Introduction
Conserving planting stock for re-introductions, rehabilitation and, in the longer term, for genetic resources conservation (especially in the face of climate change) is of paramount importance. For many plant species, this is achieved by storage of dry seeds at sub-zero temperatures. However, a significant proportion of species produce seeds that cannot be stored this way because they are metabolically active and desiccation sensitive, dying as they slowly lose water: such seeds are described as being recalcitrant or non-orthodox (Berjak and Pammenter, Reference Berjak and Pammenter2008 and references therein). Although, according to Dickie and Pritchard (Reference Dickie, Pritchard, Black and Pritchard2002), ~90% of plant species studied produce orthodox seeds, in the tropics and subtropics the proportion – particularly of tree species – producing non-orthodox/recalcitrant seeds appears to be considerably higher (Sacandé et al., Reference Sacandé, Jøker, Dulloo and Thomsen2004). Recalcitrant-seeded and poor-seeding species, and others that are primarily vegetatively propagated, require alternative means of conservation of genetic resources. It is accepted that the best way to achieve this is by cryostorage, ideally in the vapour phase above liquid nitrogen ( − 140 to − 160°C) or at − 196°C in liquid nitrogen. However, it is only rarely that entire recalcitrant seeds can be cryopreserved, as they are almost invariably too large and too hydrated for rapid partial dehydration, or to be cooled without lethal damage (Berjak and Pammenter, Reference Berjak and Pammenter2008). For conservation of genetic diversity, therefore, zygotic embryonic axes are the specimens (explants) of choice.
For successful cryostorage, very small explants must be used which need to be able to withstand rapid flash drying (Berjak et al., Reference Berjak, Farrant, Mycock and Pammenter1990; Pammenter et al., Reference Pammenter, Berjak, Wesley-Smith, Vander Willigen, Black and Pritchard2002) immediately prior to cryopreservation. This necessarily entails excision of explants, i.e. embryonic axes from seeds, or seedling apical meristems or buds, or (less ideally) somatic embryos from vegetative sources. The basic procedures applied consecutively for cryopreservation are: (1) explant excision; (2) decontamination and rinsing; (3) possible cryoprotectant treatment (omitted from the present studies); (4) flash drying; (5) cooling to cryogenic temperature; (6) cryostorage ( − 140 to − 196°C); (7) thawing and rehydration; (8) decontamination and rinsing; (9) culturing/recovering on germination medium (initially in the dark); (10) seedling/plantlet acclimation (hardening-off). However, success, in terms of seedling production rather than only expansion and greening or root production following cryopreservation, is frequently elusive.
A persistent problem with zygotic embryonic axes of several tropical/subtropical species, particularly those originating from dicotyledonous seeds having fleshy cotyledons, is that while most develop roots after retrieval from cryogenic conditions, shoots are not produced (Kioko et al., Reference Kioko, Berjak, Pammenter, Watt and Wesley-Smith1998; Goveia et al., Reference Goveia, Kioko and Berjak2004; Perán et al., Reference Perán, Berjak, Pammenter and Kioko2006; Hajari et al., Reference Hajari, Berjak, Pammenter and Watt2009). Following the suggestion of Goveia et al. (Reference Goveia, Kioko and Berjak2004) that a burst of reactive oxygen species (ROS) accompanying excision might underlie shoot meristem necrosis, we have recently linked the failure of shoot production with the production of superoxide (as a ‘marker’ ROS) upon embryonic axis excision. This is particularly the case where the cotyledonary connections (which must be severed) are in close proximity to the shoot apex of the axis (Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011).
However, not only axis excision, but also the processing steps that follow – including exposure to, and retrieval from, cryogenic storage – do, or have the potential to, lead to the generation of ROS (Benson and Bremner, Reference Benson, Bremner, Fuller, Lane and Benson2004; Roach et al., Reference Roach, Ivanova, Beckett, Minibayeva, Green, Pritchard and Kranner2008; Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010) which include the superoxide (∙O2–) and hydroxyl (∙OH) radicals and hydrogen peroxide (H2O2). Although ROS are implicated in normal metabolism under conditions where they must be strictly controlled (see below), many stresses, including wounding, are accompanied by ROS release to an extent that may well overwhelm the endogenous antioxidant capacity of small explants such as embryonic axes. This would lead to severe oxidative damage and death of all or part of the explant tissues, and consequently poor cryo-survival. Such ROS generation has been shown to accompany steps 1, 4, 6, 7 and 9, above (Touchell and Walters, Reference Touchell and Walters2000; Goveia et al., Reference Goveia, Kioko and Berjak2004; Roach et al., Reference Roach, Ivanova, Beckett, Minibayeva, Green, Pritchard and Kranner2008, Reference Roach, Beckett, Minibayeva, Colville, Whitaker, Chen, Bailly and Kranner2010; Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010; Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011) and could well also take place at all other stages.
While uncontrolled generation of ROS in plants has long been associated with an often lethal degree of tissue damage (e.g. Hendry, Reference Hendry1993 and references therein), there is now substantial evidence for the implication of these species as signal transducers, after which they are timeously and effectively quenched via the action of the high redundancy-level ROS-scavenging network (Bailey-Serres and Mittler, Reference Bailey-Serres and Mittler2006; Halliwell, Reference Halliwell2006; Van Breusegem et al., Reference Van Breusegem, Bailey-Serres and Mittler2008). As expressed by those authors, there must be a fine balance between ROS production and scavenging.
Reactive oxygen species are routinely produced as adjuncts to various intracellular metabolic pathways, the essential control being exerted by the antioxidant system that modulates intracellular ROS levels both temporally and spatially (Foyer and Noctor, Reference Foyer and Noctor2005). As indicated by those authors, antioxidant status is pivotal both in terms of removing ROS, and in response to biotic and abiotic stresses, the latter including wounding.
In contrast to the intracellular generation of ROS, in plants the apoplast is characterized by enzymes, particularly extracellular peroxidases (ECPOX), involved both in ROS synthesis and quenching (Minibayeva et al., Reference Minibayeva, Kolesnikov, Chasov, Beckett, Lüthje, Vylegzhanina, Buck and Böttger2009), and plasmalemma-bound NAD(P)H oxidases also appear be implicated (Schopfer and Liszkay, Reference Schopfer and Liszkay2006; Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010b). Extracellular production of ∙O2– and H2O2 appear to be key to events during seed imbibition, germination and seedling establishment (Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010b) and ∙OH has also been shown to be implicated in cell wall loosening during germination of cress seeds (Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009). The generation of apoplastic ROS creates a redox difference across the plasmalemma (Antunes and Cadenas, Reference Antunes and Cadenas2000; Foyer and Noctor, Reference Foyer and Noctor2005), with H2O2 apparently being the only extracellularly produced ROS crossing the membrane (Bhattacharjee, Reference Bhattacharjee2005; Pitzschke et al., Reference Pitzschke, Forzani and Hirt2006).
Important components of the ROS-scavenging network in plants are low molecular weight, non-enzymic compounds, principally reduced glutathione (GSH), ascorbic acid (AsA) and α-tocopherol (which is lipid soluble), as well as a spectrum of enzymic antioxidants (for recent reviews, see Shao et al., Reference Shao, Chu, Lu and Kang2008; Gill and Tuteja, Reference Gill and Tuteja2010).
There is a range of enzymic antioxidants in plant cells, including superoxide dismutases (SODs), catalases (CATs), ascorbate peroxidase (APX), several other peroxidases, mono- and dehydro-ascorbate reductases (MDHAR and DHAR, respectively), glutathione reductase (GR), glutathione-S-transferases (GST) and guaiacol peroxidase (GPOX) (Kranner and Birtić, Reference Kranner and Birtić2005; Gill and Tuteja, Reference Gill and Tuteja2010 and references therein).
