Autism spectrum disorder (ASD) is marked by deficits in social interaction and communication and the presence of restricted interests and/or repetitive behaviors (American Psychiatric Association, 2013). Current population-based estimates suggest that 1 in 59 children are diagnosed with ASD (Baio, Wiggins, Christensen et al., Reference Xu, Schneier, Heimberg, Princisvalle, Liebowitz, Wang and Blanco2018). The majority of these children are also diagnosed with at least one co-occurring condition. Estimates of the prevalence of co-occurring internalizing conditions (e.g., anxiety, depression) varies. Rosen, Mazefsky, Vasa, and Lerner (Reference Rosen, Mazefsky, Vasa and Lerner2018) noted that the prevalence ranged from 0.9% to 40% depending on the condition whereas others have suggested the prevalence is much higher, such that more than 50% have at least one co-occurring anxiety disorder, with estimates ranging from 42% to 79% (Kent & Simonoff, Reference Kent, Simonoff, Kerns, Renno, Storch, Kendall and Wood2017). The presence of co-occurring conditions results in significant negative effects on children's social and emotional development, and makes early detection and treatment of ASD complex, pointing to two reasons for continued work identifying biological (e.g., brain bases) and environmental (e.g., caregiving quality) factors related to the etiology, development, and maintenance of co-occurring conditions in ASD. Several researchers have highlighted the need for future work investigating mechanisms by which internalizing symptoms develop and are maintained in children with ASD (McVey, Reference McVey2019; Herrington, Parma, & Miller, Reference Herrington, Parma, Miller, Kerns, Renno, Storch, Kendall and Wood2017; Vasa & Mazurek, Reference Vasa and Mazurek2015) with some advances in this area (e.g., Bellini, Reference Bellini2006; Burrows, Timpano, & Uddin, Reference Burrows, Timpano and Uddin2017; Kerns & Kendall, Reference Kerns and Kendall2012; Wood & Gadow, Reference Wood and Gadow2010). However, extant models have not found consistent support, fail to integrate key components of other ASD models, or are missing key factors that have been identified in non-ASD models of internalizing conditions. This review aims to summarize the state of the science related to mechanisms associated with co-occurring internalizing symptoms in children with ASD and develop an integrated conceptual model.
Negative Effects of Internalizing Symptoms
Internalizing conditions such as anxiety and depression, as well as subclinical levels of internalizing conditions (i.e., anxiety, depression, social withdrawal, and somatic symptoms) are associated with concurrent and long-term negative outcomes. Longitudinal studies in youth without ASD indicate that internalizing symptoms are associated with decreased feelings of school belonging and self-efficacy (Forbes, Fitzpatrick, Magson, & Rapee, Reference Forbes, Fitzpatrick, Magson and Rapee2019), increased peer difficulties and social problems (Bierman, Kalvin, & Heinrichs, Reference Bierman, Kalvin and Heinrichs2015; Forbes et al., Reference Forbes, Fitzpatrick, Magson and Rapee2019; Kochel, Ladd, & Rudolph, Reference Kochel, Ladd and Rudolph2012), and when in conjunction with externalizing behavior, an increased risk of suicide attempt (Hart et al., Reference Hart, Van Eck, Ballard, Musci, Newcomer and Wilcox2017). Further, internalizing symptoms identified in childhood have been associated with continued internalizing symptoms in adolescence, as well as externalizing psychiatric problems such as conduct disorder (Bittner et al., Reference Bittner, Egger, Erkanli, Jane Costello, Foley and Angold2007). Finally, there is a two- to three-fold increase in the likelihood of depression during adulthood if a child experiences internalizing symptoms in adolescence (Pine, Cohen, Gurley, Brook, & Ma, Reference Pine, Cohen, Gurley, Brook and Ma1998).
Not surprisingly, children with ASD also experience negative outcomes when internalizing symptoms co-occur. When these conditions co-occur with ASD, functional outcomes are worse than when compared to children who do not have a co-occurring internalizing condition. For example, children with ASD and internalizing symptoms exhibit significantly more self-injurious behavior, more depressive symptoms, and parents report more parental stress than children with ASD but fewer internalizing symptoms (Kerns et al., Reference Kerns, Kendall, Zickgraf, Franklin, Miller and Herrington2015). Other studies have found that children with ASD and internalizing symptoms had poorer relationships with teachers, peers, and family members, as well as higher levels of aggressive behavior relative to children with ASD and no comorbid internalizing symptoms (Kim, Szatmari, Bryson, Streiner, & Wilson, Reference Kim, Szatmari, Bryson, Streiner and Wilson2000). Further, boys with internalizing symptoms use more maladaptive coping strategies and have poorer social functioning than boys without ASD and internalizing symptoms (Pouw, Rieffe, Stockmann, & Gadow, Reference Pouw, Rieffe, Stockmann and Gadow2013). Thus, investigating developmental risk and protective factors that are associated with internalizing symptoms within ASD populations is critical to prevent or reduce these negative outcomes.
Although a body of literature characterizing risk and protective factors for the development and maintenance of anxiety in non-ASD populations exists, examining this in ASD populations is important in order to elucidate whether or not there are common or differential mechanisms leading to the development and maintenance of anxiety in ASD populations. One way to assess this is through a research domain criteria framework (RDoC; Sanislow et al., Reference Sanislow, Pine, Quinn, Kozak, Garvey, Heinssen and Cuthbert2010), that is examining whether processes (e.g., negative valence systems) cut across disorders such as in anxiety and ASD (i.e., transdiagnostic). Mixed support for transdiagnostic processes relevant to anxiety has been found. For example, Herrington, Parma, and Miller (Reference Herrington, Parma, Miller, Kerns, Renno, Storch, Kendall and Wood2017) found support for ASD and anxiety sharing an underlying transdiagnostic mechanism as measured using RDoC's negative valence system. Specifically, children with ASD and co-occurring anxiety exhibited increased attentional capture by peripherally presented social information which was identified by increased amygdala activation. Alternatively, Rodriguez-Seijas et al. (Reference Rodriguez-Seijas, Gadow, Rosen, Kim, Lerner and Eaton2020) illustrated that symptoms of social anxiety and ADHD differentially manifest in children with ASD as compared to non-ASD samples. These results suggest that there may be both common and distinct mechanisms regarding the development and maintenance of anxiety in ASD samples relative to non-ASD samples.
In the remainder of the paper we first review current models of internalizing symptoms in ASD followed by outlining novel pieces of our hypothesized integrative model. Next, we provide a review of current literature in ASD and non-ASD populations that is relevant to our model to highlight areas of support, suggested areas for intervention, as well as gaps that require further testing. Given the breadth of the risk and protective factors that are included in this review, we did not take a classic systematic approach to identifying articles for inclusion in this paper, instead we provide a scoping review of the research to date. Although this was not meant to be a formal systematic review, we identified articles for inclusion in the ASD-related sections by searching using key terms in PSYCINFO, PUBMED, google scholar, as well as in the references sections of relevant articles and meta-analyses. When available, meta-analyses were included in both the ASD and non-ASD reviews. The non-ASD literature that was included was meant to provide support for our overall conceptual model and identify discrepancies or gaps in our current understanding of internalizing in ASD relative to non-ASD and was not conducted in a systematic manner.
Current Models of Internalizing in ASD
Several models of risk for internalizing conditions in the ASD literature exist and emphasize the impact of ASD-specific characteristics in the development and/or maintenance of co-occurring internalizing symptoms over time and are summarized below.
