INTRODUCTION
Toxoplasma gondii infections are widely prevalent in animals and humans worldwide (Dubey, Reference Dubey2010). Although most infections caused by this parasite are without recognized symptoms, it can cause prematurity and severe illness, eye and brain disease in congenitally infected children, and in immune-compromised individuals (Remington et al. Reference Remington, McLeod, Thulliez, Desmonts, Remington, Klein, Wilson and Baker2006). Toxoplasma gondii is the most frequent cause of infections of the back of the eye. Severe cases of toxoplasmosis have been reported in immune-competent patients and some of these are considered to be due to infection with atypical T. gondii genotypes (Ajzenberg et al. Reference Ajzenberg, Bañuls, Su, Dumètre, Demar, Carme and Dardé2004; Demar et al. Reference Demar, Ajzenberg, Maubon, Djossou, Panchoe, Punwasi, Valery, Peneau, Daigre, Aznar, Cottrelle, Terzan, Dardé and Carme2007; Elbez-Rubinstein et al. Reference Elbez-Rubinstein, Ajzenberg, Dardé, Cohen, Dumètre, Yera, Gondon, Janaud and Thulliez2009; Grigg and Sundar, Reference Grigg and Sundar2009; Delhaes et al. Reference Delhaes, Ajzenberg, Sicot, Bourgeot, Dardé, Dei-Cas and Houfflin-Debarge2010; Pomares et al. Reference Pomares, Ajzenberg, Bornard, Bernardin, Hasseine, Dardé and Marty2011).
Humans and other hosts acquire T. gondii infection by ingesting tissue cysts in undercooked meat, or by food or drink contaminated with oocysts. Mice fed oocysts can die of acute enteritis before lesions develop in extra-intestinal organs (Dubey and Frenkel, Reference Dubey and Frenkel1973). Severity of toxoplasmosis depends on many factors including dose, strain of mouse, route of inoculation, and the stage of the parasite. An oral tissue cyst mouse model has been described to study immunity and pathogenesis of orally induced toxoplasmosis (McLeod et al. Reference McLeod, Estes, Mack and Cohen1984, 1989a,b; Brown et al. Reference Brown, Hunter, Estes, Beckmann, Forman, David, Remington and McLeod1995; Liesenfeld et al. Reference Liesenfeld, Kosek, Remington and Suzuki1996; Buzoni-Gatel et al. Reference Buzoni-Gatel, Debbabi, Mennechet, Martin, Lepage, Schwartzman and Kasper2001; Johnson et al. 2002a,b; Liesenfeld Reference Liesenfeld2002; Rachinel et al. Reference Rachinel, Buzoni-Gatel, Dutta, Mennechet, Luangsay, Minns, Grigg, Tomavo, Boothroyd and Kasper2004; Heimesaat et al. Reference Heimesaat, Bereswill, Fischer, Fuchs, Struck, Niebergall, Jahn, Dunay, Moter, Gescher, Schumann, Göbel and Liesenfeld2006; Dunay et al. Reference Dunay, DaMatta, Fux, Presti, Greco, Colonna and Sibley2008; Muñoz et al. Reference Muñoz, Heimesaat, Danker, Struck, Lohmann, Plickert, Bereswill, Fischer, Dunay, Wolk, Loddenkemper, Krell, Libert, Lund, Frey, Hölscher, Iwakura, Ghilardi, Ouyang, Kamradt, Sabat and Liesenfeld2009; Schreiner and Liesenfeld Reference Schreiner and Liesenfeld2009; Dunay and Sibley Reference Dunay and Sibley2010). After oral inoculation of 100 tissue cysts, C57BL/6 mice died of acute enteritis but BALB/c mice did not, and the lesions were localized to ileum. However, the number of bradyzoites in tissue cysts varies a great deal and the inoculum is not stable at room temperature. Additionally, the infectivity of free bradyzoites by the oral route in mice is low (Dubey, Reference Dubey1997, Reference Dubey2001).
A non-infectious vaccine to prevent clinical disease would be a major advance to minimize suffering due to this parasite in humans. Various T. gondii vaccine candidates are being developed (Cong et al. Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2010, Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2011). Mice are generally used to test the protective efficacy of vaccines, because they are most susceptible, reagents are available to measure immune parameters, and they are easily managed in the laboratory.
