Significant outcomes
• The cognitive subtrait of impulsivity is associated with D2/3 receptor availability in the associative striatum that plays a critical role in cognitive processes involving attention to detail, judgement of alternative outcomes, and inhibitory control.
Limitations
• The baseline measures of D2/3 receptor availability do not necessarily account for occupancy of the receptor by endogenous dopamine, and studies using the acute depletion of dopamine are needed.
• There were fewer male than female participants, which did not allow for a full analysis of gender effects.
Introduction
Impulsivity is a multifactorial and heterogeneous concept describing a wide range of behaviours that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation, and that often result in undesirable outcomes (Reference Evenden1). It is characterised by deficits in the ability to delay immediate gratification for future rewards and is associated with behavioural disinhibition and lack of planning (Reference Lee, London and Poldrack2,Reference Chamorro, Bernardi and Potenza3). Pathological impulsivity has been implicated in various psychiatric disorders including personality disorders (Reference Buckholtz, Treadway and Cowan4), drug dependence (Reference Volkow, Wang, Fowler, Tomasi and Telang5), and attention-deficit hyperactivity disorder (Reference Tomasi and Volkow6). Individuals differ greatly in the extent of their impulsivity traits and related personality constructs, and such trait differences represent a predisposition to these disorders.
On the basis of the neurobiological concept of personality, core personality traits such as impulsivity may be heritable and are strongly associated with intrinsic properties of the monoamine neurotransmitter systems in the brain (Reference Zuckerman and Kuhlman7). Previous animal studies using behavioural tasks in specific rat strains have shown that dopamine D2/3 receptors play a critical role in impulsive behaviour (Reference Dalley, Fryer and Brichard8,Reference van Gaalen, van Koten, Schoffelmeer and Vanderschuren9). Although it is unclear whether animal models using experimental designs are equivalent to the personality trait of impulsivity in humans and whether there are differences between species, individual differences in dopamine D2/3 receptor density may be postulated to underlie the construct of self-reported impulsiveness in humans.
However, thus far, only a few studies, if any, have directly examined the relationship between dopamine D2/3 receptor density and self-reported impulsiveness in healthy human subjects, and the results are inconclusive. In one previous positron emission tomography (PET) study using [18F]fallypride, a negative relationship between striatal D2/3 receptor availability and self-reported impulsiveness measured with the Barratt Impulsiveness Scale (BIS-11) (Reference Patton, Stanford and Barratt10) was found at a weaker statistical threshold (Reference Lee, London and Poldrack2). In two other PET studies, the BIS-11 scores were negatively associated with D2/3 receptor density measured with [18F]fallypride in the substantia nigra and ventral tegmental area (Reference Buckholtz, Treadway and Cowan4), or were positively associated with ventral striatal D2/3 receptor availability measured with [11C]raclopride (Reference Reeves, Polling and Stokes11). Using a different self-reported measure of impulsivity, Oswald et al. (Reference Oswald, Wong and Zhou12) found no association between impulsivity and baseline striatal D2 receptor binding; however, they found that high impulsivity was associated with blunted right-striatal dopamine release. Related but not identical personality traits – venturesomeness, novelty seeking, and sensation seeking – were also examined using PET with different radiotracers, and the results were not consistent (Reference Bernow, Yakushev and Landvogt13–Reference Gjedde, Kumakura, Cumming, Linnet and Møller16).
Considering that impulsivity is a multidimensional construct encompassing cognitive, emotional, and behavioural aspects (Reference Evenden1), it is not surprising that the results of previous human studies are inconsistent. Moreover, it is largely unknown whether particular aspects of human impulsiveness are associated with dopamine receptor availability in specific regions of the striatum that consists of several anatomic and functional subdivisions (Reference Reeves, Polling and Stokes11,Reference Mawlawi, Martinez and Slifstein17,Reference Martinez, Slifstein and Broft18). Hence, in the present study, we investigated the relationship between self-reported cognitive impulsiveness and dopamine D2/3 receptor availability in striatal subdivisions among healthy subjects, using high-resolution PET with [11C]raclopride to better characterise the role of dopamine postsynaptic receptors in specific constructs of impulsivity. In particular, we investigated the link between them after controlling for the effects of cardinal temperament characteristics that are conceptually or empirically related to dopamine (Reference Cloninger, Svrakic and Przybeck19,Reference Yasuno, Suhara and Sudo20).
