INTRODUCTION
Inequality in lifetime reproductive rates has been described in plants, fungi and animals, including parasitic helminths (see references in Table 1). The normal pattern is that, within a population, a minority of individuals produce most of the offspring. The evolutionary and ecological significance of this inequality is enormous, since it impacts on the effective population size (N e), a key parameter in evolutionary biology. Some models have shown that populations with leptokurtic fecundity distributions (high reproductive inequality) have a faster response to selection, higher chances of genetic drift and a more rapid loss of rare alleles compared to populations with platykurtic (low reproductive inequality) fecundity distributions (Wilson and Levin, Reference Wilson and Levin1986).
Table 1. Measurements of variation in population fecundity based on the Gini coefficient
(For most organisms, fecundity was calculated by using surrogate measures such as body size, eggs produced per time interval, or size of reproductive organs.)

A Common names used for ease of interpretation.
B Smaller number was under artifically low competition; larger number under more normal conditions.
C Numbers varied by intensity; can also be manipulated by varying diet amounts of mannose.
D Numbers varied by intensity; can also be manipulated by varying diet amounts of fructose.
High variation in reproductive success (VRS) has been reported for several parasitic helminths. For example, for the pike cestode Triaenophorus crassus Forel, 1868, it was calculated that 10% of the parasite metapopulation produced about 85% of the eggs (Shostak and Dick, Reference Shostak and Dick1987). The implications of this were that the majority of offspring are contributed by small numbers of worms, which are confined to relatively few host individuals, with implications to N e and transmission dynamics. Similar results were reported for the nematode Raphidascaris acus (Block, 1779) in pike (Szalai and Dick, Reference Szalai and Dick1989), and for the tapeworm Hymenolepis diminuta and the acanthocephalan Moniliformis moliniformis in rats (Dobson, Reference Dobson1986).
The finding of high VRS in parasite populations has led to the paradigm that most populations have a much lower N e compared with the number of individuals within populations; a paradigm consistent with and required for evolution. A model constructed for macroparasite populations concluded that several features of parasite life cycles, including non-binomial variance in VRS among individuals, function together to reduce N e below that expected for comparable free-living populations (Criscione and Blouin, Reference Criscione and Blouin2005).
Recently, a study of mermithid nematodes in beach hoppers (amphipod) showed relatively low VRS (Poulin and Latham, Reference Poulin and Latham2002). These authors suggested that this result might be due to fundamental differences in the outcome of intraspecific competition in systems involving invertebrate hosts and those involving vertebrate hosts. The purpose of the present study was to investigate the VRS of another parasite with an invertebrate definitive host. Gordiids or Gordian worms are obligate parasites of insects, within which they mature and subsequently escape to mate and oviposit in a freshwater system. Mating begins within a few seconds, usually as worms are still exiting their hosts (Hanelt and Janovy, Reference Hanelt and Janovy2004). All resources a worm will use as an adult are obtained from this insect host. Gordiids can be up to several meters long (Hanelt et al. Reference Hanelt, Thomas and Schmidt-Rhaesa2005) and some hosts may harbour up to 30 worms (Hanelt and Janovy, Reference Hanelt and Janovy2004). Thus, the assumption has been that competition for resources is relatively high in these hosts, resulting in a high VRS. This assumption is re-examined using the findings of the present study. The reason for the slight size differences among gordiid worms will be explored, particularly in the context of intensity-dependence.
