INTRODUCTION
The Atlantic Forest, one of the most important biodiversity hotspots in the world (Mittermeier et al. Reference Mittermeier, Myers, Gil and Mittermeier1999), has suffered dramatic and rapid changes due to habitat loss and fragmentation, with the most intensive disturbance levels occurring in south-eastern Brazil (Dean Reference Dean1997; SOS Mata Atlântica & INPE [Instituto Nacional de Pesquisas Espaciais] 2008; Tabarelli et al. Reference Tabarelli, Aguiar, Ribeiro, Metzger and Perez2010). The unplanned expansion of both agricultural frontiers and urban areas has transformed the Atlantic Forest into agroecosystems with a patchwork of disconnected and disturbed forest remnants (SOS Mata Atlântica & INPE 2008), corresponding to < 16% of the original forest cover, with only 7.1% of the area being interior forest (SOS Mata Atlântica & INPE 2008; Ribeiro et al. Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009). The Corumbataí river basin, located in one of the most developed regions of São Paulo State (south-eastern Brazil), is a typical example of these landscape modifications, containing small (67.8% of the forest remnants in the Corumbatai river basin are < 1ha and only 0.7% are > 80ha), scattered and isolated remnants of original Atlantic Forest, with a distance of up to 1.47 km between fragments of < 5 ha, surrounded mainly by a matrix of sugar cane and pasture (Rodrigues Reference Rodrigues1999; Valente & Vettorazzi Reference Valente and Vettorazzi2003, Reference Valente and Vettorazzi2005). One of the main consequences of this fragmentation and habitat destruction is the precarious situation facing most of the endemic birds (Parker et al. Reference Parker, Stotz, Fitzpatrick, Stotz, Fitzpatrick, Parker and Moskovits1996; Goerck Reference Goerck1997), with 98 of 160 endangered bird species occurring mainly in the Atlantic Forest (Silveira & Straube Reference Silveira, Straube, Machado, Drummond and Paglia2008).
Many studies consider the effects of landscape or habitat fragmentation on biodiversity (Turner Reference Turner1996; Chiarello Reference Chiarello1999; Lynam & Billick Reference Lynam and Billick1999; Laurance et al. Reference Laurance, Lovejoy, Vasconcelos, Bruna, Didham, Stouffer, Gascon, Bierregaard, Laurance and Sampaio2002; Fahrig Reference Fahrig2003), but few consider the influence of matrix heterogeneity (Devictor & Jiguet Reference Devictor and Jiguet2007; Umetsu et al. Reference Umetsu, Metzger and Pardini2008; Prevedello & Vieira Reference Prevedello and Vieira2010). The matrix (the land cover type dominating others in area and connectivity; Forman & Godron Reference Forman and Godron1986; Forman Reference Forman1995; Metzger Reference Metzger2001), often a heterogeneous mosaic of different land cover types surrounding modified fragments in a human-dominated landscape, may exert a strong influence on vertebrate communities (Gascon et al. Reference Gascon, Lovejoy, Bierregaard, Malcon, Stouffer, Vasconcelos, Laurance, Zimmerman, Tocher and Borges1999; Laurance et al. Reference Laurance, Lovejoy, Vasconcelos, Bruna, Didham, Stouffer, Gascon, Bierregaard, Laurance and Sampaio2002; Tischendorf et al. Reference Tischendorf, Bender and Fahrig2003; Uezu et al. Reference Uezu, Metzger and Vielliard2005; Debinski Reference Debinski2006; Devictor & Jiguet Reference Devictor and Jiguet2007; Umetsu & Pardini Reference Umetsu and Pardini2007; Umetsu et al. Reference Umetsu, Metzger and Pardini2008; Hansbauer et al. Reference Hansbauer, Storch, Knauer, Pilz, Küchenhoff, Végvári, Pimentel and Metzger2010; Prevedello & Vieira Reference Prevedello and Vieira2010). The ability of species to use the matrix (Antongiovanni & Metzger Reference Antongiovanni and Metzger2005; Uezu et al. Reference Uezu, Beyer and Metzger2008), the type of matrix (Prevedello & Vieira Reference Prevedello and Vieira2010) and the matrix quality (Vandermeer & Carvajal Reference Vandermeer and Carvajal2001; Umetsu & Pardini Reference Umetsu and Pardini2007; Umetsu et al. Reference Umetsu, Metzger and Pardini2008) can be extremely important in determining the structure and persistence of vertebrate communities in heterogeneous and fragmented landscapes.
