study entry.
Participants
Participants were at least 18 years old with clinical diagnoses of psychotic or non-psychotic MDD, bipolar disorder or schizophrenia as determined by their clinician, based on the DSM-IV. Sufficient symptoms needed to be present to initiate a new medication or intolerance or inadequate benefit to a prior medication, requiring a switch from or augmentation of a current treatment. In treatment as usual, study entry was permitted with a medication change or a 24-item Brief Psychiatric Rating Scale (BPRS-24; Ventura et al. Reference Ventura, Nuechterlein, Subotnik and Gilbert1995) score within one standard deviation (1 s.d.) of the mean for the algorithm group (Rush et al. Reference Rush, Crismon, Kashner, Toprac, Carmody, Trivedi, Suppes, Miller, Biggs, Shores-Wilson, Witte, Shon, Rago and Altshuler2003). Inclusion criteria were broad and exclusion criteria minimal.
Of the 1421 evaluable participants who completed at least one follow-up assessment, 1370 completed all 10 items of the ABC (528 with MDD, 395 with bipolar disorder and 447 with schizophrenia) and were used to evaluate the assessment's psychometric properties.
Assessments
Participants' anticipated benefits of treatment were assessed at baseline with the ABC, a 10-item questionnaire used to measure patient expectations about whether they will see improved functioning if they get needed care (see Appendix 1). Each of the 10 items is rated on a scale of 1 to 5 with anchor points ‘strongly agree’, ‘agree’, ‘neutral’, ‘disagree’ and ‘strongly disagree’. Thus, higher scores indicate more negative expectations about the benefits of care. Scores can range from 10 to 50.
Research outcome assessments were conducted every 3 months for up to 2 years by independent, but unblinded, research coordinators who were not involved in treatment. Primary outcome measures were the BPRS-18 (Ventura et al. Reference Ventura, Green, Shaner and Lieberman1993) for schizophrenia, the BPRS-24 for bipolar disorder (Ventura et al. Reference Ventura, Nuechterlein, Subotnik and Gilbert1995), and the 30-item Inventory of Depressive Symptomatology – Clinician-rated (IDS-C30; Rush et al. Reference Rush, Gullion, Basco, Jarrett and Trivedi1996, Reference Rush, Carmody and Reimitz2000; Trivedi et al. Reference Trivedi, Rush, Ibrahim, Carmody, Biggs, Suppes, Crismon, Shores-Wilson, Toprac, Dennehy, Witte and Kashner2004b) for MDD.
Response was defined by a 50% reduction in the baseline IDS-C30 score for MDD, a 50% reduction from baseline BPRS-24 score for bipolar disorder, and a 25% reduction from baseline BPRS-18 score for schizophrenia.
Data analyses
Descriptive statistics were provided for sample baseline demographic and clinical characteristics. The internal consistency of the ABC was assessed by Cronbach's α (Cronbach, Reference Cronbach1951), item total correlations, and item means.
The number of factors present in the ABC was determined by parallel analysis (Horn, Reference Horn1965). Typically, the number of factors is determined by the number of eigenvalues >1. In parallel analysis, the number of factors is determined by the number of eigenvalues greater than would be expected to arise by chance. As eigenvalues measure the strength of the correlations among the variables, eigenvalues derived from data with no correlations among the variables represent those arising only by chance. To determine how large these chance eigenvalues are, we generated 1000 simulated datasets (using the same number of observations and items as our dataset) consisting of normally distributed random numbers, where correlations between all variables are expected to be zero. Then principal components analysis was applied to each simulated dataset and the eigenvalues from each analysis were averaged together. The number of factors in our dataset was defined by the number of eigenvalues that was larger than the average number derived from the simulated data eigenvalues.
The ABC's validity was evaluated by computing correlations between the ABC and symptom measures at baseline along with sociodemographic and clinical baseline characteristics. The ability of the ABC to independently predict outcomes even after adjustment for other covariates was examined using analysis of covariance (ANCOVA) to assess the effect of baseline ABC on change in symptomatic outcome from baseline to month 3. Covariates were selected using a previously described procedure (Suppes et al. Reference Suppes, Rush, Dennehy, Crismon, Kashner, Toprac, Carmody, Brown, Biggs, Shores-Wilson, Witte, Trivedi, Miller, Altshuler and Shon2003; Miller et al. Reference Miller, Crismon, Rush, Chiles, Kashner, Toprac, Carmody, Biggs, Shores-Wilson, Chiles, Witte, Bow-Thomas, Velligan, Trivedi, Suppes and Shon2004; Trivedi et al. Reference Trivedi, Rush, Crismon, Kashner, Toprac, Carmody, Key, Biggs, Shores-Wilson, Witte, Suppes, Miller, Altshuler and Shon2004a) and included baseline symptom severity, years of education, family size, disposable income, black race (yes/no) and Hispanic ethnicity (yes/no). In addition, length of illness was used as a covariate for the group with MDD, and age and gender were used as covariates for the groups with bipolar disorder and schizophrenia.