The ROS which have attracted most attention in the context of damage accompanying water stress imposed during dehydration of desiccation-sensitive plant tissues (Smirnoff, Reference Smirnoff1993) and particularly of recalcitrant seeds, are ∙O2–, ∙OH, H2O2 and 1O2 (Côme and Corbineau, Reference Côme, Corbineau, Ouédraogo, Poulsen and Stubsgaard1996; Varghese and Naithani, Reference Varghese and Naithani2002; Bailly, Reference Bailly2004; Kranner and Birtić, Reference Kranner and Birtić2005; Pukacka and Ratajczak, Reference Pukacka and Ratajczak2006; Berjak and Pammenter, Reference Berjak and Pammenter2008; Roach et al., Reference Roach, Beckett, Minibayeva, Colville, Whitaker, Chen, Bailly and Kranner2010; Varghese et al., Reference Varghese, Sershen, Berjak, Varghese and Pammenter2011). Normally ∙O2– would be effectively removed by SOD, which catalyses the dismutation of two superoxide radicals, one to H2O2 and the other to 3O2, thus preventing formation of ∙OH (Kranner and Birtić, Reference Kranner and Birtić2005; Gill and Tuteja, Reference Gill and Tuteja2010). This reaction can also proceed non-enzymically (Kranner and Birtić, Reference Kranner and Birtić2005), which is presumably the case in the apoplast where SOD appears to be absent (Gill and Tuteja, Reference Gill and Tuteja2010). However, if H2O2 dismutation, in turn, does not occur rapidly, it can be involved in ∙OH formation via the Fenton reaction, Fe2++H2O2 → Fe3++∙OH + HO– (Hendry, Reference Hendry1993; Benson and Bremner, Reference Benson, Bremner, Fuller, Lane and Benson2004), the major source of Fe2+ being reduction of Fe3+ by ∙O2– (Hendry, Reference Hendry1993). The hydroxyl radical, ∙OH, is the most damaging ROS in biological tissues, as it will react with nucleic acids, lipids and, indirectly, with proteins (Benson and Bremner, Reference Benson, Bremner, Fuller, Lane and Benson2004; Halliwell, Reference Halliwell2006). These are all potential consequences of stress, when antioxidant capacity is not able to counteract ROS generation.
Involvement of ECPOX (components of class III peroxidases) is prominent among apoplastic events which are implicated in wounding and stress responses (Minibayeva et al., Reference Minibayeva, Kolesnikov, Chasov, Beckett, Lüthje, Vylegzhanina, Buck and Böttger2009). Those authors showed that wounding of seedling roots of Triticum aestivum almost doubled the production of ∙O2– mediated by three apoplastic ECPOX, compared with levels in intact roots. These enzymes are held to switch activity from removing to producing ROS, depending on several factors, including pH when tending towards alkalinity (~7.2) and the presence of an appropriate reductant(s) (Bolwell et al., Reference Bolwell, Bindschedler, Blee, Butt, Davies, Gardner, Gerrish and Minibayeva2002). The surge in ∙O2– production is generally indicative of what is described as the ‘oxidative burst’ following wounding and other abiotic stresses, e.g. dehydration of desiccation-sensitive embryonic axes from recalcitrant seeds (Roach et al., Reference Roach, Ivanova, Beckett, Minibayeva, Green, Pritchard and Kranner2008; Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010). Evidence of an oxidative burst following recalcitrant embryonic axis excision – which is an essential prerequisite for their cryopreservation – has been obtained for sweet chestnut, Castanea sativa (Roach et al., Reference Roach, Ivanova, Beckett, Minibayeva, Green, Pritchard and Kranner2008), and the tropical/subtropical species, Trichilia dregeana (Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010; Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011), and in conjunction with severing of cotyledons entirely from axes of Syzygium cordatum, and the single cotyledon partially in the case of Amaryllis belladonna (Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011).
When the antioxidant capacity of plant tissues is inadequate to quench ROS produced, there is consequent oxidative stress (Scandalios, Reference Scandalios1993). Antioxidant levels were not sustained in axes from slowly dried T. dregeana seeds, which were characterized by elevated levels of ∙OH, decreasing viability and increasingly positive glutathione half-cell reduction potentials [EGSSG/2GSH (Kranner and Birtić, Reference Kranner and Birtić2005; Kranner et al., Reference Kranner, Minibayeva, Beckett and Seal2010a)] (Varghese et al., Reference Varghese, Sershen, Berjak, Varghese and Pammenter2011). Similarly, Cheng and Song (Reference Cheng and Song2008) showed loss of viability of recalcitrant Antiaris toxicaria seeds and axes to be associated with increasing ∙O2– production and levels of H2O2 accompanied by a decline of antioxidant enzyme activity. In terms of necrosis of the axis apical meristem, an additional factor was the proximity of the lesions resulting from severing of the cotyledons from the shoot apex. In cases where these were close to the shoot apex, necrosis appeared to be inevitable, but if separated spatially, adverse effects of the ROS burst were apparently attenuated, and shoots developed normally (Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011).
Whitaker et al. (Reference Whitaker, Beckett, Minibayeva and Kranner2010) have shown that a further ROS (∙O2–) burst accompanied rehydration following cryogen exposure of axes of T. dregeana, none of which survived. This indicates not just the potential, but the realization of additional oxidative stress, and the fact that the cumulative effects over the sequence of procedures involved are liable to be lethal. Furthermore, light appears to exacerbate the adverse effects of ROS production after cryopreservation, as illustrated for both Brassica napus shoot apices (Benson and Noronha-Dutra, Reference Benson and Noronha-Dutra1988) and excised embryos of Zizania palustris, despite enhanced production of catalase and peroxidase by the latter (Touchell and Walters, Reference Touchell and Walters2000). In cases of shoot apices and embryos/axes of recalcitrant seeds which we know often to be green, photo-oxidative damage could well ensue during a post-cryopreservation recovery phase in the light.
There are theoretically two solutions to the problems caused by damaging levels of ROS generated during procedures applied before, during and after cryostorage of recalcitrant embryonic axes (or other forms of germplasm). First, explant antioxidant potential could be enhanced either by an exogenous source of reductants, or by enhancement of endogenous antioxidant activity; and second, ROS could be quenched/scavenged at source. However, in view of the vital role of ROS in ongoing explant development (as discussed above), any treatment must be experimentally developed and judiciously applied.
Desferrioxamine, a powerful iron (Fe3+) chelator, has also been shown to have application in post-cryopreservation recovery of rice cell cultures (Benson et al., Reference Benson, Lynch and Jones1995). Iron sequestration by desferrioxamine interferes with ∙OH generation from H2O2 via the Fenton reaction (see above): this could underlie the observations of those authors that short-term provision of low concentrations of desferrioxamine (0.5–10 mg l− 1) in a cation-reduced medium, prior to and after cryopreservation, promoted post-thaw recovery of rice cells. However, the numbers of ‘embryo-like structures’ formed in flax cell cultures was reduced by incorporation of desferrioxamine in the induction medium, explained by the possibility of somatic embryogenesis being a stress response (Obert et al., Reference Obert, Benson, Millam, Pret'ová and Bremner2005). Considering that ∙OH has been implicated in cell wall loosening during germination (Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009) and may play a role in shoot induction from hypocotyl segments as well as in the formation of embryo-like structures (Obert et al., Reference Obert, Benson, Millam, Pret'ová and Bremner2005), it is clear that use of desferrioxamine (like any other substance which might modulate ROS-mediated damage) must be subject to stringent control determined experimentally, in terms of concentration, duration of application and the procedural stage(s) at which it is provided. Dimethyl sulphoxide (DMSO; Me2SO), a scavenger of ∙OH (Benson et al., Reference Benson, Harding, Johnston, Day and Stacey2007), is a commonly used, penetrating colligative cryoprotectant [10–15% (w/v) in plant vitrification solutions] but importantly, those authors reported a significant improvement in recovery of cryopreserved shoot tips when 1–2% (w/v) DMSO was used in a pre-growth medium.
