INTRODUCTION
Sexual size dimorphism is common among species from various vertebrate classes and can reflect sex differences in investment of energy or resources to growth for maximizing life-time reproductive fitness (Greenwood and Wheeler, Reference Greenwood, Wheeler, Greenwood, Harvey and Slatkin1985). Sexes also can differ in their developmental trajectories (e.g. Leclair et al. Reference Leclair, Leclair, Dubois and Daoust2000). Importantly, greater investment in growth and/or development is thought to affect the ability of individuals to invest in behavioural or somatic defences against parasites (Sheldon and Verhulst, Reference Sheldon and Verhulst1996; Moller et al. Reference Moller, Sorci and Erritzoe1998; Soler et al. Reference Soler, de Neve, Perez-Contreras, Soler and Sorci2003). The actual pattern of resource allocation over evolutionary time will be influenced not only by fitness gains of increased size or faster development rates, but also by fitness losses associated with parasitism, should parasitism occur (Schmid-Hempel, Reference Schmid-Hempel2003; Tschirren and Richner, Reference Tschirren and Richner2006).
One might expect certain species to show occurrences of sex-biased parasitism because of sexual dimorphism in investment in immune defence (Moller et al. Reference Moller, Sorci and Erritzoe1998). Testing for sex differences in susceptibility to parasites, as predicted by theory, is best done with experimental infections, where exposure to parasites is controlled (Schalk and Forbes, Reference Schalk and Forbes1997). Male-biased parasitism is often seen in mammals, where males are often the larger sex (Poulin, Reference Poulin1996a; Moore and Wilson, Reference Moore and Wilson2002). However, male-biased parasitism is not a general rule in birds (McCurdy et al. Reference McCurdy, Shutler, Mullie and Forbes1998) or arthropods (Sheridan et al. Reference Sheridan, Poulin, Ward and Zuk2000). Little is known for amphibians.
Sex differences in growth and development are expected to be detectable even at young ages, when parasites are first encountered (Tschirren et al. Reference Tschirren, Fitze and Richner2003). This is because energetic trade-offs towards faster development or greater growth at earlier life-stages can reduce the ability to control parasitic infection (Isomursu et al. Reference Isomursu, Ratti, Helle and Hollmen2006). From the viewpoint of the parasite, infecting a host with reduced potential for immune response can result in increased establishment and growth. For some species of parasitic worms, increased size in females relates to greater egg output (Poulin, Reference Poulin1996b). The fitness potential of such parasites might therefore be expected to differ between parasites infecting male and female hosts because of the existence or expression of fewer defences in one sex of host.
Anuran amphibians are a good model for exploring whether sex biases in parasitism, or measures of parasite fitness, are associated with sex biases in patterns of host growth or development. Approximately 90% of anurans display sexual size dimorphism with males being smaller than females (Shine, Reference Shine1979). However, research on growth patterns has centred on adult frogs just prior to reproductive maturity, and not at earlier stages in development (Howard, Reference Howard1985). Yet amphibian metamorphosis is a particularly vulnerable life-stage for amphibians, during which time there are gross changes in physiology and tissue development (Rollins-Smith, Reference Rollins-Smith1998; Tata, Reference Tata1999). At this stage, individuals also find themselves at the interface between aquatic and terrestrial environments that each contains novel suites of parasites. Furthermore, plasticity in development is expected to vary both between and within species (Leclair et al. Reference Leclair, Leclair, Dubois and Daoust2000); and faster development can result in increased susceptibility to trematode parasites within one anuran species (Dare et al. Reference Dare, Rutherford and Forbes2006).