The ability of the ABC to predict treatment response was also examined. A logistic regression model was fit with baseline ABC and the covariates listed above to predict response status at 3 months for each disorder. For both the ANCOVA and logistic regression analyses, the model was first fit with terms for treatment group and treatment group by ABC interaction. As these terms were not significant, they were deleted and the models were fit using data from all treatment groups combined.
Item response theory (IRT) methods (Hambleton et al. Reference Hambleton, Swaminathan and Rogers1991) allow us to explore the relationship between scores on the ABC and the unobserved (latent) construct the ABC was designed to measure. Specifically, IRT methods were used to compute the test information function (TIF; Birnbaum, Reference Birnbaum, Lord and Novick1968) of the ABC for each disorder. For each item of the instrument, IRT models (called item operating characteristic curves) were fit that relate the probability of choosing each level of response (from 1 to 5) to the construct of anticipation of benefits. The construct is scaled so that 0 represents its average level and each unit increase or decrease represents a change of 1 s.d. in the construct. The Samejima graded response model (Samejima, Reference Samejima, van Linden and Hambleton1997) was used for the item operating characteristic curves because it was the most appropriate model for instruments, such as the ABC, that have ordered categorical responses. The ‘information’ depicted by the TIF is determined by the precision with which the ABC can estimate the construct. A plot of the TIF shows the precision of the ABC in estimating anticipation of benefits across all levels of this construct. The TIF is therefore useful for determining at what level of anticipation of benefits the instrument is most sensitive and for comparing sensitivity across disorders for all levels of anticipation of benefits. All IRT models were estimated using Multilog for Windows (Thissen, Reference Thissen2003).
Results
Baseline characteristics are summarized overall and by group in Table 1. The sample had a mean age of 41 years, was 63% female, and about half of the participants were black, of another non-white race, or Hispanic.
Table 1. Baseline characteristics overall and by groupFootnote a

MDD, Major depressive disorder; SF-12, Short Form Health Survey; ABC, Anticipation of Benefits of Care; BPRS, Brief Psychiatric Rating Scale; DAI, Drug Attitude Inventory; SAFTEE, Systematic Assessment for Treatment Emergent Events; DAST, Drug Abuse Screening Test; MAST, Michigan Alcohol Screening Test.
Values are given as mean (standard deviation), or percentage.
a Sums do not always equal n because of missing values. Percentages are based on available data.
Exploratory factor analyses
The method of parallel analysis showed that the ABC was unidimensional for all patients, and also for patients with each disorder. For all patients, the average two largest eigenvalues using the simulated data of 1.13 and 1.09 were compared to the two largest eigenvalues using the real data of 6.41 and 0.78. As the first real eigenvalue was much larger than would be expected by chance (i.e. 6.41 compared to 1.13) and the second real eigenvalue was smaller (i.e. 0.78 compared to 1.09), only one factor was deemed to be present. This factor explained 85.6% of the variability of the data for the sample as a whole. For depressed patients, the largest simulated data eigenvalues of 1.22 and 1.16 were compared with real data eigenvalues of 6.28 and 0.85, which also showed the ABC to be unidimensional. This factor accounted for 83.5% of the variance. For bipolar disorder (simulated eigenvalues of 1.26 and 1.18 compared to real eigenvalues of 6.97 and 0.71), one factor accounted for 88.8% of the variance. For schizophrenic patients (simulated eigenvalues of 1.24 and 1.17 compared to real eigenvalues of 6.03 and 0.80), the single factor accounted for 84.0% of the variance.
