Introduction
Modern crop systems (monoculture crops) are fragile and ecologically unstable, so serious pest problems can be expected (Risch, Reference Risch1980). The instability of agro-ecosystems can be caused by the vegetational simplification, resulting from the adoption of vast crop monocultures (Tothill, Reference Tothill1958). There is ample evidence that vegetation diversity can have both positive and negative, and direct and indirect effects on populations of not only herbivorous insects, but also on associated natural enemies (van Emden, Reference van Emden1965; Price et al., Reference Price, Bouton, Gross, McPheron, Thompson and Weis1980). Densities of natural enemies tend to be greater in polycultures than in monocultures (Andow, Reference Andow1991), and pro-motion of biodiversity in agro-ecosystems regularly favours natural enemies, suppresses pests and, in some cases, reduces crop damage (Gurr et al., Reference Gurr, Wratten, Barbosa, Gurr and Wratten2000; Landis et al., Reference Landis, Wratten and Gurr2000).
Many pest populations may be managed by enhancing the performance and local abundance of the existing community of natural enemies, a practice which was originally recognised by van den Bosch & Telford (Reference van den Bosch, Telford and DeBac1964) and made popular as the title of a book in 1998 (Barbosa, Reference Barbosa1998) and recently has been termed ‘conservation biological control’ (Landis et al., Reference Landis, Wratten and Gurr2000). It is perhaps the oldest and most widespread form of biological pest control, with roots in many cultural practices such as vegetation diversity, mani-pulation of agro-ecosystems intercropping, use of wild plants in and around crops, trap cropping and use of row covers, which all influence the distribution and abundance of natural enemies in crops (Kean et al., Reference Kean, Wratten, Tylianakis and Barlow2003).
Habitat manipulation, a form of conservation biological control (Landis et al., Reference Landis, Wratten and Gurr2000), is an important approach that enhances the environment by making it more suitable for natural enemies, thus improving the probability of successful biological control (Rabb et al., Reference Rabb, Stinner, van den Bosch, Huffaker and Messenger1976). Conservation of natural enemies in integrated pest management (IPM) programmes is enhanced through habitat manipulation (Hopper, Reference Hopper2003). Conservation biological control involves habitat manipulations to enhance the fecundity and longevity of natural enemies (Wratten et al., Reference Wratten, Lavandero, Scarratt and Vattala2003). It includes the maintenance of ecological compensation areas, relying on the increase of plant diversity within or outside crops, and is crucial in enhancing beneficial insects' abundance for pest suppression (Rossing et al., Reference Rossing, Poehling and Burgio2003).
Habitat manipulation also involves methods like trap crops, to reduce the susceptibility to insect pest infestations in a target crop. Habitat manipulation specifically enhances the impact of arthropod natural enemies by providing: (i) alternative host/prey species; (ii) non-prey/host food (e.g. honeydew, pollen, nectar), particularly for parasitoids; and (iii) more favourable micro-climates, including overwintering sites. The use of non-crop habitats within crops which mimic natural habitats can be used to encourage the build-up of natural enemies into fields (Thomas et al., Reference Thomas, Sotherton, Coombes and Wratten1992). Field boundaries have been recognized for two decades as important reservoirs of predatory arthropod species, stemming from European research by Sotherton (Reference Sotherton1984, Reference Sotherton1985). Habitats, as found in field boundaries, differ in their suitability for predators as some non-crop plants attract more insect pests while others may favour natural enemies (predators and parasitoids) for the reasons mentioned above. Different habitats have been used to increase beneficial predator num-bers in agro-ecosystems, for example ‘beetle banks’ (Collins et al., Reference Collins, Wilcox, Chaney, Boatman and Holland1997).
The reliance on pesticides in intensively cropped areas has led to uncontrollable situations through the unwitting selection of resistant genotypes, with a high level of resist-ance readily developed in frequently sprayed contiguous populations (Prabhaker et al., Reference Prabhaker, Toscano, Castle and Henneberry1997; Simmons & McCutcheon, Reference Simmons and McCutcheon2001) and the destruction of populations of natural enemies (De Barro, Reference De Barro1995). Due to inadequate efficacy of, resistance to, and increasing environmental concerns with pesticides, there is much interest in maximising the role of biological control in the management of sap-sucking insect pests (Simmons & McCutcheon, Reference Simmons and McCutcheon2001). Most research on habitat management has been done in the colder climates of New Zealand, Europe, USA and Canada. According to Dent (Reference Dent1995), conservation of natural enemies is an approach to biological control which had not at that time received sufficient attention. Research in conservation of natural enemies is, however, gaining ground in Australia and New Zealand (Hossain et al., Reference Hossain, Gurr, Wratten and Raman2002; Gurr et al., Reference Gurr, Wratten, Kehrli and Scarratt2005). The conservation and enhancement of predators and parasitoids to suppress arthropod pests is considered one of the most important approaches in modern pest management practices (Landis et al., Reference Landis, Wratten and Gurr2000).
