Introduction
Species of the crustose lichen genus Biatora have a green-algal photobiont, biatorine apothecia and unpigmented ascopores with no or few transverse septa. This places some of them in Zahlbruckner's genus Lecidea (Zahlbruckner Reference Zahlbruckner1926), others in Bacidia sect. Weitenwebera. If Biatora were just a modern segregate of these genera, its circumscription would probably not cause much trouble. However, over the years a growing number of species have been newly described or combined into Biatora and others excluded, which for now makes a delimitation of the genus rather difficult.
Biatora was described by Elias Fries in 1817 (Fries & Sandberg Reference Fries and Sandberg1817) as a heterogeneous assemblage of taxa, some of which are today ascribed to, for example, Caloplaca, Dimerella or Pannaria. Biatora was recognized by most authors until about the middle of the 19th century, when, after a dispute about its independence, Nylander (Reference Nylander1855) subsumed it under Lecidea. With few exceptions (e.g. Räsänen Reference Räsänen1926; Choisy Reference Choisy1949), most subsequent authors followed this move. While more and more species were added to Lecidea, Biatora became one of the many groups hidden within this huge genus. By the 1860s, Lecidea had already become unmanageably large, so that several authors attempted to segregate it into subgenera and smaller taxonomic units [e.g. the “stirpes” of Fries (Reference Fries1874)]. These subgeneric systems were mainly based on ascospore characters, pigmentation of apothecia, paraphyses and hypothecium, and in some cases came close to a modern circumscription of Biatora, for example the “section Lecidea vernalis” of Vainio (Reference Vainio1934). Nevertheless, it took about 50 years until Coppins (Reference Coppins1983) and Hafellner (Reference Hafellner1984) almost simultaneously resurrected Biatora vernalis (L.) Fr., the type species of the genus, from the mass grave Lecidea.
In his revision of the European species, Printzen (Reference Printzen1995) defined the genus narrowly and excluded Biatora carneoalbida (Müll. Arg.) Coppins, B. epixanthoides (Nyl.) Diederich, B. sphaeroides (Dicks.) Körb., B. tetramera (de Not.) Coppins (today treated as Mycobilimbia, Printzen et al. Reference Printzen, Coppins, Aptroot, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009), Lecidea albohyalina (Nyl.) Th. Fr. and L. meiocarpa Nyl. because of a deviating apothecial ontogeny. In Biatora, paraphyses and excipular hyphae typically originate from the same, strongly gelatinized initial hyphae, and the first asci grow scattered among these hyphae. In Mycobilimbia and Lecidea albohyalina (L. meiocarpa was not studied), the initial gelatinized hyphae develop into the excipulum, while paraphyses and asci develop de novo in the centre of young apothecia. In this circumscription, Biatora comprised 18 species with narrowly ellipsoid unicellular ascospores and pale ochre or brownish apothecia. However, a few taxa with transversely septate ascospores [B. aegrefaciens Printzen, B. rufidula (Graewe) S. Ekman & Printzen] and with distinctly pigmented apothecia [B. mendax Anzi, B. ocelliformis (Nyl.) Arnold] were already included. Meanwhile the number of species has increased to 42, partly due to the description of new species (e.g. Printzen & Tønsberg Reference Printzen and Tønsberg1999, Reference Printzen and Tønsberg2003, Reference Printzen and Tønsberg2004; Spribille et al. Reference Spribille, Björk, Ekman, Elix, Goward, Printzen, Tønsberg and Wheeler2009) and partly to the recombination of already described species into Biatora (e.g. Printzen Reference Printzen, Nash, Ryan, Diederich, Gries and Bungartz2004). Biatora now comprises species with ellipsoid (‘lecideoid’) and elongate (‘bacidioid’), simple to multiseptate ascospores, different excipular anatomies and a large variety of secondary metabolites and insoluble pigments that result in differently coloured apothecia. As a result, it has become difficult to delimit the genus from similar or closely related taxa such as Bacidia, Cliostomum, Lecania or Mycobilimbia.
Three published molecular phylogenies that included Biatora have already found support for the monophyly of the genus, but included only few species (Reese Næsborg et al. Reference Reese Næsborg, Ekman and Tibell2007) or were based on only one or two genetic markers (Printzen & Lumbsch Reference Printzen and Lumbsch2000; Spribille et al. Reference Spribille, Björk, Ekman, Elix, Goward, Printzen, Tønsberg and Wheeler2009). The aims of this study are 1) to clarify the circumscription of Biatora by phylogenetic analyses based on three different gene loci and an extended sample of taxa (most of the currently accepted Biatora species), 2) to find out whether infrageneric evolutionary units correspond to morphologically defined groups of species, and 3) to ascertain whether some morphologically similar, and partly unnamed, taxa belong to Biatora and what their closest relatives are.