MATERIALS AND METHODS
Laboratory-reared specimens
Paragordius varius (Leidy, 1851) was maintained in the laboratory according to a previously described protocol (Hanelt and Janovy, Reference Hanelt and Janovy2004). Gryllus firmus Scudder, 1902 (crickets) were exposed to variable numbers of P. varius cysts from laboratory-reared and -exposed Physa gyrina Say, 1821 (snails). Cricket size was standardized at time of infection by only exposing females in their penultimate instar. However, cricket size, at the end of the experiment, varied directly with intensity (data not shown), since the presence of worms greatly distended the host's body cavity. Thus, host size was not included in any subsequent calculations. The worms complete their development at 25–30 days post-exposure (p.e.), at which time their cuticle darkens (Schmidt-Rhaesa, Reference Schmidt-Rhaesa2005) to a dark brown, almost black colouration. Only completely developed worms exit their host. Crickets were immersed in water to release mature worms at 30 days p.e. Upon emergence, worms were separated immediately and measured. Thereafter, crickets were dissected to monitor for immature worms, which are light yellow to white in colour. For subsequent analyses, worms were divided into 3 groups: adult worms (AW), immature worms (IW) and mixed adult worms (MAW) referring to adult worms in the same host with IW. Worm lengths of these 3 categories, were compared using Kruskal-Wallis tests followed by post-hoc pairwise comparisons using Mann-Whitney Tests. All statistics were performed on the Vassar-Stats utility available at http://faculty.vassar.edu/lowry/VassarStats.html.
Lifetime fecundity estimates
To determine whether length was an accurate measure of lifetime fecundity, the egg output of 12 females was measured. Females were isolated and paired with single, randomly chosen males in 1 litre aquaria. Each day, egg strings from each female were collected and measured; oviposition lasted from 15 to 20 days. Cumulative length and at least 15 width measurements were taken for the egg string of each female. Total fecundity was estimated by multiplying the volume of an average egg (ellipsoid volume) by the total volume of each egg string (based on total length and mean width; Hanelt and Janovy, Reference Hanelt and Janovy2002). This calculation approximated 1350 eggs per mm of egg string, and was tested by cutting a 1 mm section from each of 5 egg strings, teasing these apart upon larval maturation (15 days following ovipositioning) and manually counting developed larvae. In all cases, the counts and the calculated number of eggs were not significantly different (data not shown).
For all calculations, except the Gini coefficient, data for both males and females were included (382 AW). Since the presence of IW affects several population structure calculations (such as total length and average length), worms derived from these hosts were not included in analyses of intensity. All adult female P. varius were included in the calculations of reproductive equality (194 AW and MAW females). Since reproductive output cannot be accurately assessed for males and IW females, they were excluded from fecundity calculations. Finally, since IWs were rare, and the sample size was relatively small, analyses including this group should be interpreted with caution.
Field-collected specimens
In addition to P. varius, a dataset from a natural gordiid population, Gordius difficilis Smith, 1994, was also used in fecundity calculations (Bolek and Coggins, Reference Bolek and Coggins2002). Only adult female worms (n=119) were included in this analysis. For the calculation of G. difficilis lifetime fecundity, the assumption was made that female length correlated with fecundity. Since worms were collected after leaving their hosts, intensity data were not available. During the 2-year study period, worms were collected daily and carabid beetles were identified as the natural hosts. Five hosts contained 1 worm and 1 host contained 2 worms. Thus, nearly a third of these worms originated from hosts harbouring multiple worms (Bolek and Coggins, Reference Bolek and Coggins2002).
Calculations of fecundity variation
Fecundity variation was evaluated using the Gini coefficient (Gini, Reference Gini1912) and the Lorenz curve (Lorenz, Reference Lorenz1905). These measures are used to quantify and describe the degree of reproductive inequality within a population (see Damgaard and Weiner, Reference Damgaard and Weiner2000). The Gini coefficient (G), ranges from 0 to 1. A value of 0 (also known as the line of equality) indicates a perfect reproductive equality and a value of 1 indicates that reproduction is limited to a single individual within the population.