In Wood and Gadow (Reference Wood and Gadow2010)'s theoretical model, ASD-related stressors (social confusion, peer rejection and victimization related to autism symptoms, prevention or punishment of preferred repetitive behaviors, frequent aversive sensory experiences in daily life) due to ASD-core symptoms are thought to lead to various types of internalizing symptoms, which then lead to increased social avoidance, autism severity, behavioral problems, and personal distress/reduced quality of life. Support for this model has been mixed and comes from cross-sectional studies examining associations between ASD-core symptoms and anxiety symptoms. For example, Sukhodolsky et al. (Reference Sukhodolsky, Scahill, Gadow, Arnold, Aman, McDougle and Vitiello2008) found that within a sample of children with higher levels of internalizing symptoms also had higher levels of stereotyped behaviors. In addition, in another cross-sectional study, levels of alexithymia (a lack of emotional awareness often characteristic in ASD) and emotional acceptance mediated associations between ASD symptoms and anxiety in adults (Maisel et al., Reference Maisel, Stephenson, South, Rodgers, Freeston and Gaigg2016). The authors posit that people with ASD experience increased levels of anxiety (relative to the neurotypical population) due to their increased likelihood of reacting aversively to their emotional experiences and their difficulty identifying and understanding their emotions. These findings highlight the usefulness of interventions targeting emotional awareness for treatment of anxiety in ASD. However, other studies have not found support for links within Wood and Gadow (Reference Wood and Gadow2010)'s model. In Duvekot, van der Ende, Verhulst, and Greaves-Lord (Reference Duvekot, van der Ende, Verhulst and Greaves-Lord2018)'s longitudinal study, anxiety symptoms when children were 6.7 years old predicted social communication abilities (a core impairment in ASD) 2 years later, but social communication difficulties at age 6 did not predict later anxiety symptoms. Further, restricted, repetitive behaviors were not observed to predict later internalizing symptoms. These mixed findings suggest that there may be other mechanisms associated with the development of anxiety that are not included in this current model. Kerns and Kendall (Reference Kerns and Kendall2012) aimed to extend Wood and Gadow's (Reference Wood and Gadow2010) model by reviewing several risk factors associated with internalizing conditions in ASD such as familial risk, symptoms associated with autism (i.e., sensory sensitivities, severity of autism, social deficits), and more indirect aspects related to ASD (e.g., difficulties in emotion regulation, increased stressors), yet did not develop one clear model of risk for internalizing in ASD. Instead, the authors note that work should continue to elucidate the developmental course of anxiety related to ASD symptomatology (Kerns & Kendall, Reference Kerns and Kendall2012).
A second model that specifically focused on the development of social anxiety in ASD, posited that social anxiety develops from an early temperament of high physiological arousal (i.e., behavioral inhibition) increasing the likelihood that children/adolescents will be socially withdrawn. This withdrawal then decreases opportunities for social skill development due to avoidance of opportunities for social interaction (Bellini, Reference Bellini2006). The lack of experience in social situations leads to experiences of negative peer interactions, and due to their high physiological arousal these negative experiences lead to more adverse impacts and the development of social anxiety. The social anxiety then leads to increased social withdrawal and exacerbates the symptoms. Bellini (Reference Bellini2006) found support for his model in a sample of adolescents with ASD, albeit using cross-sectional self-report measures. This model is similar to social anxiety models in the non-ASD literature in which behavioral inhibition predicts social anxiety (e.g., Chronis-Tuscano et al., Reference Chronis-Tuscano, Degnan, Pine, Perez-Edgar, Henderson, Diaz and Fox2009).
Finally, a more recent model of risk for the development of internalizing conditions builds on a model of repetitive negative thinking as a transdiagnostic feature of internalizing symptoms (Burrows et al., Reference Burrows, Timpano and Uddin2017). Burrows et al. (Reference Burrows, Timpano and Uddin2017) posit that cognitive inflexibility combined with a predisposition to negative affect, both of which have been associated with ASD, contribute to repetitive negative thinking, placing children with ASD at risk for internalizing problems. Burrows and colleagues identify the Salience network, including the dorsal anterior cingulate, insula, and amygdala, as playing a key role in the development of internalizing symptoms in ASD. Specifically, the Salience network is involved in allocating and shifting attention between networks. Atypical connectivity within and between the Salience network and the default mode network (DMN), which is associated with self-referential thought, may lead to perseveration on negative self-referential information following a negative social interaction.
Although each of these models independently highlights important components that are related to the development of anxiety in ASD, they do not integrate key findings from each of their counterparts. Additionally, some models fail to include additional important etiological factors such as other environmental or contextual correlates (e.g., the impact parents’ stress/behavior plays) and neurobiological indices that have been found in both ASD and non-ASD literature to be associated with anxiety and/or predictive of anxiety. Developing an integrated model that includes risk and protective factors for the development of anxiety in ASD will help our understanding of whether common intervention targets in non-ASD populations are also useful for children with ASD, as well as point to additional areas of intervention targets. Finally, this will assist in early identification of children with ASD and co-occurring anxiety in order to ameliorate negative outcomes earlier.
Proposed Model of Etiological Factors Associated with the Development of Internalizing Symptoms in ASD
In developing our integrated model of risk, we assess “internalizing symptoms” rather than specific diagnoses of anxiety or depression. Internalizing symptoms refer to clinical and subclinical levels of internalizing conditions. We have decided to do this for several reasons. For one, anxiety and depression often co-occur. In a large community sample, comorbidity of anxiety and depression was estimated to be 16% in childhood, 64% in adolescence, and 57% during emerging adulthood (Essau, Lewinsohn, Lim, Ho, & Rohde, Reference Essau, Lewinsohn, Lim, Ho and Rohde2018). Second, broad measures of internalizing symptoms are often used in research, making it difficult to parse apart in extant literature. Finally, both subclinical and clinical levels of internalizing symptoms have implications for later development. Therefore, we include risk and protective factors that were identified based on both subclinical and clinical levels of internalizing symptoms in our development of an integrated model.
Our proposed model of etiological factors associated with the development of internalizing symptoms in ASD is shown in Figure 1. Our model stems from a biopsychosocial model which includes biological (i.e., genetic predisposition, structure and function of the brain, physiological regulation), environmental (i.e., parenting quality, parenting stress), and social (i.e., peer relationships) risk and protective factors. See Table 1 for a summary of key ASD-specific articles included in the review. The review includes research from the ASD literature as well as non-ASD literature to address potential risk and protective factors that were not included in previously addressed models of internalizing symptoms in ASD. Many of these risk and protective factors have bi-directional effects on children's developing internalizing symptoms through development. Further, age, sex, IQ, and ASD symptom severity are discussed as potential moderators of this proposed model.
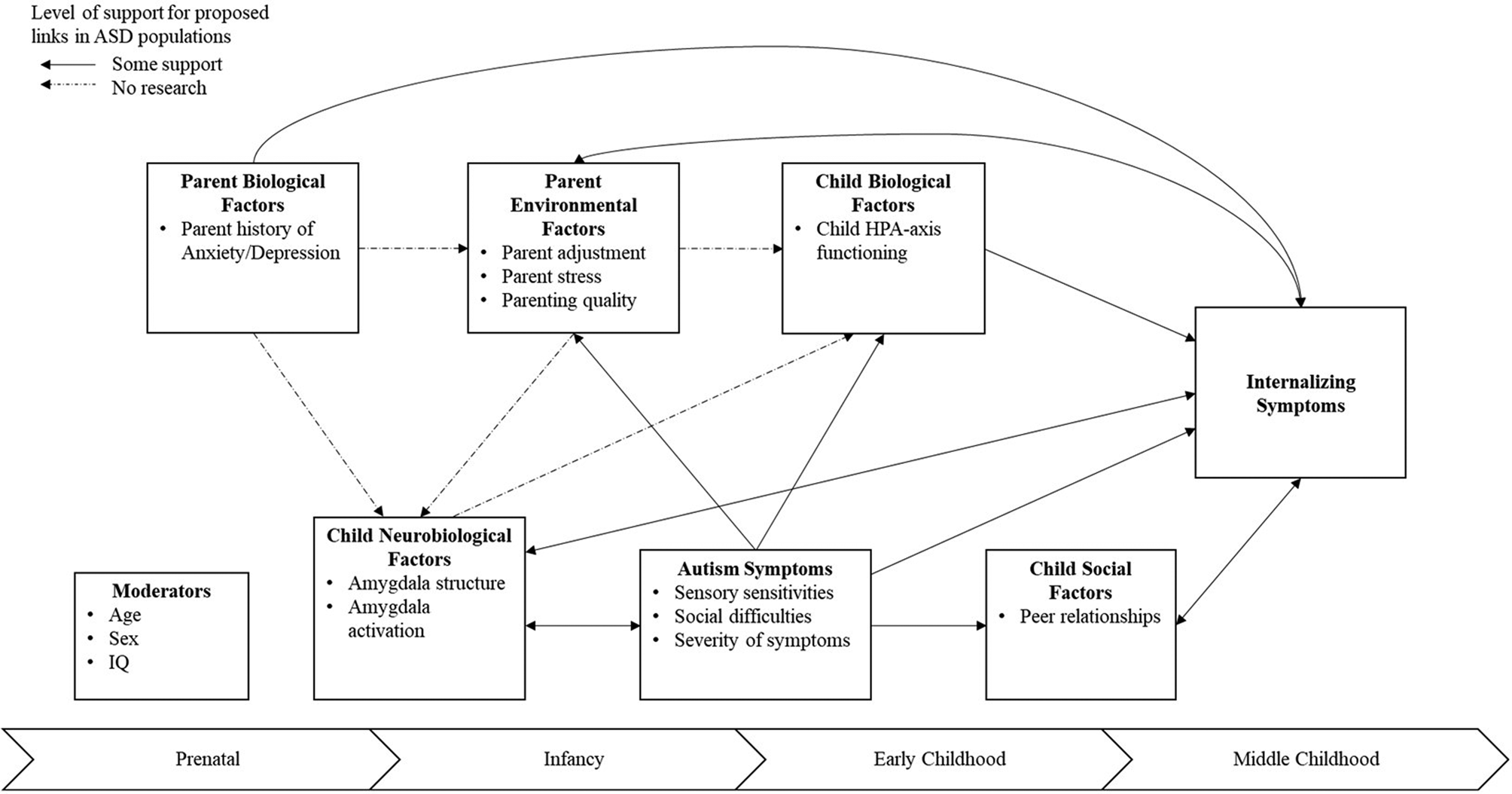
Figure 1. Illustration of known and proposed links among risk and protective factors, as well as putative developmental windows associated with internalizing symptoms in autism spectrum disorder. Note. Some support refers to links that have research support and may or may not have mixed findings in the literature.