Material and Methods
Taxon sampling
An attempt was made to include as many accepted species of Biatora as possible and superficially similar taxa that had formerly been excluded from the genus, such as Mycobilimbia and “Lecidea” albohyalina, were added to the dataset. In order to verify their taxonomic position, supposed close relatives of accepted Biatora species such as Bacidia beckhausii Körb., B. hemipolia (Nyl.) Malme and “Lecidea” ementiens Nyl. were included. The results of Reese Næsborg et al. (Reference Reese Næsborg, Ekman and Tibell2007) showed that Lecania, Bilimbia and Cliostomum were close relatives of Biatora. In order to root the tree and to detect a possible polyphyly of Biatora, some species from these groups were therefore added to the dataset as outgroup taxa. Finally, collections were added that phenotypically belonged in Biatora but could not be assigned to any described species (“B. radicicola”, Biatora species from Norway) or that resembled known species but differed in minor details (B. “orientalis”, B. “alaskana” and B. “cf. helvola” from Japan). The complete dataset comprised 59 OTUs.
DNA extraction and PCR amplification
DNA was extracted from 2–3 apothecia per thallus using the QIAquick™ Plant Mini Kit (Qiagen) according to the manufacturer's instructions. Three gene loci were amplified with the following primers: ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) for the internal transcribed spacer region of the ribosomal DNA (ITS), mrSSU1 (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999) and MSU7 (Zhou & Stanosz Reference Zhou and Stanosz2001) for part of the small subunit of the mitochondrial ribosomal DNA (mrSSU), and RPB2-5f and RPB2-7Cr (Liu et al. Reference Liu, Whelen and Hall1999) for part of the second largest subunit of RNA polymerase II (RPB2).
The 50 µl PCR reactions contained 10 µl of aqueous DNA extract, 5 µl Herculase™ reaction buffer (Stratagene), dNTPs (2·5 µM), 2·5 U polymerase (Herculase™, Stratagene), and 0·8 µM (ITS, mrSSU) or 1·4 µM (RPB2) of each forward- and reverse-primer. Some 25 µl reactions were carried out using PCR-PuReTaq Ready-to-Go Beads™ (GE Healthcare) containing 5 µl of DNA extract and 0·4 µM (ITS, mrSSU) or 1·4 µM (RPB2) of each forward- and reverse-primer. Cycling conditions for ITS and mrSSU included initial denaturation at 94°C for 5 min, 5 cycles of 94°C for 30 s, 54°C for 30 s, 72°C for 1 min, 33 cycles of 94°C for 30 s, 48°C for 30 s, 72°C for 1 min, and a final extension step at 72°C for 10 min. For RPB2, initial denaturation at 92°C for 2 min was followed by 8 cycles of 94°C for 1 min, 59°C for 1 min, 72°C for 2 min, and 33 cycles of 95°C for 30 s, 50°C for 30 s, 72°C for 2 min, and a final extension step at 72°C for 10 min. PCR products were run on agarose gels, bands cut out and purified using the QIAquick Gel Extraction Kit (Qiagen). Purified DNA was labelled with the BigDye™ Terminator Cycle Sequencing Kit (either version II or v3.1, Applied Biosystems) and cycle sequenced at 94°C for 30 s, and 29 cycles of 95°C for 15 s, 45°C for 15 s, and 60°C for 4 min using the PCR primers. Sequences were determined on ABI PRISM® 3700 or 3730 DNA Analyzers (Applied Biosystems), and either edited using SeqMan™ II, version v.5.07 (DNASTAR Inc.) and assembled in BioEdit version 7.0.9.0 (Hall Reference Hall1999), or assembled and edited using Geneious Pro, version 6.0.4 (Biomatters Inc.).
Phylogenetic analyses
BLAST searches in GenBank were performed to ascertain that all sequences used in the phylogenetic analyses originated from the lichens and not from contaminating organisms such as parasymbiotic fungi. Single gene datasets containing the sequences listed in Table 1 were aligned in Geneious Pro, version 6.0.4, using the Muscle algorithm with default settings. Regions of uncertain alignment were removed using GBlocks, version 0.91b (Castresana Reference Castresana2000), applying default settings but allowing gap positions in half of the sequences. Final alignments comprised 56 sequences, 367 bp (ITS), 47 seq., 804 bp (mrSSU) and 38 seq., 1062 bp (RPB2). These datasets were concatenated to yield an alignment of 59 sequences and 2233 bp length. The optimal partitioning scheme of the concatenated dataset and substitution models for each data partition were inferred with the help of PartitionFinder, version 1.0.1 (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012) using the Bayesian Information Criterion and the greedy algorithm (default settings) and suggesting seven data blocks (ITS1, 5.8S rDNA, ITS2, mrSSU, and three independent codon positions for RPB2). Results are shown in Table 2.
