Introduction
Sporastatia A. Massal. is a small genus comprising four currently accepted species (Wijayawardene et al. Reference Wijayawardene, Hyde, Rajeshkumar, Hawksworth, Madrid, Kirk, Braun, Singh, Crous and Kukwa2017). Species of Sporastatia are arctic-alpine lichens occurring mostly on exposed hard siliceous or weakly calcareous rocks. Representatives of Sporastatia are long-lived and play an important role in the pedogenetic process (Favero-Longo et al. Reference Favero-Longo, Castelli, Salvadori, Belluso and Piervittori2005). Sporastatia testudinea (Ach.) A. Massal. has been used in lichenometric studies (Haeberli et al. Reference Haeberli, King and Flotron1979). Two of the species, S. testudinea and S. polyspora (Nyl.) Grummann, are widely distributed throughout the Northern Hemisphere and are scattered in the Southern Hemisphere on subantarctic islands and in southern South America (Grube & Poelt Reference Grube and Poelt1993; Thomson Reference Thomson1997; Gilbert & Coppins Reference Gilbert and Coppins2009). The remaining two species, S. asiatica H. Magn. and S. subasiatica N. S. Golubk., are alpine endemics of Asia (Golubkova Reference Golubkova1973, Reference Golubkova1982). In addition, S. testudinea var. karakorina Poelt & Obermayer, described from the Karakorum in 1990, is unique within the genus in possessing thalloconidia. Grube & Poelt (Reference Grube and Poelt1993) treated these three endemic taxa as morphotypes or races of one highly variable taxon, S. testudinea and suggested additional studies of this complex to delimit taxa satisfactorily.
Sporastatia is morphologically characterized by a crustose, areolate to effigurate placodioid thallus surrounded by a distinct black prothallus, lecideine, immersed apothecia with a rough or wrinkled disc, and 100–200-spored asci with hyaline, simple, ellipsoid to globose ascospores. Several species are hosts for lichenicolous fungi or lichens, especially the species Rhizocarpon pusillum Runemark, R. asiaticum Poelt and Miriquidica invadens Hafellner et al. (Poelt Reference Poelt1990; Rambold & Triebel Reference Rambold and Triebel1992; Davydov et al. Reference Davydov, Konoreva, Andreev, Zhdanov and Dobrysh2012; Hafellner et al. Reference Hafellner, Obermayer and Tretiach2014).
The genus Sporastatia was established by Massalongo (Reference Massalongo1854) and Magnusson (Reference Magnusson1936) considered it to belong to the Acarosporaceae. The similarities between Sporastatia and Catillaria were noted by Hertel & Rambold (Reference Hertel and Rambold1988), who later placed the genus in Catillariaceae (Rambold & Triebel Reference Rambold and Triebel1992) because of its Catillaria-type ascus. Sporastatia was recently classified in a separate family, Sporastatiaceae (Bendiksby & Timdal Reference Bendiksby and Timdal2013), and was shown to be sister to Rhizocarpaceae (Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014). Several other taxa have been described but these have mostly been subsequently synonymized by different authors with the widespread species S. testudinea and S. polyspora. The taxonomic status of many taxa from the Southern Hemisphere (Dodge Reference Dodge1970) is not clear.
Sporastatia testudinea and S. polyspora are very common in the Altai Mts, especially in the upper mountain zone. This region remains underexplored (Davydov & Printzen Reference Davydov and Printzen2012; Davydov et al. Reference Davydov, Konoreva, Andreev, Zhdanov and Dobrysh2012). During fieldwork in 2014 in the Altai Mts, our attention was caught by an epilithic placodioid lichen resembling Lecanora. Anatomical investigation showed that the specimens had Catillaria-type asci with 100–200 hyaline, simple, ellipsoid to globose ascospores. Both morphological and molecular studies have independently confirmed its status as a new species, described here as Sporastatia crassulata. Sporastatia testudinea var. karakorina was also studied to determine whether it should be elevated to the level of species. In addition, the type specimen of S. subasiatica was examined.