Internal consistency
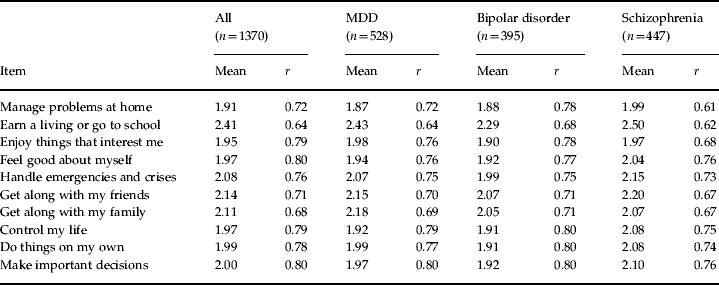
Internal consistency as measured by Cronbach's α was very good for all patients (α=0.91); and for those with MDD (α=0.90), bipolar disorder (α=0.92) and schizophrenia (0.90). Item total correlations were also good (>0.6) for all items for each disorder (Table 2).
Table 2. Item means and item total correlations (r) for all participants and by disorder

MDD, Major depressive disorder.
Item means
The mean ABC total score for all patients was 20.5 (s.d.=7.4). The means for MDD, bipolar disorder and schizophrenia were similar at 20.5 (s.d.=7.4), 19.8 (s.d.=7.7) and 21.2 (s.d.=7.1) respectively. Item means ranged from slightly less than 2 to 2.5 (Table 2). In terms of the anchor points, the average patient rated each item somewhere between ‘agree’ and ‘neutral’.
Correlation of the ABC with related measures
The ABC was not a surrogate measure for symptoms as shown by the modest correlations between ABC and symptom measures at baseline (Table 3). The ABC's correlation with the BPRS-18 was 0.22 (p=0.0000) among MDD patients, 0.10 (p=0.0484) among bipolar disorder patients and 0.15 (p=0.0015) among schizophrenic patients. The correlations between the ABC and measures of quality of life and function at baseline were also low in all groups, ranging from 0.02 to 0.18. The ABC did not correlate highly in any group with age (0.04–0.11), years of education (−0.06 to −0.08), income (−0.07 to −0.03), length of illness (−0.05 to 0.08) or number of concurrent general medical conditions (0.10–0.17).
Table 3. Correlations with baseline characteristics

MDD, Major depressive disorder; BPRS, Brief Psychiatric Rating Scale; IDS-C30, 30-item Inventory of Depressive Symptomatology – Clinician-rated; GMC, general medical condition; DAST, Drug Abuse Screening Test; MAST, Michigan Alcohol Screening Test; SF-12, Short-form Mental Health Survey.
Prediction of outcome
The ABC was used to determine if it could independently predict change in symptom status and response at 3 months after adjustment for the covariates described earlier.
Continuous outcomes
For MDD patients, the ABC at baseline was associated significantly with a change in IDS-C30 after adjustment for covariates. Each 1-point increase in the ABC resulted in a worsening of IDS-C30 scores of 0.2 points [95% confidence interval (CI) 0.06–0.37] at 3 months [F(1, 458)=7.6, p=0.0061]. For bipolar patients, the baseline ABC was not significantly associated with symptom change on the BPRS-24. Each 1-point increase in ABC resulted in a worsening of BPRS-24 scores of 0.1 point (95% CI −0.05 to 0.24) at 3 months [F(1, 340)=1.7, p=0.1972]. Bipolar patients initially presenting in a depressive episode also demonstrated a non-significant worsening of BPRS-24 score at 3 months with BPRS-24 scores decreasing by 0.08 point (95% CI −0.24 to 0.40) [F(1, 73)=0.3, p=0.6002] for each one-point increase in ABC. Similar results were obtained for schizophrenic patients, where each 1-point increase in baseline ABC resulted in a worsening of BPRS-18 scores of 0.04 points (95% CI −0.08 to 0.16) at 3 months [F(1, 383)=0.4, p=0.5387].
Binary outcomes
Table 4 shows the mean baseline ABC scores for responders and non-responders at 3 months. For depressed patients, non-responders had significantly higher (i.e. more negative) baseline ABC scores than responders. Table 4 also shows how the odds of response at 3 months change for each 5-point increase in ABC. For depression, baseline ABC was a significant predictor of response status at 3 months. For each 5-point increase in ABC, the odds of response were lowered by a factor of 0.72. The ABC was not associated with response in patients with bipolar disorder or schizophrenia, or in patients with bipolar disorder presenting in a depressive episode.
Table 4. Baseline Anticipation of Benefits of Care (ABC) scores and treatment response at 3 months

s.d., Standard deviation; OR, odds ratio; CI, confidence interval.
a Odds of treatment response given for a 5-point change in baseline ABC score.