Our first major breakthrough in obviating death of shoot apical meristems of embryonic axes from recalcitrant seeds of T. dregeana, T. emetica and Protorhus longifolia, followed the use of 1% (v/v) DMSO in conjunction with axis excision (Cassandra et al., Reference Cassandra, Benson, Berjak, Goveia and Pammenter2011). Those authors reported shoot production by 70% of T. dregeana axes, 55% of T. emetica and 60% of P. longifolia axes, whereas no axes produced shoots when the DMSO treatment was omitted. By implication from those studies, the damaging effects of the oxidative burst accompanying wounding were ameliorated (Cassandra et al., Reference Cassandra, Benson, Berjak, Goveia and Pammenter2011) – an assumption since verified by significantly lower levels of ∙O2– being measured for T. dregeana axes after the DMSO treatment, compared with those not exposed to this exogenous antioxidant (our presently unpublished data).
Conventional attempts to counteract stress-related ROS activity include the provision of exogenous synthetic (or naturally occurring) antioxidants and transition metal chelating agents. However, use of exogenous antioxidants has drawbacks: DMSO (depending on the concentration) is potentially cytotoxic; addition of naturally occurring antioxidants could disturb the endogenous balance of such compounds in explants; and chelating transition metal cations could give rise to nutrient deficiencies.
We have developed a different strategy, applying the principles of electrochemisty, i.e. cathodic protection, in the procedural stages required for cryopreservation of, and subsequent seedling/plantlet production by, explants derived from recalcitrant seeds. Having previously counteracted dry (maize) seed deterioration during storage by the application of direct cathodic protection (Pammenter et al., Reference Pammenter, Adamson and Berjak1974; Berjak, Reference Berjak1978), we have implemented the same approach during flash drying (step 4, above). Preliminary results have indicated the efficacy of direct cathodic protection during the later stages of dehydration (Ngobese et al., Reference Ngobese, Berjak and Pammenter2011). Direct provision of a cathodic field is, however, precluded when explants are hydrated prior to, and after, cryopreservation. For some of these procedural stages, cathodic water (see rationale below) has been employed, the results of which are presently reported for excised recalcitrant zygotic axes of Strychnos gerrardii N. E. Br. [an endospermous dicotyledonous species (Loganiaceae)] and, in terms of total antioxidant capacity, for excised embryonic axes of Boophane disticha (L.f.) (of the monocotyledonous family, Amaryllidaceae).
Cathodic water, generated by electrolysis of a solution containing electrolyte(s), has been shown to exert antioxidant effects (Shirahata et al., Reference Shirahata, Kabayama, Nakano, Miura, Kusumoto, Gotoh, Hayashi, Otsubo, Morisawa and Katakura1997; Hanaoka, Reference Hanaoka2001; Hanaoka et al., Reference Hanaoka, Sun, Lawrence, Kamitani and Fernandes2004; Hiraoka et al., Reference Hiraoka, Takemoto, Suzuki, Shinohara, Chiba, Shirao and Yoshimura2004). Cathodic water [electrolysed-reduced water (Shirahata et al., Reference Shirahata, Kabayama, Nakano, Miura, Kusumoto, Gotoh, Hayashi, Otsubo, Morisawa and Katakura1997)], was reported to scavenge ∙O2– and H2O2 produced in in vitro experimental systems, which was attributed to ‘active hydrogen’ in the reduced water by those authors. However, this view of the basis of the antioxidant activity of the cathodic water fraction was challenged by Hanaoka (Reference Hanaoka2001) whose results suggested that while H2O2 was scavenged as a result of activated dissolved H2 in reduced water, there were no direct ∙O2– dismutation effects; in contrast, dismutation was enhanced when a proton donor such as AsA was present in the (reduced) water, as a consequence of an increase in the dissociation activity of the water. In a subsequent publication, Hanaoka et al. (Reference Hanaoka, Sun, Lawrence, Kamitani and Fernandes2004) reported marked inhibition of conversion of supercoiled DNA to the open circular form, and suppression of single-strand breakage of DNA induced by ROS, apparently as a consequence of the effects of the changes in the ionic product of the reduced water. In addition to protection of DNA, the reduced (cathodic) water fraction has also been reported to counteract oxidative damage to RNA and protein in lymphocytes (Lee et al., Reference Lee, Kim, Ryoo, Lee and Park2006), which could have resulted from enhanced endogenous antioxidant activity. In agreement with Hanaoka (Reference Hanaoka2001), the observations of Hiraoka et al. (Reference Hiraoka, Takemoto, Suzuki, Shinohara, Chiba, Shirao and Yoshimura2004) led to the conclusion that reduced water indubitably exhibits antioxidant activity, the basis of which is molecular hydrogen.
It thus appears that the cathodic (reduced) fraction of an aqueous electrolyte solution can offer the advantage of reductant activity, whether as a direct property, or as a consequence of stimulating endogenous antioxidants, without application of any further compounds, which is the basis of the work presently reported. Also, we report on the brief application of the anodic (acidic) water fraction as a decontaminant, as it does not have the toxicity of conventionally used sodium hypochlorite (NaOCl) but apparently has potent antimicrobial activity (Huang et al., Reference Huang, Hung, Hsu, Huang and Hwang2008).
Materials and methods
Plant material
Mature fruits of S. gerrardii were collected after being shed from the trees in a conserved natural area in Durban, while those of B. disticha were harvested directly from parent plants in the Eastern Cape region of South Africa. The fruits were transported in plastic bags to the laboratory with minimum delay (0–2 d), avoiding water loss. Upon arrival in the laboratory, seeds of S. gerrardii were extracted from fruits, weighed as a batch and, after pulp removal, decontaminated for 20 min in 1% (w/v) aqueous sodium hypochlorite after which they were dried back to the original mass on paper towel, at ambient temperature. Upon receipt, seeds of B. disticha were extracted from the fruits, and similarly handled, the only difference being the duration of immersion (10 min) in NaOCl. The seeds of both species were then dusted with Benlate [active ingredient: benomyl (benzimidazole), Dupont, USA], and stored hydrated at 16°C (S. gerrardii) or 6°C (B. disticha) on grids suspended approximately 200 mm above sterile, moistened paper towel in buckets with sealing lids.
Note, for both species, that the seeds are endospermous, thus they characteristically have one (B. disticha) and two (S. gerrardii) very thin cotyledons (Fig. 1A, B).

Figure 1 Longitudinally split seeds of (A) Strychnos gerrardii and (B) Boophane disticha, both showing the embryo relative to the endosperm. The two paper-thin cotyledons of the S. gerrardii embryo can be clearly seen (A), while the position of the axis within the rolled single cotyledon in B. disticha is indicated (B, *). Scale bars = 10 mm.
Generation of electrolysed water
Water containing the electrolytes, 0.5 μM CaCl2.2H2O and 0.5 mM MgCl2.6H2O (CaMg – which we have used routinely in the non-electrolysed state for rinsing and rehydration during cryopreservation procedures), was electrolysed by provision of a 60 V potential difference using a Bio-Rad™ Powerpac (BioRad, Hercules, California, USA) modified to be equipped with platinum electrodes. Electrolysis for 1 h at room temperature yielded anodic (oxidizing) water at pH c. 2.4, and cathodic (reducing) water at pH c. 11.2, which were used within an hour of preparation in all cases. The apparatus consisted of two glass beakers, each containing 200 ml of CaMg solution, the anode being immersed in the solution in one beaker, and the cathode in the other. The circuit was completed using an agar-based salt bridge containing saturated potassium chloride.
In vitro germination
Zygotic axes were excised from wet-stored S. gerrardii seeds with 3–4 mm of each thin cotyledon attached and germinated on full-strength MS medium (Murashige and Skoog, Reference Murashige and Skoog1962) in which 0.4 g l− 1 activated charcoal was suspended. After decontamination (see below) explants were cultured in 65-mm Petri dishes containing five explants each, and maintained in the dark for 7 d before transfer to a 16 h/8 h light (52 μE m− 2 s− 1)/dark photoperiod at 23–25°C. The same procedure was used following all axis manipulations prior to, and after, cryopreservation.