Materials and Methods
Specimens and phenotypic studies
The core material for this study was collected by the authors and deposited in the herbaria ALTB and LE. Additionally, type specimens were revised in LE, GZU and S. Morphological observations were carried out using a dissecting microscope. Cross-sections of apothecia and thalli were hand cut with a razor blade and observed in water. Measurements are presented as follows: (minimum value)
![]() $$\left( {\bar{x}{\minus}{\rm SD}} \right){\minus}\bar{x}{\minus}\left( {\bar{x}{\plus}{\rm SD}} \right)$$
(maximum value), where
$$\left( {\bar{x}{\minus}{\rm SD}} \right){\minus}\bar{x}{\minus}\left( {\bar{x}{\plus}{\rm SD}} \right)$$
(maximum value), where
![]() $$\bar{x}$$
is the (arithmetic) sample mean, and SD the sample standard deviation. The two extreme values are given to the nearest 0·5 µm, and the sample mean to the nearest 0·1 µm. The description below is based on nine specimens from four localities.
$$\bar{x}$$
is the (arithmetic) sample mean, and SD the sample standard deviation. The two extreme values are given to the nearest 0·5 µm, and the sample mean to the nearest 0·1 µm. The description below is based on nine specimens from four localities.
Secondary products were analyzed by applying standard thin-layer chromatography techniques (Culberson & Kristinsson Reference Culberson and Kristinsson1970). Solvents A (toluene: 1,4-dioxane: acetic acid, 180: 45: 5), B (hexane: diethyl ether: formic acid, 140: 72: 18) and C (toluene: acetic acid, 170: 30) were used for the TLC analyses. The identification of insoluble lichen pigments follows the methods described by Meyer & Printzen (Reference Meyer and Printzen2000).
Sequences and phylogenetic reconstructions
To test the phylogenetic relationships of the putative new species, SSU mrDNA sequences of our fresh material and other sequences retrieved from the NCBI database (GenBank) were used for molecular phylogenetic analysis. We also sequenced ITS nrDNA. Sequences of S. testudinea and S. polyspora were obtained from GenBank. The Asian endemic species of Sporastatia, S. asiatica and S. subasiatica, were not included in the phylogenetic work because we were unable to obtain fresh material of these species and the specimens available in herbaria were too old to allow successful DNA extraction. Our sampling comprised 10 species of Rhizocarpales including Sporastatia, Toensbergia, and Rhizocarpon, as well as five species of Umbilicariales as an outgroup. This selection is based on the studies of Bendiksby & Timdal (Reference Bendiksby and Timdal2013) and a five-gene analysis by Miadlikowska et al. (Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014) in which Umbilicariales form a sister clade to Rhizocarpales. Information on these samples along with the GenBank Accession numbers are given in Table 1.
Table 1 Sample numbers and their GenBank Accession numbers for the phylogenetic analyses in this study. New sequences are indicated in bold

DNA extraction, amplification and sequencing followed the methods of Davydov & Yakovchenko (Reference Davydov and Yakovchenko2017). The Hasegawa-Kishino-Yano parameter with proportion of invariable sites and gamma-distribution (HKY+I+G) was inferred as the optimal substitution model. The matrix consisted of 725 sites, 203 of which were variable and used for RAxML and Bayesian analyses. Bayesian inference with the Markov chain Monte Carlo (BMCMC) method (Larget & Shimon Reference Larget and Shimon1999) was performed using MrBayes 3.2.3 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Three parallel Bayesian analyses were run in six chains and every 200th generation was sampled. Convergence of the chains was inferred by calculating the average standard deviation of split frequencies every 100 000 generations using a burn-in fraction of 0·5 and the runs terminated when the standard deviation of split frequencies dropped below 0·001. This was the case after 5·9 M generations. The first 50% of trees were discarded as burn-in and a 50% majority-rule consensus tree was calculated from the remaining trees of three runs with the sumt command implemented in MrBayes 3.2.3. Bootstrap support values and BMCMC posterior probability were noted on the best scoring tree. The most likely tree and 1000 bootstrap replicates were calculated using RAxML 8.0.26 (Stamatakis Reference Stamatakis2014) by raxmlGUI software version 1.3.1 (Silvestro & Michalak Reference Silvestro and Michalak2012), applying the GTRGAMMA model of substitution to the subsets because RAxML does not support the HKY model.