Gini coefficients and 1000 bootstrap replicates (Dixon et al. Reference Dixon, Weiner, Mitchell-Olds and Woodley1987) were calculated using a script available at www.public.iastate.edu/~pdixon/sas (P. Dixon, unpublished) using the SAS platform (SAS Institute, Cary, NC, USA). Female worm length was used in the calculations of G for both gordiid species. Since populations can have the same value of G but very different fecundity frequency distributions (Weiner and Solbrig, Reference Weiner and Solbrig1984), the distribution was represented graphically using the Lorenz curve. Curve-fitting of relationships was carried out using Curve Expert 1·37 available at www.ebicom.net/~dhyams/cmain.htm (D. Hyams, unpublished).
RESULTS
Estimates of lifetime fecundity
Fecundity of P. varius ranged from 200 000 to 5·8 million eggs (Fig. 1). The correlation between worm length and lifetime fecundity was significant, indicating that female body length, at the time of emergence from the definitive host, explained much of the variation in the total number of eggs produced. Thus, female gordiid worm length is an accurate indicator of lifetime egg output.

Fig. 1. Relationship between Paragordius varius female body length and lifetime egg production.
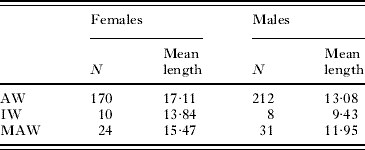
Laboratory-raised Paragordius varius
Of 95 hosts exposed, 47 survived and were infected, releasing a total of 455 worms. Three hosts that died during the second week of infection contained more than 35 IW each; these hosts most likely died due to large worm burdens. All worms, which actively exited their hosts, were light brown to black, indicating that they were adults. Upon dissection, 5 hosts contained 18 IW and 55 MAW (Table 2). The mean intensity of infection among 42 hosts releasing all worms was 9·10 and ranged from 1 to 32 worms per host (Fig. 2). The range of intensities of hosts harbouring IW and MAW suggests that these infections, with mixed developmental stages, were not a result of crowding (Fig. 2). Infections from all hosts produced marginally significantly more males (251) than females (204; binomial probabilities test; z=2·26; P=0·0309). The number of males per host (![]() =5·34) was not significantly different from the number of females (
=5·34) was not significantly different from the number of females (![]() =4·34; P=0·214; N=42). Considering both AW and MAW together, adult males (
=4·34; P=0·214; N=42). Considering both AW and MAW together, adult males (![]() =12·55 mm) were significantly shorter than females (
=12·55 mm) were significantly shorter than females (![]() =17·11 mm; P<0·001; N=437), a result normal for gordiids.
=17·11 mm; P<0·001; N=437), a result normal for gordiids.

Fig. 2. Intensity frequency distribution of Paragordius varius in Gryllus firmus. Note that IW are not limited to hosts with high or low intensities.
Table 2. Sample size and mean length (cm) of 3 groups of Paragordius varius: worms from hosts harboring only adults (AW), and worms from hosts harboring immature worms (IW) and adult worms (MAW)

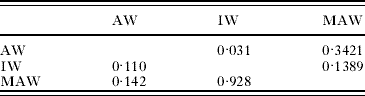
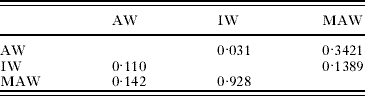
Sizes among the 3 worm categories were not significantly different (males P=0·0699; females P=0·1159). Post-hoc, pairwise tests of groups (Table 3) revealed that male IW were only marginally significantly shorter than male AW (P=0·031). AW showed a relatively normal size distribution but males tended to have a more leptokurtic distribution than females (Fig. 3A). The size structure of the female AW population was skewed towards smaller worms (left), whereas males were skewed slightly towards larger worms (right). IW appeared to be the smallest (Table 2) and the female population was skewed to the right (Fig. 3B). MAW were smaller than AW; MAW females were also heavily skewed to the left due to the remaining females (IW), not yet having matured (Fig. 3C). In hosts with both IW and MAW, all of the longest females (25–30 mm) were adults, whereas none of the shortest females (5–10 mm) had completed their development. Furthermore, worms in the most numerous size class of female IW (15–20 mm) were longer than the most numerous size class of female MAW (10–15 mm). Male MAW length was slightly reduced compared with male AW, and no MAW larger than 17 mm were recorded (a size class making up almost 10% of AW).