Assessment of desiccation sensitivity
Zygotic axes excised from seeds that had been wet-stored for 7 (S. gerrardii) or 14 d (B. disticha) were accumulated within closed Petri dishes on filter paper moistened with sterile CaMg solution. Axes were then flash-dried (Berjak et al., Reference Berjak, Farrant, Mycock and Pammenter1990; Pammenter et al., Reference Pammenter, Berjak, Wesley-Smith, Vander Willigen, Black and Pritchard2002) for various intervals, when ten were sampled for water content and a further ten rehydrated with CaMg solution, decontaminated (see below) and regenerated in vitro. The results were used to construct curves relating water content, drying times and viability, which formed the basis of all subsequent dehydration and cryopreservation studies.
Optimizing decontamination procedures for zygotic axes
Immediately after harvesting and after 3 d of hydrated storage, ten S. gerrardii zygotic axes were excised and decontaminated in: (a) 0.02% (w/v) mercuric chloride (HgCl2) for 2 min and 2% (w/v) calcium hypochlorite [Ca(OCl)2] for 5 min (Goveia, Reference Goveia2007); (b) anodic water for 5 and 10 min; and (c) anodic water for 5 and 10 min followed by 1% Cicatrin® (active ingredient, neomycin sulphate; Pfizer Consumer Healthcare, South Africa) for 2 min. Exposure to all decontaminants was followed by three rinses with sterile distilled water. Axes were then plated on germination medium (see above) in a laminar air-flow and maintained (as above) for 7 d, after which they were assessed for fungal and bacterial proliferation. The results determined the decontamination regime subsequently adopted.
Rehydration with the CaMg solution or cathodic water
In the present contribution, the non-electrolysed solution containing 0.5 μM CaCl2.2H2O and 0.5 mM MgCl2.6H2O (Mycock, Reference Mycock1999) is termed the CaMg solution, while cathodic and anodic water refer to the fractions obtained after electrolysis of the CaMg solution. Zygotic axes with attached cotyledonary segments were excised from S. gerrardii seeds that had been wet-stored for 7 and 35 d and accumulated within closed Petri dishes on filter paper moistened with sterile CaMg solution. Samples of newly excised axes and those that had been flash dried to ~0.4 g g− 1 were immersed in either CaMg solution or cathodic water for 30 min in the dark, prior to being plated out. Axes that had been similarly dried and then rapidly cooled by direct immersion in nitrogen slush ( − 210°C) and subsequently maintained in batches of five in 2-ml cryovials (Greiner™, Frickenhausen, Germany) in liquid nitrogen for up to a week, were exposed to either CaMg solution or cathodic water as follows: axes were thawed by direct immersion at 40°C for 2 min followed by rehydration for 30 min in the dark, before in vitro culture.
For each treatment combination, ten axes were scored for root and shoot development after 30 d. All experiments were repeated twice.
Water content determination
Immediately after excision or drying, ten axes from each of the non-cooled treatment combinations were weighed individually using a six-place balance (Mettler, MT5; Germany) and dried in an oven at 80°C for 48 h before being re-weighed to determine the dry mass. Water content was expressed on a dry mass (DM) basis [g H2O (g DM) − 1; g g− 1].
Assessment of extracellular superoxide production
Extracellular ∙O2– production was measured spectrophotometrically by NADH-mediated oxidation of epinephrine to adrenochrome (Misra and Fridovich, 1972) and expressed as nmol of epinephrine oxidized s− 1 (g dry weight)− 1, calculated using the molar extinction coefficient for adrenochrome (4.47 mM− 1 cm− 1). To assess excision-induced extracellular ∙O2– production, axes were incubated in an epinephrine solution (12.8 mg dissolved in 200 μl 1.0 M HCl made up to 10 ml with distilled water; pH adjusted to 7.0) immediately after excision and the oxidation of epinephrine was measured at 5-min intervals up to 30 min, the incubation medium being replaced with freshly prepared epinephrine solution at each measurement interval. To assess whether extracellular ∙O2– production was enhanced as a consequence of partial dehydration and how this was influenced by water content and rehydration, axes that had been flash dried for varying times were incubated in the epinephrine solution immediately after drying and immediately after 30 min rehydration in CaMg solution. In subsequent studies, freshly excised axes, or those flash dried to ~0.4 g g− 1, were then immersed in CaMg solution or cathodic water for 30 min in the dark, prior to ∙O2– estimation. Additionally, extracellular ∙O2– production after 4, 24, 48 and 72 h of in vitro recovery was measured for axes subjected to these procedures.
The validity of the epinephrine assay for detection of extracellular ∙O2– production (see Misra and Fridovich, 1972) in the axes investigated here was assessed by adding superoxide dismutase (from horseradish lyophilized powder; at a final concentration of 0.1 μg ml− 1) to the assay mixture before fresh, dried and germinating axes after various times in culture were incubated. In all cases, the addition of superoxide dismutase inhibited the oxidation of epinephrine by more than 50% after 15 min incubation (data not shown), validating the use of the assay in this study.
Assessment of total antioxidant activity
Total antioxidant activity was measured for embryonic axes of B. disticha only (limited seed availability precluding this analysis for S. gerrardii), according to the method of Re et al. (Reference Re, Pellegrini, Proteggente, Pannala, Yang and Rice-Evans1999), using the 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical cation decolourization assay. Immediately after the 30-min incubation in either CaMg or cathodic water, 12 freshly excised or flash-dried axes were divided into four replicated batches of three and immediately extracted for total antioxidants (after Johnston et al., Reference Johnston, Dussert, Gale, Nadarajan, Harding and Benson2006). Extracts were immediately assessed for total antioxidant activity and these assays were performed twice on the four different extracts per treatment, in the dark. A standard curve was created using the water-soluble α-tocopherol analogue, 0.05–1.0 mM Trolox™ (Sigma-Aldrich, Steinheim, Germany), in phosphate antioxidant extraction buffer. Change in absorbance of individual samples was expressed as Trolox equivalents on a fresh weight basis using the standard curve.
Statistical analysis
Extracellular superoxide production and total antioxidant activity, root length and biomass data were tested for significant inter-treatment differences across treatments by analysis of variance. Multiple comparisons were then made using least significant difference (LSD) or, in the case of total antioxidant activity, Scheffe's mean separation test. Correlations between extracellular superoxide production and water content were tested using a Pearson's correlation test. All viability, root and shoot production data were tested for significant inter-treatment differences using a one-sample T-test. For all T-test analyses percentages were arcsin transformed to conform data to parametric test assumptions. All statistical tests were performed at the 0.05 level of significance using SPSS statistical package, Version 18 (SPSS Inc., Chicago, Illinois, USA).
Results and discussion
Use of anodic water as a decontaminant
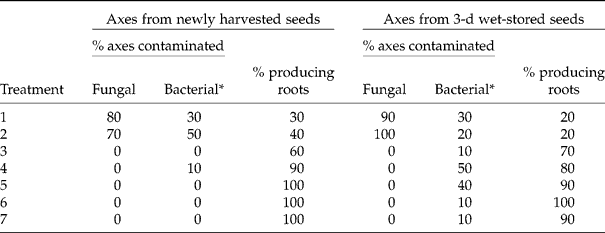
Before embarking on the main theme of the present study, a comment on the potential of a brief application of anodic water as a decontaminant for the excised axes is presented. Anodic water has considerable application in the food industry, not only for fruits, vegetables, meats and seafood, but also for sanitizing equipment (Huang et al., Reference Huang, Hung, Hsu, Huang and Hwang2008). While anodic water is oxidizing, so are conventional decontaminants used routinely for in vitro plant cultures [e.g. NaOCl and Ca(OCl2)] while others, such as HgCl2, are toxic. However, decontamination is a vital step in explant cryopreservation procedures, as virtually all recalcitrant seeds of tropical/subtropical provenance harbour fungal inoculum, which is mostly borne internally (Sutherland et al., Reference Sutherland, Diekmann and Berjak2002). In the present study the benefits of anodic water were exhibited by its potent fungicidal activity and lack of any adverse effects on root production by the axes (Table 1). However, it is inevitable that if seeds also harbour bacterial inoculum, bacteria will proliferate once the antibiotic effect of the fungi is no longer manifested. This was found to be the case for some bacteria associated with axes of S. gerrardii, although anodic water treatment has been reported to be effective against a wide range of bacteria associated with foodstuffs (reviewed by Huang et al., Reference Huang, Hung, Hsu, Huang and Hwang2008). In the present study, the anodic water was used as a decontaminant approximately 1 h after it was generated. In this regard, Huang et al. (Reference Huang, Hung, Hsu, Huang and Hwang2008) record that the solution does rapidly lose its antimicrobial activity, if not continuously recharged with electrolysed ionic products. Thus, future studies will utilize immediately generated anodic water for explant decontamination. However, in the present case when anodic water treatment was followed by application of neomycin sulphate as Cicatrin®, bacterial contamination was either eliminated or significantly reduced (Table 1).
