Introduction
Blastocystis spp. is the most frequent unicellular, anaerobic parasite found to infect the gastrointestinal tract (GIT) of nearly all species of animals as well as human beings. Its prevalence rates were higher in developing than in developed countries (Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014; Méabed et al., Reference Méabed, El-Sayed, Abou-Sreea and Roby2018).
Blastocystosis has been reported to be associated with dermatologic disorders as urticaria or many GIT manifestations, including recurrent watery or mucous diarrhoea, abdominal cramps, vomiting, and bloating (Mirza et al., Reference Mirza, Wu, Kidwai and Tan2011). The infection has been linked with acute and chronic diarrhoea, irritable bowel syndrome and possibly inflammatory bowel disease and has a high prevalence in immuno-compromised individuals. In immunocompetent individuals, Blastocystis infections are often asymptomatic or self-limiting (Abdel-Hafeez et al., Reference Abdel-Hafeez, Ahmad, Abdelgelil, Abdellatif, Kamal, Hassanin, Abdel-Razik and Abdel-Raheem2016).
Metronidazole is considered a first-line treatment for Blastocystis infection. The drug may directly affect the parasite or it may act by destroying the bacterial flora necessary for its growth or both (Dinleyici et al., Reference Dinleyici, Eren, Dogan, Reyhanioglu, Yargic and Vandenplas2011). However, there has been increasing evidence for the lack of efficacy of this treatment. Treatment failure has been reported in several clinical cases, and recent in vitro studies have suggested the occurrence of MTZ-resistant strains (Mirza et al., Reference Mirza, Wu, Kidwai and Tan2011).
The non-pathogenic yeast Saccharomyces boulardii (Sb) doesn't colonize the human intestinal mucosal cell lining. It is widely used as a probiotic, to promote health as it moves through the GIT (de Avila et al., Reference de Avila, Conceição, Telmo Pde, Dutra, de los Santos, Martins, Berne, da Silva and Scaini2012). The efficacy of Sb has been proved against various enteric pathogens, Clostridium difficile, Vibrio cholerae, Helicobacter pylori, enterohaemorrhagic and enteropathogenic Escherichia coli infections, besides several types of acute and chronic diarrhoea (Kelesidis and Pothoulakis, Reference Kelesidis and Pothoulakis2012). The beneficial effect of Sb against parasitic infections as Toxocara canis infection (de Avila et al., Reference de Avila, Conceição, Telmo Pde, Dutra, de los Santos, Martins, Berne, da Silva and Scaini2012), amoebiasis (Mansour-Ghanaei et al., Reference Mansour-Ghanaei, Dehbashi, Yazdanparast and Shafaghi2003; Dinleyici et al., Reference Dinleyici, Eren, Yargic, Dogan and Vandenplas2009), giardiasis (Castaneda et al., Reference Castaneda, Garcia, Santa Cruz, Fernandez and Monterrey1995; Besirbellioglu et al., Reference Besirbellioglu, Ulcay, Can, Erdem, Tanyuksel, Avci, Araz and Pahsa2006) and Blastocystis infection has been documented (Dinleyici et al., Reference Dinleyici, Eren, Dogan, Reyhanioglu, Yargic and Vandenplas2011). However, the precise mechanism underlying its mode of action remains unclear.
The aim was to study the therapeutic role of Sb on Blastocystis subtype-3 infected rats through assessment of parasitological cure rate, the study of histopathological effect and analysis of the level of mRNAs expressions for the proinflammatory cytokines IL-8, IL-6, TNF-α and iNOS by real-time RT-PCR.
Materials and methods
Isolation and genotyping of Blastocystis spp. cysts
Blastocystis spp. were detected in 20 diarrhoea samples collected from symptomatic cases using Lugol's iodine wet smear which was then cultured on Jones' media without starch, supplemented with 10% horse serum and kept at 37 °C for three days (Jones, Reference Jones1946). Parasites were detected by microscopy, the viability was confirmed using trypan blue stain and the mean parasite number was determined using a haemocytometer. Subculturing was performed every 3–4 days to maintain the isolates (Méabed et al., Reference Méabed, El-Sayed, Abou-Sreea and Roby2018).
Cysts were collected from positive cultures by Ficoll-Paque technique (Zaman and Khan, Reference Zaman and Khan1994). For axenization, cysts then subjected to three–four times repeated steps of washing by sterile saline and incubation for 48 h in saline supplemented with ampicillin 4 g L−1, streptomycin 1 g L−1 and colistin 1.5–4.0 mg L−1 to eliminate extracellular bacteria. To rule out the presence of any bacteria, subcultures were done on sheep-blood agar plates incubated at 36 °C for 3 days in aerobic and anaerobic conditions (Kukoschke and Müller, Reference Kukoschke and Müller1991).
DNA of Blastocystis spp. was extracted from 50 µL of the pellet of positive culture fluid after washing and centrifugation using QIAamp DNA Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer's protocol. The formerly described seven standardized subtype-specific sequences tagged site (STS) primers by Yoshikawa et al. (Reference Yoshikawa, Wu, Kimata, Iseki, Ali, Hossain, Zaman, Haque and Takahashi2004) were used to perform PCR for Blastocystis subtyping.