For this project, Wood Frog, Rana sylvatica (=Lithobates sylvaticus) metamorphs were experimentally challenged with the lungworm parasite, Rhabdias ranae (Nematoda). Our principal objectives were 3-fold. First, the relations between sex and measures of size and developmental rate for metamorphs were explored. Wood Frogs show sexual size dimorphism at sexual maturity with larger females than males (Berven, Reference Berven1982; Bastien and Leclair, Reference Bastien and Leclair1992; Leclair et al. Reference Leclair, Leclair, Dubois and Daoust2000), but it is unknown whether any sex differences in growth or development occur for larvae. Second, a measure of expected fitness for worms (maximum size attained) infecting male and female metamorphs, was examined following experimental infections with R. ranae. Third, it was determined whether hosts showed sex-specific relations between rate of development and numbers of worms establishing in the lungs. To meet these objectives, male and female R. sylvatica metamorphs were reared from larvae in outdoor mesocosms.
MATERIALS AND METHODS
General considerations
All protocols for housing frogs and for experimental challenges using nematode larvae were done in accordance with guidelines observed by Carleton University's Animal Care Committee. All statistical analyses were performed using JMP (Version 4. SAS Institute Inc., Cary, NC) and SPSS for Windows (Version 10.0.7 SPSS Inc, Chicago, IL).
Growth and development of male and female R. sylvatica
To rear frogs to metamorphosis, 10 tanks (Rubbermaid®; 132 cm×78 cm×63 cm) were established as outdoor mesocosms in a fenced-off compound. Each tank was filled with 300 litres of water and covered with 40% shadecloth to prevent oviposition by invertebrates, to prevent predation of frog tadpoles by wildlife, and also to prevent frogs from escaping. Water levels fluctuated naturally with precipitation and evaporation. On 2 occasions, water was removed to prevent overflow. Each tank was seeded with 100 g of leaf litter collected from a mixed deciduous forest stand and 8·75 g of Purina Rabbit Chow© 2 weeks prior to the introduction of the tadpoles. By this time, there also was sufficient growth of algae to provide food. Daphnia spp. were collected using D-frame dipnets from a local pond, and introduced to a ‘stock’ tank with 300 litres of water. Once Daphnia had established in the stock tank, 750 ml of water containing Daphnia were added to each rearing tank to control excessive algal growth.
Portions of 3 R. sylvatica egg masses were collected from Casselman, Ontario (45o19′N, 75o05′W) on 11 April 2005. Each egg mass was housed indoors in a separate aquarium until hatching, with pond water from their natal habitats and a bubbler. Tadpoles were fed a granular mixture of tadpole food (Ward's Nat. Sci. Tadpole Food #88V 6534) and Hagen fish flakes (Nutrafin Max Complete Flake Food #668672) until they had developed to Gosner stage 25 (Gosner, Reference Gosner1960). Five stage-25 tadpoles reared from eggs from each of the 3 egg masses were added together for a total of 15 individuals in each of the 10 tanks.
Rana sylvatica metamorphosed from 12 June to 26 June 2005, coinciding with metamorphosis of wild conspecifics in the Ottawa region (O. Dare, personal observations). The first day a metamorph was removed was recorded as day 0 to metamorphosis (DM=0). Metamorphs were removed from tanks when they developed forelimbs (Gosner stage 42). At this stage, snout-to-vent lengths (SVL) were measured to the nearest 0·1cm, and each metamorph was also measured to the nearest 0·1 g.
Parasite life-cycle and fitness metrics
Rhabdias worms are protandrous hermaphrodites (Bush et al. Reference Bush, Fernandez, Esch and Seed2001). Adult female worms fertilize eggs with sperm stored from their preceding stage as a male. Egg capsules released from the female are coughed up and swallowed by the host. Once in the host's digestive tract, some of the eggs hatch into rhabditiform (L1 stage) larvae that are passed out along with eggs in the host's faeces. These larvae undergo 4 moults and develop into free-living male and female adults in about 3 days (Goater and Vandenbos, Reference Goater and Vandenbos1997; Gendron et al. Reference Gendron, Marcogliese, Barbeau, Christin, Brousseau, Ruby, Cyr and Fournier2003). Adult free-living females become reproductively mature in another 2 days and produce eggs that are fertilized by mature males. Some eggs are laid on host faeces, while a few eggs hatch within the female and consume her tissues (matricidal endotoky) (Bush et al. Reference Bush, Fernandez, Esch and Seed2001). Filariform, infective (L3) larvae emerge from the mother and penetrate the skin of amphibians. These larvae travel through various tissues, lymph and blood towards the lungs. As they move into the lungs they first develop into adult males and then into functional females (Baker, Reference Baker1979). Thus, we used the success of larval worms penetrating hosts relative to the number of established adult worms in the lungs of hosts as a metric of fitness potential. This is equivalent to numbers infecting hosts.