Table 1 Percentage of axes of Strychnos gerrardii displaying fungal and/or bacterial contamination and percentage developing roots after various decontamination regimes. Individual axes were assessed after 7 d in culture

Treatment: 1, 5 min sterile deionized water; 2, 10 min sterile deionized water; 3, 2 min 0.02% HgCl2 followed by 5 min 1% Ca(OCl2); 4, 5 min anodic water; 5, 10 min anodic water; 6, 5 min anodic water followed by 2 min 1% (w/v) Cicatrin® suspension; 7, 10 min anodic water followed by 2 min 1% (w/v) Cicatrin® suspension.
* After sterile water treatments (1 and 2) fungal proliferation was sometimes so vigorous that it was difficult to ascertain whether axes also exhibited bacterial contamination.
Desiccation sensitivity and excision-related injury
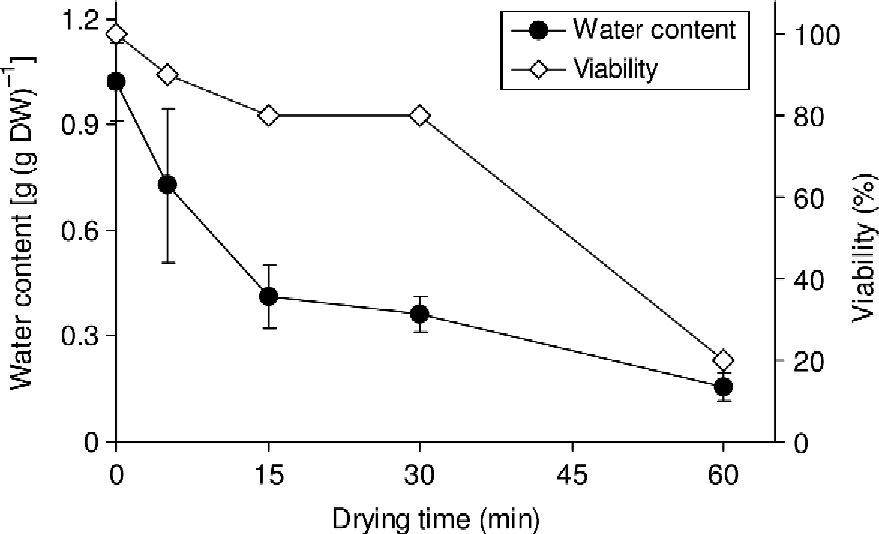
In the first instance, embryonic axes excised with cotyledonary segments attached were assessed for responses to flash drying and conventional rehydration using the CaMg solution. Water content of axes from newly shed seeds of S. gerrardii was c. 1.0 g g− 1 and, as is typical for recalcitrant-seeded species (Berjak and Pammenter, Reference Berjak and Pammenter2008), only 20% of newly excised axes survived at water contents less than 0.3 g g− 1 (Fig. 2). Hence, water contents in the range 0.3–0.41 g g− 1 were presently used for all subsequent experiments. The selection of this water content range was based on previous studies (Sershen et al., Reference Sershen, Pammenter, Berjak and Wesley-Smith2007) which have shown water contents in this range to be superior to those >0.4 g g− 1 (with occasional exceptions), in promoting post-thaw viability in desiccation-sensitive zygotic germplasm.
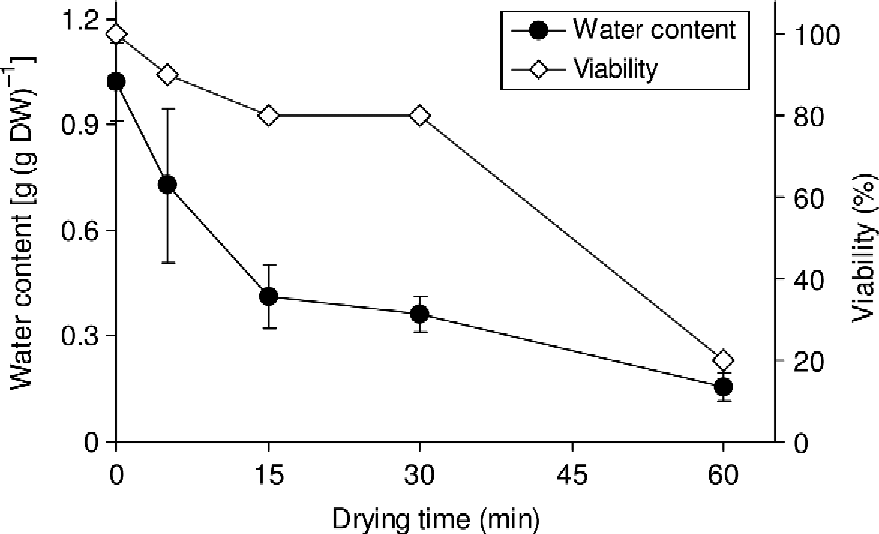
Figure 2 Changes in water content and viability during flash drying of Strychnos gerrardii embryonic axes. Viability was based on root production (n = 10). One-sample T-test for viability showed significant differences (P < 0.01). For water content, each point represents the mean of ten embryos. Vertical bars represent mean ± SD.
Since excised axes of S. gerrardii produced no shoots even at the original water content, viability data presented in Fig. 2 were based on root production only. Although the cotyledons of S. gerrardii are not fleshy (Fig. 1) and were not entirely removed, lack of shoot production was consistent with injury accompanying excision, as has been shown for axes of several other species producing recalcitrant seeds (Kioko et al., Reference Kioko, Berjak, Pammenter, Watt and Wesley-Smith1998; Goveia et al., Reference Goveia, Kioko and Berjak2004; Perán et al., Reference Perán, Berjak, Pammenter and Kioko2006; Hajari et al., Reference Hajari, Berjak, Pammenter and Watt2009).
In the first 5 min immediately following excision there was an oxidative burst indicated by substantial ∙O2– production (Fig. 3), suggesting that preclusion of shoot development may have been a consequence of excision-related injury. Similar results relating lack of shoot development to an ∙O2– burst have been reported for excised axes of T. dregeana (Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010; Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011). To ascertain whether or not this burst in the first 5 min after excision would be quenched by cathodic water, ideally axes should have been incubated immediately in epinephrine made up in cathodic water. However, the high pH of the cathodic water immediately led to the formation of adrenochrome, making it impossible to use this assay to assess the quenching effects of cathodic water.

Figure 3 Extracellular superoxide production by Strychnos gerrardii embryonic axes after excision of a portion of the cotyledon. Each point represents mean of three replicates of four axes each. Vertical bars represent mean ± SD. Superoxide levels were tested for significant differences across time intervals by one-way ANOVA and means were separated by LSD post-hoc test. Columns labelled with different letters differ significantly when compared across treatments (P < 0.001).