Three strains of Human Leukocyte Antigen (HLA) transgenic mice were used. These mice were selected because they have HLA transgenes that include HLA molecules that recognize peptide epitope supermotifs present in ∼90% of the human population (Cong et al. Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2010, Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2011). These are HLA-A*0201, HLA-A*1101, HLA-B*0702 mice. Supermotifs are defined by their binding avidity to these HLA molecules with the amino acid in the second and the ninth position of the nonamer peptide critical for anchoring the peptide into the HLA molecule binding pocket (Tan et al. Reference Tan, Mui, Cong, Witola, Montpetit, Muench, Sidney, Alexander, Sette, Grigg, Maewal and McLeod2010; Cong et al. Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2010, Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2011).These humanized HLA transgenic mice have been very useful for defining epitopes key for protection against a number of viruses and an apicomplexan parasite, eliciting protective CD8+T cells. They have been useful also in experiments to define T. gondii epitopes which can confer protection to parenteral challenges with luciferase-expressing parasites and elicit gamma interferon from CD8+T cells when administered with adjuvants and the universal CD4+T cell helper epitope PADRE (Cong et al. Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2010, Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2011).
The objective of the present study was to test susceptibility of different strains of mice (especially transgenic) to oral infection with oocysts of T. gondii strains with different genotypes to better understand pathogenesis of the infection by this route. This should provide a robust foundation for testing potential vaccines and new medicines.
MATERIALS AND METHODS
Toxoplasma gondii isolates used
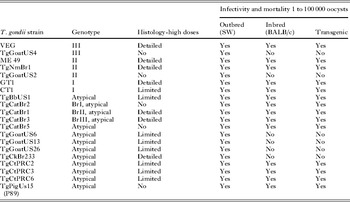
Toxoplasma gondii strains of different genotypes were used. Details of the T. gondii isolates used are given in Table 1. Because pathogenicity of T. gondii can be altered by prolonged passage in mice (Dubey, Reference Dubey and Kreier1977), we selected some strains that were recently isolated and not passaged in mice as tachyzoites or tissue cysts. Of the strains listed in Table 1, only VEG and ME49 strains had been maintained as tachyzoites or tissues cysts, before oocysts were obtained. Although, GT1, CT1, and P89 were obtained by one us (J. P. D.) many years ago these were maintained only as the tissue cyst-oocyst stage. Strains isolated in 2004 or later had been passaged in mice to obtain oocysts, and thus represented strains circulating in nature. We also selected strains from Brazil, Colombia, USA and Asia, because, in general, T. gondii strains from Brazil are more pathogenic for mice than strains from the USA (Dubey et al. Reference Dubey, Graham, Blackston, Lehmann, Gennari, Ragozo, Nishi, Shen, Kwok, Hill and Thulliez2002).
Table 1. Details of Toxoplasma gondii strains used in this study

Mouse strains used
Outbred Swiss Webster (SW) and inbred BALB/c were obtained from the National Cancer Institute (NCI). Gamma interferon gene knock out (KO) mice (C57BL/6-Ifng) were obtained from the Jackson Laboratories (Bar Harbor, ME, USA). HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice were produced at Pharmexa-Epimmune (San Diego, CA, USA) and bred at the University of Chicago, Taconic (Germantown, New York) and the Jackson laboratories (see Cong et al. Reference Cong, Mui, Witola, Sidney, Alexander, Sette, Maewal and McLeod2010). All studies were conducted according to the Institutional Animal Care and Use Committee at the USDA and University of Chicago.
Infection of mice with T. gondii oocysts
Oocysts were obtained by feeding infected tissues of mice to cats, sporulated in 2% sulfuric acid on a shaker for 1 week, and stored at 4°C until used, but not after 12 months (Dubey Reference Dubey1995, Reference Dubey2010). Most batches of oocysts had not been stored for more than 6 months. Oocysts were counted in a disposable haemocytometer and diluted 10-fold from 10−1 to 10−7 to reach an end-point of ≅1 oocyst. All 10-fold dilutions were made in 50 ml tubes with 2% sulfuric acid (5 ml aliquot+45 ml sulfuric acid), and dilutions were stored at 4°C, to avoid variability in inocula preparation. For inoculation of mice, oocysts from the designated dilution were neutralized with 3·3% sodium hydroxide with neutral red as indicator (approximately the same volume as the inoculum). The resultant mixture was inoculated orally into 5 mice for each dilution (unless indicated otherwise) via a gastric needle with a blunt bulb (22 gauge, 50 mm long, Cadence Science catalogue no. 7920), without washing to avoid variability of the inocula during washing procedures. All mice in a given experiment were inoculated at the same time. In most instances, oocysts from the last dilution were also inoculated orally into KO mice, because these mice are highly susceptible to toxoplasmosis and do not survive the infection, irresepective of the strain of T. gondii. All orally inoculated mice were housed in autoclavable rodent cages with biohazard signs to incinerate bedding and food for 10 days to avoid spread of T. gondii because some oocysts pass unexcysted in mouse feces (Dubey and Frenkel, Reference Dubey and Frenkel1973).