Preparation of live S. boulardii cells and their extract
The lyophilized Sb yeast strain kept at −20 °C in the Department of Agricultural Microbiology, Faculty of Agriculture, Fayoum University was reactivated and cultivated in the YPD (Yeast Peptone Dextrose) medium, which was incubated at 37 °C for 48 h with moderate shaking. To recover the yeast, the culture fluid was subjected to centrifugation for 10 min at 4000 g at 4 °C. The deposit was washed in phosphate buffered saline (PBS) and counted under the microscope to obtain 5 × 109 colony forming units (CFU) per 0.1 mL (de Avila et al., Reference de Avila, Conceição, Telmo Pde, Dutra, de los Santos, Martins, Berne, da Silva and Scaini2012). Yeast cells were excluded from the supernatant through filtration with 0.2 µ m Millipore membrane, and centrifugation, then kept at −20 °C until use as yeast extract (Buts et al., Reference Buts, De Keyser and De Raedemaeker1994).
Experimental rats
Forty-two healthy male Wistar rats, about 200 g of weight (7 weeks old), were kept at the Department of Parasitology, Faculty of Medicine Fayoum University at the standard hygienic care and controlled conditions (22 ± 1 °C, 65 ± 5% relative humidity), light and dark cycle. Rats had free access to water and standard rodent food. They were numbered, then simple randomization was used to assign them into six groups (Table 1). Before starting the experiment, their stool was examined to ensure their clearance of any parasitic infections. Then, 5 mL of positive Jones' media at (maximum log phase) were used to infect five groups of rats, using a gastric tube by (2 × 106) parasites (Iguchi et al., Reference Iguchi, Yoshikawa, Yamada, Kimata and Arizono2009). Treatment starts directly on the 3rd day of infection after positive detection of Blastocystis parasite in rats stool. Metronidazole 500 mg tablets (Amriya Pharm. Ind., Alexandria, Egypt) were crushed, dissolved in distilled water and used as the standard drug control (Table 1).
Table 1. The groups of rats and the treatment regimen used for each

Parasitological follow-up
Treatment efficacy was evaluated every 3rd day. Stool pellets were collected from each rat, emulsified as one pooled sample in sterile saline and examined by direct microscopy of prepared wet iodine smears, formol ethyl ether concentration for detection and enumeration of Blastocystis parasites. Three slides were prepared for each rat, and at least 20 fields were examined/slide. The value was expressed as the mean number of cells ± s.d./high power field (HPF) according to Shlim et al. (Reference Shlim, Hoge, Rajah, Rabold and Echeverria1995).
On the 12th day post-treatment (dpt) after collecting stool samples, rats were sacrificed by cervical dislocation. The abdomen was longitudinally opened, and the whole intestine was excised from the pylorus to the rectum. The intestinal lumen was washed with physiological saline and the collected material was kept for parasitological examination as described for stool samples. Negative faecal samples or intestinal wash were further cultured in Jones' medium to roll-out the presence of the parasite.
Histopathological study
Segments of rats' caecum and colon were chosen after examination by the naked eye for gross morphological changes, fixed in formalin 10% then embedded in paraffin. Haematoxylin and eosin stained slides for detection of pathological changes and Periodic Acid–Schiff (PAS) stained slides for special staining of mucin in goblet cells were prepared. Slides were coded then they were blindly examined by two pathologists. The changes were documented using an Olympus BX50F4 microscope with a digital camera and software image (Olympus Corporation, Tokyo, Japan). After examination of the slides, the effect on the number of goblet cells and the degree of colour of mucin after PAS staining were also assessed (Iguchi et al., Reference Iguchi, Yoshikawa, Yamada, Kimata and Arizono2009). The degrees of cellular infiltration, oedema as well as the intestinal gland brush border damage were graded into five degrees; nil, minor, mild, moderate and marked (Erben et al., Reference Erben, Loddenkemper, Doerfel, Spieckermann, Haller, Heimesaat, Zeitz, Siegmund and Kühl2014).
Assessment of mRNA gene expression by real-time RT-PCR
The rest of rats' caecum and colon were scrapped to collect mucus and kept at −20 °C until analysed for local expressions of mRNAs for the proinflammatory cytokines; IL-8, IL-6, TNF-α and iNOS by real-time RT-PCR. Total RNA was extracted from mucosal scraping (~30 mg) using the SV total RNA Isolation kits (Promega, USA), according to manufacturer's instructions. The extracted RNA was dissolved in 30 µL nuclease-free distilled water and stored at −20 °C. The concentration and purity of RNA were determined by Nanodrop Spectrophotometer (Thermo Scientific, USA). Two hundred nanograms of total RNA was converted to cDNA in a thermal cycler PTC-100 using random hexamers [Applied Biosystems (AB), Weiterstadt, Germany].
Real-time PCR and relative quantification were performed according to Yakoob et al. (Reference Yakoob, Abbas, Usman, Sultana, Islam, Awan, Ahmad, Hamid and Jafri2014). The primers used in this study were listed in the Supplementary material. The PCR was performed in UltraFastLabChip Real-time PCR G2-3 System (NanoBioSys Inc., Korea).