Forest specialist species are among the taxa most vulnerable to the conversion of forest into agriculture landscapes, as most struggle to exist in small and isolated forest remnants (Giraudo et al. Reference Giraudo, Matteucci, Alonso, Herrera and Abramson2008; Martensen et al. Reference Martensen, Pimentel and Metzger2008; Boscolo & Metzger Reference Boscolo and Metzger2011). While some species may be restricted to the remaining forest fragments, others may be able to survive in different anthropogenic habitat types to various degrees (Hansbauer et al. Reference Hansbauer, Storch, Knauer, Pilz, Küchenhoff, Végvári, Pimentel and Metzger2010). The reduction and isolation of habitat patches can lead to a local loss of forest-dependent species, favouring habitat generalists (Willis Reference Willis1979; Turner Reference Turner1996; Stratford & Stouffer Reference Stratford and Stouffer1999; Willis & Oniki Reference Willis and Oniki2002; Antunes Reference Antunes2005; Giraudo et al. Reference Giraudo, Matteucci, Alonso, Herrera and Abramson2008). The dispersal capacity of some bird species between isolated patches can be limiting (Moore et al. Reference Moore, Robinson, Lovette and Robinson2008; Boscolo & Metzger Reference Boscolo and Metzger2011), especially in a landscape with a consolidated matrix (>40 years old), as the case in the Corumbataí river basin. Improving matrix connectivity and the potential for species dispersal within this landscape should be prioritized in conservation and environmental planning; habitat area has been considered an important predictor of bird species occurrence (Cerezo et al. Reference Cerezo, Perelman and Robbins2010; Mortelliti et al. Reference Mortelliti, Fagiani, Battisti, Capizzi and Boitani2010, Smith et al. Reference Smith, Fahrig and Francis2011).
Considering the importance of knowing the impacts of anthropogenic landscapes on vertebrate communities, this study aimed to evaluate the environmental suitability of a highly fragmented agricultural landscape in the Atlantic Forest (south-eastern Brazil) for forest-dependent bird species using species distribution modelling (SDM) techniques. SDM was also used to evaluate a simulated scenario assuming the expansion of forest remnants along all riparian zones (such as the buffer strips surrounding rivers and streams) on private landholdings, improving landscape connectivity for species distribution and dispersal, as required by current Brazilian forest legislation (Código Florestal 2001). This is particularly important, as Brazil risks suffering its worst environmental setback in half a century (Metzger et al. Reference Metzger, Lewinsohn, Joly, Verdade, Martinelli and Rodrigues2010), with the ongoing reform of its forest legislation (Brazilian Forest Act) condemning old-growth remnants and forest regrowth in private landholdings, potentially leading to irreversible loss of tropical biodiversity (Michalski et al. Reference Michalski, Norris and Peres2010).
METHODS
Study area
We undertook the study in the Corumbataí river basin (1710 km2), in São Paulo state (22°04′–23°41′S, 47°26′–47°56′W; Fig. 1). The study area comprises eight municipalities, containing c. 530 000 inhabitants. The topography of the region is moderately undulating. The most important river is the Corumbataí river, which originates in the cuesta zone (1058 m at the headwaters), reaching the Piracicaba river (470 m at the discharge) after crossing Rio Claro city, the most important municipality in the basin (Garcia et al. Reference Garcia, Antonello and Magalhães2006).

Figure 1 Location of the Corumbataí river basin (C) in São Paulo State (B), Brazil (A). Forest remnants and study sites are indicated (C).
The study area is characterized by a landscape composed of sparse and scarce Atlantic Forest fragments surrounded by a matrix of sugar cane or pasture. After intensive and persistent anthropogenic landscape modifications, c.12% of original Atlantic Forest remains in the river basin in highly fragmented condition; most remnants follow the drainage network (Valente & Vettorazzi Reference Valente and Vettorazzi2003). The landscape is a heterogeneous mosaic encompassing mixed cultivated fields, urban areas, pasture, forest remnants and eucalyptus plantations. Sugar cane (c. 26%) and pasture (c. 44%) are now the dominant land uses in the Corumbataí river basin (Valente & Vettorazzi Reference Valente and Vettorazzi2003; Fig. 2).