Cucurbit crops, such as cantaloupe (Cucumis melo L.), cucumber (C. sativus L.) and squash (Cucurbita pepo L.), have generally been found to be more attractive to sap-sucking insects than other crops (Tonhasca et al., Reference Tonhasca, Palumbo and Byrne1994). Silverleaf whitefly Bemisia tabaci (Gennadius) biotype B (Homoptera: Aleyrodidae) (SLW), as a prime example of a sap-sucking insect pest, has emerged as a key pest of many crops during the past decade. It was first detected in Australia in October 1994 after being recorded in the Berrima region near Darwin, Northern Territory, on both nursery species and horticultural crops belonging mainly to the Cucurbitaceae (Gunning et al., Reference Gunning, Byrne, Conde, Connelly, Hergstrom and Devonshire1995). In common with other sap-sucking insect pests, it is primarily a phloem feeder and survives in habitats ran-ging from temperate through to tropical. It causes damage through direct feeding, which may induce irreversible physiological disorders and crop yield decline, and through excretion of honeydew and virus transmission (De Barro, Reference De Barro1995). Honeydew encourages the growth of sooty mould on the leaves, thereby inhibiting photosynthesis and causing cosmetic damage.
Numerous natural enemies of insect pests are known in different parts of the world and on various crops, but the biology of these natural enemies still requires study, as does their efficacy in aiding the control of sap-sucking insects. Natural enemies of sap-sucking insect pests can be classified into three groups: predators, parasitoids and entomopathogens (Gerling, Reference Gerling and Gerling1990). Predators are the primary biological control agents in central Queensland due to dry and hot weather conditions of the region, especially during the time of year when cucurbits are cultivated. Only a few groups of insects represent predators of the major sap-sucking insect pests of cucurbits in Queensland, namely whiteflies and aphids: Coleoptera (mainly ladybirds); Heteroptera (bugs essentially belonging to the families Miridae and Anthocoridae); Neuroptera (Lacewings); and Diptera (Gerling, Reference Gerling and Gerling1990; Vasquez Moreno, Reference Vasquez Moreno1997). These groups include some well-known generalist predators that prey on sap-sucking insects, including Chrysoperla species larvae (lacewings), Orius species (minute pirate bugs) and Geocoris species (bigeyed bugs). Several coccinellid species are specialist insect predators, such as Delphastus catalinae (LeConte) and Nephapsis oculatus (Blatchely) (Fasulo et al., Reference Fasulo, Allen, Bellows, Evans, Flint, Goodell, Liu, Nichols, Norman, Perring, Riley, Sparks, Stansly and Toscano1995). Aphids and whiteflies are attacked by several species of predators that can act together to suppress or delay the outbreak of damaging populations (Sechser et al., Reference Sechser, Ayoub and Monuir2003). Populations of insect pests can also be reduced by predation of mites and spiders and some other minor insect taxa.
This study, therefore, aimed to manipulate the habitat of cucurbit crops in a tropical setting to increase the popu-lations of beneficial insects and spiders, so as to control major sap-sucking insect pests such as SLW and aphids. Further, the habitat was identified that best supported an introduced lacewing (Mallada signata (Schneider)) population, commercially available through the Australian company ‘Bugs for Bugs’ and often used by vegetable growers for bio-control of sap-sucking insect pests. Four potential conservation bio-control treatments were assessed:
(i) ‘Goodbug Mix’ (GBM) – a proprietary seed mixture produced by the Australian seed company ‘Green Harvest’. GBM contains self-sowing annual and perennial flowers, including red clover (Trifolium pretense F.); lucerne (Medicago sativa L.); sweet alice/Sweet alyssum (Lobularia maritima (L.) Desv.); dill (Anethum graveolens L.); caraway (Carum carvi L.); coriander (Coriandrum sativum L.); buckwheat (Fagopyrum esculentum Moench); baby's breath (Gypsophila elegans Bieb.); Queen Anne's Lace (Ammi majus L.); marigolds (Tagetes patula L.); and cosmos, (Cosmos bipinnatus Cav.), and reputedly enhances pollen and nectar resources utilised by predators and parasitoids.