Results
ITS and mtSSU sequences were successfully obtained from two specimens of the putative new species, described below as Sporastatia crassulata; mtSSU sequences were used for reconstruction of the phylogeny in Rhizocarpales. As the Bayesian 50% majority-rule consensus tree had the same topology as RAxML, both phylograms are combined in Fig. 1.

Fig. 1 Maximum likelihood phylogeny of selected Rhizocarpales mtSSU. Numbers at nodes indicate bootstrap values of ML (left of slash) and BMCMC posterior probabilities (right of slash) (only values≥50% and≥0·50, respectively, are depicted). Thicker branches indicate when the bootstrap value of ML is≥70% or the BMCMC posterior probability is≥0·95. GenBank Accession numbers are given as OTU names (see Table 1). New sequences are in bold.
Sequences of Sporastatia crassulata clustered with the sequences of S. testudinea and S. polyspora in a clade with 78/0·97 support values (bootstrap/posterior probability) in the phylogenetic tree (Fig. 1). Therefore, it is highly likely, based on DNA results as well as morphology, that S. crassulata belongs in Sporastatia.
The two mtSSU sequences of S. crassulata are identical. The genetic difference between sequences of S. crassulata and S. testudinea (including gap vs. non-gap) is 10 residues in mtSSU excluding gap versus non-gap or 24 residues including gaps (2·8%). Sporastatia crassulata is closest morphologically to S. testudinea but clearly differs in thalline and apothecial characters, as described below.
Sporastatia crassulata Yakovch. & Davydov sp. nov.
MycoBank No.: MB 824361
Thallus squamulose, placodioid, uneven, thick (1–2 mm), pale to medium brown, delimited by the well-developed black hypothallus; squamules usually ascending at the central part of the thallus, convex, rotund, usually marginally pruinose, surface rugose; apothecia lecideine, sunken, fertile areoles with ascending margins wrapping the disc forming a pseudolecanorine margin; epihymenium Cinereorufa-green with Atra-red patches.
Type: Russia, Republic of Tuva, Mongun-Taiginsky District, Mongun-taiga massif, right side of the Khairykan River valley at 4 km upstream from its mouth (Mugur River), 50°18'11"N, 90°11'55"E, alt. 2540 m a.s.l., stonefields within alpine meadows and mountain tundra, on stones, 11 July 2014, E. A. Davydov 14814 & L. S. Yakovchenko (LE-L13175—holotype; ALTB—isotype).

Fig. 2 Sporastatia crassulata. A, type locality, Mongun-Taiga Massif, Khaiyrkan River basin; B, type specimen (field photograph); C, section of thallus showing the continuous algal layer; D, section of apothecium with Cinereorufa-green (white arrows) and Atra-red (black arrows) pigments; E, multispored ascus of Catillaria-type (in KI). Scales: B=1 cm; C & D=50 µm; E=20 µm. In colour online.
Life habit lichenized, not lichenicolous. Thallus squamulose, placodioid, round to irregular in outline, up to 5 cm in diam., 1–2 mm thick, uneven, beige, greyish-yellow, pale-brown to medium brown, paler at margins, colour unchanged when wet, prothallus usually distinct, black, forming a rim around the thallus and sometimes visible between the squamules, vegetative propagules absent. Squamules in the central part of the thallus convex, rotund or irregular in outline (but not angular), usually ascending, (0·25–)0·65–1·00–1·35(–2·35) mm (n=74). Fertile squamules larger and prominent, with margins wrapping the disc forming a pseudolecanorine margin. Marginal squamules elongated, convex, attached, extended and notched at the ends, (1·10–)1·65–2·00–2·50(–3·75) mm (n=67). Surface rimose, wrinkled, rugose to scabrose, rarely ±smooth, matt to shiny, with white pruina forming a rim around each squamule and heavy on the periphery of the thallus, rarely the whole thallus pruinose or entirely without pruina. Upper cortex (37·5–)42·5–70·5–98·0(–125·0) µm thick (n=13), hyaline to brownish yellow, opaque due to the presence of crystals, sometimes covered by an epinecral layer (7·5–)14·5–17·5–20·5(–25·0) µm tall (n=11). Algal layer continuous, (62·5–)74·0–98·0–122·0(–187·5) µm, tall (n=10). Photobiont: Myrmecia sp., algal cells up to 12·5 µm diam. Medulla up to 1–2 mm tall, grey with unclear structure, granular, with perpendicular thick-walled hyphae (3·5–)4·5–5·0–5·5(–7·5) µm thick (n=10).