The reaction consisted of 8 µL master mix including the polymerase and the dNTPs (SYBR Green I Real-Time PCR Master Mix Kit, NanoBioSys Inc., Korea), 1.6 µL of the sample cDNA, primers forward or backward 10 µ m in 1.6 µL to a final concentration of 0.4 µ m and 3.2 µL of distilled water. The PCR profile was adjusted to software protocol (pre-denaturation at 95 °C for 8 s then 30 cycles at 95 °C for 8 s and 72 °C for 14 s).
The relative fold change in mRNA expression of the target gene was calculated with the 2 − ΔΔCT method, normalized to the mRNA expression of β-actin genes that was used as the housekeeping gene. Each biopsy sample was examined twice, and the average of the two Ct values was used in this study.
Statistical analyses
Data were presented as means ± standard deviation (s.d.). Statistical difference between groups was evaluated with one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test to determine the degree of significance between every two groups using SPSS version 2017. All P values were two-sided. P values of ⩽0.05 and ⩽0.001 were considered significant and highly significant, respectively.
Results
All the Blastocystis positive samples used in the study proved to belong to subtype 3; Sb 227 (526 bp) for ST3.
Results of parasitological examination were presented (Table 2, Fig. 1). At the 3rd day post infection, stool examination of infected rats showed Blastocystis count ranged from 4 to 8/HPF, mean 5.52 ± 1.13 (P value = 0.785), while the negative-CG was negative for the parasite. Vacuolar forms were the most encountered. Introduction of treatment started at this level of infection and then follow-up of groups by stool examination was done. The stool parasite count of the placebo-G increased gradually till 12.2 ± 0.4 at the 12th dpt.
Table 2. Mean ± s.d. number per HPF of Blastocystis forms detected by stool and intestinal wash fluid examination and percent reduction compared to the infected placebo-treated group

One-way ANOVA test was used to compare between different groups at each day of stool examination or after intestinal wash examination. P-value is the degree of significance between different groups (F-test).

Fig. 1. The Mean number/HPF of Blastocystis forms detected by stool examination compared to the infected placebo-treated group at different days post treatment. The error bars represent s.d.
At the 3rd dpt, co-MTZ/Sb-G and MTZ-G, showed early 58.3 and 51.4% decrease of the stool parasite count, respectively. The co-MTZ/Sb-G induced early 100% stool clearance effect at 9th dpt, while MTZ-G reached to 83.6% stool clearance at the 12th dpt. Both Sb-G and Ex-G induced early lower rates of stool clearance till the 6th dpt, then Sb-G increased till reached 100% clearance at 12th dpt, while the Ex-G showed a relatively stable rate of parasite clearance up to 55.7% at 12th dpt. The intestinal wash parasite reduction rates were 100 and 92% for co-MTZ/Sb-G and Sb-G, respectively. The complete negative samples were confirmed negative after culture. The MTZ-G and Ex-G showed 76.4 and 45% parasite clearance by intestinal wash fluid examination, respectively. A statistically significant difference in treatment efficacy was detected between different groups at each day of stool examination or after intestinal wash examination (P values ⩽0.001, Table 2).
Results of histopathological examination were presented (Table 3, Figs 2–4). The negative-CG showed the normal intestinal structure with normal mucosa, columnar epithelia, lumen and crypts, submucosa, muscularis mucosa and lamina propria. The placebo-G and Ex-G showed marked and moderate degrees of degeneration, respectively. The MTZ-G showed some improvement of intestinal epithelium which was still different from the normal. The Sb-G and co-MTZ/Sb-G showed clear improvement of intestinal tissues. PAS staining cleared the number and the degree of colour of secreted mucin in each group. The placebo-G and MTZ-G showed goblet cell mucin depletion, while Sb-G and co-MTZ/Sb-G showed goblet cell mucin hyperplasia.
Table 3. Assessment of pathological changes in different groups

Scoring system: nil = 0%, minor = 1–10%, mild = 11–25%, moderate = 26–50%, marked or severe ⩾51%.
Definitions: crypt hyperplasia is the increase in the epithelial cells numbers in longitudinal crypts in comparison with normal level (crypt elongation), epithelial erosion is the loss of surface epithelium, cryptitis is the presence of neutrophils in between crypt epithelial cells, crypt abscess is the presence of neutrophils in the crypt lumen.
P value is the degree of significance between groups (F-test) as regard the mean goblet cell count using the one-way ANOVA test. *The star means significant difference between this group and the negative control group by post hoc test.

Fig. 2. Histopathological examination results of the negative–CG (panels A and B) and placebo-G (panels C and D). Panel A: H&E-stained section ×10 demonstrating normal negative-CG with intact mucosa (black arrow, normal goblet cell appearance (yellow arrow). Panel B: PAS-stained section ×20 demonstrating normal negative-CG with the average amount of goblet cell mucin secretion. Panel C: H&E-stained section ×20 demonstrating infected placebo-G; black arrows show inflammatory infiltrate (mucosal and submucosal or cryptitis), the red arrow shows epithelial hyperplasia, the yellow head arrow shows epithelial erosion, the blue arrow shows oedema and the green arrow shows crypt abscess. Panel D: PAS-stained section ×20 demonstrating depletion of goblet cell mucin secretion in the placebo-G.