Table 1. Empirical articles investigating risk and protective factors associated with internalizing symptoms in people with autism spectrum disorder (ASD)
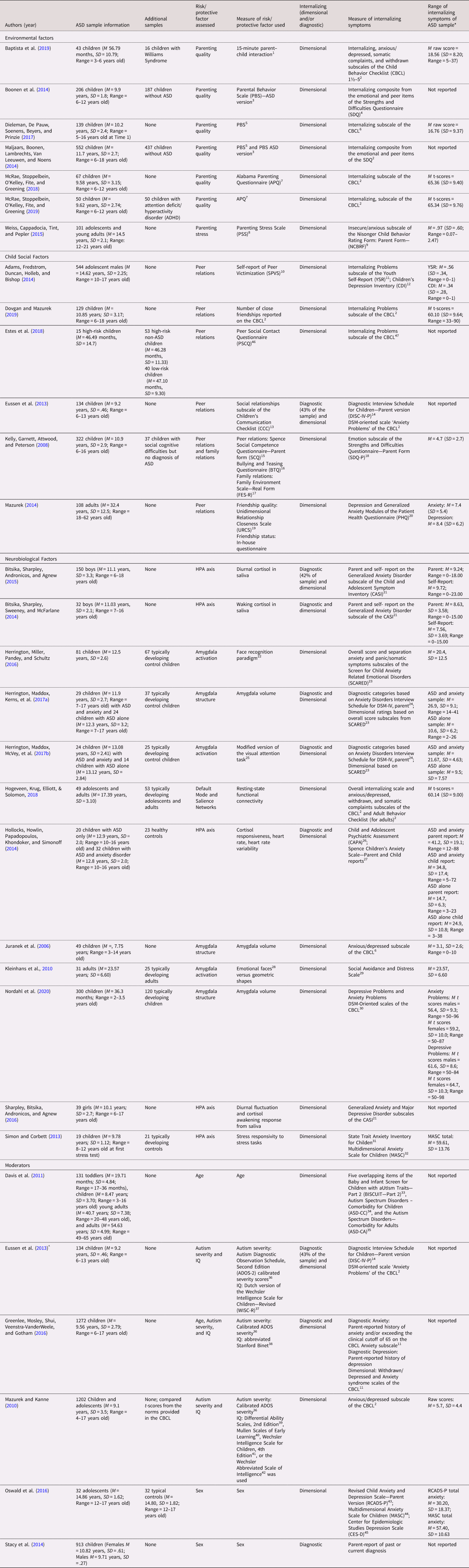
* When multiple measures were used to assess symptoms, the broad-band measure of internalizing symptoms is reported.
^ Article included in both Child Social Factors and Moderators sections.
Note. This table does not include information regarding meta-analyses or chapters that were referenced in the paper. 1Ainsworth (Reference Ainsworth, Blehar, Waters and Wall1978); 2Achenbach and Rescorla (Reference Achenbach2000); 3Van Leeuwen & Noens (Reference Van Leeuwen and Noens2013); 4Goodman (Reference Goodman1997); 5Van Leeuwen & Vermulst (Reference Van Leeuwen and Vermulst2004, Reference Van Leeuwen and Vermulst2010); 6Achenbach (Reference Achenbach1991); 7Frick (Reference Frick1991); 8Bonds, Gondoli, Sturge-Apple, & Salem (Reference Bonds, Gondoli, Sturge-Apple and Salem2002); 9Aman, Tasse, Rojahn, & Hammer (Reference Aman, Tass_e, Rojahn and Hammer1996); 10Schwartz et al. (Reference Schwartz, Farver, Chang and Lee-Shin2002); 11Achenbach, Reference Achenbach2001; 12Kovacs, Reference Kovacs1992; 13Bishop (Reference Bishop1998); 14Shaffer, et al. (Reference Shaffer, Fisher and Lucas2000); 15Spence (Reference Spence1995); 16Rigby (Reference Rigby1996); 17Moos & Moos, Reference Moos and Moos1994; 18 Goodman (Reference Goodman1997); 19Dibble et al. (Reference Dibble, Levine and Park2012); 20Spitzer et al. (Reference Spitzer, Kroenke and Williams1999); 21Gadow & Sprafkin (Reference Wood and Gadow2010), Gadow et al. (Reference Gadow, Sprafkin, Carlson, Schneider, Nolan, Mattison and Rundberg-Rivera2002); 22Herrington et al. (Reference Herrington, Taylor, Grupe, Curby and Schultz2011); 23Birmaher et al. (Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997); 24Silverman, Saavedra, & Pina (Reference Silverman, Saavedra and Pina2001); 25Vuilleumier (Reference Vuilleumier2002), Vuilleumier, Armony, Driver, & Dolan, Reference Vuilleumier, Armony, Driver and Dolan2001, Vuilleumier, Richardson, Armony, Driver, & Dolan, Reference Vuilleumier, Richardson, Armony, Driver and Dolan2004; 26Angold & Costello (Reference Angold and Costello2000); 27Spence (Reference Spence1998); 28Ekman & Friesen (Reference Ekman and Friesen1976); 29Watson & Friend (Reference Watson and Friend1969); 30Achenbach (Reference Achenbach2014); 31Spielberger (Reference Spielberger1973); 32Seligman & Ollendick (Reference Seligman and Ollendick2011); 33Matson, Boisjoli, & Wilkins (Reference Matson and González2007); 34Matson & Gonzalez, Reference Matson and González2007; 35Matson, Terlonge, & Gonzalez (Reference Matson, Terlonge and González2006); 36Gotham et al. (Reference Gotham, Pickles and Lord2009); 37Wechsler (Reference Wechsler1974); WISC-R Project Group, Reference Van Haasen, De Bruyn, Pijl, Poortinga, Spelberg, Van der Steene, Coetsier, Spoelders-Claes and Stinissen1986; 38Bart & Peterson (Reference Bart, Peterson and Salkind2008); 39 Elliot (Reference Elliot2007); 40Mullen (Reference Mullen1995); 41Wechsler (Reference Wechsler2003); 42Wechsler (Reference Wechsler1999); 43Chorpita et al. (Reference Chorpita, Yim, Moffitt, Umemoto and Francis2000); 44March et al. (Reference March, Parker, Sullivan, Stallings and Conners1997); 45Radloff (Reference Radloff1977); 46Guralnick (Reference Guralnick1997); 47Achenbach (Reference Achenbach2009)
Genetic predisposition for internalizing symptoms
Parent history of anxiety/depression
In non-ASD samples, a genetic predisposition is one biological risk factor for the development of internalizing symptoms, with heritability estimates ranging from 40% to 73%, depending on the specific internalizing condition (American Psychiatric Association, 2013). In parents of children with ASD, high rates of depression and anxiety are present with median meta-analytic proportions found to be 31% and 33% for depressive and anxiety disorders, respectively (Schnabel et al., Reference Schnabel, Youssef, Hallford, Hartley, McGillivray, Stewart and Austin2019). Importantly, research has shown that parents’ internalizing symptoms are associated with children's internalizing symptoms in ASD populations (e.g., Conner, Maddox, & White, Reference Conner, Maddox and White2013; Mazefsky, Conner, & Oswald, Reference Mazefsky, Conner and Oswald2010). However, these studies did not assess for the presence of internalizing conditions in parents prior to birth or during pregnancy leaving questions as to whether parents had a history of anxiety or depression prior to the time of the study.