Materials and methods
Subjects
The data were collected from subjects who participated in a previous study on the relationship between harm avoidance and dopamine D2/3 receptor availability (Reference Kim, Son and Kim21). The subjects were studied as part of ongoing investigations of individual differences in neuroreceptor availability. Details of the study procedure have been described elsewhere (Reference Kim, Son and Kim21). Twenty-one healthy normal subjects [eight men, 13 women; mean age: 34.6 (SD = 8.8) years; mean years of education: 14.8 (SD = 1.5)] participated in the study after giving their written informed consent. Subjects underwent complete medical, neurological, and psychiatric examinations to ensure the absence of diseases. The psychiatric evaluation included lifetime and current DSM-IV diagnoses that were determined by the Structured Clinical Interview for DSM-IV Disorders (Reference First, Spitzer, Gibbon and Williams22). None of the subjects were taking any medication known to affect dopaminergic neurotransmission. All subjects had normal 3-T magnetic resonance imaging (MRI) scans as evaluated by a radiologist. The study protocol was approved by the Institutional Review Board, and all procedures used in the study were performed in accordance with international ethical standards, Declaration of Helsinki.
Assessment of impulsivity
The personality trait of impulsiveness was measured using the BIS-11 (Reference Patton, Stanford and Barratt10), which is a widely used 30-item self-administered questionnaire that assesses control of thoughts and behaviour. Each of the items is scored on a four-point Likert-type scale (1 = rarely/never and 4 = almost always/always), representing increasing levels of impulsivity. The BIS-11 consists of three subscales: non-planning impulsiveness, motor impulsiveness, and attentional impulsiveness. To control for the potentially confounding effects of general temperament characteristics that are conceptually or empirically related to dopamine, the Temperament and Character Inventory (TCI) (Reference Cloninger, Svrakic and Przybeck19) was administered for measuring the biogenetic temperament of harm avoidance and novelty seeking. The TCI is a 240-item self-rating questionnaire developed on the basis of the psychobiological model of temperament and character (Reference Cloninger, Svrakic and Przybeck19). In the present study, we used the subscale scores for harm avoidance and novelty seeking, which have been reported to be associated with dopamine receptors in human PET studies (Reference Zald, Cowan and Riccardi15,Reference Gjedde, Kumakura, Cumming, Linnet and Møller16,Reference Yasuno, Suhara and Sudo20).
High-resolution PET and MRI imaging
All subjects completed PET scanning using the high-resolution research tomograph (HRRT) system (Siemens Molecular Imaging, Knoxville, TN, USA). Emission data were collected as listmode data in the three-dimensional (3D) mode during the 60 min after [11C]raclopride injection. The tracer [11C]raclopride was synthesised as previously described by methylation of the desmethyl precursor using [11C]iodomethane (Reference Fei, Mock and DeGrado23). A saline solution of 527.25 (SD = 37.00) MBq [11C]raclopride with a specific activity at time of injection of 62.16 (SD = 24.42) GBq/μmol was injected as a bolus into an intravenous line. Transmission scans using 137Cs were used to correct for attenuation of the emission scans. The 3D ordinary Poisson ordered-subset expectation maximisation algorithm accelerated by the parallelised computations (Reference Hong, Chung and Kim24) was used for the reconstruction. The 19 frames (2 × 30 s, 4 × 60 s, 2 × 90 s, 2 × 210 s, 9 × 300 s) were reconstructed from the listmode data. The 207 planes covering an axial field of view of 25.2 cm (axial sampling of 1.22 mm) were generated for each time frame. The in-plane and axial resolutions were 2.52 and 2.57 mm full-width at half-maximum at the centre of the field of view, respectively. Attenuation, scatter, and decay corrections were carried out and applied for each frame.