In R. sylvatica, adult fecund R. ranae worms can be found in the lungs after about 1 week post-infection (Baker, Reference Baker1979; Goater and Vandenbos, Reference Goater and Vandenbos1997). The number of females present in the lungs ⩾1 week post-infection gives an indication of the success of worms in establishing and developing to their reproductive potential. Since developmental rate influences the time of first reproduction and potentially length of egg laying over the parasite's life-time, any delay in becoming a female could reduce the parasite fitness potential. Another measure of parasite fitness is the size of fecund females. High mortality during juvenile stages should result in strong selection for high egg output and thus larger body sizes of adult females (Poulin and Morand, Reference Poulin and Morand1997). In nematodes, female worm length is a strong predictor of fecundity (Goater, Reference Goater1992; Marcogliese, Reference Marcogliese1997; Tompkins and Hudson, Reference Tompkins and Hudson1999). Thus, maximum female size attained in hosts after a set period of time can index potential fecundity for those individuals. These two measures of parasite fitness, developmental rate and size attained, are known to be independent of each other in Rhabdias ranae (Gendron et al. Reference Gendron, Marcogliese, Barbeau, Christin, Brousseau, Ruby, Cyr and Fournier2003).
Host sex and metrics of parasite success
Of the initial 150 tadpoles reared, only 93 metamorphs were used for the experiment, due in part to mortality, but more to logistic limitations on the numbers of lungworm larvae available for infection. Metamorphs were brought indoors into a quiet room. Frogs were housed in square Ziploc© plastic containers (12cm×12cm×5·5cm (ca. 946 ml)) with perforated lids. The bottom of each plastic container was lined with a wet paper towel to provide traction and prevent drowning of individuals.
To obtain parasites for controlled challenges, 12 wild adult R. sylvatica were caught in Bishops Mills Ontario (44o53′N 75o40′W) and brought back to the laboratory. Each frog was housed individually in 2·5 litre glass aquaria in the laboratory. Each aquarium was lined with a damp paper towel and an overturned Petri dish was placed at one end to provide a dry post. Temperature was maintained between 23°C and 26°C. Each frog was fed 3–5 large crickets daily. The crickets were dusted in amphibian multivitamin mix (Herptivite, Rep-Cal Research Labs, CA, USA) every other day.
Infective-stage R. ranae larvae were cultured in a manner similar to that described by Goater and Vandenbos (Reference Goater and Vandenbos1997). Briefly, faeces from the wild-caught adult frogs were incubated at room temperature (23–25°C) in Petri dishes lined with moist, unbleached, coarse-grade coffee filter paper (#2 Presidents Choice Green coffee filters®) for 3–5 days. When infective larvae were observed (a minimum of 72 h), the contents of the Petri dish were rinsed with water, and the larvae were isolated and counted using a dissecting microscope.