Figure 2 Land use/land cover map and environmental suitability models for eight forest bird species, as modelled for both (a) actual and (b) simulated landscapes (assuming riverine forest corridors in compliance with the mandatory permanent protection area required by Brazilian Federal Law).
Bird survey
We selected 122 study sites for bird surveys at random in order to spatially cover the river basin (Fig. 1). Study sites encompassed native forest (fragments and corridors), eucalyptus forest, pasture, sugar cane, perennial crops and urban areas. The same observer recorded all bird species that were seen or heard throughout the study. As we only used presence records, we surveyed birds using unlimited radius point counts (Ralph et al. Reference Ralph, Sauer, Droege, Ralph, Droege and Sauer1995) during 20 min. in the early morning, April–September 2006 and January–March 2009. Sampling effort was the same for all sampling sites.
From the total 169 recorded species, for our model, we preferentially selected eight forest-dependent bird species (Sick Reference Sick1997; Willis & Oniki Reference Willis and Oniki2003; Sigrist Reference Sigrist2006; Table 1) for which we had recorded a reasonable number of observations. Species more vulnerable to fragmentation, such as Odontophorus capueira, Hypoedaleus guttatus, Drymophila ferruginea and Pyriglena leucoptera did not provide suitable sample sizes for modelling. Despite their low to medium sensitivity to human disturbances, the species analysed are considered forest-dependent, and we had previously established that their populations were suffering from the impacts of habitat loss and fragmentation. Thus, these eight species should act as appropriate indicators of the environmental suitability of the anthropogenic landscape for forest-dependent species.
Table 1 Characteristics of forest bird species used in models. Sensitivity to human disturbance obtained from Parker et al. (Reference Parker, Stotz, Fitzpatrick, Stotz, Fitzpatrick, Parker and Moskovits1996), biomes obtained from Parker et al. (Reference Parker, Stotz, Fitzpatrick, Stotz, Fitzpatrick, Parker and Moskovits1996), Sick (Reference Sick1997) and Sigrist (Reference Sigrist2006). *Endemic species from Atlantic Forest (according to Parker et al. Reference Parker, Stotz, Fitzpatrick, Stotz, Fitzpatrick, Parker and Moskovits1996). **With occurrence in secondary forest.

Modelling procedures
We used the species presence records and six landscape predictors with a spatial resolution of 20 m (Table 2) as our variables in the SDM. We produced two predictions for the potential species distributions. One set was modelled on the actual landscape, based on a 2003 land use/land cover map (Valente & Vettorazzi Reference Valente and Vettorazzi2003; Fig. 2). The other set was modelled for a future simulated landscape, assuming that forest remnant areas had increased in compliance with current Brazilian forest legislation, requiring the set-aside of all riparian forest buffer strips along rivers and streams as a ‘Permanent Protection Area’ (Código Florestal 2001; Fig. 2). For the present study, we assumed this simulated landscape to contain forest buffer strips of 30 m width for any river or stream <10 m wide, and 50 m width for rivers and streams between 10 and 50 m wide. The consequent simulated land cover map assumed a 48% increase in forest corridors in the river basin, connecting fragmented forest cover over the whole drainage network. Landscape diversity and distance from the nearest forest fragment were calculated for both the actual and simulated land cover maps, and used for each model.
Table 2 Description of landscape predictors used in the modelling.

We used Maxent for our SDMs (see URL http://www.cs.princeton.edu/~schapire/maxent/; Phillips et al. Reference Phillips, Dudík and Schapire2004, Reference Phillips, Anderson and Schapire2006, Reference Phillips, Dudík, Elith, Graham, Lehmann, Leathwick and Ferrier2009; Phillips & Dudík Reference Phillips and Dudík2008). Maxent is a modelling technique that achieves high predictive accuracy. In maximum entropy density estimation, the true distribution of a species is represented as a probability distribution over the set of sites in the study area. This probability assigns a non-negative value to every site in the study area and respects a set of constraints derived from the occurrence data. The constraints are expressed in terms of simple functions of the environmental variables (features). Specifically, the mean of each feature must be close to the empirical average over the presence sites (Phillips & Dudík Reference Phillips and Dudík2008).