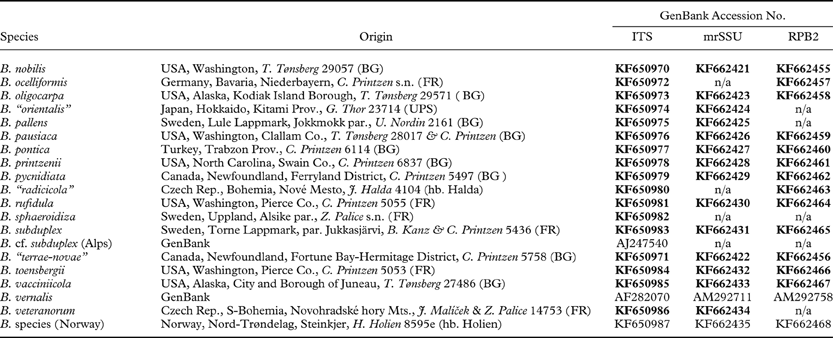
Table 1. Taxa, geographical origin of samples and GenBank numbers of sequences used in this study. GenBank numbers in bold indicate newly generated sequences

Table 2. Optimal partitioning scheme and substitution models for each data partition inferred by PartitionFinder, version 1.0.1, and used in the phylogenetic analyses
Phylogenetic trees for the single gene datasets were inferred with the Markov chain Monte Carlo (MCMC) approach implemented in MrBayes, version 3.2 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012), applying the substitution models and partitioning schemes inferred with PartitionFinder. Default settings of MrBayes were used with the following exceptions: a proportional model on partition-specific rates, gamma-distributed site-specific rates modelled as six rate categories with an exponential prior of mean 1 and an unconstrained, exponential branch length prior. The mean of the branch length prior was inferred by calculating ML trees for all single gene datasets and the concatenated dataset using raxmlGUI version 0.9 beta 2 (Stamatakis Reference Stamatakis2006; Silvestro & Michalak Reference Silvestro and Michalak2010), and applying either an unpartitioned GTRGAMMAI model (mrSSU) or a partitioned GTRGAMMAI model (ITS, RPB2) and 10 runs. The mean branch lengths of the ML trees were then used as means of the exponential distributions for branch length priors. Parameters of substitution models were unlinked between data partitions. MrBayes was set to sample every 500th tree out of 100 million generations using three independent runs, each with four chains that were incrementally heated by 0·15. To infer convergence of the Markov chains, the average standard deviation of bipartition frequencies among runs was calculated every 1 million generations, discarding the first 50% of the trees sampled as burn-in and including only those bipartitions with a frequency of at least 10%. The analyses were stopped when the standard deviation dropped below 0·01.
The resulting single gene MCMC trees were compared to identify conflicting phylogenetic signal between datasets (see , available online). Four supported conflicts were detected, which concerned the positions of B. chrysantha (Zahlbr.) Printzen, B. pontica Printzen & Tønsberg, B. printzenii Tønsberg and Lecania brialmontii (Vain.) Zahlbr. In mrSSU, B. chrysantha was the sister taxon of B. cf. helvola from Japan, in ITS it was the closest relative of B. vernalis. Biatora pontica formed a well-supported clade with B. printzenii and B. hertelii Printzen & Etayo in RPB2, but grouped with B. bacidioides Printzen & Tønsberg and “Lecidea” ementiens in mrSSU, where B. printzenii appeared as sister of Biatora sp. from Norway. In the ITS analysis, L. brialmontii was the closest relative of the two Lecidea albohyalina collections, which in mrSSU grouped with Mycobilimbia. The four problematic taxa were removed from the dataset and all analyses repeated with the reduced datasets, after which no conflict was found between single-gene phylogenies. The three datasets were then combined into a single alignment for further analysis using the above-mentioned partitioning scheme, substitution models and settings for the Markov chains. The inferred branch length prior for the MCMC analysis of this dataset followed an exponential distribution with mean 1/20. The analysis was stopped after 9 million generations when the standard deviation had dropped below 0·01. A ML bootstrap tree with 1000 replicates was calculated for the concatenated dataset using the ‘rapid bootstrap’ option in raxmlGUI and unlinked GTRGAMMAI models for the five partitions inferred by PartitionFinder. Newly generated DNA sequences were submitted to Genbank (Table 1), the concatenated dataset used in the final analyses, and the ML tree and the consensus tree of the MCMC analysis were submitted to Treebase (http://purl.org/phylo/treebase/phylows/study/TB2:S15023).