Apothecia lecideine, (0·25–)0·60–0·80–1·10(–1·65) mm (n=71), common, numerous in the centre of the thallus, sunken, 1–3 per squamule, rarely separated from the thallus by cracks; disc black to dark brown-black, colour unchanged when wet, plane, angular, rarely roundish, cracked to roughened, slightly pruinose or without pruina; margin thin, black, at the same level as disc or higher, sometimes excluded. Exciple extended in uppermost part, (22·5–)37·5–55·0–72·5(–87·5) µm thick (n=15), brown-black, of rounded cells (5·0–)6·5–7·5–8·5(–10·0) µm diam. (n=12), lateral exciple (12·5–)16·5–19·5–22·5(–25·0) µm thick (n=11), of elongated cells, hyaline to brown, basal exciple indistinct and scarcely distinguishable from the hypothecium, brown, (12·5–)13·0–30·0–47·0(–62·5) µm thick (n=10). Hypothecium (25·0–)46·0–68·5–91·0(–112·5) µm thick (n=15), hyaline, ochraceous to brown toward the exciple, I+ blue. Hymenium (75·0–)88·0–99·0–110·0(–137·5) µm thick (n=15), hyaline, always with Atra-red lines of sterile hyphae, I+ blue. Epithecium Cinereorufa-green with Atra-red patches (see Meyer & Printzen Reference Meyer and Printzen2000). Paraphyses septate, simple to weakly branched, (1·6–)1·6–1·8–2·0(–2·4) µm thick (n=9) in mid-hymenium with cylindrical or narrowly clavate tips, (2·1–)2·3–2·6–2·9(–3·2) µm thick (n=11). Asci Catillaria-type, 100–200-spored, clavate, unitunicate, thick-walled, (75·0–)76·0–79·0–84·5(–90·0)×(25·0–)24·5–26·5–28·5(–30·0) µm (n=16); ascospores (3·5–)3·7–3·9–4·2(–4·5)× (3·2–)3·2–3·4–3·6(–4·0) µm (n=35), hyaline, simple, broadly ellipsoid to globose, thin walled, without a distinct perispore.
Pycnidia not seen.
Chemistry. Thallus K−, С+ red, KС+ red; gyrophoric and lecanoric acids by TLC.
Etymology. The name refers to the thick squamulose thallus.
Ecology. Sporastatia crassulata was found abundantly in dry conditions on exposed siliceous rocks in the upper mountain belt (c. 2400–3100 m a.s.l.).
Distribution. Sporastatia crassulata is currently known from several localities in the Altai Mountains, both in South Siberia and in adjacent territory of China (Fig. 3). The distance between the two most remote localities is c. 500 km.