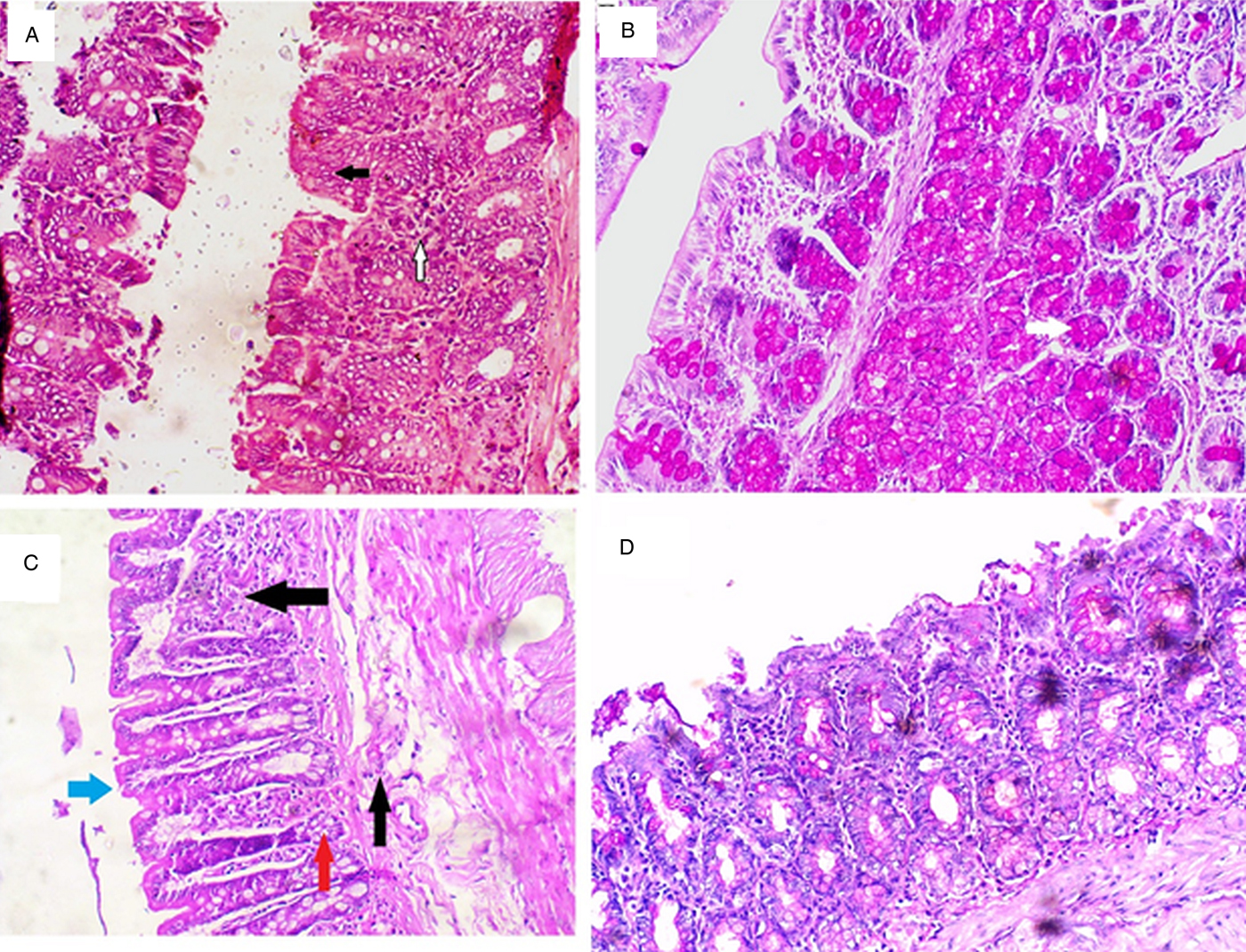
Fig. 3. Histopathological examination results of the Sb-G (Panels A and B) and Ex-G (Panels C and D). Panel A: H&E-stained section ×20 demonstrating the Sb-G showing epithelial hyperplasia (black arrow) and minor inflammatory infiltrate (white arrow). Panel B: PAS-stained section ×20 demonstrating increased goblet cells mucin secretion in Sb-G. Panel C: H&E-stained section ×20 demonstrating Ex-G showing mucosal and submucosal inflammatory cellular infiltrate (black arrows), moderate epithelial damage (blue arrow) and minor crypt abscess (red arrow). Panel D: PAS-stained section ×20 of Ex-G showing some light coloured goblet cell mucin secretion.