Metamorphs were challenged only after they had completely resorbed their tails, i.e. at Gosner stage 46. Thus the stage of development was controlled, although the metamorphs developed at different rates and achieved different sizes by this stage. Each metamorph was placed in a Petri dish (10·0 cm×1·5 cm) and exposed to 30 infective larvae (10 larvae from each of 3 adult frogs) for 24 h. This exposure in a Petri dish was done to minimize parasite avoidance by metamorphs and thereby enable worm larvae to find their host. After 24 h, the Petri dishes and damp filter paper were rinsed thoroughly 3 times and any remaining larvae were counted to index the numbers of worms that penetrated. A period of 10–11 days was allowed before the metamorphs were killed by an overdose of MS-222, and later necropsied. Thus, infections were staggered across all individuals and done for each individual according to its timing of metamorphosis.
Following challenges, metamorphs were each fed 2 or 3 crickets (from ca. 3·2–6·4 mm) every day. The number of crickets fed to each individual was determined by satiety. Crickets were dusted with amphibian multivitamin mix every other day. The containers housing metamorphs were rinsed every 3 days with water and 100% EtOH.
During necropsies, the number of worms in the lungs and body cavity were counted, and samples preserved in 70% glycerol. Each worm was identified as subadult or adult. Adult worms were identified to sex based on morphological features (ovaries and characteristic darkened digestive tube and the sheath on adult females, Fig. 1). The length of the largest female worm from each infected male and female metamorph was measured using the scaling function of a digital microscope (Zeiss Axioplan 2ie), which is accurate to ±0·01 mm. Although this measurement does not represent the mean or median length of worms in the population within a host, this length was used to index the maximum potential reproductive output of a worm, because the size of the worm likely relates to fecundity. An accurate measure of egg output per day or per gramme of feces as a direct measure of worm fecundity in Rhabdias was not possible. Some eggs hatch as they move out from the lung into the intestines before they are shed as L1-stage larvae along with the feces. At present, it is not known if there is substantial variation in proportions of larvae versus eggs that are voided. Specimen vouchers of subadults, males and females were catalogued at the Canadian Museum of Nature, Ottawa, Ontario (voucher numbers: CMNPA 2005 0011–0019).

Fig. 1. Representative stages of Rhabdias ranae. (A) L3 infective larva; (B) subadult; (C) male adult; (D) female adult. L3 larvae were obtained from fecal samples contributed by wild-caught Rana sylvatica. Subadults were removed from the body cavity, while male and female adult worms were removed from the lungs of infected R. sylvatica metamorphs. Females were identified by the presence of ovaries, darkened digestive tube and sheath, all shown in the photograph. The ranges of lengths of the worms (mm) measured are indicated on each stage.
The sex of each metamorph was identified during necropsy by the presence of either ovaries or testes. Because our necropsies were done 10–11 days later and sex was determined last during necropsy, our parasite counts and later our measures of parasite size, were taken blind relative to sex of the hosts. It was determined whether the numbers of worms that successfully penetrated hosts varied with host sex. The number of worms that established successfully in lungs as females was also compared, given that numbers penetrating were not different between male and female hosts (see Results section). Median abundance and median intensity of worms (sensu Bush et al. Reference Bush, Laffery, Lotz and Shostak1997) was used, and also a mean number of established worms was computed for each sex on a per tank basis, examining whether the sexes differed in these mean numbers of established worms using a paired t-test. Unlike the original counts, means are amenable to parametric tests. The mean sizes of the largest female worms obtained from male and female hosts were compared (using Anova) and also the differences in mean sizes of the largest female worms from males and females, on a per tank basis were compared (using a paired t-test).
Sex-specific patterns of infection in relation to developmental trajectories
Our initial questions were whether there were sex differences in host developmental trajectories and also sex differences in measures of parasite success. Therefore it was examined whether differences in developmental rates within a sex were related to differences in establishment of female worms. These analyses were done to determine whether there were sex-specific patterns of worm establishment in relation to developmental rate.