In desiccation-sensitive tissue if a stress induces a metabolic disorder it takes time for the damage consequent upon that disorder to accumulate (Walters et al., Reference Walters, Pammenter, Berjak and Crane2001). In most cases the damage incurred may be evident only after the system has rehydrated fully and metabolism has been re-initiated. In this context, evolution of ∙O2– was assessed for axes immediately after excision from 7-d-stored S. gerrardii seeds (Fig. 4; first datum point [□]), immediately upon sampling after dehydration to various water contents (Fig. 4; □), and for material sampled as above, but then subjected to 30 min rehydration in CaMg solution or cathodic water (Fig. 4, first datum points; △,■). Superoxide production increased with decreasing water content in S. gerrardii axes, and the Pearson's correlation coefficients between water content and ∙O2– production of − 0.77 for dehydrated axes and − 0.88 for those rehydrated with CaMg solution after dehydration validated this trend (Fig. 4). Note that ideally ∙O2– production by axes rehydrated in cathodic water should have been assessed in parallel at all water contents (Fig. 4), but this was precluded by the limitation in seed numbers. Hence, these values for ∙O2– are shown (Fig. 4; ■) for undried axes after 30 min immersion in cathodic water and those similarly rehydrated after dehydration to the water content (~0.4 g g− 1) selected for subsequent experiments. Exposure of undried (□) and axes dehydrated (to ~0.4 g g− 1) to cathodic water (■) did not decrease ∙O2– levels relative to those exposed to the CaMg solution (△) (Fig. 4).

Figure 4 Extracellular superoxide production after flash drying of Strychnos gerrardii embryonic axes to various water contents (□), and those rehydrated in the CaMg solution (△) or cathodic water (■) after flash drying to the same water contents. The first datum points represent undried axes immediately after excision (□), or those exposed to the CaMg solution (△) or cathodic water (■) for 30 min. Note that for dehydrated axes exposed to cathodic water, the superoxide level is given only for the water content (■, ~0.4 g g− 1) relevant for cryopreservation. Each point represents the mean of three replicates of five axes each. Bars represent mean ± SD. Pearson's correlation coefficients between water content and superoxide production were: r = − 0.77 for dried axes and − 0.88 for dried axes that were subsequently rehydrated with CaMg solution. Superoxide levels were tested for significant differences across treatments, within time intervals by one-way ANOVA and means were separated using LSD post-hoc test, as given in the table beneath the figure.
It should be noted that because of the direct effect of cathodic water on epinephrine (see above), axes treated with cathodic water were removed from this medium and briefly rinsed with distilled water immediately prior to immersion in an epinephrine solution (made up in distilled water). Effectively, ∙O2– production was measured immediately following, rather than concomitantly with, treatment with cathodic water.
If axes had not been rehydrated, there was an increase in ∙O2– production once water contents were reduced below 0.36 g g− 1. We attribute this to desiccation damage sensu stricto, i.e. damage ensuing when most of the intracellular water remaining is structure-associated (Pammenter et al., Reference Pammenter, Greggains, Kioko, Wesley-Smith, Berjak and Finch-Savage1998; Walters et al., Reference Walters, Pammenter, Berjak and Crane2001). The ∙O2– at these water contents was accompanied by marked loss of viability (compare Figs 2 and 4).
Incubation in CaMg or cathodic water for 30 min significantly reduced ∙O2– production by undried S. gerrardii axes (see first datum points [△,■], Fig. 4). This suggests that the initial levels of ∙O2– measured for undried S. gerrardii axes (first datum point [□], Fig. 4) may still have been a consequence of excision damage. The ∙O2– levels are considered less likely to have been a reflection of the natural metabolic status as, unlike recalcitrant seeds of other species (Berjak and Pammenter, Reference Berjak and Pammenter2008), those of S. gerrardii showed no signs of germination in hydrated storage over 8 weeks (data not shown).
The dual role of ROS (as indicated by ∙O2–) and effects of rehydration medium
The role of ROS in seeds has been documented by Müller et al. (Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009), who implicated ∙OH in cell wall loosening during germination and seedling establishment, while Kranner et al. (Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010b) identified ∙O2– and H2O2 production as key events in these developmental phases and also during imbibition. Additionally, Liszkay et al. (Reference Liszkay, van der Zalm and Schopfer2004) showed that root extension was suppressed when endogenous ∙OH formation was inhibited by scavengers of ∙O2– or ∙OH. It has been established, however, that these same ROS, and others, are central to the damage associated with dehydration of recalcitrant seeds or embryonic axes (Côme and Corbineau, Reference Côme, Corbineau, Ouédraogo, Poulsen and Stubsgaard1996; Varghese and Naithani, Reference Varghese and Naithani2002; Pukacka and Ratajczak, Reference Pukacka and Ratajczak2006; Berjak and Pammenter, Reference Berjak and Pammenter2008; Roach et al., Reference Roach, Beckett, Minibayeva, Colville, Whitaker, Chen, Bailly and Kranner2010; Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010; Varghese et al., Reference Varghese, Sershen, Berjak, Varghese and Pammenter2011). The difference between ROS activity and developmental events on the one hand, and ROS activity and damage on the other, is a matter of strict antioxidant control, or its breakdown (e.g. Foyer and Noctor, Reference Foyer and Noctor2005; Kranner and Birtić, Reference Kranner and Birtić2005; Bailey-Serres and Mittler, Reference Bailey-Serres and Mittler2006; Van Breusegem et al., Reference Van Breusegem, Bailey-Serres and Mittler2008).
The relatively low production of ∙O2– initially recorded for undried axes of S. gerrardii after 30 min immersion in the CaMg solution (○, Fig. 5) was in agreement with the decline in level 30 min after excision, as shown in Fig. 3, which records the time course of ∙O2– production following excision. Immediately prior to being plated on the recovery medium [zero time (first datum points) Fig. 5] irrespective of whether immersed in the CaMg solution or cathodic water, S. gerrardii axes flash dried to water content ~0.4 g g− 1, showed elevated ∙O2– levels relative to undried axes [Fresh+CaMg, (○) Fig. 5]. Both undried (fresh) axes and those rehydrated in CaMg solution after dehydration exhibited a burst of ∙O2– after 4 h in culture (Fig. 5). In contrast, after 4 h in culture, not only was this burst absent in axes rehydrated with cathodic water, but ∙O2– level was significantly lower that that recorded for similarly treated axes at zero time in culture (■, Fig. 5). These observations imply that a second burst of ∙O2– occurred, i.e. in addition to the excision-related burst [which subsided within 30 min (Fig. 3)], within 4 h in culture of the undried and dried axes rehydrated in the CaMg solution. As this second ∙O2– burst was significantly higher for axes that had been partially dehydrated before CaMg-rehydration, it is probable that it reflects a combined stress response to both excision and drying, but to excision alone for the undried axes. These observations are in agreement with those of Roach et al. (Reference Roach, Ivanova, Beckett, Minibayeva, Green, Pritchard and Kranner2008), who suggested that the pattern of ∙O2– production by desiccation-sensitive Castanea sativa axes is the product of a complex interaction between excision and subsequent drying.

Figure 5 Extracellular superoxide production during various stages of in vitro recovery in freshly excised and partially dried embryonic axes of Strychnos gerrardii. Freshly excised axes (○) were immersed in the CaMg solution for 30 min prior to plating out on germination medium. Partially dried axes (water content ~0.4 g g− 1) were rehydrated for 30 min in either the CaMg solution (△) or cathodic water (■) prior to being set out to germinate. Zero time readings refer to material rehydrated in either CaMg solution or cathodic water, prior to placing on germination medium. Points represent the mean of three replicates of five axes each. Vertical bars represent mean ± SD. Superoxide levels were tested for significant differences across treatments, within time intervals by one-way ANOVA and means were separated using LSD post-hoc test, as given in the table beneath the figure. Treatments with different letters in each column differ significantly at the given P value.
The effect of cathodic water in terms of reduced ∙O2– production by partially dried axes was not immediately apparent [zero time (■), Fig. 5], but was manifested after 4 h in culture. This suggests that ∙O2– was not quenched at source, but that subsequent antioxidant activity was displayed. While this could have been a direct effect of the cathodic water, as suggested by Shirahata et al. (Reference Shirahata, Kabayama, Nakano, Miura, Kusumoto, Gotoh, Hayashi, Otsubo, Morisawa and Katakura1997), it is likely that the activity of endogenous antioxidants was enhanced (Hanaoka, Reference Hanaoka2001).
