Article contents
Greenlizardite, (NH4)Na(UO2)2(SO4)2(OH)2·4H2O, a new mineral with phosphuranylite-type uranyl sulfate sheets from Red Canyon, San Juan County, Utah, USA
Published online by Cambridge University Press: 28 February 2018
Abstract
The new mineral greenlizardite (IMA2017-001), (NH4)Na(UO2)2(SO4)2(OH)2·4H2O, was found in the Green Lizard mine, Red Canyon, San Juan County, Utah, USA, where it occurs as a secondary alteration phase. It is associated with ammoniozippeite, boussingaultite and dickite. It forms as light green-yellow blades up to ~0.3 mm long. The mineral is vitreous and transparent with a white streak. It fluoresces greenish blue in 405 nm light. Mohs hardness is ~2. Crystals are brittle with irregular fracture and two cleavages: perfect {001} and good {2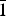 $\bar 1$0}. Greenlizardite is easily soluble in room-temperature H2O. The calculated density is 3.469 g cm–3. Optically, it is biaxial (+) with α = 1.559(1), β = 1.582(1) and γ = 1.608(1) (measured in white light). The measured 2V is 88(1)°; the calculated 2V is 87.8°. Dispersion is moderate, r < v. Pleochroism is X = very pale yellow green, Y = pale yellow green and Z = light yellow green; X < Y < Z. The optical orientation is X ≈ c, Y ≈ a and Z ≈ b*. The Raman spectrum exhibits bands attributable to both sulfate and uranyl groups. Electron probe microanalyses (with H2O based on the crystal structure) yielded (NH4)0.98Na1.00U1.96S2.04O18.00H10.02. Greenlizardite is triclinic, P
$\bar 1$0}. Greenlizardite is easily soluble in room-temperature H2O. The calculated density is 3.469 g cm–3. Optically, it is biaxial (+) with α = 1.559(1), β = 1.582(1) and γ = 1.608(1) (measured in white light). The measured 2V is 88(1)°; the calculated 2V is 87.8°. Dispersion is moderate, r < v. Pleochroism is X = very pale yellow green, Y = pale yellow green and Z = light yellow green; X < Y < Z. The optical orientation is X ≈ c, Y ≈ a and Z ≈ b*. The Raman spectrum exhibits bands attributable to both sulfate and uranyl groups. Electron probe microanalyses (with H2O based on the crystal structure) yielded (NH4)0.98Na1.00U1.96S2.04O18.00H10.02. Greenlizardite is triclinic, P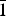 $\bar 1$, a = 6.83617(17), b = 9.5127(3), c = 13.8979(10) Å, α = 98.636(7), β = 93.713(7), γ = 110.102(8)°, V = 832.49(8) Å3 and Z = 2. The crystal structure (R1 = 2.39% for 2542 I > 2σI) contains edge-sharing dimers of UO7 pentagonal bipyramids. The dimers link by sharing corners with SO4 groups to form a [(UO2)2(SO4)2(OH)2]2– sheet based on the phosphuranylite anion topology. Zig-zag edge-sharing chains of NaO6 octahedra link adjacent [(UO2)2(SO4)2(OH)2]2– sheets, forming thick slabs. NH4 bonds to O atoms in adjacent slabs linking them together. H2O groups occupy channels in the slabs and space between the slabs.
$\bar 1$, a = 6.83617(17), b = 9.5127(3), c = 13.8979(10) Å, α = 98.636(7), β = 93.713(7), γ = 110.102(8)°, V = 832.49(8) Å3 and Z = 2. The crystal structure (R1 = 2.39% for 2542 I > 2σI) contains edge-sharing dimers of UO7 pentagonal bipyramids. The dimers link by sharing corners with SO4 groups to form a [(UO2)2(SO4)2(OH)2]2– sheet based on the phosphuranylite anion topology. Zig-zag edge-sharing chains of NaO6 octahedra link adjacent [(UO2)2(SO4)2(OH)2]2– sheets, forming thick slabs. NH4 bonds to O atoms in adjacent slabs linking them together. H2O groups occupy channels in the slabs and space between the slabs.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Stuart Mills
References
- 10
- Cited by