Whether time to metamorphosis related to establishment of female worms was examined for all metamorphs, separately by sex, and whether SVL or mass related to establishment of female worms for all metamorphs, separately by sex was also examined. These analyses were done using non-parametric correlation. A mean time to Metamorphosis for males and females was calculated in each tank separately, as well as mean abundance of worms. Comparison was made as to whether these two measures were related for each sex, weighted by the number of individuals used to calculate each mean. Data were analysed by sex separately (1-way Anova) because sexes differed in developmental rate and this precluded other approaches (e.g., analyses of covariance with time to metamorphosis as a covariate, following Zar, Reference Zar1996).
Numbers of male and female metamorphs produced by each tank were compared to test whether certain tanks might differentially contribute individuals of one sex to subsequent analyses. In studies such as this one, there is often significant variation in variables among rearing tanks, despite the tanks having identical rearing regimens. An effect of tank on time to metamorphosis for both males and females was tested in order to address the potential problem of pseudoreplication. If certain tanks were more likely to contribute early metamorphs of one sex, then subsequent sex differences in developmental rate might be reasonably ascribed to tank effects. Finding no significant tank effects, frogs in tanks were considered to be quasi-replicates and sexes across all tanks were compared for differences in mean SVL, mean mass, and mean time to metamorphosis, using Anova. As a control for the potential problem of pseudoreplication the tank means of SVL, mass, and time to metamorphosis for each sex were tested for differences between sexes in these measures, using paired t-tests. These considerations of tank effects also were assessed prior to other analyses examining relations between host sex and metrics of parasite success.
RESULTS
Growth and development of male and female R. sylvatica
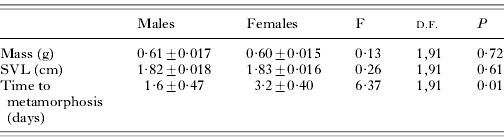
It was found that there was no propensity for tanks to contain a particular sex (pooled S.E.=0·58, F=2·53, D.F.=1,9; P=0·13). When all 10 tanks were emptied, 40 male and 53 female stage 46 metamorphs were removed (a result that is not statistically different from 50:50, P>0·05, sign test). At metamorphosis, male mass and SVL were very similar to females (Table 1). However, males and females did differ in the average time it took to metamorphose, with females taking almost 2 days longer than it took males (Table 1). Thus, although males and females were not size dimorphic at metamorphosis, the sexes still showed different developmental trajectories with males showing more rapid development. No effect of tank on time to metamorphosis was found for males (F=1·04, D.F.=9,30; P=0·43) or for females (F=1·41, D.F.=9,43; P=0·21). Using mean time to metamorphosis per tank for each sex and comparing these grand means, it was still found that males took significantly shorter duration to reach metamorphosis (paired t=2·7, D.F.=9, P=0·02). This sex difference in developmental time on a per tank basis occurred, again despite males not being different from females in terms of mean mass or mean SVL (paired t-values ranged from 1·2 to 2·0, D.F.=9, P ranged from 0·07 to 0·28).
Table 1. Developmental trajectories of male and female Rana sylvatica
(Means±S.E. of mass (g), snout-vent length (SVL) (cm) and time to metamorphosis (days) are presented. Time to metamorphosis is the number of days for larvae to develop to metamorphosis following the appearance of the first metamorph (day 0 was the first day in which a metamorph was seen). F-values (F), associated degrees of freedom (D.F.) and P-values (P) are also presented. The text also describes matched analyses based on means for each sex from each tank.)
Host sex and metrics of parasite success
On average, in both males and females approximately 20 of the 30 infective larvae penetrated (males: 20·2±0·95; females: 20·1+0·83; F=0·009, D.F.=1,91; P=0·92). In total, 15 worms were subadults, found in either lung (8) or in the body cavity (7). In comparison, 90·6% of 159 worms dissected out during necropsies were adults. Of these 144 worms, 11 were males, carried by 7 metamorphs (5 females and 2 males); male worms are not considered further. No effect of tank was found on numbers of worms establishing in male metamorphs (F=0·42, D.F.=9,30; P=0·91) or in females, although this latter result approached significance (F=1·96, D.F.=9,43; P=0·07).