To achieve accurate delineation of the brain regions for data analysis, each subject underwent an MRI scan using a 3-T scanner (Magnetom Verio; Siemens, Munich, Germany). A magnetisation-prepared rapid acquisition gradient echo 3D T1-weighted sequence with 1 mm thickness was performed. The MRI scan of each subject was co-registered to his or her PET scan using statistical parameter mapping software (SPM8). Spatial normalisation of the co-registered MRI images of each subject was performed on the Montreal Neurological Institute template using SPM8, and the estimated transform was applied to the corresponding PET images. Regions of interest (ROIs) were drawn on each individual's MRI. The striatum was divided into five anatomic ROIs, such as the ventral striatum (VST), the pre-commissural dorsal caudate (pre-DCA), the pre-commissural dorsal putamen, the post-commissural caudate (post-CA), and the post-commissural putamen (post-PU), following the guidelines specified in the studies by Mawlawi et al. (Reference Mawlawi, Martinez and Slifstein17) and Martinez et al. (Reference Martinez, Slifstein and Broft18). Figure 1 shows representative MRI and PET images indicating the precise location of the striatal ROIs. The cerebellum was used as the region of reference for the analysis without arterial blood sampling. Time-activity curves were generated for each ROI by averaging the dynamic PET images, which were co-registered to the corresponding MRI images. Activities from the left and right regions were averaged. The distribution volume ratio (DVR) for [11C]raclopride was obtained on the basis of the Logan graphical method (Reference Logan, Fowler and Volkow25). The DVR is linearly related to the binding potential with respect to the non-displaceable compartment (BPND) and the relationship between DVR and BPND is as follows: DVR = BPND + 1 (Reference Joshi, Fessler and Koeppe26).

Fig. 1 Representative magnetic resonance imaging and positron emission tomography images showing the precise location of the striatal regions of interest (ROIs), that is, the pre-commissural dorsal caudate (pre-DCA), the pre-commissural dorsal putamen (pre-DPU), the ventral striatum (VST), the post-commissural caudate (post-CA), and the post-commissural putamen (post-PU). Averaged distribution volume ratios (DVRs) within the ROIs were obtained for use in data analysis.
Statistical methods
To evaluate the relationship between self-reported impulsiveness and D2/3 receptor availability in striatal subdivisions after controlling for the effects of cardinal temperament characteristics that are conceptually or empirically related to dopamine, Pearson's partial correlation analysis was performed between BIS-11 scores and D2/3 receptor availability in subregions of the striatum, controlling for the influence of the harm avoidance and novelty seeking scores measured by the TCI. The level of statistical significance was defined as p < 0.05 (two-tailed).
Results
The mean BIS-11 scores were 18.5 (SD = 3.2; range: 13–25) on the non-planning impulsiveness subscale, 13.7 (SD = 2.3; range: 9–18) on the motor impulsiveness subscale, and 14.0 (SD = 2.3; range: 10–18) on the attentional impulsiveness subscale. The mean BIS-11 total score was 46.2 (SD = 6.1; range: 35–60). With regard to the relationship between demographic variables and dopamine D2/3 receptor availability, age showed a significant negative correlation with the DVR value of [11C]raclopride in the whole striatum (r = −0.46, p = 0.04) and in the post-PU (r = −0.48, p = 0.03). No significant correlations were observed between gender and [11C]raclopride DVR values in any subregion (ρ = −0.03 to 0.21, p > 0.05). The [11C]raclopride DVR values also had no significant correlations with years of education (r = 0.06 to 0.37, p > 0.05) or smoking status (ρ = −0.26 to 0.01, p > 0.05).
The BIS-11 scores were not significantly correlated with age (non-planning: r = 0.15, p = 0.50; motor: r = −0.01, p = 0.97; attentional: r = 0.26, p = 0.26), gender (non-planning: ρ = 0.17, p = 0.46; motor: ρ = 0.23, p = 0.33; attentional: ρ = 0.34, p = 0.14), or years of education (non-planning: r = −0.17, p = 0.45; motor: r = −0.24, p = 0.29; attentional: r = −0.35, p = 0.12). Regarding the relationship between the BIS-11 scores and the temperament characteristics assessed by the TCI, there was a tendency towards positive correlation between motor impulsiveness and novelty seeking (r = 0.37, p = 0.09).
Table 1 presents the [11C]raclopride DVR for each subregion of the striatum in high and low BIS-11 score groups, which were divided on the basis of the median value of the BIS-11 total score for all subjects. Mann–Whitney U-test showed that the DVR value of [11C]raclopride in the pre-DCA was significantly higher in the high BIS-11 score group than in the low score group (p = 0.04). To evaluate the relationship between impulsiveness and D2/3 receptor availability in striatal subdivisions, Pearson's partial correlation analysis was performed, controlling for the influence of age and temperament scores on the TCI. The analysis revealed that the non-planning and attentional impulsiveness subscale scores of the BIS-11 had significant positive correlations with the DVR value of [11C]raclopride in the pre-DCA (non-planning: r = 0.65, p = 0.004; attentional: r = 0.61, p = 0.007) (Table 2, Fig. 2). There was a tendency towards positive correlation between the non-planning impulsiveness score and the [11C]raclopride DVR value in the post-CA (r = 0.43, p = 0.075; Table 2). No significant correlations were observed between the motor impulsiveness score and the [11C]raclopride DVR values in any subregion (Table 2).