Initiation of axis elongation of S. gerrardii occurred between 24 and 48 h in culture (results not shown). Accompanying this phase, ∙O2– levels rose significantly in axes that had not been dried (○), and in those partially dried axes which had been rehydrated in cathodic water (■), relative to those rehydrated in the CaMg solution (△) (Fig. 5). Noting that exposure to either the CaMg solution or cathodic water was confined to a 30-min period prior to axis culture, it is probable that ∙O2– production after 48 h was indicative of the normal role of ROS during germination. These results are in agreement with those of others, which showed elongation during germination and seedling establishment to be associated with controlled activity of ROS, including ∙O2– (Liszkay et al., Reference Liszkay, van der Zalm and Schopfer2004; Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009; Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010b). The 48-h peak in ∙O2– (Fig. 5) was highest for undried axes, probably because they had not been subjected to any desiccation stress.
What is particularly relevant is that shoot production was promoted after both undried and partially dried axes had been exposed to cathodic water, while no shoots were produced by undried or CaMg-rehydrated axes which had been excised from 7-d-stored S. gerrardii seeds (Fig. 6a; specimens on the left of Fig. 7a, b). Additionally, root production by undried axes and those flash dried to ~0.4 g g− 1 was enhanced by immersion in cathodic water (Figs 6a, 7a, 7b). Following the observations of Goveia et al. (Reference Goveia, Kioko and Berjak2004) that the state of recalcitrant axis development may be an important factor in responses to excision, presently axes were excised from a batch of S. gerrardii seeds that had been wet-stored for 35 d. The beneficial effects of cathodic water were even more apparent in terms of both shoot and root production by these axes (Fig. 6b). These observations suggest that cathodic water had both protective and promotive effects in alleviating consequences of stress-related ROS generation.

Figure 6 Percentage of Strychnos gerrardii axes producing a root and shoot after excision only, and excision followed by partial dehydration and partial dehydration and freeze thawing (cryo). Axes were excised from seeds stored hydrated for either (a) 7 or (b) 35 d. Partially dried and cryopreserved embryos were rehydrated with CaMg solution or cathodic water before in vitro recovery. Means are based on three replicates of ten axes each. One-sample T-test was used to test for inter-treatment differences in root and shoot production for axes excised from seeds stored for 7 d (roots: P < 0.001; shoots: P = not significant) and 35 d (roots: P < 0.001; shoots: P < 0.05).

Figure 7 Seedlings formed by variously treated axes of Strychnos gerrardii excised from 7-d-stored seeds are illustrated after 30 d recovery in vitro. Freshly excised axes exposed to: (a) CaMg solution (left) and cathodic water (right) immediately after excision; (b) partial dehydration and subsequent rehydration with CaMg solution (left) and cathodic water (right); and (c) partial dehydration, rapid cooling and subsequent thawing and rehydration with CaMg solution (two specimens, left) and cathodic water (two specimens, right). Note that although callus formed on the hypocotyl surface in both cases, the appearance and relative amounts were different in relation to the thawing/rehydration medium. Scale bar = 10 mm.
These positive effects of cathodic water were manifest after cryopreservation of axes of S. gerrardii at water content ~0.4 g g− 1, when it was used as the thawing and rehydration medium (Figs 6a, 6b, 7c). After retrieval from cryostorage and thawing and rehydration in cathodic water, roots and shoots, respectively, were produced by 55 and 35% of axes excised from 7-d-stored seeds (Fig. 6a; specimens on the right of Fig. 7c). When seeds had been wet-stored for 35 d, 83% of the axes produced roots, and shoots were developed by 70% (Fig. 6b). Whitaker et al. (Reference Whitaker, Beckett, Minibayeva and Kranner2010) showed that pronounced production of ∙O2– accompanied rehydration of axes of T. dregeana retrieved from cryostorage, none of which survived. Although shortage and seasonality of S. gerrardii seeds precluded assessment of ∙O2– production after cryopreservation in the present study, it is highly likely that similar ROS production occurs, considering the similar patterns of ∙O2– evolution upon excision and drying of axes of this species and those recorded by Whitaker et al. (Reference Whitaker, Beckett, Minibayeva and Kranner2010) for T. dregeana. The most likely explanation, therefore, for the ameliorative effects of cathodic water as a thawing and rehydration medium resides in its recorded ability to counteract ROS-mediated oxidative stress (Shirahata et al., Reference Shirahata, Kabayama, Nakano, Miura, Kusumoto, Gotoh, Hayashi, Otsubo, Morisawa and Katakura1997; Hanaoka, Reference Hanaoka2001; Hiraoka et al., Reference Hiraoka, Takemoto, Suzuki, Shinohara, Chiba, Shirao and Yoshimura2004).
The beneficial effects of exposure of axes to cathodic water after dehydration and following cryopreservation continued to be manifest in terms of the biomass of seedlings harvested after 30 d in vitro. Seedlings developed from axes that had been exposed to cathodic water after partial drying only, or cryopreservation after dehydration, were characterized by significantly higher biomass than those recovered in the CaMg solution (Fig. 8). It is presently difficult to explain why the highest biomass recorded was for seedlings produced in vitro from cryopreserved axes after exposure to cathodic water (Fig. 8), except perhaps in terms of more vigorous growth in combination with the callus that had formed (Fig. 7c).

Figure 8 Biomass of Strychnos gerrardii axes (n = 10) after excision only, and excision followed by partial dehydration and partial dehydration and freeze thawing (cryo). Data were tested for significant differences across treatments by one-way ANOVA and means were separated by LSD post-hoc test. Bars labelled with different letters differ significantly when compared across treatments (P < 0.001).
Assessment of total antioxidant status
Ideally, this assay should have been carried out on axes of S. gerrardii, but the limitation of seed numbers made this presently impossible. However, similar studies to these reported here for S. gerrardii are currently under way with axes of Boophane disticha. The studies with B. disticha have provided evidence of enhanced antioxidant capacity of excised embryonic axes following exposure to cathodic water relative to the CaMg solution, and hence are justified to be reported here (Fig. 9). The enhancement in antioxidant capacity shown by B. disticha was manifest 4 h after the axes were cultured and not immediately after the 30-min exposure to cathodic water. This coincides with the lowest ∙O2– emission by the S. gerrardii axes after 4 h in culture, when they had previously been exposed to cathodic water for 30 min (Fig. 5). This argues that the positive effects of cathodic water on the explants were most likely to have been the consequence of enhancement of activity of endogenous antioxidants, as proposed by Hanaoka (Reference Hanaoka2001).

Figure 9 Total antioxidant potential of fresh and partially dried axes (grey and black histogram bars, respectively) of Boophane disticha during in vitro recovery after having been immersed in CaMg solution or cathodic water. Each point represents mean of six replicates. Bars represent mean ± SD. Total antioxidant levels were tested for significant differences across treatments by one-way ANOVA and means were separated by Scheffe's post-hoc test. Bars labelled with different letters differ significantly when compared across treatments (P < 0.001).
Concluding considerations
Bursts of ROS typified by ∙O2– production have been shown to accompany wounding of seedling roots (Minibayeva et al., Reference Minibayeva, Kolesnikov, Chasov, Beckett, Lüthje, Vylegzhanina, Buck and Böttger2009), axis excision from recalcitrant seeds of various species (Pammenter et al., Reference Pammenter, Berjak, Goveia, Sershen, Kioko, Whitaker and Beckett2011) and excision and dehydration of recalcitrant axes of Antiaris toxicaria (Cheng and Song, Reference Cheng and Song2008) and Castanea sativa (Roach et al., Reference Roach, Ivanova, Beckett, Minibayeva, Green, Pritchard and Kranner2008). It has been shown that uncontrolled ROS bursts occurring repeatedly during procedures for recalcitrant embryonic axis cryopreservation – as is the case upon excision, flash drying, and during thawing and rehydration – are associated with lethal consequences (Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010).