Fig. 3. Frequency distribution of Paragordius varius lengths from (A) hosts (N=42) harboring only adults (AW); and hosts (N=5) harboring both (B) immature worms (IW) and (C) adult worms (MAW).
Table 3. Post-hoc pairwise comparisons of worm lengths (P-values) for 3 worm types
(Upper right part of matrix is data for males; lower left part of matrix is for females.)
The total number of worms per host correlated significantly with mean worm length (P<0·001; R 2=0·265; N=42). In addition, when considering sexes separately, mean worm length of AW correlated significantly with number of worms per host (Fig. 4A). The correlation coefficient for males was higher than for females, suggesting that males are more likely to vary their size according to intensity than females. By correlating intensity with total worm length, a sexually dimorphic response to crowding was apparent (Fig. 4B). The correlation between total worm length and intensity for females revealed a better fit with a linear model (R 2=0·6785, S=30·78) than an exponential model (R 2=0·5848, S=34·97), whereas, for males, the fit was better using an exponential model (R 2=0·6701, S=21·36) than a linear model (R 2=0·6027, S=23·47). These data suggest that, while total male length reaches a maximum at approximately 150 cm per host, females do not appear to limit their growth at any intensity.

Fig. 4. Mean (A) and total length (B) of male and female Paragordius varius as a function of the number of male or female worms per host. Note that the relationship of total worm length and intensity for females is best fit by a linear model and for males is best fit by an exponential model.
Fecundity variation
The Lorenz curve for P. varius shows only a slight degree of concavity (Fig. 5A). The Gini coefficient (G=0·1999; 95% ci: 0·1863–0·2174) indicates clearly that VRS is extremely low in this population. To test whether these results might have been affected by the rearing and maintenance of the parasite in the laboratory, a field population was subjected to the same analyses. The average length of G. difficilis females was 24·55 cm, and the size structure of the female population was slightly skewed toward larger worms (Fig. 6). The Lorenz curve for G. difficilis (Fig. 5B) and its Gini coefficient (G=0·1523; 95% ci: 0·1319–0·1730) indicate that, within this population, VRS is even lower than the laboratory-reared P. varius. This result clearly indicates that the small Gini coefficient calculated for P. varius is not an artefact of the rearing and maintenance of the worm under laboratory conditions.

Fig. 5. Lorenz curves for gordiids. Cumulative worm lengths plotted against cumulative number of worms, which were first ranked by size, increasing from left to right. (A) Paragordius varius, (B) Gordius difficilis. Dashed lines represent the line of equality (G=0).

Fig. 6. Frequency distribution of female Gordius difficilis worm lengths from a natural population (n=119).
DISCUSSION
The correlation between lifetime fecundity and female length in gordiids is not surprising. Gordiids are known not to feed outside of their hosts due to a degraded, non-functional gut and a plugged mouth. All nutrients that females pass to the next generation are assembled from the definitive host and are most likely absorbed directly through the cuticle of the juvenile worm (Inoue, Reference Inoue1959; Kirjanova, Reference Kirjanova1959); this digestive mechanism seems to be similar to that in tapeworms. Juveniles have a single permeable cuticle (composed of several layers) and moult just before emerging from the host (Müller et al. Reference Müller, Jochmann and Schmidt-Rhaesa2004). Adults replace this thin juvenile cuticle with a second, darker and thicker cuticle composed of 21–22 layers (Schmidt-Rhaesa, Reference Schmidt-Rhaesa2005). Between 25 and 30 days p.e., the cuticle thus changes its function from ‘nutrient uptake’ to ‘protection’, and feeding ceases in adult worms, regardless of whether they have been released from their host. Thus, after moulting the lifetime resource amount available to the worm is ‘locked-in’, making gordiids an excellent model for the study of life-history measures, including fecundity.