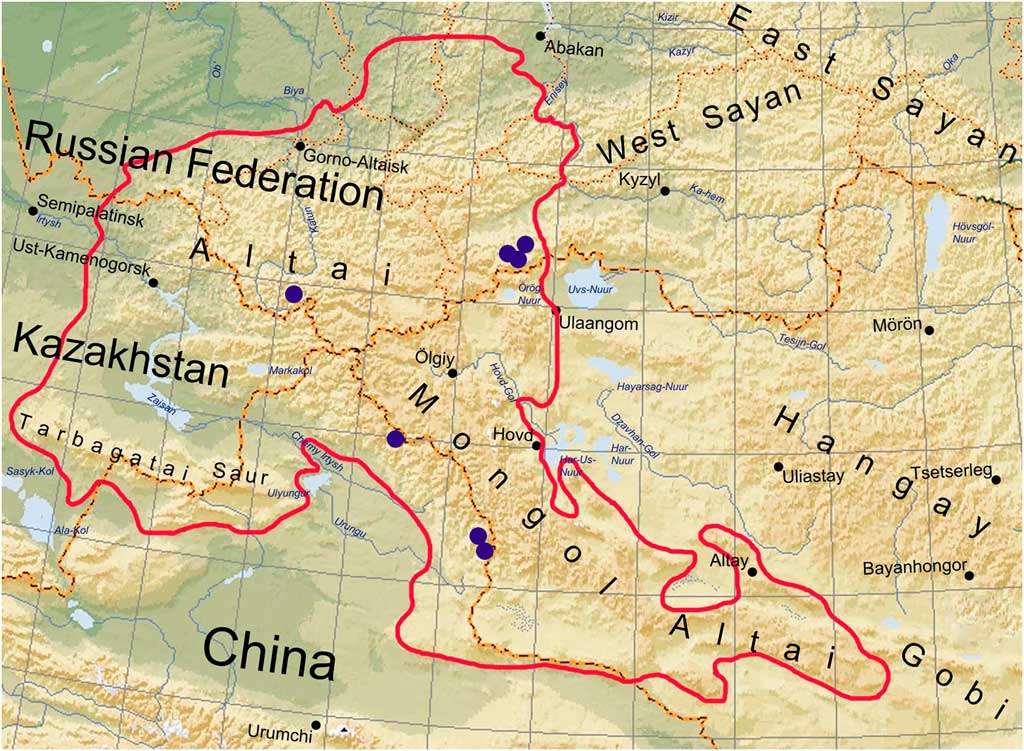
Fig. 3 Distribution of Sporastatia crassulata. Solid line indicates the outline of the Altai region. In colour online.
Additional material examined. Russia: Republic of Altai: Ust′-Koksinsky District, Katunsky Range, upper reaches of the Ioldo River, 49°49'37·5''N, 86°15'58·7''E, alt. 2574 m a.s.l., stonefields and rocks, on stones, 2008, E. A. Davydov 10677 (ALTB). Republic of Tuva: Mongun-Taiginsky District, Mongun-taiga Massif, right side of the Khairykan River valley at 3 km upstream from its mouth (Mugur River), 50°18'3''N, 90°12'23''E, alt. 2400–2500 m a.s.l., alpine meadows and mountain tundra with stones, on stones, 2014, E. A. Davydov 14812 & L. S. Yakovchenko (ALTB); same massif, left side of the Toolaity River valley at 2·7 km upstream from the Eski-Toolaity lake, 50°10'1''N, 90°09'05''E, alt. 2670 m a.s.l., mountain tundra, stonefield, on stones, 2014, E. A. Davydov 14813 & L. S. Yakovchenko (ALTB); same valley, 5 km upstream from the Eski-Toolaity lake, 50°11'3''N, 90°08'46''E, alt. 2550 m a.s.l., mountain tundra, stonefield, on stones, 2014, E. A. Davydov 16521 & L. S. Yakovchenko (ALTB).— China: Xinjiang: Mongol′sky Altai Range, SE slope of Mt. Keshtau (3511 m), 46°45'18''N, 90°50'15''E, alt. 3136 m a.s.l., gravelly tundra, on rocks, 2007, E. A. Davydov 17335 (ALTB); same range, upper reaches of Khara-Belchir-he, west slope of Kara-Balchigtau Mt. (3215 m), 46°42'0''N, 90°56'49''E, 2656 m a.s.l., Larix sibirica forest, steppe slopes with Juniperus spp., on rocks, 2007, E. A. Davydov 17336 (ALTB); same range, Kungeytytau Mts, upper reaches of Yelt-gol, 15 km up from the mouth of Duntsa-he, 48°03'37''N, 88°51'25''E, alt. 2800–3040 m a.s.l., subalpine and alpine meadows, near the snow, on rocks, 2007, E. A. Davydov 17337 (ALTB).