Table 1 [11C]raclopride DVRs in striatal subdivisions
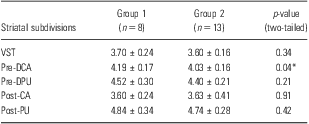
BIS, Barratt Impulsiveness Scale; DVR, distribution volume ratio; post-CA, post-commissural caudate; post-PU, post-commissural putamen; pre-DCA, pre-commissural dorsal caudate; pre-DPU, pre-commissural dorsal putamen; VST, ventral striatum.
Values are presented as mean ± standard deviation. The p-values are for Mann–Whitney U test. Group 1 represents a high BIS-11 score group. Group 2 represents a low BIS-11 score group.
*p < 0.05.
Table 2 Correlation coefficients between the BIS-11 scores and the [11C]raclopride DVR values in striatal subregions, controlling for age and TCI scores
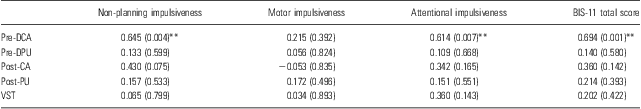
BIS, Barratt Impulsiveness Scale; DVR, distribution volume ratio; post-CA, post-commissural caudate; post-PU, post-commissural putamen; pre-DCA, pre-commissural dorsal caudate; pre-DPU, pre-commissural dorsal putamen; TCI, Temperament and Character Inventory; VST, ventral striatum.
The p values are presented in parentheses.
**p < 0.01.

Fig. 2 Scatter plots showing correlations between the non-planning and attentional impulsiveness scores on the Barratt Impulsiveness Scale (BIS-11) and the [11C]raclopride distribution volume ratios (DVRs) in the pre-commissural dorsal caudate (pre-DCA).
Discussion
In the present study, we found that the non-planning and attentional impulsiveness subscale scores on the BIS-11 had significant positive correlations with D2/3 receptor availability in the pre-DCA. The non-planning impulsiveness score also tended to be positively correlated with D2/3 receptor availability in the post-CA at a weaker threshold. On the basis of the organisational scheme of the striatum, the pre-DCA and post-CA constitute a large and important part of the associative striatum (Reference Haber and McFarland27). Therefore, the results of the present study suggest that the cognitive subtrait of impulsivity is strongly associated with D2/3 receptor availability in the associative striatum. To the best of our knowledge, this is the first report using high-resolution PET on the association of the cognitive subtrait of impulsivity with dopamine receptor availability in specific striatal subregions.
Previous studies have emphasised cognitive aspects of impulsivity, in that individual differences in impulsivity reflect differences in the mechanisms of allocating attention (Reference Evenden1,Reference Dickman and Meyer28,Reference Stanford, Mathias and Dougherty29). Dickman and Meyer (Reference Dickman and Meyer28) suggested that insufficient focusing of attention or concentration leads to impulsivity, and Barratt (Reference Barratt30) proposed the concept of the planning subtrait of impulsivity, which involves the process of attention to detail. Moreover, it was reported that individuals with executive dysfunction scored higher on the BIS-11 (Reference Whitney, Jameson and Hinson31) and that the BIS-11 score was significantly associated with measures of cognitive dysfunction, which were also correlated with anterior cingulate cortical activation (Reference Garavan, Ross, Murphy, Roche and Stein32). The activation of dorsolateral prefrontal cortex and anterior cingulate cortex is crucial in tasks that require inhibition of automatic responses (Reference Stanford, Mathias and Dougherty29). On the other hand, the pre-DCA and post-CA are the critical striatal subregions that have reciprocal connections with the dorsolateral prefrontal cortex, the internal segment of the globus pallidus, and the ventral anterior thalamic nuclei, forming an associative neural circuit and serving segregated as well as integrative cognitive (Reference Abi-Dargham33,Reference Kegeles, Abi-Dargham and Frankle34). Therefore, our results suggest that the cognitive subtrait of impulsivity, as measured by the BIS-11, is associated with D2/3 receptor availability in the associative striatum that plays a pivotal role in cognitive processes involving attention to detail, judgement of alternative outcomes, and inhibitory control.