Bioassay of T. gondii in mice
Mice were observed daily for 8 weeks. All mice were examined for T. gondii infection. Impression smears of tissues (usually mesenteric lymph nodes or lungs) were examined microscopically for tachyzoites. Survivors were bled 6–8 weeks later and 1:25 dilutions of their sera were examined for T. gondii antibodies using the modified agglutination test (MAT) as described (Dubey and Desmonts, Reference Dubey and Desmonts1987). The last infective dilution was considered to have 1 viable organism for data presentation. The inoculated mice were considered infected with T. gondii when tachyzoites or tissue cysts were found in tissues. Seroconversion at 6 weeks was considered as indication of the presence of live parasites in the inocula. However, the brains of all mice that survived 6 weeks were examined for tissue cysts, irrespective of serological results (Dubey, Reference Dubey2010). With the strains of T. gondii used here, tissue cysts were found in all seropositive mice.
Tissue cysts were enumerated in mice that survived 8 weeks post-inoculation (p.i.). For this, whole mouse brain was homogenized with 1 ml of saline (0·85% NaCl) and tissue cysts were counted microscopically in 50 μl of the homogenate, and the count was multiplied by 20 to obtain the number of tissue cysts per brain. If cysts were not found, another 50 μl was examined in the same manner.
Experimental design
Table 2 summarizes information on T. gondii infections in mice.
Table 2. Experimental design
Pathogenesis of acute toxoplasmosis in mice
Histogenesis of lesions and parasitism in mice orally inoculated with oocysts was studied herein using different strains of T. gondii, and different strains of mice (Table 3). Most mice were inoculated orally using 100 000 or more oocysts. The primary objective was to document the development of early lesions, particularly in the small intestine. As mentioned in the Introduction, after oral inoculation of 100 tissue cysts, C57BL/6 mice died of acute enteritis but BALB/c mice did not, and the lesions were localized to the ileum. However, it is not known whether early lesions developed in other parts of small intestine of C57BL/6 mice after feeding approximately 100 tissues cysts that might contain 100 000 bradyzoites. Actually, early lesions were not seen in SW mice inoculated orally with millions of T. gondii bradyzoites (Dubey, Reference Dubey1997). Therefore, an unusually high dose of oocysts was selected to study early events in small intestine.
Table 3. Histological study of mouse tissues fed large doses of Toxoplasma gondii oocysts

In the present study, detailed observations were made using 3 strains of T. gondii (VEG, Type III, ME49, Type II, and GT1, Type I), in SW, BALB/c, and transgenic mice (Table 4). Additionally, data on 15 other strains of T. gondii were added to show the pattern of lesion; not all time-points were examined for each strain of T. gondii in each strain of mouse. Subsequently, data on transgenic mice (HLA-A*1101, HLA-B*0702) orally inoculated with 1–100 oocysts were added (Table 5). Mice were euthanized starting 6 h p.i. and up to 14 days p.i. The entire intestine was studied histologically. For this, the small intestine (approximately 25 cm) was stretched on a paper towel, and divided into 5 equal portions (nos. 1–5). The large intestine constituted the sixth segment (Table 4). Samples from the remaining organs, including the mesenteric lymph nodes, spleen, liver, lungs, heart, pancreas, skeletal muscle, tongue, uterus, and eyes were fixed in 10% buffered formalin along with the intestines. One day later, 3 mm thick sections were processed for paraffin embedding. The intestines were embedded on end, like a tube. Virtually all portions of intestines were processed for histology. Paraffin-embedded sections were cut at 5 μm thickness, and examined microscopically after staining with Haematoxylin and Eosin (HE). Immunohistochemistry for T. gondii was performed on paraffin-embedded sections using reagents and methods described previously (Dubey, Reference Dubey2010). Lesions and T. gondii tachyzoites were graded in mice euthanized at 1–14 days p.i.
Table 4. Histopathology of lesions of acute toxoplasmosis in BALB/c mice fed oocysts

a T. gondii not seen, no lesion. 1–5, small intestine, 6-large intestine.
b + Few T. gondii, no lesion.
c ++ T. gondii, few lesions.
d +++ Nearly all villi affected or severe lesions.
e no lesions, no T. gondii.
f M.L., mesenteric lymph nodes.
g B, brain; E, eye; H, heart; K, kidneys; Li, liver; Lu, lungs; M, skeletal muscle; Sp, spleen; St, stomach; T, tongue.
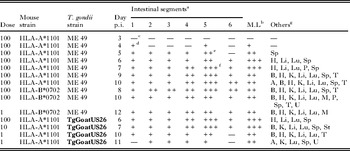
Table 5. Histogenesis of lesions of acute toxoplasmosis in transgenic mice fed few ME 49 (Type II) or TgGoatUS26 (Atypical) oocysts
a 1–5, small intestine, 6-large intestine.
b Mesenteric lymph nodes.
c no lesions, no T. gondii.
d Few T. gondii, no lesion.
e Nearly all villi affected or severe lesions.
f B, brain; E, eye; H, heart; K, kidneys; Li, liver; Lu, lungs; M, skeletal muscle; P, pancreas; Sp, spleen; St, stomach; T, tongue; U, uterus.