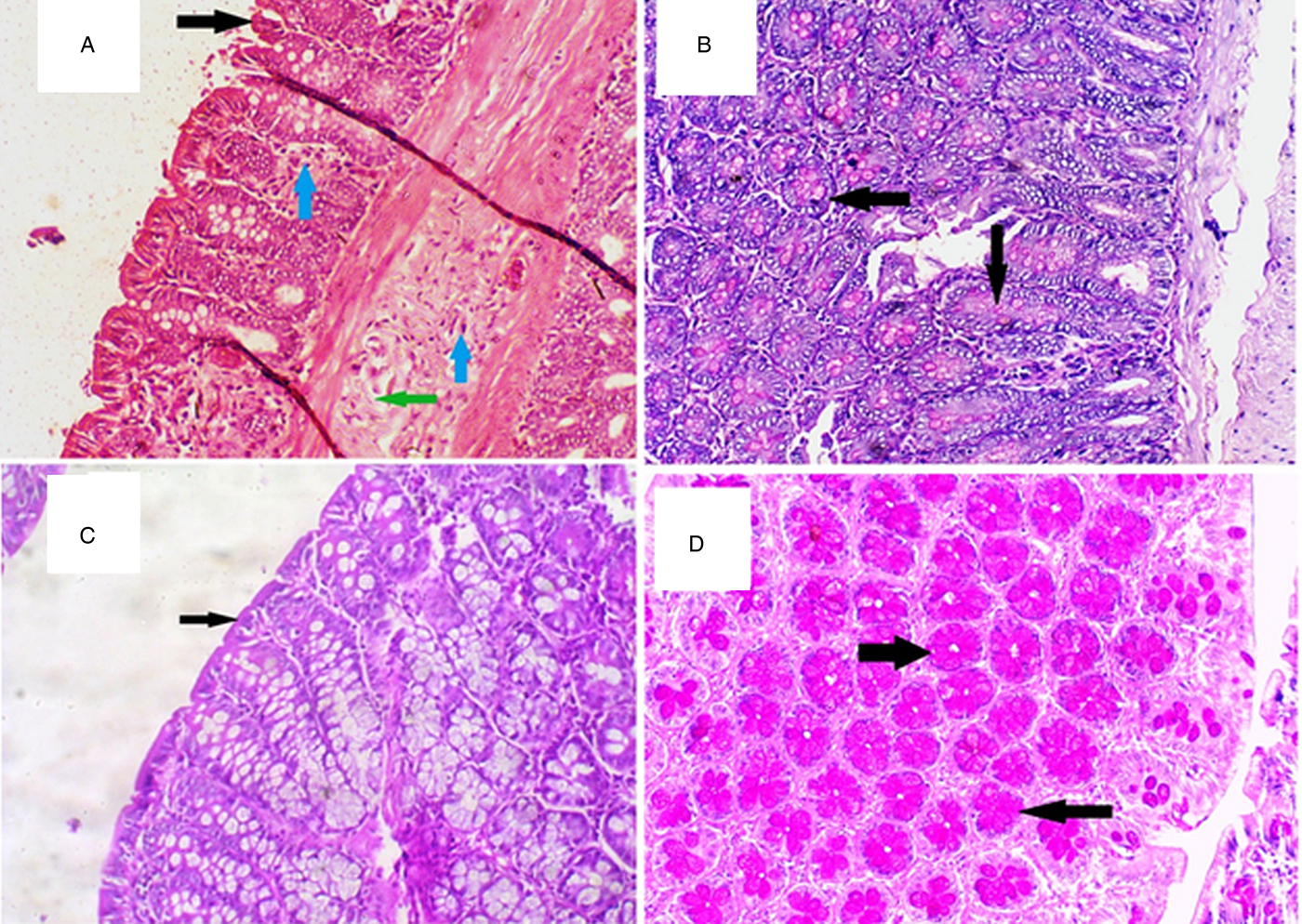
Fig. 4. Histopathological examination results of the MTZ-G (Panels A and B) and co-MTZ/Sb-G (Panels C and D). Panel A: H&E-stained section ×10 demonstrating MTZ-G shows mild to moderate mucosal and submucosal inflammatory cellular infiltrate (blue arrows), minor brush border damage (black arrow) and mild oedema (green arrow). Panel B: PAS-stained section ×20 demonstrating MTZ-G showing light goblet cell mucin secretion. Panel C: H&E-stained section ×20 demonstrating co-MTZ/Sb-G showing best results with the healing of epithelial surface (arrow), no inflammatory infiltrate. Panel D: PAS-stained section ×20 demonstrating of co-MTZ/Sb-G showing the dense goblet cell mucin secretion (arrows).
In comparison with the negative-CG level, all the measured pro-inflammatory cytokines and iNOS genes were significantly more expressed in the colonic mucosa of placebo-G (P values ⩽0.001, Table 4). Treatment by MTZ alone or Sb extract decreased the cytokines and iNOS gene expression level than placebo-G to levels below those of the placebo group. Treatment by live Sb alone or by co-MTZ/Sb showed the significant lowest values of the cytokines and iNOS gene expression below the negative control level (P values ⩽0.001).
Table 4. The mRNA gene expression level of IL-6, IL-8, and TNF-α and iNOS detected in the mucosal scraping of rats

Values are presented as mean ± s.d. fold of increase of gene expression level compared to the negative control level.
One-way ANOVA test was used to compare between different groups. P-value is the degree of significance between different groups (F-test).