Conceptually, there are two approaches to modulating the damaging effects of ROS bursts: treatments to reduce their production, or the exogenous application of antioxidants which may exert a direct effect, or may operate by enhancing endogenous antioxidant activity. Although not in the context of cryopreservation, numerous references in the literature describe evaluation of medicinal plant extracts for antioxidant activity (e.g. Hazra et al., Reference Hazra, Sarkar, Mandal, Biswas and Mandal2009), or application of such extracts to animal systems, particularly liver (e.g. Tripathi et al., Reference Tripathi, Mohan and Kamat2007; Samarth et al., Reference Samarth, Panwar, Kumar, Soni, Kumar and Kumar2008; Gutiérrez et al., Reference Gutiérrez, Alvarado, Presno, Pérez-Veyna, Serrano and Yahuaca2010), and Masella et al. (Reference Masella, Di Benedetto, Varì, Filesi and Giovannini2005) suggested that dietary polyphenols could stimulate antioxidant and detoxification defence systems. Beneficial effects in terms of modulation of oxidative stress were reported in all those cases. The work of Sirdaarta and Cock (Reference Sirdaarta and Cock2008) showed that pre-treatment of Artemia franciscana nauplii with low doses of vitamin E and Trolox™ (vitamin E analogue) reduced the toxicity of Aloe barbadensis juice, suggestedly by protecting against oxidative stress induced by components in the juice. As an example of a different approach, work with animal cells showed that derivatized (and hence water-soluble) fullerenes (hollow nano-particles comprised of 60 or more carbon atoms), and particularly those of 82 carbon atoms incorporating gadolinium, are potent scavengers of the spectrum of ROS, with the authors emphasizing the biomedical potential of such fullerenes (Yin et al., Reference Yin, Lao, Fu, Warner, Zhao, Wang, Qiu, Sun, Xing, Dong, Liang and Chen2009).
In a plant system, application of low concentrations of desferrioxamine [which sequesters iron, thus interfering with ∙OH generation from H2O2 (Fenton reaction)] in a cation-reduced medium prior to, and after, cryopreservation, promoted post-thaw recovery of rice cells (Benson et al., Reference Benson, Lynch and Jones1995). Cassandra et al. (Reference Cassandra, Benson, Berjak, Goveia and Pammenter2011) have shown that shoot production was significantly promoted in the case of excised zygotic embryonic axes of T. dregeana and P. longifolia, by treatment with 1% (v/v) DMSO, a potent ∙OH scavenger (Benson et al., Reference Benson, Harding, Johnston, Day and Stacey2007) prior to complete severing of cotyledons, followed by 30 min post-excision soaking in this solution. In the case of T. emetica, shoots were produced by a greater proportion of axes if post-excision exposure to DMSO was omitted. The effects of prior application of DMSO have also been found to improve recovery of shoot tips significantly after cryopreservation (Benson et al., Reference Benson, Harding, Johnston, Day and Stacey2007).
As shown in the present study, exposure of S. gerrardii axes to cathodic water successfully promoted shoot development (Figs 6 and 7), which we had not been able to achieve previously for similar explants excised from seeds of tropical and subtropical species [including those of S. gerrardii (Goveia, Reference Goveia2007)] – except when DMSO was used (Cassandra et al., Reference Cassandra, Benson, Berjak, Goveia and Pammenter2011) as described above. In the present study, shoot production was associated with a reduction in the ROS burst, as indicated by ∙O2– levels 4 h after axes were placed in culture. Current work on the other species, B. disticha, showed that relative to axes immersed in the CaMg solution, after exposure to cathodic water a significant increase in total antioxidant activity occurred in both fresh and dried axes after 4 h in culture (Fig. 9).
In view of the role of ROS in germination and seedling development (Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009; Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010b), the timing of the use of cathodic water must be carefully controlled – as must be any treatment that might modulate ROS activity of excised embryonic axes. In the present study, axes were exposed to cathodic water for just 30 min following procedures known to generate damaging oxidative bursts. While this treatment significantly lowered ∙O2– levels in the early stages of axis recovery in culture, it appeared not to have had any adverse effects later, when elevated ∙O2– levels (Fig. 5) were correlated with visible axis elongation after 48 h in vitro. This view is supported by the data for root and shoot production (Fig. 6) and total biomass (Fig. 8), even after retrieval from cryopreservation.
There is general agreement that the ∙O2– burst is the consequence of activity of apoplastic peroxidases (ECPOX) (Minibayeva et al., Reference Minibayeva, Kolesnikov, Chasov, Beckett, Lüthje, Vylegzhanina, Buck and Böttger2009; Roach et al., Reference Roach, Beckett, Minibayeva, Colville, Whitaker, Chen, Bailly and Kranner2010; Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010), which exhibit such activity when extracellular conditions tend towards alkalinity (pH ~7.2) associated with influxes of Ca2+ and protons and efflux of K+ (Bolwell et al., Reference Bolwell, Bindschedler, Blee, Butt, Davies, Gardner, Gerrish and Minibayeva2002). However, the effects of a substantially elevated pH (~11.2, as provided by the use of cathodic water) were presently found to reduce ∙O2– production. While four classes of ECPOX have been found to be associated with cell walls of T. dregeana axes, two of these, loosely bound by hydrogen and ionic binding, respectively, were associated with most of the stress-related ∙O2– production associated with procedures for cryopreservation (Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010). While the effects of extremely elevated pH on the ECPOX presently remain to be ascertained, one may speculate that these enzymes, which appear to have pH optima at or around 7.2 (Bolwell et al., Reference Bolwell, Bindschedler, Blee, Butt, Davies, Gardner, Gerrish and Minibayeva2002), are inactivated. However, the present data indicate that after 15 min of flash drying, similar levels of ∙O2– are produced whether axes were imbibed for 30 min with the CaMg solution or with cathodic water (Fig. 5), which does not support this contention.
However, cathodic water has been shown to have strongly antioxidative effects, whether by direct scavenging of a variety of ROS (Shirahata et al., Reference Shirahata, Kabayama, Nakano, Miura, Kusumoto, Gotoh, Hayashi, Otsubo, Morisawa and Katakura1997) or by selectively scavenging H2O2, but generally acting to enhance the activity of (endogenous) antioxidants (Hanaoka, Reference Hanaoka2001). In this regard, the latter author showed that cathodic (reduced) water significantly increased the antioxidant activity of ascorbic acid (AsA) in terms of ∙O2– dismutation in an in-vitro system. Lee et al. (Reference Lee, Kim, Ryoo, Lee and Park2006) reported that human lymphocyte resistance to H2O2-induced strand breaks of DNA was enhanced when the cells were exposed to cathodic water. Those authors also provided evidence that the antioxidative activity of AsA in reduced water was approximately threefold higher than when dissolved in non-electrolysed, de-ionized water.
Plants contain high concentrations of AsA which is described as the most abundant and powerful of antioxidants (Gill and Tuteja, Reference Gill and Tuteja2010), located intracellularly and apoplastically (Shao et al., Reference Shao, Chu, Lu and Kang2008). It is thus suggested that the basis of the efficacy of cathodic water in counteracting ROS-associated damage during the procedures preceding and following cryostorage of embryonic axes, as presently demonstrated for S. gerrardii, might include substantially enhanced activity of AsA. Ascorbic acid is a water-soluble redox buffer (Foyer and Noctor, Reference Foyer and Noctor2005), which reacts readily with ∙O2–, ∙OH and 1O2 (Halliwell and Gutteridge, Reference Halliwell and Gutteridge1999) forming monodehydroascorbate (MDHA) and then dehydroascorbate (DHA) from which AsA must be rapidly regenerated. This generally proceeds in vivo via the AsA–GSH cycle at the expense of NADPH (Kranner and Birtić, Reference Kranner and Birtić2005). However, in the strongly reducing milieu of cathodic water as presently applied to axes after cryopreservation, AsA regeneration may be promoted spontaneously.
While a spectrum of questions that require investigation has been generated by the present study, there is no doubt about the potential of cathodic water to ameliorate stress responses related to cryopreservation procedures and, potentially, those associated with a variety of other in-vitro procedures with plant tissues.
Acknowledgements
This work is based upon research supported by the National Research Foundation (South Africa). Thanks are due to the University of KwaZulu-Natal, for providing a stimulating research atmosphere, and colleagues, especially Richard Beckett, for acting as a sounding board.