(ii) Lablab (Lablab purpureus (L.) Sweet) – a fast growing, drought-tolerant, annual, summer forage legume. In a crop rotation program, it can significantly improve soil nitrogen levels by nitrogen fixation or by incorporation in soil as a green manure crop. Lablab is tolerant of drought and heat. In NSW, lablab is adapted to slopes and plains with a minimum annual rainfall of 500 mm, and to coastal or irrigated areas. Lablab does very well on a wide variety of soils – from light, sandy soils through to well-drained, heavier-textured soils. Lablab's performance on heavy soils is greatly superior to that of other legumes. Lablab is resistant to phytophthora root rot (Mullen, Reference Mullen1999). Sixteen species of natural enemies belonging to the Trichogrammatidae, Braconidae, Ichneumonidae, Sarcophagidae, Coccinellidae, Chrysopidae and Eumenidae were recorded on lablab in Tamil Nadu, India (Srinivas & Jayaraj, Reference Srinivas and Jayaraj1989).
(iii) Lucerne (Medicago sativa L.) – a medic which harbours a rich arthropod fauna. Unharvested refuge strips of lucerne are known to improve the distribution and activity of natural enemies of sap-sucking insect pests within the field (Hossain et al., Reference Hossain, Gurr, Wratten and Raman2002) by providing suitable microclimates (Pinter et al., Reference Pinter, Hadley and Lindsay1975) and an alternative food source as pollen of lucerne flowers (Kevan & Baker, Reference Kevan, Baker, Huffaker and Rabb1984). Further, lucerne crops in Australia harbour a rich spider fauna (Bishop & Holtkamp, Reference Bishop and Holtkamp1982), which respond favourably to unharvested refuge vegetation (Hossain et al., Reference Hossain, Gurr, Wratten and Raman2002); and
(iv) Niger (Guizotia abyssinica (L.f.)) – is reported to support high densities of prey insect and provide protective leafy canopies which supply shelter especially during the winter months (Grundy & Maelzer, Reference Grundy and Maelzer2003). Niger has the tendency to be a more successful refuge treatment than some brassicae and legume species. It has an abundance of yellow flowers that were found to be attractive to pollinating insects, serving as supplementary prey on which the predatory bug Pristhesancus plagipennis (Walker) were observed to feed (Grundy & Maelzer, Reference Grundy and Maelzer2003).
Materials and methods
Trial design and treatments
Four treatments were arranged on each of three 90-m beds (each treated as a block), 2 m apart, in a randomized complete block design on a central Queensland vegetable farm near Rockhampton, Queensland, Australia (23°22'S, 150°32'E) in 2005–06. Each bed comprised four plots (2×20 m) with a buffer of 3 m of hay mulch between plots. The four treatments comprised the potential boundary crops of GBM, lablab (var. High Worth), lucerne (var. Sequel Lucerne) and niger. The GBM mixture consisted 80% by seed number of clover, lucerne and buckwheat and the remainder as the seven other species. Seeds were sown on 10 October 2005 at the recommended rates of 10 kg ha−1 GBM, 15 kg ha−1 lablab, 15 kg ha−1 lucerne and 10 kg ha−1 niger and were irrigated with an overhead system. Pumpkin and watermelon crops, both good hosts for SLW, were grown on the east (2 m distance) and south (10 m distance) sides of the trial, while nothing was grown on the north and west sides.
Two hundred second instar green lacewing (Mallada signata (Schneider)) larvae from ‘Bugs for Bugs’, Australia, were released into each plot on 22 November 2005.
Sampling
Insect and spider densities were sampled weekly from 2 December 2005 to 3 February 2006 by counting a sample of individuals on a random selection of approximately ten exposed leaves within the canopy of each plot during the early hours of the morning when the insects were least active. Insects and spiders in each plot were counted by carefully turning leaves over and counting the number of adult individuals present (larvae and adults for lacewing) taking two minutes to complete. Also, one minute was spent for visual observation of insects and spiders on the upper side of exposed leaves of each plot so the total time spent on each plot was three minutes. We could not find evidence of parasitism in whitefly nor aphids during the sampling periods.
Data analysis
The total number of each insect or spider species observed across all weekly sampling during the sampling period was computed, and the data were analysed by standard analysis of variance. Distributional assumptions for all analyses were assessed by visual inspection of residual and normal probability plots with no major departures being observed, so no transformations were necessary. Pairwise comparison of means was performed using a protected least significant difference test with GenStat 8th Edition (2005).
Results
Beneficial insects
The density of European bees (Apis mellifera Linnaeus) differed (P<0.001) among treatments with more European bees for GBM compared with other treatments (table 1). GBM also hosted the largest number of spiders. The population of spiders and European bees remained higher throughout the trial in the GBM than the other treatments (table 1).