Response was defined by a 50% reduction in the baseline Inventory of Depressive Symptomatology – Clinician-rated (IDS-C) score for major depressive disorder (MDD), 50% reduction from baseline in the 24-item Brief Psychiatric Rating Scale (BPRS-24) score for bipolar disorder, and a 25% reduction from baseline in the 18-item BPRS (BPRS-18) score for schizophrenia.
TIFs
Figure 1 shows the TIF for MDD, bipolar and schizophrenic patients. In this figure, ‘theta’ represents a unitless measure of anticipation of benefits estimated from the IRT model. Theta is scaled so that zero represents an average level of anticipation of benefits, +1 represents 1 s.d. above average (i.e. lower anticipation of benefits), and −1 represents 1 s.d. below average (i.e. greater anticipation of benefits). It can be seen that the precision of the ABC is relatively better for patients between 1 and 2 s.d. above average in theta (i.e. below average in anticipation of benefits). This makes it somewhat more effective at distinguishing degree of concern among patients with more concerns about anticipated benefits of treatment. The instrument also provides somewhat greater sensitivity for bipolar patients than MDD and schizophrenic patients.

Fig. 1. Test information functions for the Anticipated Benefits of Care by disorder: ––––, Major depressive disorder; - - - -, bipolar disorder; ......., SCZ, schizophrenia.
Discussion
The ABC has acceptable psychometric properties and clear utility in evaluating patient expectations about the benefits of treatment on everyday functioning. It has very good internal consistency and is unidimensional for patients with MDD, bipolar disorder and schizophrenia. The ABC is relatively independent of disease severity and is not associated with age, years of education or length of illness. With only 10 items in a Likert format, the ABC measure is a straightforward self-report, easy for clinicians to administer and score and for patients to understand and complete.
More negative anticipation of the benefits of care was significantly associated with treatment non-response for participants with MDD. Positive expectancies about treatment outcome have been related to improved treatment outcomes in depression, bipolar disorder and schizophrenia (Martin et al. Reference Martin, Guhr, Hunter and Acree1977a, Reference Martin, Moore, Sterne and Lindseyb; Sotsky et al. Reference Sotsky, Glass, Shea, Pilkonis, Collins, Elkin, Watkins, Imber, Leber, Moyer and Oliveri1991; Krell et al. Reference Krell, Leuchter, Morgan, Cook and Abrams2004; Gaudiano & Miller, Reference Gaudiano and Miller2006) and across chronic illnesses (Mondloch et al. Reference Mondloch, Cole and Frank2001). Placebo response rates have been reported as 35%, 32% and 24% in recent reviews of clinical trials with patients with depression, bipolar mania and schizophrenia respectively (Sysko & Walsh, Reference Sysko and Walsh2007; Girardi et al. Reference Girardi, Pompili, Innamorati, Mancini, Serafini, Mazzarini, Del Casale, Tatarelli and Baldessarini2009; Leucht et al. Reference Leucht, Arbter, Engel, Kissling and Davis2009). However, in this study, anticipation about the benefits of treatment on functioning was not related to symptom change or treatment response in bipolar or schizophrenia patients or bipolar patients presenting in a depressive episode.
It is possible that the link between more negative anticipated benefits of care and treatment non-response among depressed patients is related to the cognitive features of MDD, such as negative and pessimistic attitudes about self, others and the future. These cognitions are difficult to resolve during treatment and may disrupt a patient's ability to effectively manage behavioral symptoms of depression, such as passivity and inactivity (Gortner et al. Reference Gortner, Gollan, Dobson and Jacobson1998), or their engagement in their own treatment and self-care (Fournier et al. Reference Fournier, de Ridder and Bensing2002), contributing to a poorer outcome. These cognitions may not play the same role in bipolar disorder or schizophrenia, where more negative anticipation about treatment's effect on functioning does not seem to be related to symptom outcome. Negative and pessimistic thoughts may also be less prominent in the bipolar group, given the inclusion of patients with mania and mixed symptoms in addition to those with depression, and may be less consistently present in bipolar patients presenting initially with a depressive episode. However, the association between depression symptom severity at baseline and negative expectations about treatment outcome among the patients with MDD was low.