Discussion
Sporastatia crassulata is unique within the genus in having a distinctly squamulose, thick, uneven thallus composed of convex, not angular squamules irregularly ascending in the central part of the thallus. It is also unusual among Sporastatia species in that its apothecia are below the thallus level and the prominent fertile squamules have ascending margins sometimes surrounding the disc as a pseudolecanorine margin. Thus, in the field S. crassulata resembles a species of Lecanora s.l. Sporastatia crassulata shows wide morphological variability throughout its distribution. Russian populations characteristically have an effigurate thallus, delimited by a well-developed black prothallus with squamules ascending in the central part of the thallus, and with the upper surface matt, pruinose, wrinkled, and rugose to scabrous. The Chinese populations of the species differ by their less well-developed prothallus, marginal lobes weakly elongated (1·1–1·5(–2·5) mm) and central squamules weakly ascending, and by the darker, glossier, non-pruinose, ± smooth upper surface. These regional differences are within the variability of the species and appear to be due merely to climatic factors. Sporastatia crassulata and the widely distributed species S. testudinea are both brown-coloured with a radiate thallus delimited by a black, well-developed prothallus. However, the thallus of S. testudinea is thin, crustose-areolate, composed of plane, angular areoles with apothecia at the same level as the thallus. Sporastatia crassulata clearly differs from S. polyspora by its squamulose radiate (vs. non-effigurate), brown (vs. grey), thick and uneven (vs. thin and plain) thallus. Sporastatia crassulata may slightly resemble S. asiatica (Fig. 4E) and S. subasiatica (Fig. 4F) in the presence of pruina, and by having an uneven, rugose to scabrous upper surface (distinctions given below).

Fig. 4 A, Sporastatia testudinea, Altai Mts (field photograph); B, S. polyspora, Altai Mts (field photograph); C, S. karakorina, holotype; D, S. karakorina, holotype, note the thalloconidial rims around areoles; E, S. asiatica, holotype; F, S. subasiatica, holotype. Scales: A & B=1 cm; C, E & F=0·5 cm; D=0·5 mm. In colour online.
Sporastatia crassulata is currently known from several localities throughout the Altai Mountains (South Siberia and adjacent China). In Russia, this species occurs in the Katunsky Range (Central Altai) and in five localities through the high mountain massif Mongun Taiga (south-east Altai) (Fig. 3). In China, it is known from the Mongolian Altai Range. Sporastatia crassulata grows together with S. testudinea and S. polyspora at all localities. We have seen intermixed populations of the first two taxa in the Altai where the differences in thallus morphology cannot be the result of different ecology. The distribution of S. crassulata in the Altai appears to be restricted to alpine environments at elevations of 2400–3100 m a.s.l. Other endemic species of Sporastatia also occur in high Asian mountains. Mountainous Central Asia therefore appears to be the centre of species diversity and endemism for Sporastatia.
Additional Species Reported
Sporastatia karakorina (Obermayer & Poelt) Davydov & Yakovch. comb. & stat. nov.
MycoBank No.: MB 824362
Sporastatia testudinea (Ach.) Massal. var. karakorina Obermayer & Poelt, Herzogia 8: 278 (1990); type: China, Xinjiang Prov., Karakorum, östlich des K2-Gletschers oberhalb der Einmündung des Siang-kiang-Gletchers (35°58'N, 76°27'E), c. 490 m, gröβer Sandstein/Quarzit- Block, Exp. Nordwest 80°, 24 Sept. 1986, B. Dickoré Nr. F24 (GZU!—holotype).