Discussion
This is the first report proving the efficacy of Sb on Blastocystis infection using experimental animals. Rats were chosen since the duration of Blastocystis infection is influenced by the animal model. Rats are usually stably infected for longer periods of several weeks and can shed cysts. While older mice are refractory to infection, young mice have acute self-limiting course of infection and usually do not shed cysts (Ajjampur and Tan, Reference Ajjampur and Tan2016). In addition, there is a proved similarity of the bacterial phyla described in the rat gut microbiota to those in human after a large-scale survey of bacterial 16S rRNA genes in caecal or faecal contents (Tomas et al., Reference Tomas, Langella and Cherbuy2012). This study was performed on Blastocystis ST3 since there is no previous data on the efficacy of Sb on this subtype which is the commonest subtype in humans (Souppart et al., Reference Souppart, Sanciu, Cian, Wawrzyniak, Delbac, Capron, Dei-Cas, Boorom, Delhaes and Viscogliosi2009). In Egypt, more than 60% of cases presented with ST3 (Souppart et al., Reference Souppart, Moussa, Cian, Sanciu, Poirier, El-Alaoui, Delbac, Boorom, Delhaes, Dei-Cas and Viscogliosi2010). So it was import to get the efficacy of this simple treatment from yeast that may solve the problem of many diarrhoea patients and to compare Sb efficacy with MTZ which is the standard treatment for this parasite especially that STs 1, 3, and 5 are susceptible to MTZ (Girish et al., Reference Girish, Kumar and Aminudin2015), while STs 4 and 7 are resistant to it (Mirza et al., Reference Mirza, Wu, Kidwai and Tan2011).
In this study Blastocystis ST3 infection showed marked degeneration of intestinal epithelium, oedema and marked inflammatory cellular infiltration. This agreed with previous studies that Blastocystis infection can induce pathology that differs in its degree according to parasite subtype (Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014). Intense secretion of inflammatory cytokines has been reported higher in ST-7 more than ST-4 in mice (Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014), and in human colon cells by ST3 (Ragavan et al., Reference Ragavan, Kumar, Chye, Mahadeva and Shiaw-Hooi2015). Intense infiltration of inflammatory cells was reported in infected mice and human colon (Moe et al., Reference Moe, Singh, Howe, Ho, Tan, Chen, Ng and Yap1997; Yakoob et al., Reference Yakoob, Abbas, Usman, Sultana, Islam, Awan, Ahmad, Hamid and Jafri2014; Pavanelli et al., Reference Pavanelli, Kaneshima, Uda, Colli, Falavigna-Guilherme and Gomes2015).
The current results showed a rise of pro-inflammatory cytokines and iNOS in the colon after Blastocystis infection this agreed with previous studies (Chandramathi et al., Reference Chandramathi, Suresh and Kuppusamy2010a, Reference Chandramathi, Suresh, Mahmood and Kuppusamy2010b, Reference Chandramathi, Suresh, Shuba, Mahmood and Kuppusamy2010c; Yakoob et al., Reference Yakoob, Abbas, Usman, Sultana, Islam, Awan, Ahmad, Hamid and Jafri2014), and were attributed to Blastocystis antigens, and its enzymes such as hyaluronidase and proteases (Chandramathi et al., Reference Chandramathi, Suresh, Mahmood and Kuppusamy2010b; Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014). Blastocystis antigens are absorbed through the epithelium paracellular or transcellular pathway into the lamina propria to come in contact with the immune cells (Iguchi et al., Reference Iguchi, Yoshikawa, Yamada, Kimata and Arizono2009; Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014). The enterocytes or immune cells, including macrophages, monocytes, neutrophils, and lymphocytes secrete proinflammatory cytokines, such as IL-1β, IL-6, IL-8, TNF-α, IL-12, and interferon-gamma IFNγ that contribute to mucosal inflammation in response to Blastocystis antigens (Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014).
Blastocystosis proved to induce upregulation of the proinflammatory cytokines in different types of studies either in vitro, in human colonic epithelium or in infected animals as IL-8 genes (Long et al., Reference Long, Handschack, König and Ambrosch2001; Puthia et al., Reference Puthia, Lu and Tan2008; Chandramathi et al., Reference Chandramathi, Suresh and Kuppusamy2010a, Reference Chandramathi, Suresh, Mahmood and Kuppusamy2010b; Ragavan et al., Reference Ragavan, Kumar, Chye, Mahadeva and Shiaw-Hooi2015), TNF-α genes (Iguchi et al., Reference Iguchi, Yoshikawa, Yamada, Kimata and Arizono2009; Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014; Abdel-Hafeez et al., Reference Abdel-Hafeez, Ahmad, Abdelgelil, Abdellatif, Kamal, Hassanin, Abdel-Razik and Abdel-Raheem2016) and IL-6 gene (Chandramathi et al., Reference Chandramathi, Suresh and Kuppusamy2010a, Reference Chandramathi, Suresh, Mahmood and Kuppusamy2010b; Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014).