One way to assess for risk of anxiety or depression prenatally, in order to begin to investigate genetic heritability of internalizing conditions, is to investigate antidepressant use during pregnancy. Maternal antidepressant use including selective serotonin reuptake inhibitors (SSRIs) during pregnancy has been associated with risk for ASD (Croen, Grether, Yoshida, Odouli, & Hendrick, Reference Croen, Grether, Yoshida, Odouli and Hendrick2011), with one study only seeing the association for males with ASD (Harrington, Lee, Crum, Zimmerman, & Hertz-Picciotto, Reference Harrington, Lee, Crum, Zimmerman and Hertz-Picciotto2014). Although these studies did not assess risk for internalizing symptoms in offspring, results support the hypothesis that parents may already have an internalizing condition prior to a child's ASD diagnosis and points to genetic heritability as a potential mechanism through which risk for internalizing conditions in offspring with ASD may occur. To our knowledge, there are no known genetic studies investigating the heritability of internalizing conditions in ASD-specific populations to date. However, as discussed in Vasa and Mazurek's (Reference Vasa and Mazurek2015) paper on anxiety in youth with ASD, small family studies have identified several potential genes associated with risk of anxiety in ASD including the dopamine transporter gene, the D4 receptor gene, monoamine oxidase A (MAOA) gene, and the glutamate transporter genes. Therefore, given the high heritability of internalizing conditions in non-ASD conditions and the high rates of internalizing conditions in parents of children with ASD, genetic risk should be included in models of risk for co-occurring internalizing conditions in children with ASD.
Environmental factors associated with internalizing symptoms
Parenting stress
Parenting stress, or psychological distress due to the demands of raising a child, is common for all parents (Deater-Deckard, Reference Deater-Deckard1998). Parenting stress has been associated with both parenting quality and internalizing symptoms in non-ASD samples, such that higher levels of parenting stress is associated with children's internalizing symptoms (Costa, Weems, Pellerin, & Dalton, Reference Costa, Weems, Pellerin and Dalton2006; Rodriguez, Reference Rodriguez2011). Parenting a child with a neurodevelopmental disorder, such as ASD, comes with unique stressors, such as additional behavioral challenges and stress that may arise from, for example, navigating the additional services needed. Parents of children with ASD consistently report having higher stress levels than parents of children without ASD (Hayes & Watson, Reference Hayes and Watson2013).
The impact parenting stress has on internalizing conditions in children with ASD is an emerging area of research. In one study, maternal stress moderated associations between bullying victimization and anxiety in adolescents with ASD, such that the strongest associations between bullying victimization and anxiety were observed when parents reported higher levels of stress (Weiss et al., Reference Weiss, Cappadocia, Tint and Pepler2015). This study noted the importance of parental warmth in undermining these associations, pointing to another piece of our conceptual model and place for potential intervention. Additionally, relations among parent stress, child characteristics such as behavior problems and increased rates of anxiety or depression in parents has been observed (e.g., Rezendes & Scarpa, Reference Rezendes and Scarpa2011), underscoring the importance of providing intervention strategies for parents in order to help manage stress.
Parenting quality
The quality of care a child receives has long-term effects on children's functioning across all domains of development including cognitive (e.g., Raby, Roisman, Fraley, & Simpson, Reference Raby, Roisman, Fraley and Simpson2015), language (Tamis-LeMonda, Kuchirko, & Song, Reference Tamis-LeMonda, Kuchirko and Song2014), physical (e.g., Bernier, Calkins, & Bell, Reference Bernier, Calkins and Bell2016), and socio-emotional (e.g., Rothbaum & Weisz, Reference Rothbaum and Weisz1994). Caregiving quality has also been identified as a predictor of internalizing symptoms in non-ASD populations (e.g., McLeod, Weisz, & Wood, Reference McLeod, Wood and Weisz2007; Yap & Jorm, 2015). Importantly, parenting quality is not associated with the emergence or development of ASD, instead risk for ASD is due largely to genetic factors, with some estimates indicating the median heritability of ASD is 80% (Bai et al., Reference Bai, Yip, Windham, Sourander, Francis, Yoffe and Sandin2019), as well as environmental influences (Gardener, Spiegelman, & Buka, Reference Gardener, Spiegelman and Buka2011; Modabbernia, Velthorst, & Reichenberg, Reference Modabbernia, Velthorst and Reichenberg2017). However, given the strong links between parenting quality and internalizing in the non-ASD literature, parenting quality may be a contributing factor in the development or maintenance of internalizing symptoms in ASD, but relatively little research has examined these associations.
Aspects of positive and negative parenting and their associations with internalizing symptoms have only just begun to be investigated as protective or risk factors in ASD, respectively. Positive parenting includes responsive parenting (i.e., parents that are accepting, supportive, sensitive, and warm), behavioral control (i.e., setting clear and consistent expectations), and autonomy granting (i.e., allowing children to choose activities and make decisions independently). Negative parenting includes harsh discipline or control (i.e., physical or verbal punishment) and psychological control (i.e., manipulating children's experiences and emotions). When investigating parental adjustment (i.e., parent-reported symptoms of anxiety/depression), parenting quality (warm/supportive parenting and harsh discipline), and the presence of internalizing or externalizing symptoms in a structural equation model, higher levels of warm/supportive parenting predicted fewer reported internalizing symptoms (β = −.49, p <.01; (McRae et al., Reference McRae, Stoppelbein, O'Kelley, Fite and Greening2018). Additionally, harsh discipline predicted increased rates of externalizing symptoms (β = .59, p <.01) within a sample of children with ASD. Notably, there was also a direct effect from parental adjustment to child internalizing symptoms (β = .32, p <.01), indicating that higher levels of concurrent parental internalizing symptoms is also a risk factor for child's internalizing symptoms. This is consistent with our previous discussion related to parenting stress and again points to parental adjustment/stress as a putative intervention target. Adding support to positive parenting as a protective factor against child internalizing, a similar association between fewer warm/supportive parenting behaviors and borderline to clinically significant levels of internalizing symptoms in children with ASD was found in another study by McRae et al. (Reference McRae, Stoppelbein, O'Kelley, Fite and Greening2019). Further, in a longitudinal study, negative parental control when children were approximately 10 years old was associated with increased internalizing symptoms 6 years later (β = .15, p < .05; Dieleman et al., Reference Dieleman, De Pauw, Soenens, Beyers and Prinzie2017).
However, it is important to note that other studies investigating associations between positive parenting and internalizing symptoms in children have yielded no association. For example, Maljaars et al. (Reference Maljaars, Boonen, Lambrechts, Van Leeuwen and Noens2014) and Boonen et al. (Reference Boonen, Maljaars, Lambrechts, Zink, Van Leeuwen and Noens2014), reported no statistically significant associations between variability in parenting quality and level of internalizing symptoms (r = .06, p > .05) in children with ASD. Further, only one study published to date investigated associations between positive parenting in a small sample (n = 43) of younger children (i.e., approximately 4.5 years old) with ASD and internalizing symptoms, yielding no statistically significant associations (Baptista et al., Reference Baptista, Sampaio, Fachada, Osório, Mesquita, Garayzabal and Soares2019). Thus, it is unclear whether positive parenting serves as protective factor for internalizing symptoms in ASD populations or not.