Results and Discussion
For this study, 104 new DNA sequences were generated. Figure 1 shows the midpoint-rooted maximum likelihood tree inferred by raxmlGUI with ML bootstrap values and posterior probabilities. The length of this tree was 0·0487 and that of the Bayesian consensus tree 0·0361, indicating that branch length estimates of the MCMC tree are biologically plausible (Brown et al. Reference Brown, Hedtke, Lemmon and Moriarty Lemmon2010). If not stated otherwise, anatomical, chemical and geographical data for the species discussed below are taken from Printzen (Reference Printzen1995, Reference Printzen, Nash, Ryan, Diederich, Gries and Bungartz2004), Printzen & Tønsberg (Reference Printzen and Tønsberg1999, Reference Printzen and Tønsberg2003, Reference Printzen and Tønsberg2004) and Printzen et al. (Reference Printzen, Holien and Etayo1998, Reference Printzen, Lumbsch and Orange2001).
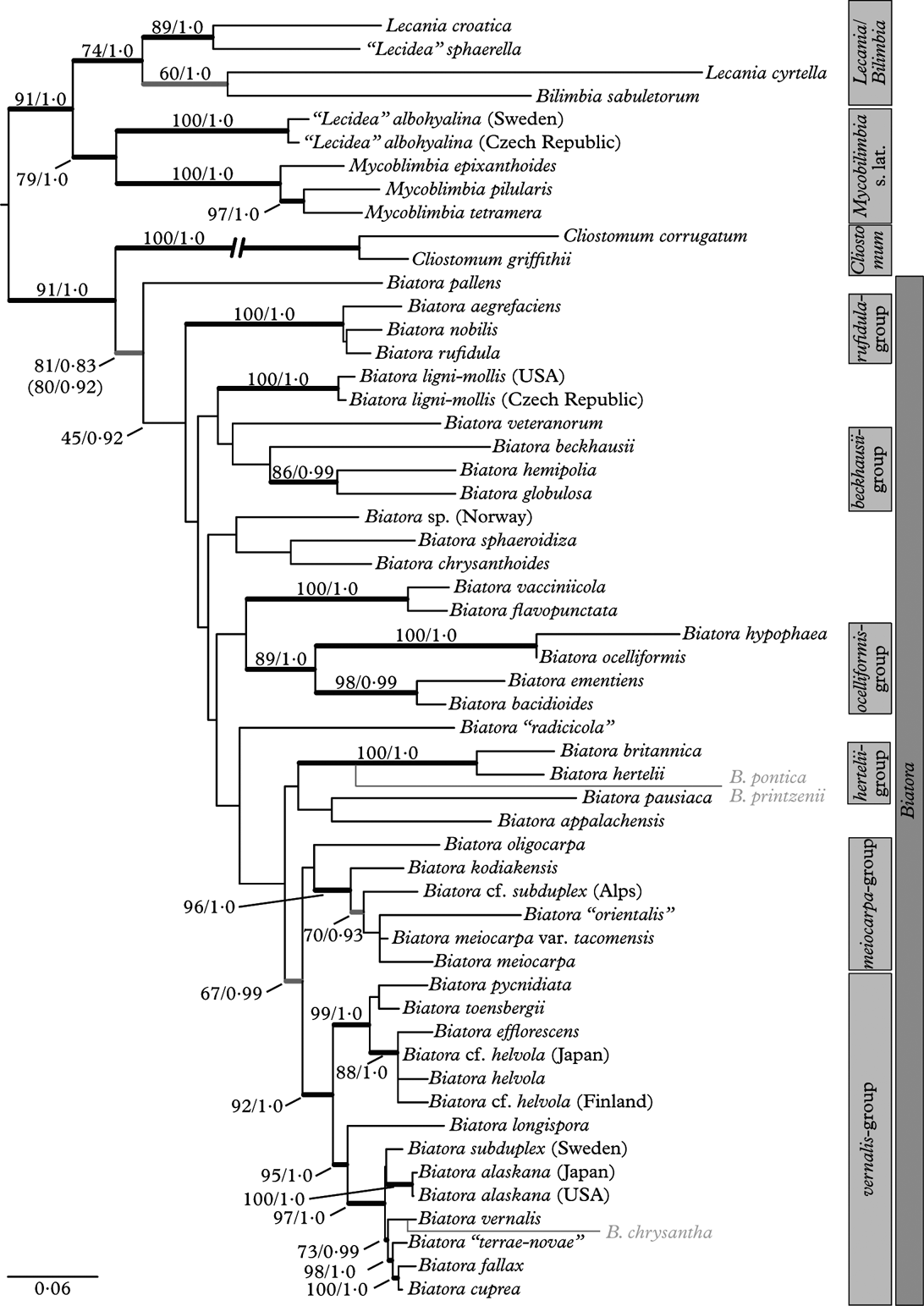
Fig. 1. Midpoint-rooted Maximum Likelihood phylogenetic tree of Biatora. Species of Bilimbia, Cliostomum, Mycobilimbia and Lecania were added to root the tree and to ascertain the phylogenetic position of unnamed taxa included in the analysis. Bold branches received ML bootstrap support ≥70% and posterior probability ≥0·95. Bold grey branches were only supported by one of the analyses. Exact support values above or left of branches. Support values in brackets underneath the branch leading to Biatora indicate those of an analysis including species with conflicting signal. The position of these species (grey) in the extended analysis is indicated to the right of the tree. The branch leading to Cliostomum is reduced to half its length.