The number of worms that established in lungs of hosts ranged from 0 to 10 for females and 0 to 13 for males. A large number of male and female hosts did not have any worms establish (20 of 40 males and 27 of 53 females). For this reason, it was first examined whether there were differences in median abundance of worms between males and females. It was found that 20 of 40 male frogs had worms above the median number of 0·5 worms as compared to a near equal proportion (25 of 53) of females (Z=0·27, P=0·78, Median test, Table 2). Then only hosts that were infected with adult female worms were examined. The results here were clear. It was found that 11 of 20 male frogs had worms above the median number of 2 worms as compared to 4 of 25 females (Z=−2·72, P<0·01, Median test, Table 2). Mean numbers of female worms established for each host sex per tank were compared using a paired t-test, which required only that the differences of matched pairs be normally distributed (Zar, Reference Zar1996). It was found that males again averaged higher numbers of worms (paired t=−2·76, P=0·02) for the 8 tanks where individuals of both sexes were infected with female worms.
Table 2. Median abundance and intensity of female worms (interquartile ranges in parentheses) established in male and female metamorphs
(T refers to the test statistic. For tests comparing abundance and intensity between host sexes, the Z-value is the test statistic (N=93 for abundance and N=45 for intensity). For tests comparing lengths of the largest female worms established in host sexes, the t- test is used with 36 degrees of freedom. P-values (P) are also presented. The text also describes matched analyses based on mean numbers of female worms established for each sex from each tank.)

Finally, we found that the largest female worm was, on average, larger in size in male metamorphs than in female metamorphs, despite male hosts harbouring more worms than female hosts (Table 2). However, when we examined mean lengths of the largest female worms for each sex on a per tank basis, we found that there was only a trend for larger female worms in male metamorphs (paired t=−1·81, P=0·11). There was significant variation among male metamorphs in sizes of female worms that were attributable to tank effects (F=4·30, D.F.=1,9, P=0·02), but not so for female metamorphs (F=1·18, D.F.=1,9, P=0·38). Worm intensity did not influence the size of the largest female worm (F=0·91, D.F.=1,35, P=0·35).
Sex-specific patterns of infection in relation to developmental trajectories
Time to metamorphosis was unrelated to number of female worms that established for either male or female metamorphs, when individuals were considered replicates (Spearman rho ranged from −0·14 to −0·18, P values ranged from 0·19 to 0·33). However, other patterns emerged between mean time to metamorphosis and mean number of female worms that established for each host sex, on a per tank basis. For males, we found that these two variables were related positively to one another (r 2=0·52, F=8·97, P=0·02) whereas they were inversely related for females (r 2=0·39, F=5·12, P=0·05) (Fig. 2). Thus, males that developed slower on average compared to other males, and females that developed faster compared to other females, had higher numbers of established worms. No significant nor consistent relations were found between either SVL or mass and number of female worms that established for either male or female hosts (Spearman rho ranged from 0·056 to 0·26, P values ranged from 0·10 to 0·73).

Fig. 2. Relationships between developmental rate (mean number of days to metamorphosis) and parasite establishment (mean numbers of female worms that established in lungs) for male and female metamorphs, on a per tank basis. Standard errors of the means are also shown indicating that there was much within-tank variation in both time to metamorphosis and parasite abundance for each sex. A significant positive correlation between the two variables was found for males and a significant negative correlation for females (when weighted by numbers of individuals used to obtain mean values such that single observations received little weight). These results suggested slight, but nonetheless, important sex-specific differences in relations between development and susceptibility to infection by lung nematodes. See text for further details.