The lack of association between positive parenting and internalizing symptoms in the aforementioned samples may be, in part, due to issues in measurement. For example, two of the three studies that found no support (Boonen et al., Reference Boonen, Maljaars, Lambrechts, Zink, Van Leeuwen and Noens2014; Maljaars et al., Reference Maljaars, Boonen, Lambrechts, Van Leeuwen and Noens2014) used the Strengths and Difficulties Questionnaire (SDQ; Goodman, Reference Goodman1997) to assess internalizing symptoms, which is a brief (20-item) screening measure assessing externalizing behaviors (e.g., conduct, hyperactivity), emotional symptoms, and peer problems. Although it is highly correlated with the broad internalizing scale on the Child Behavior Checklist (CBCL) (r = .74; Goodman & Scott, Reference Goodman and Scott1999), it does not provide a more specific breakdown of which types of internalizing symptoms (e.g., anxious/depressed, withdrawn/depressed, somatic complaints). The use of brief screening measures of symptoms may lead to inconclusive evidence because they may not be as sensitive to differentiate between anxiety-specific problems and other ASD-related problems (e.g., social difficulties).
Another potential reason for the inconclusive findings may be related to the timing of when parenting is measured. One theoretical model, the enduring effects model, posits that early (i.e., during infancy or early childhood) caregiving experiences leave lasting impacts on children's development (Haltigan, Roisman, & Fraley, Reference Haltigan, Roisman and Fraley2013). Indeed, in non-ASD literature this has been the case, such that caregiving experiences during infancy were a protective factor of internalizing problems later in life (Faure et al., Reference Faure, Habersaat, Harari, Müller-Nix, Borghini, Ansermet and Urben2017; Kok et al., Reference Kok, Linting, Bakermans-Kranenburg, van IJzendoorn, Jaddoe, Hofman and Tiemeier2013; Wagner, Propper, Gueron-Sela, & Mills-Koonce, Reference Wagner, Propper, Gueron-Sela and Mills-Koonce2016). No studies have been published to date assessing the enduring effect caregiving quality during infancy has on children's development of internalizing conditions over time in an ASD population, highlighting another area of future research. Thus, the impact early caregiving has on the development of internalizing conditions in ASD and parenting quality later in life has once internalizing conditions have already developed needs empirical testing.
Parental expressed emotion
One aspect of the parent–child relationship that has been studied in the ASD literature is parental expressed emotion (a construct assessing the emotional climate between parents and children with distinct domains including warmth, the intensity of feeling by which the parent describes the child, critical statements about their child, and over-involvement). Romero-Gonzalez, Chandler, and Simonoff (Reference Romero-Gonzalez, Chandler and Simonoff2018) conducted a meta-analysis that investigated the different domains of parental expressed emotion and their association with co-occurring psychopathology in children with ASD, suggesting that there were associations between high levels in the domain of criticism and externalizing problems and inconclusive results with associations with internalizing conditions. However, although the gold-standard approach of assessing the domains of expressed emotion is through the Camberwell Family Interview (CFI; Leff & Vaughn, Reference Leff and Vaughn1985) as identified and accepted in the field (Hooley & Parker, Reference Hooley and Parker2006), most of the studies in the meta-analysis derived the domains of expressed emotion from the five-minute speech sample (FMSS; Magaña et al., Reference Magaña, Goldstein, Karno, Miklowitz, Jenkins and Falloon1986) or self-report measures of feelings toward their child. The FMSS approach involves parents talking about their child for five minutes and is later coded regarding descriptions of their child. Another limitation of this meta-analysis and focusing solely on the construct of expressed emotion is that most of the studies included were derived from the parents’ descriptions rather than observed or objective behaviors of the parent–child relationship. Therefore, both parenting quality (i.e., positive parenting, negative parenting) and aspects of the parent–child relationship (e.g., expressed emotion) may be important to pursue as risk or protective factors for internalizing symptoms in children with ASD.
Child social factors
Presence and quality of peer relationships
As children get older, peer relationships become increasingly more important. The presence of positive peer relationships in non-ASD samples is associated with positive outcomes, more adaptive functioning, and lower levels of internalizing symptoms (Chu, Saucier, & Hafner, Reference Chu, Saucier and Hafner2010; Hodges, Boivin, Vitaro, & Bukowski, Reference Hodges, Boivin, Vitaro and Bukowski1999; Ladd, Reference Ladd1990). In ASD samples, similar protective effects have been observed in some samples, but not others. For example, a better quality of social relations was associated with less anxiety in children aged 6–13 years old and more anxiety was reported for children who engaged in less social contact (Eussen et al., Reference Eussen, Van Gool, Verheij, De Nijs, Verhulst and Greaves-Lord2013). In adults with ASD, the quality and quantity of peer relationships was inversely related to loneliness and, in a separate model the number of peer relationships was associated with internalizing symptoms (Micah O Mazurek, Reference Mazurek2014). In preschool-aged children with ASD who also had a sibling with ASD, having no peers was associated with significantly higher levels of internalizing scores on the CBCL relative to children with no diagnosis of ASD and siblings of children with an ASD diagnosis who themselves had no diagnosis (Estes et al., Reference Estes, Munson, John, Dager, Rodda, Botteron, Hazlett, Schultz, Zwaigenbaum, Piven and Guralnick2018). These results highlight the protective effect positive peer relationships have on emotional functioning throughout development for people with ASD. Alternatively, Dovgan and Mazurek (Reference Dovgan and Mazurek2019) found that children and adolescents aged 6–17 years old who participated in more activities (e.g., sports, hobbies, etc.) were more likely to have at least one friend; however, the presence of friendships or participation in activities did not relate to internalizing symptoms in this sample. Alternatively, negative peer relations and interactions are associated with increased internalizing symptoms. For example, when controlling effects of age, family income, and symptom severity, experiences of peer victimization during adolescence were associated with increased rates of internalizing symptoms (Adams et al., Reference Adams, Fredstrom, Duncan, Holleb and Bishop2014). Interestingly, one study investigating associations among family cohesion, positive peer relationships, internalizing symptoms, and ASD symptomatology found no significant associations between positive peer relationships and internalizing symptoms (Kelly et al., Reference Kelly, Garnett, Attwood and Peterson2008). However, family cohesion predicted lower levels of internalizing symptoms in this model, again pointing to the potential buffering effect of family relationships and the need to examine putative risk and protective factors together.
Neurobiological risk factors for internalizing conditions
Research in children and adolescents with ASD has recently begun to focus on the neural correlates of ASD and internalizing symptoms, again pointing to an important area for further exploration (Vasa & Mazurek, Reference Vasa and Mazurek2015). The amygdala is an area of the brain that is involved in emotion processing, negative valence systems including anxiety and fear, reward learning, approach motivation (Hennessey, Andari, & Rainnie, Reference Hennessey, Andari and Rainnie2018), and salience or novelty (Todd, Evans, Morris, Lewis, & Taylor, Reference Todd, Evans, Morris, Lewis and Taylor2010; Todorov, Reference Todorov2012).
Amygdala volume
Amygdala volumes have been associated with individual differences in internalizing symptoms in non-ASD samples, but with mixed findings, such that some studies point to increased amygdala volumes (Albaugh et al., Reference Albaugh, Nguyen, Ducharme, Collins, Botteron, D'Alberto and Hudziak2017; De Bellis et al., Reference De Bellis, Casey, Dahl, Birmaher, Williamson, Thomas and Ryan2000; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Casey2010; van der Plas, Boes, Wemmie, Tranel, & Nopoulos, Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010; Weems, Klabunde, Russell, Reiss, & Carrión, Reference Weems, Klabunde, Russell, Reiss and Carrión2015) and other studies point to decreased amygdala volumes (McQueeny et al., Reference McQueeny, Padula, Price, Medina, Logan and Tapert2011; Milham et al., Reference Milham, Nugent, Drevets, Dickstein, Leibenluft, Ernst and Pine2005; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013; Strawn et al., Reference Strawn, Hamm, Fitzgerald, Fitzgerald, Monk and Phan2015; Warnell, Pecukonis, & Redcay, Reference Warnell, Pecukonis and Redcay2018) relating to greater internalizing symptoms. Recent work investigating amygdala volume and internalizing symptoms during middle childhood found that there were no group differences evident between amygdala volumes when comparing typically developing (TD) children and children with ASD until they subdivided children with ASD into those with and without co-occurring internalizing conditions (i.e., anxiety; Herrington, Parma, & Miller, Reference Herrington, Parma, Miller, Kerns, Renno, Storch, Kendall and Wood2017). Those with co-occurring internalizing conditions had a decrease in amygdala volume relative to TD children. Additionally, three empirical studies to date have investigated associations between amygdala volume and internalizing symptoms in children with ASD yielding mixed results. Herrington, Parma, and Miller (Reference Herrington, Parma, Miller, Kerns, Renno, Storch, Kendall and Wood2017) found a trend (r (55) = −.21, p = .09) between right amygdala volume and total anxiety symptoms as measured using the parent-report version of the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997) and the association was strongest between the panic/somatic subscale (r (55) = −.30, p = .02). Further, children with ASD and internalizing symptoms exhibited decreased amygdala volumes relative to children with ASD and no anxiety symptoms and typically developing controls. However, in a large sample (n = 300) of pre-school-aged children with ASD, Nordahl et al. (Reference Nordahl, Iosif, Young, Hechtman, Heath, Lee, Libero, Reinhardt, Winder-Patel, Amaral, Rogers, Solomon and Ozonoff2020) demonstrated a significant association between right amygdala volume and internalizing symptoms on the CBCL such that larger amygdala volumes were associated with higher levels of internalizing symptoms, although the association was observed only in females and not males. Further, Juranek et al. (Reference Juranek, Filipek, Berenji, Modahl, Osann and Spence2006) found a positive association between elevated internalizing symptoms, as measured using the Anxious/Depressed parent-report subscale of the CBCL, and total amygdala volumes (i.e., sum of left and right; r (40) = .39, p = .01) and right amygdala volumes (r (40) = .47, p < .01). These studies are cross-sectional in nature and therefore do not provide information regarding the developmental course of amygdala development and internalizing symptoms over time. Therefore, it is not possible to determine whether internalizing symptoms cause changes in the amygdala or vice versa.