Enumeration of T. gondii tachyzoites in the ileum of acutely infected mice
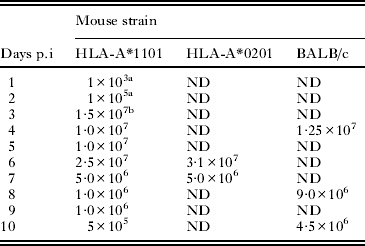
Results of the experiment in Table 4 indicated that the terminal part of the ileum is the most heavily affected portion of the small intestine. In order to determine whether parasite multiplication could account for intestinal lesions, HLA-A*1101 mice were orally inoculated with graded doses of oocysts of the GT1 strain, which is lethal for mice. For this, 2–3 mice (Table 6) were euthanized at 1–10 days p.i., their ileum (terminal 10 cm) weighed, homogenized in blender in 50 ml of saline at full speed for about 1 min, filtered through gauze (suspension A), centrifuged for 10 min at 1400 g, and the supernatant discarded. The sediment was suspended in 5 ml of antibiotic saline, and this homogenate was considered as the 10−1 dilution (suspension B). Tachyzoites were counted in 50 μl of the 10−1 dilution in a haemocytometer. Further, 10-fold dilutions were made of the homogenate until an end-point was achieved. Aliquots from different dilutions were bioassayed by subcutaneous inoculation in to 5 SW mice; bioassay data were used if the tachyzoites were not detected by microscopic examination. Data from HLA-A*0201, and BALB/c mice euthanized on days 4–8 were added to supplement evidence obtained with HLA-A*1101 mice (Table 6).
Table 6. Density of Toxoplasma gondii in ileum of mice fed GT1 oocysts
a By bioassay.
b Counted.
ND, no data.
To further study colonization of the ileum by tachyzoites after feeding oocysts, 27 HLA-A*0201 (Table 7) mice were orally inoculated with 3 strains of T. gondii (genotype I, II, III) and 3 mice (for each T. gondii strain) were euthanized on days 3, 4, and 5, and parasites enumerated as in Table 6.
Table 7. Concentration of Toxoplasma gondii tachyzoites in ileum of HLA-A*0201 mice fed three genetic Types (III, II and I) strains of oocysts

RESULTS
Overview
Each narrative statement applies to data in a specific table. Also, a variety of different experiments were performed for different purposes. These were performed to clarify relative susceptibility of the different strains of mice to different strains of oocysts. Survival, parasite burden measured with bioassay of tissues, cyst number, and in histopathology, and kinetics were characterized. Humanized HLA transgenic mice were of particular interest. Oocysts from 21 strains of parasites were characterized in outbred SW mice. Then, 15 of these strains of parasite oocysts from different locales throughout the world also were studied in BALB/c, C57/BL6, HLA-A*1101 and IFN-gamma knockout mice on a C57BL/6 background. C57BL/6 mice are the parental strain for HLA-A*0201 and HLA-A*1101 transgenic mice. BALB/c by C57BL/6 mice backcrossed through many generations are the parental strain for the HLA-B*0702 mice, which have 3-B*0702 transgenes. Then, Type I (GT1) (Table 9), Type III (Tg Goat US4) and Type II (ME49, TgNmBr1) (Table 11) were compared in all 3 strains of the HLA supermotif transgenic mice.
Pathogenicity of different T. gondii strains in various strains of mice
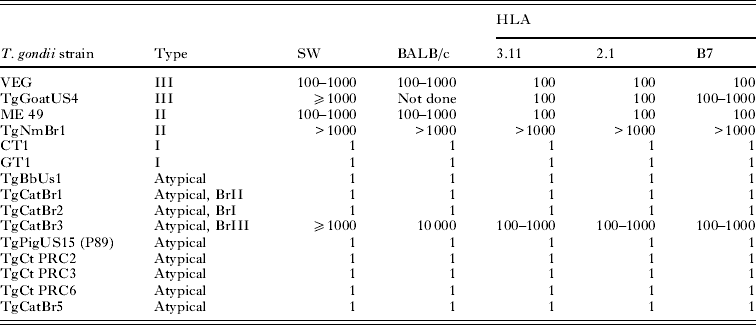
Based on a 100% lethal dose, data are summarized in Table 8. The Type I strains (GT1, CT1) and 6 atypical strains (TgBbUs1, TgCatBr1, TgCatBr3, TgCatBr5, TgPigUS15, TgCtPr6) were lethal for all mice, irrespective of the strain and the dose; mice that received the last dilution, calculated to have no oocysts, remained healthy, did not develop antibody to T. gondii, and had no demonstrable T. gondii in their tissues. Not all atypical T. gondii strains were lethal for mice, irrespective of their country of origin. For example, the TgCatBr3 was only mildly pathogenic, whereas TgCatBr1 was highly virulent; both of these isolates are from cats from Brazil. TgBbUs1, an atypical strain from a bear from Alaska, USA, was the most virulent, irrespective of the strain of mouse.