Our model parameters were: a convergence threshold of 10−5 with 500 iterations and with 10 000 background points, auto features, and analysis of variable importance measured by jackknife, response curves and random seed. We defined two different partitioning methods, depending on the number of presence records of each species. We sampled datasets having at least 15 presence records by bootstrapping with 10 random replicates with replacement setting 70% of the dataset for training and 30% for testing models (Pearson Reference Pearson2007). Datasets with less than 15 presence points were sampled by a jackknife (or ‘leave-one-out’) procedure, where each observed locality was removed once from the set of data and we constructed the model using the remaining (n – 1) localities (Pearson et al. Reference Pearson, Raxworthy, Nakamura and Peterson2007). We assessed the predictive performance of each model on their ability to predict the single locality excluded from the training data set.
The logistic output format was used, which results in each grid cell in the map having values ranging continuously from 0 (least suitable) to 1 (most suitable). These values can be interpreted as indicating the environmental suitability for the target species (Phillips et al. Reference Phillips, Dudík and Schapire2004; Veloz Reference Veloz2009). We made the distinction between suitable and unsuitable areas, necessary for model validation and interpretation, by setting the ‘maximum test sensitivity plus specificity’ as a decision threshold rule. Sensitivity (Se) and specificity (Sp) are conditional probabilities widely used in SDM. Se is the probability that the model correctly predicts an observation of a species at a site, and Sp is the probability that a known absence site is correctly predicted (Liu et al. Reference Liu, White and Newell2011). Both measures can be used to assess the overall prediction success of SDMs. The sum of Se and Sp can be maximized to give a better threshold (Manel et al. Reference Manel, Williams and Ormerod2001; Liu et al. Reference Liu, Berry, Dawson and Pearson2005), which is equivalent to finding a point on the receiver operating characteristic (ROC) curve whose tangent slope is equal to 1 (Cantor et al. Reference Cantor, Sun, Tortolero-Luna, Richards-Kortum and Follen1999); the ROC curve characterizes the performance of a model under all possible thresholds, and is used to identify those areas with highest suitability (where the sum of Se and Sp is maximized), reducing the risk of choosing unsuitable sites for species (Pearce & Ferrier Reference Pearce and Ferrier2000).
The final model chosen was that based on the average produced by Maxent software (version 3.3.3e), which presented the mean value for each pixel based on the suitability values, for each of the 10 replicates used. We evaluated the models by calculating the area under the curve (AUC), a threshold-independent measure of overall model performance (Fielding & Bell Reference Fielding and Bell1997); the AUC is the probability that a randomly chosen presence site will be ranked above a random site, where a random ranking has, on average, an AUC of 0.5, and a perfect ranking achieves the best possible AUC of 1.0, although, when true presences and random points are used to calculate AUC, its maximum value is always <1. SDMs were evaluated by the omission error (false negative predictions) (Fielding & Bell Reference Fielding and Bell1997). We evaluated the significance of models generated by the bootstrapping method by the one-tailed binomial test (Anderson et al. Reference Anderson, Lew and Peterson2003), and models generated with the jackknife procedure by a p value (Pearson et al. Reference Pearson, Raxworthy, Nakamura and Peterson2007).
RESULTS
Potential distribution areas for forest bird species were concentrated at and close to forest remnants (Fig. 2). The SDMs predicted an average of 24.41 ± 6.31% of the anthropogenic landscape as suitable for forest birds. These areas encompassed forest remnants (fragments and corridors), pasture and a small portion of sugar cane. Highly suitable areas (≥0.7 suitability) represented no more than 2% of the area (ranging from 0 to 1.81%), encompassing only small portions of forest remnants for most species.
Simulated landscapes resulted in a low increase in the availability of total suitable areas for most of the species (averaging 43.16 ± 6.14%), except for Thamnophilus caerulescens and Basileuterus hypoleucus, and also in the area of suitable native forest (averaging 23.69 ± 6.95%) (Table 3).