Notes. Examination of the type material of Sporastatia testudinea var. karakorina and its description in the protologue suggested that this taxon required recognition at the species level. In morphology it resembles S. testudinea but differs by having globose ascospores (i.e. length/width ratio: 1·0–1·05(–1·1) (vs. ellipsoid to broadly ellipsoid, length/width ratio 1·2–1·7)) and possessing characteristic mitosporic dispersal units, so-called ‘thalloconidia,’ on the prothallus (Fig. 4D). Thalloconidia as defined by Hestmark (Reference Hestmark1990) may develop in various lichen families (Hasenhüttl & Poelt Reference Hasenhüttl and Poelt1978; Poelt & Obermayer Reference Poelt and Obermayer1990) but they are most commonly formed by members of the Umbilicariaceae. Thalloconidia in various taxa were shown to be non-homologous and to have a highly species-specific morphology (Hasenhüttl & Poelt Reference Hasenhüttl and Poelt1978; Hestmark Reference Hestmark1991; Davydov et al. Reference Davydov, Peršoh and Rambold2017 b). Within Sporastatia, true thalloconidia are so far known only in Sporastatia karakorina. Poelt & Obermayer (Reference Poelt and Obermayer1990) mentioned that some specimens of S. testudinea, collected higher than 2500 m in the Alps, show a tendency to produce thalloconidia but true thalloconidia were not observed. In the Altai Mountains, specimens collected above 2800–3000 m also have a better developed rough prothallus but never produce thalloconidia. The presence of thalloconidia alone is not necessarily a distinguishing trait at the species level (Poelt & Obermayer Reference Poelt and Obermayer1990; Davydov et al. Reference Davydov, Pérez-Ortega and Printzen2017 a) but as this trait is geographically localized and correlated to other characters (in this case globose ascospores), we propose the species level for this taxon.
Sporastatia subasiatica N. S. Golubk.
Nov. Sist. Niz. Rast. 10: 202 (1973); type: [Tadjikistan], East Pamir, Murgabsky District, valley of Kul’-Nambed River, alt. 4200 m a.s.l., on siliceous boulder with lime intrusions, 26 July 1966, Golubkova N. S. 809 (LE-L497!—holotype).
(Fig. 4F)
Sporastatia subasiatica was described from the Pamir Mountains (Golubkova Reference Golubkova1973). This alpine species occurs at elevations up to 4200 m a.s.l. on siliceous rocks partly with calcareous modification. It is characterized by its thin, crustose, placodioid, whitish thallus, composed of plane angular areoles (central 0·3–0·9 mm diam., marginal 1·2–1·8×0·4–0·9 mm) with a pruinose scabrous to echinate upper surface, and delimited by a black prothallus. Apothecia are at the same level as the thallus, 0·2–0·7 mm diam., black, lecideine, pruinose, with a thin proper margin and rugose disc. Other characters include: green-black exciple, hyaline hymenium, (130–)145–170 µm tall with violet epihymenium, hyaline to pale-brown hypothecium, 100–145 µm tall, and Catillaria-type asci with 100–200 hyaline, simple, ellipsoid ascospores, 5–6×2·5–3·0 µm. Thallus C+ red (gyrophoric and lecanoric acids), K−.
Sporastatia subasiatica differs from other Sporastatia species mainly by the presence of a violet epihymenium, green-black exciple, hymenium (130–)145–170 µm high and a pale, whitish thallus (largely due to the presence of dense pruina). The pigments in the epithecium (unknown in the classification of Meyer & Printzen (Reference Meyer and Printzen2000)) differ from those in S. testudinea and S. crassulata. They are brownish with a purple tint, KOH+ (purple intensive) – HCl (black to brown); HNO3 (purple clearing) – KOH+ (black to purple) – HCl (brown).

We are grateful to Dr Christian Printzen for the opportunity to sequence a part of the material in his laboratory, and Dr W. Obermayer (GZU) for help with herbarium specimens and providing photographs of S. karakorina. The authors thank the following colleagues for organizing expeditions: Dr G. E. Insarov, Dr T. V. Yashina and Dr A. N. Kuksin to the Katunsky Nature Reserve (Altai) and the Ubsunur Nature Reserve (Tuva), and Dr Wen-Li Chen, Dr Bing Liu and Dr D. German to China. We also thank the directors and staff of the mentioned institutions for their considerable support during these expeditions. The photograph of S. asiatica was taken from the website of the Swedish Museum of Natural History (S, http://herbarium.nrm.se). We are grateful to two anonymous reviewers and Dr Alan Fryday for their valuable comments and suggestions, and for improving the text.