DISCUSSION
The salient results of this study are 4-fold. First, sexual differences in developmental rate can occur without an associated sex difference in size or mass (a result supported by tests based on individuals as replicates, and also tests based on tank means for each sex). Second, male and female hosts did not differ in the numbers of worms that penetrated, but male hosts harboured more female worms in their lungs than did female hosts in both types of tests. Third, the size of the largest adult female worms was larger in male than female hosts, but this result was restricted to tests where individuals were considered replicates and was only a strong trend when tank means of worm lengths for male and female metamorphs were compared with paired tests. Fourth, patterns of worm establishment in relation to developmental rate differed between sexes and between tests. Relations using individuals as replicates were not significant. However, relations based on tank means showed that as average time to metamorphosis increased for males, more female worms established. The opposite pattern was seen for females. Qualitatively similar results were found for susceptibility to parasites and its relation to developmental rate for the sexes when transformed data were used: i.e., arcsin √(proportion of penetrating worms that established as females, data not presented).
Although several species of amphibians display sexual size dimorphism at adulthood, there is still some uncertainty about how the differences in sizes of adult males and females are reached. In R. sylvatica, males achieve reproductive maturity faster, but have lower longevity than females (Leclair et al. Reference Leclair, Leclair, Dubois and Daoust2000). The time-point in development at which a noticeable divergence in growth rate and/or size between sexes occurs is still largely unknown for sexually size dimorphic amphibians. However, the examination of natural populations of R. sylvatica (Howard, Reference Howard1985) has revealed that size-specific growth rates of adult males (just prior to reproductive maturity) are slower than females. Furthermore, females continue to grow for approximately a year longer than males before achieving the minimum size for reproduction. Our study demonstrates that differences in developmental rate occur between males and females of R. sylvatica, as early as metamorphosis (consistent with earlier maturity of adult males). Developmental rate is a life-history trait that is thought to trade off against avoidance of parasites or investment in immunity (e.g. Lochmiller and Deerneberg, Reference Lochmiller and Deerenberg2000). This idea can be tested experimentally with amphibians at early life-stages.
Most anurans take 2–4 years to achieve reproductive maturity, thus, it can be difficult to establish appropriate laboratory protocols for experimental infections using adults. Most controlled studies may necessitate the use of juveniles. Importantly, amphibians experience the highest rate of mortality during the aquatic phase of their life-cycle. For instance, Rana pipiens survivorship in the first stages of life is usually very low with less than 10% of oviposited eggs achieving metamorphosis (Hine et al. Reference Hine, Les and Hellmich1981). Rapid development through larval stages might confer survival advantages for terrestrial juvenile and adult stages (Werner, Reference Werner1986). Thus, amphibians may adopt the strategy of rapid development to metamorphosis for predation avoidance, or as an adaptation to ephemeral breeding ponds. For our study species there was still a reasonable amount of variation in developmental rate against which susceptibility to parasitism could be examined, even though this species can display highly synchronous, rapid development to metamorphosis (Waldman, Reference Waldman1982).
For adult amphibians, we might expect that increased allocation to larger size would result in inhibited immune function. For anurans, females should therefore carry higher parasite burdens than conspecific males, and larger individuals within a sex should carry higher burdens than smaller individuals of the same sex. The same prediction should apply to rate of development, that is, individuals that invest in faster rates of development should show greater susceptibility to parasite infection. Alternatively, the relationship between investment and immune function may be positive, whereby individuals of superior fitness invest more in faster rates of development and also display heightened resistance to parasitic infection.
For larval R. sylvatica, there are no differences in body size or mass at metamorphosis. However, sex-specific patterns of development appear to exist. This might explain why males were generally more susceptible to parasites and on average carried female worms of larger size, when the largest worm in each host was considered. Among infected males, those that developed the fastest seem to be less parasitized than other males that developed more slowly. Whereas for females, those with the slowest development had the lowest worm burdens.