The results of the present study are partly in line with those of the previous study reporting that non-planning impulsivity, as measured by the BIS-11, was significantly positively correlated with the [11C]raclopride BPND in the limbic striatum (Reference Reeves, Polling and Stokes11). Because our study used high-resolution PET instrumentation (i.e., HRRT), which has the highest resolution and uses the specific reconstruction algorithm to preserve the high-intrinsic spatial resolution and to account for the scanner's specific geometry (Reference Hong, Chung and Kim24), the method of the present study seems quite adequate for an analytical approach that captures the precise subregions of the striatum. In our statistical analyses, we controlled for the confounding effects of temperament characteristics that are conceptually or empirically related to dopamine, although we did not include the measure for the level of socially desirable responding, which was adjusted for in the previous study (Reference Reeves, Polling and Stokes11).
The outcome measure used for receptor availability represents a combined parameter of the concentration of receptors available to bind with radiotracer and the affinity of the radiotracer for the receptor. Therefore, higher D2/3 receptor availability in individuals with higher scores on the BIS-11 could result from either an increase in D2/3 receptor density or greater affinity of the [11C]raclopride for the D2/3 receptor. However, significant individual differences in the affinity of the D2/3 receptor for an antagonist such as raclopride are unlikely. Another possibility is lower baseline synaptic dopamine levels occupying fewer D2/3 receptors, resulting in higher D2/3 availability among subjects with higher scores on the BIS-11. Studies using the acute depletion of dopamine are needed to address this issue (Reference Martinez, Orlowska and Narendran35).
In our study, if higher D2/3 receptor availability in the associative striatum results from increased D2/3 receptor expression rates in that specific region as a genuine trait, it seems that high levels of cognitive impulsivity may be associated with increased dopamine transmission in the associative striatum. This speculation is in line with recent reports that higher levels of impulsivity were associated with greater amphetamine-induced dopamine release in the striatum (Reference Buckholtz, Treadway and Cowan4). This is also in agreement with the suggestion that persistently elevated striatal dopaminergic transmission may promote the development of pathological impulsive behaviours and that impulsivity is associated with cortico-striatal dysfunction (Reference Dagher and Robbins36). Moreover, prospective studies have reported an association between impulse control disorders and the use of dopamine agonists in patients with Parkinson's disease (Reference Weintraub37).
Although there was a significant relationship between impulsiveness and D2/3 receptor availability in the present study, the correlation coefficients were somewhat modest and the predictive value was not high. Therefore, other mechanisms are thought to be involved in impulsivity, which include other neurotransmitter systems such as serotonin and noradrenaline (Reference Dalley, Mar, Economidou and Robbins38).
The interpretation of the results of the present study should be considered in light of some limitations. The PET data were not corrected for partial volume effects. However, we used the HRRT system, which has been reported to consistently improve the quantification of dopamine transmission parameters owing to a reduced partial volume effect compared with conventional scanners (Reference Varrone, Sjöholm and Eriksson39). This improvement was reported to be more important in small brain structures (Reference Leroy, Comtat and Trébossen40). In a recent study using HRRT, the BPND values were found to be consistent before and after partial volume correction and it was suggested that partial volume correction may not be necessary with HRRT in healthy subjects (Reference Uchida, Chow and Mamo41). As our HRRT-PET imaging system has the highest resolution and the point spread function reconstruction capability, the present data may have a smaller partial volume effect than those acquired with other PET systems. In our study, there were fewer male than female participants, which did not allow for a full analysis of gender effects.
In conclusion, the results of the present study suggest that the cognitive subtrait of impulsivity is associated with D2/3 receptor availability in the associative striatum that plays a critical role in cognitive processes involving attention to detail, judgement of alternative outcomes, and inhibitory control. These results also suggest that particular aspects of human impulsiveness may be associated with dopamine receptor availability in specific subregions of the striatum composed of several anatomic and functional subdivisions.
Acknowledgements
For J.H. Kim, this work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A070001), and by the Gachon University Gil Medical Center Research Fund.
Authors’ Contributions
J.H. Kim was involved in study design, enrolment of subjects, data analysis, data interpretation, and manuscript writing. Y.D. Son carried out PET scanning and data analysis, and helped to draft the manuscript. H.K. Kim carried out PET scanning and data analysis. S.Y. Lee synthesised radiotracers and carried out PET imaging. Y.B. Kim and Z.H. Cho coordinated the study and revised the manuscript for important intellectual content.