NO is important for epithelial barrier modulation and generation of cellular defence against microbes. NO induction of Blastocystis damage through apoptosis has been suggested, since increased NO levels was detected in the colonic mucosal epithelial cells of mice experimentally infected with Blastocystis (Eida et al., Reference Eida, Hussein, Eida, El-Moamly and Salem2008; Abdel-Hafeez et al., Reference Abdel-Hafeez, Ahmad, Kamal, Abdellatif and Abdelgelil2015), and in vitro study (Mirza et al., Reference Mirza, Wu, Kidwai and Tan2011). Increased NO levels indicate inflammation and, in turn, the intensity of the disease.
In this study, Blastocystis infection was accompanied by depletion of goblet cells mucin in the intestinal brush border in accordance with Yakoob et al. (Reference Yakoob, Abbas, Usman, Sultana, Islam, Awan, Ahmad, Hamid and Jafri2014). While other previous studies reported goblet cell hyperplasia in a mouse model of Blastocystis spp. infection (Iguchi et al., Reference Iguchi, Yoshikawa, Yamada, Kimata and Arizono2009; Abdel-Hafeez et al., Reference Abdel-Hafeez, Ahmad, Abdelgelil, Abdellatif, Kamal, Hassanin, Abdel-Razik and Abdel-Raheem2016). This may be related to the difference in the parasite species between these studies. Results of this study showed goblet cell hyperplasia in the groups treated with live Sb (Sb-G or CO-MZ/Sb-G). Goblet cell hyperplasia increases mucin and fluid secretion that enhances the mucous layer which acts as a barrier providing immunity against parasitic infection and collaborates with increased peristalsis for the expulsion of lumenal parasites (Iguchi et al., Reference Iguchi, Yoshikawa, Yamada, Kimata and Arizono2009). Also, mucus acts as a trap inhibiting parasite motility and feeding capacity. In addition, goblet cells have a role in immune activation by antigen presentation to lamina propria dendritic cells (Abdel-Hafeez et al., Reference Abdel-Hafeez, Ahmad, Abdelgelil, Abdellatif, Kamal, Hassanin, Abdel-Razik and Abdel-Raheem2016).
The result of this study showed that live Sb significantly improved the colonic mucosa, increased goblet cells and downregulated dramatically the proinflammatory cytokines IL-8, IL-6, TNF-α and iNOS in the colonic mucosa. These results were in accordance with previous studies that Sb can modulate host systemic and local immune responses, secretion of mucin from intestinal goblet cells (Girard et al., Reference Girard, Pansart and Gillardin2005; Lee et al., Reference Lee, Kim, Chi, Joo and Kim2009; Kelesidis and Pothoulakis, Reference Kelesidis and Pothoulakis2012). Previous studies showed that Sb treatment decreases IL-8 mRNA expression by producing a soluble factor that blocks IL-8 gene expression in colonocytes (Sougioultzis et al., Reference Sougioultzis, Simeonidis, Bhaskar, Chen, Anton, Keates, Pothoulakis and Kelly2006). Live Sb can cancel the TNF-α induced activation of IL-8 (Lee et al., Reference Lee, Kim, Chi, Joo and Kim2009). The secreted protein level of IL-8 using the enzyme-linked immunosorbent assay (ELISA) reported 57% decrease following the treatment with Sb, in comparison with controls that were correlated with the treatment doses of Sb (Lee et al., Reference Lee, Kim, Chi, Joo and Kim2009). Oral treatment with Sb decreased TNF-α and IFNγ levels in Candida albicans infected mice (Jawhara and Poulain, Reference Jawhara and Poulain2007). There is evidence that Sb inhibits inducible nitric oxide synthase (Girard et al., Reference Girard, Pansart, Lorette and Gillardin2003; Soyturk et al., Reference Soyturk, Saygili, Baskin, Sagol, Yilmaz, Saygili and Akpinar2012).
Administration of Sb showed slow efficacy till the 6th dpt, that reached to 100% stool clearance at the 12th dpt, but with still positive intestinal wash fluid, meaning it needs continuous administration for a long time to produce its effect. This was in accordance with Jawhara and Poulain (Reference Jawhara and Poulain2007) that stopping administration of Sb had led to recovery and reactivation of C. albicans colonies. They explained this phenomenon by the inability of Sb to permanently colonize the GIT. Elmer et al. (Reference Elmer, McFarland, Surawicz, Danko and Greenberg1999b) revealed that Sb achieves steady-state concentration within three days of oral intake and is cleared within 3–5 days after its discontinuation. This result agreed with a previous small clinical study proving the efficacy of Sb compared to MTZ for treatment of Blastocystis infection in children who reported parasitological cure rate on day 15 of 80% in the group given standard doses of MTZ vs 72.2% in the group given Sb (P > 0.05), and at the end of the first month the rates were 94.4 vs 93.3%, respectively (P > 0.05) (Dinleyici et al., Reference Dinleyici, Eren, Dogan, Reyhanioglu, Yargic and Vandenplas2011).