Phylogenetic relationships of Biatora
The overall topology of the tree agrees with the phylogeny of Lecania and related genera presented by Reese Næsborg et al. (Reference Reese Næsborg, Ekman and Tibell2007), with Mycobilimbia s. lat. as sister to a clade formed by Lecania and Bilimbia species. With one exception (the relationship between Bilimbia sabuletorum and Lecania cyrtella), all branches in the basal part of the tree receive strong support. The position of Mycobilimbia and “Lecidea” albohyalina as close relatives of Lecania and Bilimbia and the exclusion of these species from Biatora are thus strongly supported. A close relationship between M. pilularis (Körb.) Hafellner & Türk (as Biatora sphaeroides) and Lecania was already inferred by Ekman (Reference Ekman2001), based on ITS sequences and a wide taxonomic sample from the Ramalinaceae.
The monophyly of Cliostomum griffithii (Sm.) Coppins and C. corrugatum (Ach.) Fr. and their position as sister to Biatora is strongly supported in the present analysis. Such a relationship has so far not been reconstructed, although both genera are anatomically similar (Printzen Reference Printzen1995). Ekman (Reference Ekman2001) found C. griffithii as sister to Ramalina, while Reese Næsborg et al. (Reference Reese Næsborg, Ekman and Tibell2007) found a close relationship between C. tenerum (Nyl.) Coppins & S. Ekman and Lecania furfuracea Vězda. These differences are perhaps explained by different taxon sampling. Ramalina was excluded from the present dataset, because preliminary analyses inferred a group in which R. fastigiata was firmly embedded between C. corrugatum and C. griffithii (not shown). The relationship between Ramalina and Cliostomum needs further clarification, but is not the focus of this contribution. Cliostomum tenerum, on the other hand, differs from all other currently accepted Cliostomum species by having a lecanorine margin and might not belong in this genus.
The remaining 44 collections included as known or putative members of Biatora form a sister group to Cliostomum but the monophyly of this group was only supported in the ML analysis. Surprisingly, the posterior probability of a monophyletic Biatora was considerably higher when the three conflictory taxa B. chrysantha, B. pontica and B. printzenii were included in the analysis, while the ML bootstrap support remained unaffected (Fig. 1, support values in brackets).
Infrageneric species groups within Biatora
The infrageneric systematics of Biatora in its modern circumscription, that is excluding older and much wider interpretations such as that of Körber (Reference Körber1855) or “Lecidea subgenus Biatora” (Fries Reference Fries1874), has not been explicitly dealt with before. Because data on crucial characters such as ascus apical structure, apothecial ontogeny or secondary metabolites were lacking, early authors often joined largely similar species into groups. Fries (Reference Fries1874), for example, combined B. cuprea, B. vernalis and B. helvola into a “stirps L. vernalis” which also included the remotely related Lecidea gibberosa sensu Th. Fr. [=Puttea exsequens (Nyl.) Printzen & Davydov], but excluded L. atroviridis (Arnold) Th. Fr. (=B. ocelliformis). Printzen (Reference Printzen1995) noted similarities between B. aegrefaciens and B. rufidula, as well as between B. flavopunctata and B. subgilva, but otherwise did not propose any subgeneric groupings.
Figure 1 shows that some well-supported clades can be distinguished within Biatora. Because the relationships among these groups are largely unsettled, no attempt is made to assign them any systematic rank. The crown group (here called the “vernalis-group”) combines species with a typical Biatora anatomy: hyaline, light or reddish brown apothecial tissue and a strongly gelatinized excipulum with cylindrical cell lumina. The most common secondary metabolites in Biatora are found in species from this group; gyrophoric acid in B. helvola Hellb. and B. chrysantha, argopsin in B. efflorescens (Hedl.) Räsänen, B. pycnidiata Printzen & Tønsberg, B. “terrae-novae” B. toensbergii Holien & Printzen and B. cuprea (Sommerf.) Fr., and both in B. fallax Hepp. The vernalis group falls into two major clades, one of which combines the corticolous B. efflorescens, B. helvola, B. pycnidiata and B. toensbergii. The other comprises all ‘non-corticolous’ Biatora species (except L. ementiens, see below), which either overgrow bryophytes (B. chrysantha, B. vernalis) or rotten bark (B. fallax). Biatora cuprea and B. subduplex (Nyl.) Printzen prefer detritus in (sub)arctic tundra. Biatora alaskana Printzen & Tønsberg and B. longispora (Degel.) Lendemer & Printzen are the only species in this group restricted to bark.