Finally, in this highly socio-economically disadvantaged group with substantial severity and persistence of illness treated in public sector clinics (Suppes et al. Reference Suppes, Rush, Dennehy, Crismon, Kashner, Toprac, Carmody, Brown, Biggs, Shores-Wilson, Witte, Trivedi, Miller, Altshuler and Shon2003; Miller et al. Reference Miller, Crismon, Rush, Chiles, Kashner, Toprac, Carmody, Biggs, Shores-Wilson, Chiles, Witte, Bow-Thomas, Velligan, Trivedi, Suppes and Shon2004; Trivedi et al. Reference Trivedi, Rush, Crismon, Kashner, Toprac, Carmody, Key, Biggs, Shores-Wilson, Witte, Suppes, Miller, Altshuler and Shon2004a), treatment response was the exception rather than the rule in all three groups, with particularly modest gains in the bipolar and schizophrenia groups (Suppes et al. Reference Suppes, Rush, Dennehy, Crismon, Kashner, Toprac, Carmody, Brown, Biggs, Shores-Wilson, Witte, Trivedi, Miller, Altshuler and Shon2003; Miller et al. Reference Miller, Crismon, Rush, Chiles, Kashner, Toprac, Carmody, Biggs, Shores-Wilson, Chiles, Witte, Bow-Thomas, Velligan, Trivedi, Suppes and Shon2004), which may have made it more difficult to identify an association between anticipation of benefits and symptom improvement or treatment response in the bipolar and schizophrenia groups. Attitudes such as skepticism, concerns about the overuse or harmfulness of antidepressants, and ambivalence about persistence in medication treatment have been associated with medication non-adherence or discontinuation in depressed patients (Brown et al. Reference Brown, Battista, Bruehlman, Sereika, Thase and Dunbar-Jacob2005; Aikens et al. Reference Aikens, Nease and Klinkman2008; Warden et al. in press). Positive expectancies about symptom improvement were related to treatment retention in bipolar patients (Gaudiano & Miller, Reference Gaudiano and Miller2006), and perceived benefits of treatment were related to adherence in schizophrenic patients (Adams & Scott, Reference Adams and Scott2000). In this sample, however, although percentage adherence to medication was not reported, patient anticipation of the benefits treatment would have on everyday functioning was not related to treatment attrition in any of the groups (data not shown).
There is no other measure currently available that assesses patient expectations about the impact of treatment on domains of functioning. The ABC fills a current gap in available assessments. It can be used in research evaluating the association between anticipation of benefits and adherence, health outcomes or health-care utilization. It can also be used in clinical settings to identify specific negative expectations about treatment, which may be modifiable with clinician intervention.
There are several limitations of this study. The ABC's sensitivity to change over time and its performance in predicting utilization or quality of life or function was not assessed. Its association with symptomatic outcomes or treatment continuation in less psychosocially disadvantaged groups and in groups with other psychiatric disorders or medical illnesses has yet to be determined.
Attitudes and expectancies are important in research and clinical practice given their association with symptomatic outcomes, adherence and utilization (Andersen, Reference Andersen1995; Gaudiano & Miller, Reference Gaudiano and Miller2006; Warden et al. Reference Warden, Trivedi, Wisniewski, Lesser, Mitchell, Balasubramani, Fava, Shores-Wilson, Stegman and Rush2009). The ABC is the first assessment that measures the anticipated benefits of health care on everyday functioning, filling an important gap in available assessments. It offers the first opportunity to measure one of the factors patients may weigh in balancing costs and benefits of a treatment being considered. It is valid in patients with varied psychiatric disorders. Assessment of its association with outcome and utilization in less disadvantaged samples is needed.
Appendix 1. The anticipated benefits of care
Please indicate if you agree with each of the following statements:

This scale is in the public domain and can be copied and used at no cost, but acknowledgment of this publication is appreciated.