Table 1. Treatment averages of the numbers of various beneficial insects observed during three minutes of sampling per plot across all the weekly samples during sampling from December 2005 to February 2006.

Means within a column followed by common letters are not significantly different (P=0.05).
1 Significance of treatment effect; ***, P<0.001 (treatment effect from ANOVA); 2SEM, standard error of the mean.
There were more than twice as many ladybird beetles (Coccinella transversalis Fabricius) and lacewing (Mallada signata Schneider) larvae and adults, for lablab compared with all other treatments (table 1; P<0.001), although the population of lacewing larvae in lablab decreased over time, whereas the numbers of lacewing larvae remain relatively constant over time in all other treatments (fig. 1). The population of lacewing larvae and adult and ladybird beetles tended to be relatively higher in the lablab than the other treatments throughout the trial period (table 1). The number of spiders also differed (P<0.001) among treatments with a greater number of spiders for GBM, lablab and lucerne (table 1) compared with niger.

Fig. 1. Number of lacewing larvae observed during three minutes in four treatments (⋄, lablab; ▪, lucerne; ▴, GBM; and ![]() , niger) from 2 December 2005 to 3 February 2006.
, niger) from 2 December 2005 to 3 February 2006.
Harmful insects
The density of cotton stainers (Graptostethus servus Fabricius), grasshoppers (Chortoicetes terminifera (Walker)) and heliothis (Heliothis armigera Hübner) differed (P<0.001) among treatments, with more of these insects for lablab compared with other treatments (table 2). The greatest density of aphids (Aphis gossypii (Glover)) and Myzus persicae (Sulzer)) were observed for lucerne, while the least number was observed for niger (table 2; P<0.001); and the population of aphids in lucerne tended to increase over time, whereas the population of aphids remain relatively constant in all other treatments (fig. 2). The number of vegetable weevils (Listroderes difficillis (Germar)) was greater (P<0.001) for niger compared with all other treatments (table 2). In terms of harmful insects, GBM harboured only grasshoppers in any substantial numbers, and the population trend was similar and almost equal to that of lablab (table 2).

Fig. 2. Number of aphids observed during three minutes in four treatments (⋄, lablab; ▪, lucerne; ▴, GBM; and ![]() , niger) from 2 December 2005 to 3 February 2006.
, niger) from 2 December 2005 to 3 February 2006.
Table 2. Treatment averages of the numbers of various harmful insects observed during three minutes of sampling per plot across all the weekly samples during sampling from December 2005 to February 2006.
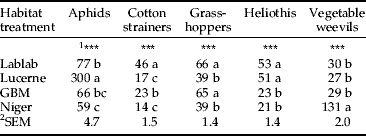
Means within a column followed by common letters are not significantly different (P=0.05).
1 Significance of treatment effect; ***, P<0.001 (treatment effect from ANOVA); 2SEM, standard error of the mean.
Discussion
Lablab hosted large numbers of ladybird beetles and spiders and best supported the population of introduced lacewing larvae and adults with their numbers greater than all other treatments (table. 1). Hence, lablab may be used as a crop to enhance the population of natural enemies for cucurbit pests but requires further field testing. The decline in lacewing larvae numbers in lablab with time may reflect movement out of the lablab into adjacent crops, or it may be due to a less attractive aspect of old lablab plants. Although lablab also hosted large numbers of cotton stainers, grasshoppers and heliothis, these are not considered serious insect pests of cucurbits in the central Queensland region. Predators, such as ladybird beetles, are more aggressive, typically longer-lived, may attack more prey and generally have greater consumption requirements (Kean et al., Reference Kean, Wratten, Tylianakis and Barlow2003) than parasitoids so may be hosted by lablab due to the availability of food in the form of insect pests present. Predacious lady beetles are known to be one important group of whitefly and/or aphid predators (Hodek & Honek, Reference Hodek and Honek1996; Dixon, Reference Dixon2000). Although there may be a short-term increase of insect pests, any short-term increase will lead to a growth and reproductive response of natural enemies (Luff, Reference Luff1983), resulting in lower densities of the pest insects (Hossain et al., Reference Hossain, Gurr and Wratten2000). Once the food source in the crop (refuge species) is decreased, predators such as lacewings, ladybird beetles and spiders will move to adjacent crops (cucurbit crops) in search of food.