With the exception of B. subduplex and B. vernalis, the species within the vernalis group are morphologically and chemically easily distinguished. The extremely short branches within this group indicate that the species are genetically closely related and probably diverged from each other recently. In spite of these short branches, the vernalis group is the only one with a well-resolved and supported internal topology. The only branches with insufficient statistical support are the ones that combine B. alaskana and B. subduplex, and B. pycnidiata and B. toensbergii.
The meiocarpa-group is closely related to the vernalis group. Species of this group have more rounded excipular cell lumina and apically broadened paraphyses, but otherwise the two groups are very similar. The close relationship between both groups has a high posterior probability but slightly less than 70% bootstrap support. With the exception of B. kodiakensis Printzen & Tønsberg, with gyrophoric acid, species of the meiocarpa group do not produce secondary metabolites. The phylogenetic tree allows no conclusion whether B. oligocarpa Printzen & Tønsberg belongs in the group or not, but the anatomical similarities between this species and B. meiocarpa (Nyl.) Arnold make it likely.
Four further groups can be distinguished in the basal part of the Biatora-tree: the hertelii-group, the ocelliformis group, the beckhausii group and the rufidula group. Among these are spread numerous (pairs of) taxa with unclear relationships. The hertelii group comprises B. britannica Printzen et al. and B. hertelii. In the extended dataset, B. pontica and B. printzenii appear as closely related to these two. The group is mainly characterized by the production of insoluble pigments rarely found outside the group and a preference for Tertiary relict areas (Macaronesia, south-eastern North America; see e.g. Tiffney Reference Tiffney1985). Biatora britannica and B. hertelii contain Hertelii-green (Meyer & Printzen Reference Meyer and Printzen2000), otherwise only known from the thallus of Lecania leprosa Reese Næsborg & Vondrák (Reese Næsborg Reference Reese Næsborg2008). Biatora hertelii has up to now only been collected on Madeira. The ascogenous hyphae of B. pontica are surrounded by Pontica-blue and Pontica-red (Printzen & Tønsberg Reference Printzen and Tønsberg2003), so far not known from any other lichen. Biatora printzenii is only known from eastern North America, while B. pontica is also found in East Asia and the eastern Black Sea Region.
The ocelliformis group consists of the closely related and anatomically very similar B. hypophaea and B. ocelliformis, and a well-supported clade formed by the Arctic “Lecidea” ementiens and B. bacidioides, which is so far only known from north-eastern Turkey. This last relationship is not supported by anatomical or chemical characters. Biatora bacidioides has long, bacidioid ascospores and produces the pigment Sedifolia-grey, which rather suggests a relationship with Bacidia beckhausii as claimed by Printzen & Tønsberg (Reference Printzen and Tønsberg2003).
A close relationship between Bacidia beckhausii and Bacidia hemipolia was postulated by Ekman (Reference Ekman1996). In the present analysis, both species group together with Biatora globulosa (Flörke) Fr. Although the beckhausii-group as a whole is unsupported, the relationship between B. globulosa and B. hemipolia was reconstructed with high confidence. The peculiar position of B. bacidioides and the occurrence of Cinereorufa-green and Sedifolia-grey in both groups might indicate a closer relationship between the ocelliformis group and the beckhausii group. Within Biatora, Cinereorufa-green is otherwise only produced by B. sphaeroidiza (Vain.) Printzen & Holien and the undescribed B. “radicicola”, both with an undecided phylogenetic position.
The rufidula group is highly supported and morphologically clearly distinguished by broadly ellipsoid, rather thick-walled, 3-septate ascospores and an exciple in which individual hyphae can be discerned. Typically, the exciple in Biatora consists of a gelatinous matrix, in which only the cell lumina appear as ‘holes’.
The sorediate species B. flavopunctata (Tønsberg) Hinteregger & Printzen and B. vacciniicola (Tønsberg) Printzen appear as closely related taxa. Both species are typically found on twigs of shrubs in (sub)arctic-alpine situations and may grow side by side. Biatora flavopunctata shows the most complex chemical pattern of all Biatora species, including the β-orcinol para-depside atranorin, β-orcinol depsidones from the stictic acid complex, the dibenzofurans usnic and isousnic acid and an unknown terpenoid. The only other species producing usnic and isousnic acid is B. subgilva (Arnold) Hinteregger, which is so far only known from twigs of Rhododendron ferrugineum in the Eastern Alps (Hinteregger Reference Hinteregger1994). Because recent collections were lacking it was not included in the present analysis, but it is likely that it belongs in the same group.
Phylogenetic position of single species within Biatora
A number of taxa appeared in surprising positions on the tree. This affects already described taxa as well as collections of uncertain identity. In order to facilitate communication about these taxa, I have here given them informal names within quotation marks.