Co-administration of both MTZ/Sb showed the best regimen that induced an early and complete stool and intestinal wash clearance, normal mucosa, and a significant decrease of proinflammatory cytokines and iNOS, while administration of MTZ was less effective. The results agreed with previous studies on amoebiasis that co-MTZ/Sb administration significantly improved the symptoms of intestinal amoebiasis and enhanced the GIT clearance of the cysts as compared to MTZ alone (Mansour-Ghanaei et al., Reference Mansour-Ghanaei, Dehbashi, Yazdanparast and Shafaghi2003; Dinleyici et al., Reference Dinleyici, Eren, Yargic, Dogan and Vandenplas2009), and on giardiasis (Castaneda et al., Reference Castaneda, Garcia, Santa Cruz, Fernandez and Monterrey1995; Besirbellioglu et al., Reference Besirbellioglu, Ulcay, Can, Erdem, Tanyuksel, Avci, Araz and Pahsa2006).
Sb extract induced weak parasite clearance with moderate improvement of the colonic mucosa associated with a relative increase of pro-inflammatory cytokines and iNOS in agreement with West et al. (Reference West, Stanisz, Wong and Kunze2016) that Sb supernatants helped in the treatment of acute stress-related gut dysmotility. This weakness may be explained by the absence of the direct effect exerted by live Sb on intestinal epithelium and the bacterial flora. Sb live cells have many direct effects. Sb re-establishes the beneficial gut flora and inhibits the action and reproduction of pathogenic microorganisms by several mechanisms. It has a trophic action on intestinal mucosa with induction of enzymatic activity favouring absorption and nutrition. It modulates the host local and systemic immunity (de Avila et al., Reference de Avila, Conceição, Telmo Pde, Dutra, de los Santos, Martins, Berne, da Silva and Scaini2012; Kelesidis and Pothoulakis, Reference Kelesidis and Pothoulakis2012).
Immune recognition of Sb is most likely mediated by the cell wall, a highly complex structure that mediates responses to external stresses including anaerobic conditions as well as pH and osmotic changes. The cell wall contains many immunomodulatory components. Mannoproteins, for example, compose the outer layer of the yeast cell wall and bind galectin 3, Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN), Toll-like receptors (TLR4) and others. β-glucans, which constitute the middle layer, ligate Dectin-1 and TLRs 2 and 6 and can stimulate Langerin positive DCs in small intestinal Peyer's patches. Chitin, a minor component of the innermost cell wall layer, binds the mannose receptor. Administration of yeast cell wall fragments such as β-glucans has been found to stimulate mucosal immune responses and recapitulate some effects of whole probiotics (Hudson et al., Reference Hudson, McDermott, Stewart, Hudson, Rios, Fasken, Corbett and Lamb2016).
Another hypothesis that may explain these results and needs another designed study for confirmation that the effect of both MTZ and Sb is related to an indirect effect on the gut microbiota. In healthy rats, the most common microbiota are Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria which are similar to those in human intestine (Tomas et al., Reference Tomas, Langella and Cherbuy2012), and the yeast content of <0.1%, C. albicans is the most prominent yeast inhabitant (Czerucka et al., Reference Czerucka, Piche and Rampal2007). MTZ can cause undesirable side-effects and changes in the gut microbiota (Lepczyńska et al., Reference Lepczyńska, Białkowska, Dzika, Piskorz-Ogórek and Korycińska2017). MTZ treatment in healthy rat exerts an antioxidant effect by reducing the colonic protein carbonyl levels and increases both enterobacterial and bifidobacterial colonic populations (Pélissier et al., Reference Pélissier, Vasquez, Balamurugan, Pereira, Dossou-Yovo, Suau, Pochart and Magne2010).
Meanwhile, Sb is not competitive enough to displace the healthy microbiota, and the number of Sb during healthy intestinal passage may possibly not rise above the administered dose (Moré and Swidsinski, Reference Moré and Swidsinski2015). Sb doesn't seem to alter the composition of the healthy microbiota, except perhaps for a certain reduction of the rather minor prevalence of Enterobacteriaceae like E. coli (Akil et al., Reference Akil, Yilmaz, Kurutepe, Degerli and Kavukcu2006). Sb inhibits C. albicans hyphae formation, adhesion and blocks initial stages of biofilm formation which is necessary for adherence to the host mucosal surface (Krasowska et al., Reference Krasowska, Murzyn, Dyjankiewicz, Łukaszewicz and Dziadkowiec2009).
Sb can create a favourable growth environment for the beneficial intestinal microbiota while constituting extra protection to the host mucus layer and mucosa.
In a dysbiosis, as during diarrhoea, the main microbial population (especially Lachnospiraceae, Ruminococcaceae, Bacteroidaceae and Prevotellaceae) is known to collapse by at least one order of magnitude. This gap generally leads to transient increases in Enterobacteriaceae, Bifidobacteriaceae and Clostridiaceae. That treatment with Sb in dysbiosis either in humans or animals leads to the faster reestablishment of a healthy microbiome (Moré and Swidsinski, Reference Moré and Swidsinski2015).
Author ORCIDs
Eman M. H. Méabed, 0000-0003-1673-4327.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019000696
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors report no conflicts of interest.
Ethical standards
The study was performed in the Parasitology Department, Faculty of Medicine, Fayoum University, from the period from March to September 2016. The study was ethically approved by the Faculty Ethical Committee and all animal procedures were done according to the Declaration of Helsinki.