Acknowledgments
This research was supported by the National Institute of Mental Health (NIMH) Grant MH-53799 (A.J.R.), MH-5R01MH064062-05 IMPACTS/CDSS (M.H.T.), AHRQ/Centerstone MH-1R18HS017189-01 (M.H.T.), the Robert Wood Johnson Foundation, the Meadows Foundation, the Lightner-Sams Foundation, the Nanny Hogan Boyd Charitable Trust, the Texas Department of Mental Health and Mental Retardation, the Center for Mental Health Services, the Department of Veterans Affairs, Health Services Research and Development Research Career Scientist Award (RCS92-403) (T.M.K.), the Betty Jo Hay Distinguished Chair in Mental Health (A.J.R.), the Rosewood Corporation Chair in Biomedical Science (A.J.R.), the United States Pharmacopoeia Convention, Inc., Mental Health Connections, a partnership between Dallas County Mental Health and Mental Retardation (MHMR) and the Department of Psychiatry of the University of Texas Southwestern Medical Center, which received funding from the Texas State Legislature and the Dallas County Hospital District, the University of Texas at Austin College of Pharmacy, the Southwestern Drug Corporation Centennial Fellowship in Pharmacy (M.L.C.), and the National Alliance for Research on Schizophrenia and Depression (D.W.). The following pharmaceutical companies provided unrestricted educational grants: Abbott Laboratories, AstraZeneca, Bristol–Myers Squibb, Eli Lilly & Company, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Novartis, Organon, Pfizer, Inc. and Wyeth–Ayerst Laboratories, Inc. These sponsors and organizations performed no role in the design and conduct of the study nor in the collection, management, analysis and interpretation of the data, and they did not participate in the preparation, review or approval of the manuscript.
Declaration of Interest
D. Warder has owned stock in Pfizer, Inc. and Bristol–Myers Squibb Company within the past 5 years. J. K. Gollan has received research support from the National Institute of Mental Health; National Alliance for Research in Schizophrenia and Depression; and American Foundation of Suicide Prevention. She has received royalties from the American Psychological Association and Guilford Press. She has owned shares of Pfizer and Bristol–Myers Squibb stock. She has received a speaker honoraria from AstraZeneca. She currently works as a consultant for Prevail, Inc. M. H. Trivedi has been a consultant for Abbott Laboratories Inc.; Akzo (Organon Pharmaceuticals Inc.); AstraZeneca; Bayer; Bristol–Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Fabre-Kramer Pharmaceuticals, Inc. Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Johnson & Johnson PRD; Eli Lilly & Company; Meade Johnson; Neuronetics; Parke-Davis Pharmaceuticals, Inc.; Pfizer, Inc.; Pharmacia & Upjohn; Sepracor; Solvay Pharmaceuticals, Inc.; VantagePoint; and Wyeth–Ayerst Laboratories. He has served on speakers bureaus for Abdi Brahim; Akzo (Organon Pharmaceuticals Inc.); Bristol–Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Eli Lilly & Company; Pharmacia & Upjohn; Solvay Pharmaceuticals, Inc.; and Wyeth–Ayerst Laboratories. He has also received grant support from Bristol–Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Merck; National Institute of Mental Health; National Alliance for Research in Schizophrenia and Depression; Novartis; Pfizer Inc.; Pharmacia & Upjohn; Predix Pharmaceuticals; Solvay Pharmaceuticals, Inc.; and Wyeth–Ayerst Laboratories. M. L. Crismon, at present or during the past 3 years, has received research grant or unrestricted grant funding (through The University of Texas at Austin) from Barriere County Mental Health Authority, Cyberonics, Inc., Eli Lilly and Company, Jackson Evidence Based Partnership, MHMRA of Harris County, Seton Health Network, Shire Pharmaceuticals, the Texas Department of State Health Service, the University of Hawaii, and the Hawaii Department of Mental Health. At present or during the past 3 years, Dr Crismon has served as a consultant or on an advisory board for Bristol–Myers Squibb, Cyberonics, Inc., Eli Lilly and Company, Forest Laboratories, The Reach Institute, and Shire Pharmaceuticals. Dr Crismon's wife (C. Hemlock) has significant stock ownership in Pfizer Inc. and Cephalon Inc. A. J. Rush has received research support from the National Institute of Mental Health, and the Stanley Medical Research Institute; has been on the advisory boards and/or consultant for Advanced Neuromodulation Systems, Inc., AstraZeneca, Best Practice Project Management, Inc., Bristol–Myers Squibb/Otsuka Company, Cyberonics, Inc., Forest Pharmaceuticals, Gerson Lehman Group, GlaxoSmithKline, Jazz Pharmaceuticals, Magellan Health Services, Merck & Co., Inc., Neuronetics, Novartis Pharmaceuticals, Ono Pharmaceutical, Organon USA Inc., Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., Transcept Pharmaceuticals, Urban Institute, and Wyeth–Ayerst Laboratories Inc.; has been on the speaker's bureau for Cyberonics, Inc., Forest Pharmaceuticals, Inc., and GlaxoSmithKline; has equity holdings (exclude mutual funds/blinded trusts) in Pfizer Inc.; and has royalty income affiliations with Guilford Publications and Healthcare Technology Systems, Inc.