Amygdala activation
In non-ASD populations, including both non-clinical populations and in those diagnosed with social anxiety disorder (SAD) or generalized social phobia, increased amygdala activation in response to faces is well established. This has been observed in adults (Bishop, Duncan, & Lawrence, Reference Bishop, Duncan and Lawrence2004; Brühl, Delsignore, Komossa, & Weidt, Reference Brühl, Delsignore, Komossa and Weidt2014; Phan, Fitzgerald, Nathan, & Tancer, Reference Phan, Fitzgerald, Nathan and Tancer2006) and in children and adolescents (Killgore & Yurgelun-Todd, Reference Killgore and Yurgelun-Todd2005; Monk et al., Reference Monk, Telzer, Mogg, Bradley, Mai, Louro and Pine2008), with results pointing to right amygdala activation being uniquely associated with internalizing symptoms. For example, in participants with SAD relative to controls, those with SAD showed increased right amygdala activation and decreased left amygdala activation to neutral faces relative to oval stimuli (Cooney, Atlas, Joormann, Eugène, & Gotlib, Reference Cooney, Atlas, Joormann, Eugène and Gotlib2006). Further, amygdala activation to interpersonal threat (i.e., fearful faces) has been found to be positively correlated with severity of social anxiety symptoms (Phan et al., Reference Phan, Fitzgerald, Nathan and Tancer2006). However, the type of internalizing condition and stimulus may determine the direction or presence of activation. For example, Hattingh et al. (Reference Hattingh, Ipser, Tromp, Syal, Lochner, Brooks and Stein2013)'s meta-analysis demonstrated that social anxiety disorder is associated with increased amygdala activation in response to emotional faces. Alternatively, in a meta-analysis assessing amygdala activation to faces in depressed patients, increased activation to negative stimuli and decreased activation for positive stimuli was observed (Groenewold, Opmeer, de Jonge, Aleman, & Costafreda, Reference Groenewold, Opmeer, de Jonge, Aleman and Costafreda2013). Interestingly, in a meta-analysis of youth with depression, amygdala activation was not indicated as being associated with response to emotional faces (Miller, Hamilton, Sacchet, & Gotlib, Reference Miller, Hamilton, Sacchet and Gotlib2015).
The amygdala, including size (e.g., Cody, Pelphrey, & Piven, Reference Cody, Pelphrey and Piven2002; Mosconi et al.,Reference Mosconi, Cody-Hazlett, Poe, Gerig, Gimpel-Smith and Piven2009; Nordahl et al., Reference Nordahl, Scholz, Yang, Buonocore, Simon, Rogers and Amaral2012; Schumann, Barnes, Lord, & Courchesne, Reference Schumann, Barnes, Lord and Courchesne2009) and activation (e.g., Di Martino et al., Reference Di Martino, Ross, Uddin, Sklar, Castellanos and Milham2009), is one area of the brain that has been consistently implicated in ASD, independent of accounting for the presence of internalizing symptoms. This association is theorized to be due to the amygdala's role in the core autism symptoms including deficits in social communication and interaction as evidenced by difficulties in social cognition and emotion recognition (Baron-Cohen et al., Reference Baron-Cohen, Ring, Bullmore, Wheelwright, Ashwin and Williams2000; Dziobek, Fleck, Rogers, Wolf, & Convit, Reference Dziobek, Fleck, Rogers, Wolf and Convit2006). Recent research has pointed to how co-occurring internalizing symptoms may be associated with amygdala size and activation in ASD samples. For example, in adults with ASD, increased social anxiety was associated with increased right amygdala activation in response to emotional faces (Kleinhans et al., Reference Kleinhans, Richards, Weaver, Johnson, Greenson, Dawson and Aylward2010). Children with ASD and low levels of anxiety exhibited decreased amygdala activation relative to controls (Herrington et al., Reference Herrington, Miller, Pandey and Schultz2016). Anxiety symptoms in general were positively associated with amygdala activation across the full sample of children with ASD and core symptoms of ASD were negatively correlated with amygdala activation. These results were validated in a second, separate sample (Herrington, Parma, & Miller, Reference Herrington, Parma, Miller, Kerns, Renno, Storch, Kendall and Wood2017). Therefore, emerging evidence suggests that amygdala hyperactivation may be a marker of co-occurring anxiety symptoms.
Amygdala–medial prefrontal cortex (mPFC) (functional connectivity)
Functional connectivity is the temporal correlation of activation between brain regions and can be in response to a task or task-independent (i.e., resting-state functional connectivity). In adults with generalized anxiety disorder (GAD) relative to adults without GAD, GAD adults showed greater resting-state functional connectivity between areas of the brain including the amygdala, insula, putamen, thalamus, and posterior cingulate cortex (Qiao et al., Reference Qiao, Li, Cao, Wang, Sun and Xu2017). Additionally, effective connectivity from the amygdala to the prefrontal cortex and basal ganglia was decreased in GAD. Further, Hahn et al. (Reference Hahn, Stein, Windischberger, Weissenbacher, Spindelegger, Moser and Lanzenberger2011) found decreased resting-state functional connectivity between the left amygdala and the medial orbitofrontal cortex and amygdala and posterior cingulate cortex/precuneus. Functional connectivity was negatively associated with severity of anxiety. In healthy adults, the anterior insula–basolateral amygdala connectivity during resting-state was identified as being associated with both state and trait anxiety (Baur, Hänggi, Langer, & Jäncke, Reference Baur, Hänggi, Langer and Jäncke2013). According to a recent meta-analysis, task-related functional connectivity studies of adults with SAD generally exhibit increased connectivity between the amygdala and mPFC (Brühl et al., Reference Brühl, Delsignore, Komossa and Weidt2014). In children and adolescents, right amygdala and right ventrolateral prefrontal cortex showed strong negative coupling to masked angry faces (Monk et al., Reference Monk, Telzer, Mogg, Bradley, Mai, Louro and Pine2008). This association was weaker in youth with GAD relative to TD.
In two meta-analyses of adult and child ASD samples, the majority of studies pointed to reduced connectivity or “under connectivity” among brain regions including the prefrontal cortex (Müller et al., Reference Müller, Shih, Keehn, Deyoe, Leyden and Shukla2011; Rane et al., Reference Rane, Cochran, Hodge, Haselgrove, Kennedy and Frazier2015). However, some studies have continued to indicate increased connectivity, indicating that other individual characteristics may explain the differences observed in connectivity. Further, some studies are finding no differences in functional connectivity when accounting for the effects of head motion (e.g., He, Byrge, & Kennedy, Reference He, Byrge and Kennedy2019). Importantly, studies to date have not yet examined the role anxiety may play in the aberrant connectivity, pointing to an area of future investigation, especially given the associations observed in non-ASD, anxiety-laden samples.