High rates of VRS, as calculated by the Gini coefficient, are well known in plants and fungi, and usually vary from 0·5 to 1·0. In animals, G is also generally above 0·5, but the manipulation of environmental factors has led to artificially low G values in some species. For example, if New Zealand mudsnails are maintained under artificial conditions of low competition, G can decrease to 0·25; however, under more ‘normal’ conditions, G can increase significantly to 0·46 (Richards and Cazeir-Shinn, Reference Richards and Cazeir-Shinn2004). Similarly, Hymenolepis diminuta and Moliniformis moliniformis can be manipulated experimentally to G<0·3 by increasing the amount of sugar in the host's diet, thus decreasing competition among worms (Dobson, Reference Dobson1986).
In this study, cricket hosts containing more than 35 worms died before the parasites could mature, leading to the safe assumption that intensities reported here span the range of worm loads (1–32 worms per host). Surprisingly, although P. varius was placed in environments (high intensity infections) where keen competition was expected, along with high VRS, the data clearly indicate that gordiids have an extremely low rate of fecundity inequality.
Usually, inequalities in parasite fecundity have been explained by differences in age of parasites, environment (such as resources and intensity of infection), and individual genetic variation (Shostak and Dick, Reference Shostak and Dick1987). However, some of these factors seem to have had little influence on the worms in this study.
Age can influence fecundity rates of organisms that are long-lived and vary their reproductive output, usually as a direct result of age. Since gordiids amass all of their energy from the definitive host before leaving, and it was possible to measure their lifetime reproductive output, the age structure of the population could not have had an impact on the results reported here. The intensity of infection, and thus the resources available to the worms, varied greatly for laboratory-exposed P. varius. In crickets with heavy worm burdens, parasite biomass is often greater than host biomass (as measured by wet mass; B. Hanelt, unpublished observations). After releasing worms, crickets are usually hollow, with most organs barely visible (or recognizable). The host's severe resource limitation would seemingly be an environment of strong parasite competition, which should have greatly increased the fecundity inequality.
Although, the data showed that the size of worms correlates negatively with intensity, the correlation coefficient for females was relatively low, indicating that intensity does not greatly influence fecundity. However, the correlation coefficient was higher for males, suggesting that intensity has a stronger effect on the regulation of size in males than females. Within the range of intensities recorded in this study (1–32), males were limited in their growth when more than 25 worms per host were present, whereas females continued growing linearly throughout the intensity range (Fig. 4B). The mechanism for this is unclear. However, whatever limits males but not females is likely to be the result of interference competition, since exploitative competition should have an equal affect on both sexes.
The effect of resource limitation and intensity of infection on G. difficilis is more difficult to quantify. Worms were collected in the free-living, adult phase and, thus, the intensity of these worms in their hosts was unknown (Bolek and Coggins, Reference Bolek and Coggins2002). Gordius difficilis worms were collected every 24 h (Bolek and Coggins, Reference Bolek and Coggins2002), making size selective mortality during these intervals unlikely. Collections of hosts at this site showed that 2 of 7 worms, or nearly 30%, of G. difficilis worms originated from hosts with multiple infections; a proportion consistent with other gordiid species (Poulin, Reference Poulin1995; Thomas et al. Reference Thomas, Schmidt-Rhaesa, Martin, Manu, Durand and Renaud2002). Thus, a third of adult G. difficilis worms was likely the result of competitive interactions. However, as in P. varius, these competitive interactions do not appear to be reflected in the G values of these populations.