Table 8. Lethal dose (100%) of oocysts of various Toxoplasma gondii strains in different strains of mice*
* Five mice per group.
Histogenesis of lesions
The pattern of lesions and tissue parasitization were the same, irrespective of the strain of mouse or T. gondii used. An example of lesions seen in BALB/c mice after feeding VEG (Type III), ME 49 (Type II), and the GT1 (Type I) is shown in Table 4; this strain of mouse was chosen here because it is considered resistant to clinical toxoplasmosis, based on feeding 100 tissue cysts. Mice fed approximately 100 000 or more oocysts died of acute toxoplasmosis within 6 days. The last 2/5 of the small intestine was the most parasitized tissue. The extent of lesions in the intestine varied with dose and the strain of T. gondii, lesions being most severe in the last 10 cm of the ileum. After feeding oocysts, T. gondii parasites were seen in histological sections of the entire small intestine, but initial multiplication was the highest in the ileum and the mesenteric lymph nodes. Mice that died on day 4 after feeding oocysts did so because of severe transmural enteritis, particularly of the ileum (Fig. 1). Tachyzoites multiplied in most cells in the lamina propria causing parenchymal necrosis (Fig. 1B); many times the surface enterocytes were minimally affected (Fig. 1B). Death was precipitated by sloughing of the contents of the small intestine into the lumen. Subsequently lesions and tachyzoites were seen in other regions of small intestine and also in the cecum and rectum; however, at any given time the ileum was most parasitized among 5 regions of small intestine (Table 4). Immunohistologically, tachyzoites were seen in descending order of density in the intestine, mesenteric lymph nodes, spleen, lungs of mice examined 6 days p.i. Subsequently, tachyzoites were seen in the liver, heart, and brain. Mice that died of acute toxoplasmosis during the first week after infection did so because of enteritis and mesenteric lymph node necrosis. During the second and the third week p.i., mice died primarily of acute interstitial pneumonitis, with demonstrable tachyzoites.

Fig. 1. Section of ileum of a Swiss Webster mouse, 96 h after feeding VEG strain oocysts. Note extensive parasitization of the lamina propria. All red-stained bodies are tachyzoites. Immunohistochemical staining with rabbit Toxoplasma gondii polyclonal antibodies. (A) Low magnification showing cross-section. Scale bar=250 μm. (B) Higher magnification of a villus showing numerous tachyzoites and spilled antigen (arrow) in the lamina propria. A few tachyzoites (arrow heads) are present in enterocytes but the surface epithelium is intact. Scale bar=25 μm.
A similar pattern of lesions observed with the high dose of oocysts was seen in transgenic mice (HLA-A*1101, B7) after feeding a low dose (1–100) of oocysts (Table 5). Mice fed 100 oocysts were ill by 5–6 days p.i.; T. gondii was not detectable in histological sections of the mouse killed on 3 day p.i., and only few parasites were seen on day 4 p.i. Tachyzoites first multiplied in the small intestine and the ileum was the most parasitized tissue.
Density of T. gondii in the ileum of mice fed large doses of oocysts
Parasitaemia was detected in mice euthanized at 6 h p.i. by bioassay of blood removed from the orbital sinus (data not shown). By 3 day p.i. more than 10 million T. gondii were present in 10 cm of ileum (Table 6). The results were similar, irrespective of the strain of mouse or T. gondii genetic type (Table 7).
Mortality pattern and the number of tissue cysts in mice infected with different T. gondii strains
Examples of mortality rates are shown in Tables 9–11. With GT1 (Type I) strain, all inoculated mice died within 13 days p.i., irrespective of the dose and the mouse strain (Table 9).
Table 9. Toxoplasma gondii GT1 strain (Type I) oocyst infectivity to various mouse strains
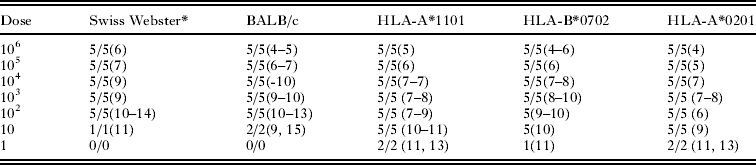
* Five mice per group. First figure is no. of mice infected with T. gondii. Second figure is no. of mice dead. Figure in parenthesis is day of death.