An ITS-sequence of a collection identified as Biatora subduplex and growing on Rhododendron ferrugineum in the Italian Alps appears to be a member of the meiocarpa group, while another specimen growing on detritus in northern Sweden is a member of the vernalis-group (see above). Four highly-supported branches separate the two specimens, which rules out any possibility that both can be conspecific. The question is whether there is a distinction between corticolous specimens and those growing on detritus, or whether arctic and alpine populations belong to different species. It is necessary to reinvestigate the morphology of what was until now regarded as Biatora subduplex to see whether unobserved phenotypic differences would support the molecular distinction.
Another interesting taxon in this respect is Biatora “orientalis”, which is also assigned to the meiocarpa group but closely resembles B. vernalis. It is distinguished from this species by more narrowly elongate ascospores and has so far been found in eastern North America, East Asia and the eastern Black Sea Region, where it tends to grow directly on bark rather than over bryophytes, the typical substratum for B. vernalis. Its placement in the meiocarpa group shows that even extremely subtle morphological differences are important for species recognition in Biatora.
A third case concerns two collections tentatively identified as B. helvola but lacking gyrophoric acid. One of the collections is from Finland (named “B. pseudohelvola” in Printzen & Lumbsch Reference Printzen and Lumbsch2000), the other from Japan. In this case the three samples are closely related to each other but the comparatively long branches between them indicate that superficially similar but distinct species might be involved. It is necessary to investigate the chemical variability of B. helvola in more detail to solve this question. Finally, Biatora “terrae-novae” is similar to B. pycnidiata, the most common Biatora species on Newfoundland, but has more convex apothecia. The phylogeny shows that it is not closely related to this species but rather belongs in the vicinity of B. fallax. On the other hand, a collection from Japan identified as B. alaskana with unusually long ascospores proved to be conspecific with North American material. Biatora alaskana was so far regarded as endemic to north-western North America. This finding therefore extends the known range of the species considerably.
Biatora “radicicola” contains the pigment Cinereorufa-green and is somewhat similar to B. ocelliformis, but in contrast to this species the pigment is concentrated in the interior of the apothecium. Again, the analysis shows that it is not conspecific or closely related to B. ocelliformis.
The phylogenetic position of Biatora pallens (Kullh.) Printzen, a species with 3-septate ascospores, remains unresolved between the rufidula group, likewise with 3-septate ascospores, and Cliostomum. Interestingly, B. pallens was treated as a member of Cliostomum by Ekman (Reference Ekman1997). Cliostomum corrugatum and C. griffithii are clearly distinguished from Biatora by their large, pigmented pycnidia and the anatomy of the exciple. However, C. vitellinum has unpigmented pycnidia and in those of C. flavidulum the pigmentation is restricted to a narrow zone (Ekman Reference Ekman1997). An analysis including additional species of Cliostomum is necessary to resolve the exact delimitation of both genera and the position of B. pallens.
Four sorediate Biatora species with gyrophoric acid as the only secondary metabolite are currently known: B. appalachensis Printzen & Tønsberg from the southern Appalachian Mountains, the circumboreal B. chrysantha, and B. chrysanthoides Printzen & Tønsberg and B. kodiakensis, both known from western North America and Central Norway (Spribille et al. Reference Spribille, Björk, Ekman, Elix, Goward, Printzen, Tønsberg and Wheeler2009; Holien & Tønsberg Reference Holien and Tønsberg2012). Sterile collections of these species can be difficult to distinguish. However, the phylogenetic analysis shows that despite their morphological and chemical similarity, they have obviously not evolved from the same ancestor.
Sorediate taxa in general are scattered rather randomly across the tree, some as sister taxa to other sorediate species (B. flavopunctata, B. vacciniicola), some basal to clades of non-sorediate species (B. kodiakensis, B. oligocarpa), and some as sisters of esorediate taxa (B. britannica, B. hertelii). Biatora fallax, sister to B. cuprea, can be called a facultatively sorediate species, in which the squamules of the thallus are sometimes dissected to the point of becoming sorediate in appearance. With one exception, Biatora hertelii and B. britannica, there are always additional characters to distinguish these from their closest relatives. They therefore do not fit the original concept of “species pairs” as introduced by Du Rietz (Reference Du Rietz1924), in which two closely related taxa are only distinguished by the presence or absence of vegetative propagules. Because several recent studies involving molecular datasets (e.g. Buschbom & Mueller Reference Buschbom and Mueller2006; Tehler et al. Reference Tehler, Irestedt, Bungartz and Wedin2009) have found no evidence for the existence of species pairs, this concept now seems to be obsolete.