The highest numbers of spiders and European bees and similar numbers of other beneficial insects were observed in the GBM mixture compared with the other species. Further, GBM hosted relatively small numbers of harmful insects, although grasshoppers were quite prevalent. The GBM mixture could, therefore, be used as a potential companion or field boundary crop in cucurbits to enhance beneficial insect populations for the control of sucking insect pests, especially SLW. Leite et al. (Reference Leite, Picanco, Jham and Moreira2006) reported that spiders were limiting factors for population increases of SLW.
An important management technique is the provision of floral foods (nectar and pollen) for use by predators and parasitoids (Landis et al., Reference Landis, Wratten and Gurr2000). The Australian proprietary seed mixture, GBM, is also a mixture of colourful flower species developed for the build-up of beneficial insects, spiders and natural enemies of arthropod pests. The GBM mixture hosted the largest numbers of spider fauna. Previously, the role of spiders in population regulation of pests was not fully known (Wheeler, Reference Wheeler1973), but they were believed to play a significant role in limiting some herbivore populations (Yeargan, Reference Yeargan1975). Spiders have been shown to be one of the aggressive predators and have the ability to move greater distance at a faster pace than other generalist pre-dators (Bishop & Riechert, Reference Bishop and Riechert1990), and their collective predation lowers pest populations across a number of crop species (Nyffeler et al., Reference Nyffeler, Sterling and Dean1994).
Lucerne hosted relatively larger numbers of spiders as predators; but, at the same time, lucerne hosted the highest numbers of aphids, and these numbers increased over time (fig. 2). The greater number of spiders may be due to the availability of sufficient food in the form of aphids, for aphid numbers per leaf increased over time. Lucerne has been reported to harbour a rich arthropod fauna. As many as 600 arthropod species were recorded in the state of New York, USA (Pimentel & Wheeler, Reference Pimentel and Wheeler1973) and 250 species in New South Wales, Australia (Bishop & Holtkamp, Reference Bishop and Holtkamp1982). Most of these arthropods are predators and parasitoids, while only a few of these species, including Heliothis spp. (Bishop, Reference Bishop1984), are pests that seriously affect lucerne production (Anonymous, 1985). In particular, of all the crops assessed, lucerne hosted the largest number of aphids, which are one of the most harmful insect pests of cucurbit crops. They also characteristically colonise a range of other horticultural crops and cotton. Lucerne and lablab hosted the highest numbers of heliothis than the other two crops, which is why lucerne alone may not be the best option for habitat manipulation for the enhancement of natural enemies in cucurbit crops.
Niger showed little promise in hosting beneficial insects and spiders, and hosted the highest numbers of vegetable weevils throughout the trial (tables 1 and 2) although Grundy & Maelzer (Reference Grundy and Maelzer2003) recorded significantly higher (P<0.05) numbers of adults of the predatory assassin bug, Pristhesancus plagipennis (Walker) (Homoptera: Reduviidae), on niger than on canola (Brassica napus L.), red salvia (Salvia coccinea P.J. Buchoz ex Etlinger), linseed (Linum usitatissimum L.), lupins (Lupinus angustifolius L.) and lucerne (Medicago sativa L.).
Modern pest management practices include the encouragement and enhancement of predators and parasitoids to suppress arthropod pests (Landis et al., Reference Landis, Wratten and Gurr2000). If the full potential of natural enemies is to be realised in an integrated pest management programme, it is necessary to understand their population dynamics over time and the factors that influence them, including the role of refuges (Wratten et al., Reference Wratten, Gurr, Landis, Irvin, Berndt and Hoodle2000) and of the interference through intraguild predation (Lang, Reference Lang2003). Although habitat manipulation can take various forms, van Emden & Dabrowski (Reference van Emden and Dabrowski1997) suggested that focus should be on provision of non-host foods for natural enemies.
Therefore, lablab could be the best option to grow alongside cucurbits or as a field boundary crop to host beneficial insects and spiders, especially generalist predators such as lacewings, ladybird beetles and spiders to control sap-sucking insect pests such as SLW and aphids. The population of introduced lacewing adults and larvae was greater in lablab than in other treatments, suggesting that it could be used to encourage survival of the predator species. The GBM mixture may be used as an alternative for better habitat manipulation in cucurbit crops. Further field studies are needed to evaluate the effectiveness of promotion of predators and suppression of sap-sucking insect pests, especially SLW and aphids, in cucurbit crops grown alongside lablab before widespread use is recommended.
Acknowledgements
The authors are grateful to Dr Bob Newby and Professor Kerry Walsh (Central Queensland University, Australia), for their helpful comments on earlier drafts of this manuscript, to Dr Richard Sequeira and Dr Siva Subramaniam (DPI&F) for their technical advice and to Rob Lowry (Central Queensland University, Australia) for his technical assistance.