Table 10. TgCatBr3 (atypical) oocyst infectivity to various mouse strains
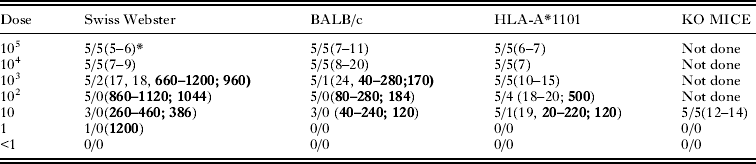
* Five mice per group. First figure is no. of mice infected with T. gondii. Second figure is no. of mice dead. Figure in parenthesis is day of death. Figures in bold are number of tissue cysts, with the first numbers the range; and the second number after the semicolon as average per infected mouse.
Table 11. Infectivity of low numbers of Type III (TgGoatUS4) and Type II (ME 49, TgNmBr1) strains of Toxoplasma gondii oocysts to mice
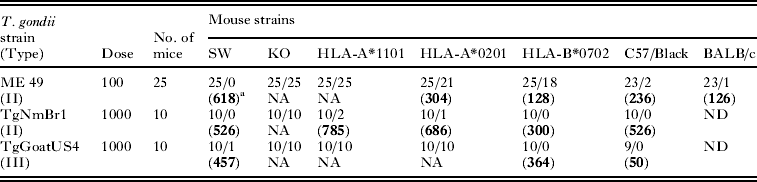
a No. of mice T. gondii infected/no. died (average no. of tissue cysts).
b ND, not done; NA, not applicable, mice died.
Note: Data for VEG and other strains shown in Table 8. 100–1000 VEG are lethal to these strains of mice.
With a relatively non-virulent atypical Brazilian strain (TgCatBr3), the decreasing order of virulence was KO, HLA-A*1101, SW, and BALB/c (Table 10). It is noteworthy that all KO mice fed an infective dose of oocysts died within 14 days p.i. Among the mice that survived, the lowest numbers of tissue cysts were found in BALB/c mice (Table 10).
In the previous experiments, 5 mice were inoculated per dose. In another experiment, 10–25 mice were inoculated using 100–1000 infective oocysts of 3 strains of T. gondii (Table 11). The infectivity of the dose was determined previously by bioassays in mice, including KO mice. The mortality and the number of oocysts are shown in Table 11. With the ME 49 strain, oocysts were most pathogenic to KO, HLA-A*1101, HLA-A*0201, HLA-B*0702, C57/black, BALB/c, and SW—in decreasing order of pathogenicity, the number of tissue cysts were lowest in BALB/c mice. A similar trend was noted with other strains of T. gondii, although not all strains were used to infect large numbers of mice.
DISCUSSION
The early events in the mouse intestine after feeding oocysts initially described in out-bred Swiss Webster mice orally inoculated with VEG strain (Dubey et al. Reference Dubey, Speer, Shen, Kwok and Blixt1997; Speer and Dubey, Reference Speer and Dubey1998) were confirmed here using different strains of mice and T. gondii strains of different genotypes. These events are important biologically because ingestion of oocysts is a major route of transmission in animals and humans (Boyer et al. Reference Boyer, Hill, Mui, Wroblewski, Karrison, Dubey, Sautter, Noble, Withers, Swisher, Heydemann, Hosten, Babiarz, Lee, Meir and McLeod2011; Hill et al. Reference Hill, Coss, Dubey, Wroblewski, Sautter, Hosten, Muñoz-Zanzi, Mui, Withers, Boyer, Hermes, Coyne, Jagdis, Burnett, McLeod, Morton, Robinson and McLeod2011). After ingestion of oocysts, T. gondii sporozoites excyst in the small intestine, and can invade entrocytes as early as 30 min p.i. (Dubey et al. Reference Dubey, Speer, Shen, Kwok and Blixt1997). Sporozoites are carried to the lamina propria by an undefined mechanism, but not by intraepithelial cells (Speer and Dubey, Reference Speer and Dubey1998). The isolation of viable organisms as early as 4 h p.i. (Dubey et al. Reference Dubey, Speer, Shen, Kwok and Blixt1997) and 6 h (present study) from the peripheral blood of mice fed oocysts indicates that sporozoites circulate in the body early in infection but initial multiplication occurs in the mesenteric lymph nodes and the intestinal lamina propria. Toxoplasma gondii multiplies in all cell types (except red blood cells) of the lamina propria and secondarily invade surface enterocytes (Speer and Dubey, Reference Speer and Dubey1998). Although sporozoites can excyst throughout the small intestine, they preferentially invade the distal part of the small intestine (Dubey et al. Reference Dubey, Speer, Shen, Kwok and Blixt1997). A similar pattern of infection was found in the present study using at least 2 other strains of T. gondii and other strains of mice. Mice died early after infection with sporozoites in association with a massive infection of the intestine with intestinal necrosis. This was most prominent in the terminal ileum. The exact cause of this acute enteritis was not the focus of this research but finding of as many as 10 million tachyzoites in 10 cm (<1 g) of the terminal ileum indicates that the parasite-induced cell death is important in the oocyst model. Interferon gamma conferred some protection, as seen by the greater susceptibility of the interferon gamma knock out versus the wild type BALB/c