Default mode and salience networks
As previously discussed, Burrows et al. (Reference Burrows, Timpano and Uddin2017)'s proposed model of risk for internalizing symptoms identified the DMN and salience networks as additional putative neural mechanisms for the development of internalizing conditions in ASD. Although an area of emerging research, one study has investigated these theorized mechanisms, finding that the resting-state connectivity among the salience network and DMN was associated with higher levels of internalizing symptoms in adolescents and young adults with ASD (Hogeveen et al., Reference Hogeveen, Krug, Elliott and Solomon2018). Specifically, the connectivity between the anterior insula and the retrosplenial cortex showed overconnectivity in ASD. Age was not associated with these findings and no other connections among the salience network, DMN, or frontoparietal network were significantly associated with internalizing symptoms.
Parenting quality and amygdala development
In non-ASD populations, parental warmth and support has been identified as one form of parenting behavior that is associated with brain development and functioning, and may buffer children from developing internalizing symptoms. A longitudinal study of parenting found that a higher rate of observed maternal warmth and support when children were 12 years old predicted a slower growth in the right amygdala four years later, as compared to children whose parents were observed to engage in a lower rate of maternal warmth and support (Whittle et al., Reference Whittle, Simmons, Dennison, Vijayakumar, Schwartz, Yap and Allen2014). Further, maternal warmth and support is associated with decreased amygdala activation when observing fearful faces in healthy adolescents (Romund et al., Reference Romund, Raufelder, Flemming, Lorenz, Pelz, Gleich and Beck2016). To the best of our knowledge, no published studies have examined parenting quality's association with amygdala structure or function in an ASD sample, a clear path for future research in order to examine whether the amygdala serves as a mechanism between environmental influences and the development of internalizing symptoms.
Hypothalamic–pituitary–adrenal (HPA) axis
Another potential biological marker of anxiety in ASD is cortisol, an end product of the HPA axis. The HPA axis maintains a diurnal rhythm of cortisol, such that cortisol is elevated in the morning, peaking approximately 30 minutes post-awakening (i.e., the cortisol awakening response), and declines throughout the day (Russell & Shipston, Reference Russell and Shipston2015). In addition to its production throughout the day, cortisol is also produced as part of the HPA axis stress response, peaking approximately 20 minutes after exposure to an acute stressor, although age, sex, and other factors can impact the magnitude of cortisol reactivity (Foley & Kirschbaum, Reference Foley and Kirschbaum2010). The amygdala plays a role in activation of the HPA axis through the detection of threat and stress (e.g., Pruessner et al., Reference Pruessner, Dedovic, Pruessner, Lord, Buss, Collins and Lupien2010). Experiences of acute and/or chronic stress can impact both the diurnal production of cortisol and cortisol produced as part of the HPA axis stress response (Russell & Shipston, Reference Russell and Shipston2015).
In ASD samples, a blunted cortisol response appears to exist in response to both chronic and acute stress. For example, Bitsika et al. (Reference Bitsika, Sharpley, Sweeney and McFarlane2014) found significant correlations between autistic boys’ self-report of anxiety symptoms and morning levels of diurnal cortisol; however, parent-report of children's anxiety symptoms were not significantly associated. Bitsika et al. (Reference Bitsika, Sharpley, Andronicos and Agnew2015) in a larger sample of boys identified an interaction between age, diurnal cortisol, and anxiety. Specifically, children's afternoon cortisol was significantly correlated with parent- and self-reports of internalizing symptoms, but there was no significant association between these variables for adolescents in the study. Further, in Sharpley et al.'s (Reference Sharpley, Bitsika, Andronicos and Agnew2016) study of girls with co-occurring internalizing symptoms, the normative cortisol awakening response was not observed in this sample. Instead, more than half of the participants evidenced either a flat slope or a decrease in cortisol from waking to shortly after waking and this dysregulation was associated with self-reported internalizing symptoms, specifically those related to major depressive disorder. For acute stressors results have been mixed with regards to whether co-occurring internalizing symptoms in ASD is associated with cortisol response. For example, Hollocks et al. (Reference Hollocks, Howlin, Papadopoulos, Khondoker and Simonoff2014) found that children and adolescents (aged 10–16 years) exhibited a blunted cortisol response after participation in an adapted Trier Social Stress Test (TSST) relative to ASD without co-occurring anxiety and TD children. Further, cortisol responsiveness was also associated with increased anxiety symptoms. Alternatively, (Simon & Corbett, Reference Simon and Corbett2013) found no association with internalizing symptoms and the cortisol response to the TSST, a mock magnetic resonance imaging scan, or a playground social stress paradigm. In sum, research points to atypical HPA axis functioning and associations with anxiety, although more research should continue to clarify these associations.
Gender dysphoria
Gender dysphoria is “the distress that may accompany the incongruence between one's experienced or expressed gender and one's assigned gender” (American Psychiatric Association, 2013, p. 451). While this is a newly emerging area of research, a growing body of literature is pointing to high rates of gender dysphoria in people diagnosed with ASD (van der Miesen, Hurley, Bal, & de Vries, Reference van der Miesen, Hurley, Bal and de Vries2018; van der Miesen, Hurley, & De Vries, Reference van der Miesen, Hurley and De Vries2016). Recent work by George and Stokes (Reference George and Stokes2018) found that people with ASD (Mage = 31.01, SD = 11.37) and gender dysphoria and/or nonheterosexuality had poorer mental health (including more internalizing problems) and poorer overall well-being relative to typical controls. Further work should continue to investigate the impact gender dysphoria and nonheterosexuality has on the development of internalizing symptoms in ASD populations.
Potential moderators
Additional factors that may moderate associations between risk and protective factors and internalizing symptoms in children with ASD are described below. However, as summarized below, the associations among these variables and internalizing symptoms is complex and no clear picture has emerged yet.
Age
The developmental course of internalizing symptoms in ASD remains unclear. One cross-sectional study of internalizing symptoms across the lifespan, which included participants 17 months through 65 years old, found a nonlinear trajectory of internalizing symptoms, such that the level of reported anxiety in the sample was elevated during childhood (ages 3–16 years), followed by a decline in young adulthood (ages 20–48 years), and an increase again in adulthood (ages 49–65 years; Davis et al., Reference Davis, Hess, Moree, Fodstad, Dempsey, Jenkins and Matson2011). This nonlinear trajectory could relate to developmental transitions when peers and/or significant others become more important than relations with parents or when there are significant biological changes (e.g., puberty, brain development). In a large sample of children aged 6 to 17 years, 20% of adolescents were reported as having a previous depression diagnosis compared to only 4.8% of children (Greenlee et al., Reference Greenlee, Mosley, Shui, Veenstra-VanderWeele and Gotham2016). Both of these studies involved investigating age related between-person change, rather than within-person change longitudinally, pointing to a necessary step in future work in order to truly characterize the developmental course of internalizing symptoms in ASD.
As discussed earlier, the amygdala may be associated with internalizing symptoms in ASD and non-ASD populations. Additionally, age has been identified as being associated with the development of the amygdala, such that there is a nonlinear growth trajectory in amygdala volumes. Human studies assessing the growth trajectory of the amygdala suggest that the amygdala continues to grow throughout the lifespan, but with the most rapid change during early and middle childhood (Ostby et al., Reference Ostby, Tamnes, Fjell, Westlye, Due-Tønnessen and Walhovd2009). Recent work in macaque monkeys demonstrated significant linear changes in amygdala volumetric growth as well, with nearly 50% of the increases observed within the first 5 years of life, which although not formally assessed in this study is equivalent to after or around puberty (Schumann, Scott, Lee, Bauman, & Amaral, Reference Schumann, Scott, Lee, Bauman and Amaral2019). These age effects have been observed in research looking at the associations between internalizing conditions and amygdala volume in non-ASD samples. For example, Warnell et al. (Reference Warnell, Pecukonis and Redcay2018) found that the association between amygdala volume and internalizing symptoms was moderated by age such that children who were 11 years or older no longer exhibited an association. Similar to non-ASD research, the age at which relations between amygdala volume and internalizing symptoms are assessed may impact whether or not significant associations are observed. This is due to differing growth trajectories that have been observed in children with ASD relative to TD samples (Schumann et al., Reference Schumann, Barnes, Lord and Courchesne2009), albeit studies of amygdala volumes have mixed results with regards to whether or not people with ASD have smaller, larger, or no difference in amygdala size compared to TD individuals (Cody et al., Reference Cody, Pelphrey and Piven2002). For example, Schumann et al. (Reference Schumann, Barnes, Lord and Courchesne2009) found larger left, right, and overall amygdala volumes in toddlers later diagnosed with ASD as compared to toddlers not diagnosed with ASD. Further, faster amygdala growth was observed in children with ASD (Nordahl et al., Reference Nordahl, Scholz, Yang, Buonocore, Simon, Rogers and Amaral2012) and Mosconi et al. (Reference Mosconi, Cody-Hazlett, Poe, Gerig, Gimpel-Smith and Piven2009) identified that amygdala volume was enlarged by age 2 years relative to controls. However, these studies were not designed to investigate whether co-occurring internalizing conditions, which may explain differences observed.