Conclusions
The published phylogenies of Biatora have so far been based on rather small datasets involving one or two gene loci and 11–22 taxa (Printzen & Lumbsch Reference Printzen and Lumbsch2000; Spribille et al. Reference Spribille, Björk, Ekman, Elix, Goward, Printzen, Tønsberg and Wheeler2009). Even with an extended three-gene dataset, the circumscription and delimitation of Biatora remains somewhat uncertain. As delimited here, it comprises crustose lichen species with a green-algal photobiont, biatorine apothecia, an exciple of more or less parallel, anticlinal hyphae, asci of the Biatora-type containing eight colourless, more or less thin-walled ascospores of variable shape and septation, strongly gelatinized apothecial tissues, and inconspicuous, immersed pycnidia with bacilliform conidia (an exception is B. meiocarpa, for which long, sausage-shaped conidia have been reported). The genus Cliostomum is very similar in most respects but has more irregularly branched excipular hyphae, and more conspicuous, often dark pigmented pycnidia containing ellipsoid to ovoid conidia (when C. pallens and C. tenerum are excluded). Biatora comprises several evolutionary lineages that are also supported by phenotypic differences. However, the rather high number of taxa that cannot be attributed to any of these groups at present precludes attempts to split Biatora into smaller genera. Using the name Biatora in the broad circumscription suggested here, necessitates a few nomenclatural changes, as outlined below.
Several unidentified samples included in this study proved to belong to so far undescribed species. Because descriptions of new taxa are outside the scope of this study, these will be published separately. The confounding phylogenetic placement of many similar species in different clades (e.g. the two collections of B. subduplex or B. orientalis and B. vernalis) suggests that the diversity of Biatora is not yet fully explored. This is, however, not surprising considering that most Biatora species occur in boreal coniferous forests and that the huge Asian part of its range has not so far been monographed.
Nomenclature
Biatora beckhausii (Körb.) Tuck.
Syn. N. Amer. Lich. 2: 46 (1888).
Bacidia beckhausii Körb., Parerga lichenol.: 134 (1860).—Secoliga beckhausii (Körb.) Stizenb., Nova Acta Acad. Leopoldin.-Carolin. 30: 21 (1863).—Patellaria beckhausii (Körb.) Müll. Arg., Flora 57: 485 (1879).—Micarea beckhausii (Körb.) Vězda, in Poelt, Bestimmungsschlüssel europ. Flechten, Ergänzungsheft 1: 162 (1977); type: Germany, “Westphalen”, Beckhaus (L-910.137 1363—lectotype, selected by Coppins Reference Coppins1983: 196).
Biatora ementiens (Nyl.) Printzen comb. nov.
MycoBank No.: MB805892
Lecidea ementiens Nyl., Flora 67: 222 (1884); type: [Russia: Chukchi], “Fretum Behring, Konyambay, fjellet i öster.”, [28–30 vii] 1879, E. Almquist (H-Nyl 20912!—lectotype, selected here).
Biatora hemipolia (Nyl.) S. Ekman & Printzen comb. nov.
MycoBankNo.: MB805810
Lecidea arceutina * hemipolia Nyl., Flora 56: 294 (1873).—Lecidea hemipolia (Nyl.) Nyl., Lich. Envir. Paris: 84 (1896).—Bacidia hemipolia (Nyl.) Malme., Bot. Notiser 1895: 140 (1895); type: Tavastia, Hollola, Manskivi, ad cort. alni, 1863, legit J. P. Norrlin 244 (H-Nyl. 17975—lectotype, selected by S. Ekman in sched. and designated here; H—isolectotype).
Note
The name “Lecidea arceutina f. hemipolia Nyl.” was first published in Flora 52: 413 (1869) but, as already mentioned by Fries (Reference Fries1874: 353), without any diagnostic characters. Arnold (1870: 472) only lists “Bacidia arceutina f. hemipolia N.” without any description. The earliest diagnosis of the taxon appears to be that mentioned above.
I dedicate this article to Brian Coppins on the occasion of his 65th birthday. Our discussions on the circumscription of Biatora and his (and Sandy's) hospitality during a visit to Edinburgh helped me greatly when my Ph.D. thesis was still in its infancy, and a worn-out copy of his Micarea- monograph on my bookshelf testifies to its importance for everyone dealing with non-saxicolous lecidoid lichens.
I am indebted to the following collectors who made fresh samples available to me: Håkon Holien, Fredrik Jonsson, Mikko Kuusinen, Philip May, Ulrika Nordin, Zdeněk Palice, Toby Spribille, Göran Thor and Tor Tønsberg. I am very grateful to Stefan Ekman who allowed me to use several DNA samples and unpublished sequences for this study and to formally publish his lectotypification of Biatora hemipolia. Technical support by the staff of the Grunelius Möllgaard laboratory, especially Heike Kappes, is also gratefully acknowledged. Parts of this study received financial support from the Research Council of Norway through the Strategic University Programme ‘Applications of molecular techniques in systematic biology’. Field trips of the author were partly financed by the Grolle Olsen fund and the Felix-Ungerer-Stiftung.
Supplementary Material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0024282913000935