Table 3 Total suitable area (km2), suitable native forest (km2) and increment (%) in suitable areas for forest bird species in both actual and simulated landscapes.

All predictive models were statistically significant, with high AUC values and low omission errors (Table 4). Distance from forest was the highest contributor variable for all model predictions, although landscape diversity also explained the predicted distributions of Leptotila verreauxi, Picumnus albosquamatus and Synallaxis spixi (Table 5). In general, environmental suitability decreased as distance from fragments increased, and increased as landscape diversity increased (Fig. 3).
Table 4 AUC scores, test and training omission, binomial probability (based on bootstrapping method presented by Anderson et al. Reference Anderson, Lew and Peterson2003) and p values (based on jackknife technique presented by Pearson et al. Reference Pearson, Raxworthy, Nakamura and Peterson2007). Threshold: maximum test sensitivity plus specificity. – = no value.
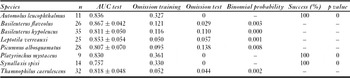
Table 5 Per cent contribution of main landscape predictors to forest species models.


Figure 3 Logistic regression curves for the probability of occurrence against main landscape descriptors: (a) distance from forest and (b) landscape diversity for forest bird species.
DISCUSSION
SDMs revealed the agricultural and fragmented landscape was only of low suitability for forest bird species in both current and simulated landscapes. As found across the entire Atlantic Forest region (Ribeiro et al. Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009), the Corumbataí river basin, one of the most developed regions in south-eastern Brazil (Garcia et al. Reference Garcia, Antonello and Magalhães2006), is at a critical stage of the fragmentation process, with only c. 12% of the original Atlantic Forest remaining, represented by small and isolated forest fragments. More than 90% of remaining fragments in the river basin cover <5 ha (Valente & Vettorazzi Reference Valente and Vettorazzi2005). Thus, the availability of suitable ‘natural’ habitats for forest species in the region is restricted (as for most of the Atlantic Forest in Brazil), as confirmed by our SDMs. Although the species modelled present medium to low sensitivity to human disturbance (Parker et al. Reference Parker, Stotz, Fitzpatrick, Stotz, Fitzpatrick, Parker and Moskovits1996), the areas of their potential distributions were small, reflecting the restricted availability of suitable habitat in the river basin.
The SDMs revealed that suitable areas included most of the forest remnants and also a small portion of the surrounding agricultural matrix. Thus, the increase in total suitability for most of species (averaging 43.16%) generally resulted from an increase in pasture and sugar cane area considered as suitable in the final models, excluding Basileuterus hypoleucus and Thamnophilus caerulescens. However, as all species are forest dependent (Sick Reference Sick1997; Willis & Oniki Reference Willis and Oniki2003; Sigrist Reference Sigrist2006), they may not actually occur in matrix habitats such as sugar cane and pasture. This apparent suitability of the agricultural matrix may be an artefact of commission errors resulting from presence points located at the fragment edge being characterized as sugar cane or pasture when the location database was overlaid with the land cover maps.
The simulated land cover model assumed linear forest remnants (forest corridors) were distributed along the drainage network, as required by Brazilian federal law (Código Florestal 2001), predicting a small increase in suitable native forest (average of 23.69%; Table 3) for forest bird species occurrence. Narrow riparian forest corridors are a predominant feature of many deforested landscapes in Brazil, as current forest legislation requires that (1) all riparian zones on private landholdings are maintained as permanent reserves, and (2) riparian forest buffers fixed minimum width are retained alongside rivers and perennial streams (Lees & Peres Reference Lees and Peres2008). Maintaining suitable corridor widths is crucial for biodiversity conservation, as the effects of fragmentation are striking within 100 m of forest edges (Laurance et al. Reference Laurance, Lovejoy, Vasconcelos, Bruna, Didham, Stouffer, Gascon, Bierregaard, Laurance and Sampaio2002), and narrow remnant corridors may therefore fail to provide suitable habitat for many forest vertebrate species, retaining only a relatively depauperate vertebrate assemblage typical of deforested habitats (Lees & Peres Reference Lees and Peres2008).