mice. Enteritis associated with an immune response to SAG 1 was described in C57BL/6 mice fed tissues cysts (Rachinel et al. Reference Rachinel, Buzoni-Gatel, Dutta, Mennechet, Luangsay, Minns, Grigg, Tomavo, Boothroyd and Kasper2004). It is of interest that the HLA transgenic mice were more susceptible than the parental C57BL6/J and BALB/c strains but the mechanisms are not known. Whether and if so how often T. gondii infections might cause gastrointestinal symptoms in humans is unknown. Historically, enteritis was not recognized as cause of death in experimentally infected mice until the discovery of the resistant stage of T. gondii, the oocyst in late 1960. Mice fed cat feces (containing oocysts) died within 8 days p.i. but diagnosis was difficult because intestines were not examined (Hutchison et al. Reference Hutchison, Dunachie and Work1968). Subsequently, it was found that the cause of death in mice fed large numbers of oocysts was enteritis (Frenkel et al. Reference Frenkel, Dubey and Miller1970; Dubey and Frenkel, Reference Dubey and Frenkel1973; Dubey et al. Reference Dubey, Speer, Shen, Kwok and Blixt1997). Initially, these studies were performed in out-bred albino mice, and now are extended to in-bred and transgenic mice herein.
Our data demonstrate that, at least in mice, T. gondii infection can cause severe and lethal enteritis, irrespective of the genetic background. In animals, oocyst-induced infections are more severe than tissue cyst-acquired infections (Dubey, Reference Dubey2010). Schreiner and Liesenfeld (Reference Schreiner and Liesenfeld2009) reviewed reports of toxoplasmosis in many species of animals and concluded that orally-induced infections can cause severe immunopathology in the gut and other viscera. Whether there is a parallel disease in humans remains to be determined. Lymphadenopathy in nodes draining the terminal ileum has been confused with appendicitis, but whether there was such severe enteritis in any humans is unknown.
In the present study in mice fed high doses of oocysts, necrosis and hyperinfection of the terminal ileum seems to be a pathogenic hallmark of these infections for unknown reasons. Histopathology and time to death are associated and follow the same pattern. As the Tables illustrate, the histopathology and time to death are dose related for the relatively more resistant strains of mice. Whether there is a receptor or type of cell there that causes this localization is unknown. Further, the progression of enteritis associated with early deaths there and later deaths due to pneumonia occur whether the infection is with 100 or 100 000 oocysts; parasite multiplication is responsible, at least in part, for cause of death because numerous tachyzoites can be demonstrated in smears made from intestines and lungs. It is of interest that the TgNmBr1 (Type II) strain is much less virulent. TgGoatUS 4 (Atypical) also is less virulent. The genetic differences in these parasite strains from ME49 or other type II or atypical strains will be of interest to determine in the future. Similarly, crosses of these parasites as previously created for other strains may provide powerful tools to further characterize this difference in the future.
Since an acutely infected cat can excrete up to 500 million oocysts in a period of a few days it does not seem unreasonable to consider whether an incidental host like a mouse or human might encounter 100 or even 100 000 oocysts. Whether this happens in nature is unknown. It is of interest that more than 70% of the mothers of children in the National Collaborative Congenital Toxoplasmosis Study in USA have antibody to oocysts (Hill et al. Reference Hill, Coss, Dubey, Wroblewski, Sautter, Hosten, Muñoz-Zanzi, Mui, Withers, Boyer, Hermes, Coyne, Jagdis, Burnett, McLeod, Morton, Robinson and McLeod2011; Boyer et al. Reference Boyer, Hill, Mui, Wroblewski, Karrison, Dubey, Sautter, Noble, Withers, Swisher, Heydemann, Hosten, Babiarz, Lee, Meir and McLeod2011), and the source of exposure is unknown for many. Most of these mothers did not have recognized symptoms and most of the infants had moderate or severe signs of infection. Epidemics of toxoplasmosis have been linked epidemiologically to ingestion of oocysts from the environment (see Hill et al. Reference Hill, Coss, Dubey, Wroblewski, Sautter, Hosten, Muñoz-Zanzi, Mui, Withers, Boyer, Hermes, Coyne, Jagdis, Burnett, McLeod, Morton, Robinson and McLeod2011).