Our data suggest that in amphibians, time to metamorphosis may be a determinant of susceptibility to worm infection (although different relations may exist within the sexes). This existence of apparent sex-specific relations between development and susceptibility to parasitism is intriguing. It appears that individuals that do not have a developmental trajectory characteristic of their sex, are the ones most susceptible to parasitism. This finding is somewhat at odds with theoretical predictions that different developmental trajectories shown by different sexes can result in differential susceptibility to infection between sexes. It is important that there is still considerable within-tank variation in both time to metamorphosis and susceptibility to nematodes for both males and females.
Susceptibility to parasitism is a host-centred metric, but also relates to the parasite's expectation of fitness. The largest female worm from each host was, on average, larger in male metamorphs than in female metamorphs. It was not possible to isolate live female worms from the lungs to determine egg output or establish fecundity; however, larger worms might reasonably be expected to have higher fecundity. Several studies have reported a positive relationship between per capita fecundity and per capita worm size (see Tompkins and Hudson, Reference Tompkins and Hudson1999). Larger-sized helminths in male hosts may be a generality; it has been observed in the majority of studies in which helminth size has been measured (Poulin, Reference Poulin1996b). Furthermore, egg production by individual worms infecting male hosts has been observed to be higher than that observed in female hosts (Molan and James, Reference Molan and James1984; Swanson et al. Reference Swanson, Falvo and Bone1984). Thus studies examining sex-bias in parasitism should include lengths of worms obtained from among male and female hosts.
The nematodes studied herein are expected to exact fitness costs on their hosts. Each female worm spanned approximately 2/3 the length of a host's lung (O. Dare-unpublished observations), thereby occupying a significant portion of the lung cavity. Also, adult female R. ranae acquire bloodmeals from their host. Thus, these worms may inhibit the mechanical functioning of the lung, may cause damage to the lining of the lung or cause blockages of the blood vessels and therefore impose a considerable debilitating effect on hosts if even few worms are carried (see Goater et al. Reference Goater, Semlitsch and Bernasconi1993). As many as 17 adult female worms have been observed in the lungs of a single wild R. sylvatica individual (O. Dare-unpublished observations). Rana bufonis, a congener lungworm of similar natural history and infective characteristics, is also known to cause anorexia and reduce survival, growth and physical performance of the toad, Bufo bufo (Goater and Ward, Reference Goater and Ward1992). Similarly, it has been suggested that infection with R. ranae may impede chorusing ability in male R. sylvatica during the breeding season and reduce reproductive success (Goater and Vandenbos, Reference Goater and Vandenbos1997). Intensity of infection in the host did not affect the size of the largest female worms, or developmental rate, likely because of the low infection dosage used in our experimental protocol. We suspect increased susceptibility to infection in males might contribute to the higher mortality levels observed in wild male amphibians (Leclair et al. Reference Leclair, Leclair, Dubois and Daoust2000).
In summary, our study demonstrates that differential developmental trajectories between sexes can occur in an amphibian species independently of differences in size. We also show that variation in host development is associated with variation in parasite establishment, maximum size attained by worms and their potential reproductive output. One epizootiological implication of sex-bias in immune response is that parasite burdens may be over-dispersed within hosts of a particular sex because of that sex's increased susceptibility to infection. As such, individuals of that sex may become the main contributors of subsequent cohorts of parasites, and may drive the dynamics of parasite transmission and establishment throughout a host population. This problem has been suggested by others (Ferrari et al. Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004; Skorping and Jensen, Reference Skorping and Jensen2004). Such ecological implications will of course depend on exposure of individuals of each sex to parasites, and whether there is disparate pathology or mortality in the sex with overdispersed parasite burdens. Such sex differences in exposure and impact of parasites are worthy of further investigation.
We would like to thank Lynne Rasmussen who assisted with the technical and logistical components of this project, and Arash Rashed for helpful editorial contributions. Funding for this project was provided by a NSERC PGS-B Scholarship to O.D. and by a NSERC discovery grant and Petro-Canada Young Investigators Award to M. R. F. Four anonymous referees made many helpful suggestions that aided presentation, analysis, and interpretation.