Most of the riparian forest corridors in south-eastern Brazil are narrow remnant riparian buffers set aside following deforestation; these are typically highly degraded and of low conservation value. As tropical landscapes become increasingly human-dominated, deforested and fragmented, riparian corridors are becoming disproportionately important in connecting and harbouring populations of tropical forest organisms (Sekercioglu Reference Sekercioglu2009). A revision of the Brazilian Forest Act, the main Brazilian environmental legislation for privately-owned land, proposes reductions in the area of forest that must be retained along rivers and streams, and is currently awaiting approval by Congress (Metzger et al. Reference Metzger, Lewinsohn, Joly, Verdade, Martinelli and Rodrigues2010). If approved, this revision could lead to an irreversible loss of tropical biodiversity (Michalski et al. Reference Michalski, Norris and Peres2010), aggravating the critical situation facing the conservation of biodiversity in Atlantic Forest remnants.
Distance from forest was the most critical variable in our model predictions. We used this continuous variable in place of the corresponding categorical land cover variable to avoid an increase in the number of variables required in our models. Landscape diversity, distance from streams, slope and aspect were also important in predicting the potential distribution of some species, suggesting that the distribution patterns for the forest-dependent species (excluding Automolus leucophthalmus) occurring in this agricultural landscape depended on other environmental descriptors besides the extent of forest cover. Proximity to forest fragments and landscape diversity (heterogeneity) increased environmental suitability for all forest bird species.
Heterogeneity and extent of habitat cover are often positively correlated with the richness of taxonomic assemblages (Radford et al. Reference Radford, Bennett and Cheers2005; Bennett et al. Reference Bennett, Radford and Haslem2006; Devictor & Jiguet Reference Devictor and Jiguet2007; Haslem & Bennett Reference Haslem and Bennett2008), while the composition of the habitat mosaic (based on the proportions of elements present) is associated with the species composition (Bennett et al. Reference Bennett, Radford and Haslem2006). However, the role of heterogeneity in species distribution patterns, especially in modified and heterogeneous landscapes, still remains unclear. The results of this study highlighted the importance of quantifying and including landscape variables as descriptors in modelling species distributions.
The heterogeneous mosaic of the agricultural landscape in south-eastern Brazil could be related to the amount of available critical resources and surrounding habitats for the local biodiversity. According to Kennedy et al. (Reference Kennedy, Marra, Fagan and Néel2010), the structure, composition and land-use disturbance regimes in matrix areas have an overall impact on the habitat quality in landscapes by potentially mediating resource availability inside as well as outside of forest habitats. The population's persistence for many species in agriculturally dominated landscapes depends not only on the amount of surrounding habitats, but also on the existence of favourable habitats within the adjacent matrix (Devictor & Jiguet Reference Devictor and Jiguet2007).
CONCLUSIONS
The SDM proved to be an efficient tool for modelling species distributions in a small region with a fine spatial resolution, as all models were biologically relevant and statistically significant, with high AUC scores and low omission errors. Spatial scale can play an important role in the application of species’ distribution models (Pearson Reference Pearson2007). In general, SDMs have been used at continental scales, using datasets that cover large extents with a coarse resolution. Ideally, as pointed out by Pearson (Reference Pearson2007), the data resolution should be relevant to (1) the species under consideration, (2) the study question and (3) the desired application.
The use of landscape structure variables in the SDMs contributed significantly to the accuracy of our species distribution predictions. Most SDMs are generated using environmental data, which describe the region where the species occur, represented by climate and topographical variables (see Pearson Reference Pearson2007); landscape structure variables have been used relatively rarely. The incorporation of landscape variables is strongly encouraged for future similar studies; they may better explain habitat suitability, and are particularly critical for species distributions over small extents and at fine scales.
ACKNOWLEDGEMENTS
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (2008/03500-6, 2006/04878-7, 2005/00405-4) for scholarships and financial support for field activities, and the Forest Science Department (ESALQ/USP) for logistic help in the development of this research. We thank Jefferson Polizel for his logistic support, Rodrigo da Silva Matos for helping with modelling, and Juliana Mesquita for figures artwork. We thank the anonymous reviewers for valuable contributions to the manuscript.