Certain HLA gene products bind peptides with specific motifs for >90% of the human population, hence these 3 HLA haplotypes are called HLA supermotifs. They have been used productively in model studies to vaccinate against hepatitis, influenza and malaria, among other diseases. This approach to identify supermotif-binding protective epitopes for a variety of diseases is now progressing with the identified peptides or their gene sequences in DNA vaccines to human clinical trials. This indicates that these HLA supermotif mice provide a robust model for testing vaccines for humans. Thus, further work testing protective preparations against toxoplasmosis in these mice, including challenges with oocysts, holds promise for future studies.
It is noteworthy that the introduction of the transgenes increases the susceptibility of the mice to certain parasite strains but not to others. This is reminiscent of the increased susceptibility seen in other transgenic mice earlier (Brown and McLeod, Reference Brown and McLeod1990). Possible mechanisms are the competing MHC molecules from the human transgene that diminish the response of the murine MHC thus confering some protection (Brown et al. Reference Brown, David, Khare and McLeod1994, Reference Brown, Hunter, Estes, Beckmann, Forman, David, Remington and McLeod1995; Mack et al. Reference Mack, Johnson, Roberts, Roberts, Estes, David, Grumet and McLeod1999; McLeod et al. 1989a,b, Reference McLeod, Brown and Mack1993; Johnson et al. Reference Johnson, Suzuki, Mack, Mui, Estes, David, Skamene, Forman and McLeod2002a,Reference Johnson, Roberts, Pope, Roberts, Kirisits, Estes, Mui, Krieger, Brown, Forman and McLeodb; Jamieson et al. Reference Jamieson, de Roubaix, Cortina-Borja, Tan, Mui, Cordell, Kirisits, Miller, Peacock, Hargrave, Coyne, Boyer, Bessieres, Buffolano, Ferret, Franck, Kieffer, Meier, Nowakowska, Paul, Peyron, Stray-Pedersen, Prusa, Thulliez, Wallon, Petersen, McLeod, Gilbert and Blackwell2008, Reference Jamieson, Peixoto-Rangel, Hargrave, de Roubaix, Mui, Boulter, Miller, Fuller, Wiley, Castellucci, Boyer, Peixe, Kirisits, Elias, Coyne, Correa-Oliveira, Sautter, Smith, Lees, Swisher, Heydemann, Noble, Patel, Bardo, Burrowes, McLone, Roizen, Withers, Bahia-Oliveira, McLeod and Blackwell2010; Lees et al. Reference Lees, Fuller, McLeod, Boulter, Miller, Zakrzewski, Mui, Witola, Coyne, Hargrave, Jamieson, Blackwell, Wiley and Smith2010; Witola et al. Reference Witola, Mui, Hargrave, Liu, Hypolite, Montpetit, Cavailles, Bisanz, Cesbron-Delauw, Fournie and McLeod2011). Alternatively the human MHC could introduce a harmful/lethal immune response because it is robust but damaging.
The data herein demonstrate that host and parasite genes interact in profoundly important and unpredictable ways in these murine models, and showed that not all type 2 or type 1 or 3 lineage parasites behave in the same manner in these models. More than one parasite allele must be critical. Variation among alleles/epitopes in parasites of differing and the same lineages have been noted in the past.
The work described herein demonstrates that many strains of T. gondii oocysts are highly pathogenic for mice. There is variation in pathogenicity of oocysts, which depends in part on the strain of mouse: SW<BALB/c,<HLA-B*0702,<HLA-A*0201,<HLA-A*1101,<C57BL6/J background gamma knockout. In addition, these data demonstrate that certain strains of oocysts are much less pathogenic providing the opportunity in the future to understand what parasite protein(s) are different between these strains of parasites. This may provide insight into what is causing this remarkable pathology. The pathology the oocysts cause is intestinal necrosis with parasite proliferation. This pathology is associated with mouse death in a dose-related and time-dependent manner. The pathology occurs early after infection before adaptive immunity would be functioning, but interferon gamma clearly plays a role based on the data in the gamma knockout compared to parental control mice. This murine model is suitable for further understanding the pathogenesis. This includes understanding the parasite molecules involved because of the informative parasite strain differences and providing a robust and harmful challenge, setting the bar high for prevention and protection in future studies. This is for testing both vaccines that depend on human HLA molecules that bind and present protective epitopes, and antimicrobial agents.
The model developed herein should prove to be extremely useful for testing vaccines because it is possible to very accurately quantitate a consistent challenge inoculum, test the response to different strains of T. gondii using the same preparations of oocysts which are stable for up to a year, and to have a very reproducible response to the infection. This should be a robust model for testing vaccine preparations in these HLA transgenic mice. This model is relevant to the human infection since CD8 T cells are protective against this infection.
ACKNOWLEDGEMENTS
This work was supported by NIAID U01AI077887 (RM, JPD), the Rooney-Alden Family, and the Dominique Cornwell and Mann Family Foundation. We would like to thank Dr. Kamal El Bissati for illustrations.