Sex
Sex differences in the prevalence of internalizing conditions exist with females generally showing higher rates of internalizing conditions than males including depression (Nolen-Hoeksema, Reference Nolen-Hoeksema2001; Piccinelli & Wilkinson, Reference Piccinelli and Wilkinson2000) and anxiety disorders (McLean, Asnaani, Litz, & Hofmann, Reference McLean, Asnaani, Litz and Hofmann2011; Xu et al., Reference Xu, Schneier, Heimberg, Princisvalle, Liebowitz, Wang and Blanco2012). The emergence of sex differences in depression has been suggested to occur during early adolescence (Nolen-Hoeksema & Girgus, Reference Nolen-Hoeksema and Girgus1994) with one study noting the greatest difference between the prevalence of depressive symptom occurred between the ages of 15 and 18 years (Hankin et al., Reference Hankin, Abramson, Moffitt, Silva, McGee and Angell1998). The emergence of sex differences in anxiety disorders may occur during early childhood as Lewinsohn, Gotlib, Lewinsohn, Seeley, and Allen (Reference Lewinsohn, Gotlib, Lewinsohn, Seeley and Allen1998) note that females at age 6 years were twice as likely as males to have experienced an anxiety disorder.
The presence of sex differences in ASD more broadly has been examined frequently, such that males are more likely to be diagnosed with ASD, yet it is unclear whether there are sex differences in the presence of co-occurring internalizing symptoms. Mixed results have emerged with regard to the presence of internalizing symptoms in males relative to females. In a nationally representative sample of children, Stacy et al. (Reference Stacy, Zablotsky, Yarger, Zimmerman, Makia and Lee2014) found that females with a current ASD diagnosis were less likely than males to be diagnosed with a past anxiety disorder; however, this association was no longer significant after adjusting for race and mother's education. Alternatively, in one study of adolescents with and without ASD, females with ASD exhibited more internalizing symptoms than males with ASD and their TD counterparts in early adolescence, which was hypothesized to relate to biological (e.g., genetic, hormonal) and psychosocial factors that increase risk for internalizing symptoms during adolescence (Oswald et al., Reference Oswald, Winter-Messiers, Gibson, Schmidt, Herr and Solomon2016). Future work should continue to characterize the prevalence of internalizing symptoms in ASD and their associations with sex.
Autism severity and IQ
A complex and inconsistent picture emerges regarding the severity of autism symptoms and a child's IQ as potential moderators of internalizing symptoms in ASD. For example, in a large sample of children aged 4 to 17 years with ASD and a high prevalence of co-occurring internalizing symptoms, higher ASD severity scores and a lower IQ was associated with fewer internalizing symptoms (Mazurek & Kanne, Reference Mazurek and Kanne2010). Alternatively, Eussen et al. (Reference Eussen, Van Gool, Verheij, De Nijs, Verhulst and Greaves-Lord2013)'s study of children 6 to 13-years-old identified global levels of ASD symptom severity as having the strongest association with internalizing symptoms when controlling for other variables in the model including quality of social relations and IQ. These relations were true for all aspects of ASD symptomatology. Additionally, in this sample, IQ was not associated with internalizing symptoms in either direction. Yet, Greenlee et al. (Reference Greenlee, Mosley, Shui, Veenstra-VanderWeele and Gotham2016)'s cross-sectional study indicated that a history of depression diagnosis was associated with a higher IQ and Asperger's diagnosis (suggesting less symptom severity). Thus, it is currently unclear when ASD symptom severity or IQ is associated with the presence of internalizing symptoms.
Integrated Theoretical Model: Hypothesized Links and Future Directions
Our theorized conceptual model of risk and protective factors for internalizing symptoms in ASD (Figure 1) includes both direct and indirect links to internalizing symptoms and both child and parent factors. Further, the risk and protective factors outlined may be bidirectional and/or interact with other risk and protective factors identified, as well as with the moderators discussed, and other factors not included in this review. Below we outline a few proposed links organized in terms of developmental emergence in order to help identify areas for future research.
Parent factors (i.e., genetic heritability and parent history of internalizing symptoms) may predispose children to the development of internalizing symptoms. These parent internalizing symptoms, as well as parent stress that may be related or unrelated to their internalizing, may be associated with differences in parenting quality. Further, variations in parenting quality, especially during infancy and early childhood, in turn, could serve as moderators and/or risk or protective factors in the development and/or maintenance of internalizing symptoms in children; however, differences in child characteristics (e.g., temperament, gene–environment correlations), as well as the bidirectional relations between parent and child behavior should also be considered. Additionally, variations in parenting quality during infancy and early childhood can impact children's developing neurobiology (i.e., amygdala), which then influences children's developing stress responses (e.g., HPA axis functioning), and subsequently the development and/or maintenance of internalizing symptoms.
A child's diagnosis of ASD can lead to greater susceptibility to the development of internalizing symptoms through several potential pathways. First, this could be through neurodevelopmental effects on a child's neurobiology (e.g., effects on the amygdala and other brain networks) that are associated with a diagnosis of ASD (whether cause or consequence). Second, differences in social processing and cognition associated with an ASD diagnosis may hinder the development of social relationships or serve as a catalyst for increased internalizing symptoms related to social interactions. For example, difficulties with reading social cues may lead to the experience of negative social interactions which could lead to the development of anxiety related to future social interactions. Peer rejection, either due to atypical social interaction or other behavioral characteristics such as the presence of repetitive behaviors and/or restricted interests that may be immature for the child's age, may lead to peers making negative comments to or about the child with ASD. Finally, the presence of ASD and behaviors associated with ASD (e.g., insistence on sameness, difficulty with transitions) may influence the parenting environment leading to variations in parent stress, parenting quality, and parent's own mental health, which subsequently impacts children's development and/or maintenance of internalizing symptoms.
Summary
Internalizing conditions are among the most common co-occurring conditions in people diagnosed with ASD (Kent & Simonoff, Reference Kent, Simonoff, Kerns, Renno, Storch, Kendall and Wood2017; Rosen et al., Reference Rosen, Mazefsky, Vasa and Lerner2018). The presence of co-occurring internalizing symptoms in ASD samples leads to more functional impairment than in samples without co-occurring internalizing symptoms (Kerns et al., Reference Kerns, Kendall, Zickgraf, Franklin, Miller and Herrington2015; Kim et al., Reference Kim, Szatmari, Bryson, Streiner and Wilson2000; Pouw et al., Reference Pouw, Rieffe, Stockmann and Gadow2013) and may also be a contributing factor to risk for suicide (Richa, Fahed, Khoury, & Mishara, Reference Richa, Fahed, Khoury and Mishara2014). Therefore, identifying risk and protective factors of internalizing conditions in ASD is critical in order to continue to develop and refine prevention and intervention programs aimed at preventing and ameliorating internalizing symptoms. This paper summarized the current literature regarding environmental and biological risk and protective factors related to the etiology, development, and maintenance of internalizing symptoms in ASD. Further, we aimed to develop an integrative bio-psycho-social model of risk and protective factors pulling from the literature in both ASD and non-ASD populations.
Given the heterogeneity in ASD and development in general, no one path will explain the development of internalizing conditions in ASD for all individuals. However, this review emphasized several areas that are apt for future research and potential intervention in order to elucidate potential mechanisms and mitigating factors. Future studies should aim to include multiple levels of analysis (e.g., biological, environmental, social) and utilize longitudinal samples in order to delineate the developmental course of internalizing symptoms in ASD.
Conflicts of Interest
None.