A factor that remains unknown for either worm population is genetic similarity. Relatedness could impact fecundity by altruism; more related individuals are assumed to compete less with each other than less related ones (Hamilton, Reference Hamilton1963, Reference Hamilton1964). It is possible that the gordiid populations studied here were closely related, leading to more equal fecundity. Relatedness may have had a larger impact on the P. varius population, since it had been reared in the laboratory for multiple generations, during which several bottlenecks may have occurred. In the future, it may be fruitful to establish the degree of relatedness within these populations, for example by microsatellite analyses (e.g. Shrivastava et al. Reference Shrivastava, Gower, Balolong, Wang, Qian and Webster2005).
Two additional factors should have increased G in the natural population, which may have been masked by the controlled laboratory rearing of P. varius. First, since gordiid hosts (beetles in the case of G. difficilis) show a substantial variation in size under natural conditions, both among and between sexes (e.g. Desender, Reference Desender1994), host resource variation should increase gordiid VRS. Second, infections in nature may not be simultaneous, which could lead to a competitive advantage of worms that colonize earlier. However, since the G value of this natural population was lower than the laboratory-raised P. varius, it appears that neither of these factors influenced significantly worm size and thus fecundity.
Finally, several factors of the life cycle and ecology of gordiids might have contributed to their low VRS. First, worms mate immediately following the exit from their host. It is possible that worms sharing the same host refrain from competition in order to avoid retarding the development of a potential future mate. Second, the 5 hosts containing IW and MAW acted and appeared normal, and it is very likely that the IW would have continued development either with or without the host having released the adult worm cohort. Unlike most other parasite systems, if a gordiid worm fails to develop (or complete development), due to competition, crowding, or developmental differences, gordiids may have the chance to wait for their environment (cricket) to be vacated by the winning competitors. Similarly, after 25 days p.e., when the winning competitors moult, and are no longer able to absorb nutrients, they relinquish the remaining resources to the losing competitors. Thus, one explanation for the low VRS in this system is that immature worms may have a chance to ‘catch up’ once adult worms are done using the host. This hypothesis is supported by the size structure of the adult and immature worms from hosts containing both of these developmental stages. Although female worm lengths did not vary significantly between IW and MAW, the worms in the smallest size groups remained immature but a majority of female IW were much larger than most fully developed worms from the same hosts. This size-structuring suggests that immature worms are not necessarily competitively excluded; they might be developmentally delayed. Since the 5 hosts containing IW and MAW did not contain unusually high or low intensities, compared with other hosts, it appears that crowding itself could not have been the only cause leading to the retarded development of worms. Further testing of these immature worms could be useful to determine whether this developmental delay is due to a genetic component.
After worms complete development, gordiid hosts are manipulated to enter water (Thomas et al. Reference Thomas, Schmidt-Rhaesa, Martin, Manu, Durand and Renaud2002, Reference Thomas, Ulitsky, Augier, Dusticier, Samuel, Strambi, Biron and Cayre2003). It is unknown how or when this manipulation begins in hosts containing mature and immature worms. However, considering the advantages to each ‘player’, host manipulation likely starts when the first group of worms is ready to emerge. Hosts would benefit by releasing a majority of the parasite biomass; developed worms would benefit by being released into the environment, where mating begins without delay; and immature worms would benefit by gaining access to more resources (the cricket host would no longer use its energy to carry a large biomass of worms). However, it is safe to assume that host manipulation, and its result of the cricket entering water, can also be disadvantageous to the host. These disadvantages include the possibilities of drowning and systemic bacterial infections from the damage inflicted by escaping worms. It would be interesting to investigate the timing of host manipulation, based on the proportion of immature worms and their relatedness.
Though the exact mechanisms leading to the low VRS rates are unknown, gordiids appear to be distinct from helminths of vertebrates, as females contribute offspring relatively equally to the next generation. Further investigations are needed to explore the mechanisms leading to this equality and to test whether this is a common feature of parasitic helminths with invertebrate definitive hosts.
I would like to thank Matt Bolek for allowing me access to his G. difficilis dataset, and Sara V. Brant and two anonymous reviewers for comments, which greatly improved this manuscript.