1. Introduction
The appearance of the complex, mineralized Cambrian fauna has fascinated scientists for decades. There exists a general consensus that the Ediacara biota marks the advent of metazoans (Fedonkin & Waggoner, Reference Fedonkin and Waggoner1997; Bobrovskiy et al. Reference Bobrovskiy, Hope, Ivantsov, Nettersheim, Hallmann and Brocks2018; Dunn et al. Reference Dunn, Liu and Donoghue2018), though their specific phylogeny remains controversial (Bonner, Reference Bonner1998; Budd, Reference Budd2008; Dececchi et al. Reference Darroch, Rahman, Gibson, Racicot and Laflamme2017). The Cambrian fauna, however, is more easily assigned to various phylogenetic ranks (Conway Morris, Reference Conway Morris1979; Erwin et al. Reference Erwin, Valentine and Jablonski1997; Davidson & Erwin, Reference Davidson and Erwin2006; Chen, Reference Chen2009; Budd & Jackson, Reference Budd and Jackson2016). With few similarities in constructional morphologies, the relationship between the Ediacaran and Cambrian biotas remains enigmatic (Droser & Gehling, Reference Droser and Gehling2015). This has led many researchers to question what caused this faunal turnover, commonly referred to as the ‘trigger’ to the ‘Cambrian Explosion’. The proposed hypotheses are numerous (for reviews see Conway Morris, Reference Conway Morris2000; Marshall, Reference Marshall2006; Zhang et al. Reference Zhang, Shu, Han, Zhang, Liu and Fu2014; Darroch et al. Reference Darroch, Smith, Laflamme and Erwin2018; Sperling & Stockey, Reference Sperling and Stockey2018) and are broadly categorized into genetic, ecological and environmental causes (Erwin, Reference Erwin2015). There is a growing consensus that bioturbation may have played a key role in this evolutionary event (e.g. Mángano & Buatois, Reference Mángano and Buatois2014; Hantsoo et al. Reference Hantsoo, Kaufman, Cui, Plummer and Narbonne2018; Kaufman, Reference Kaufman, Sial, Gaucher, Ramkumar and Ferreira2018; Lenton & Daines, Reference Lenton and Daines2018). During the Ediacaran–Cambrian transition and early Cambrian Period there exists a marked infaunalization, and a switch from an Ediacaran-style matground ecology to a Cambrian-style mixground ecology (Seilacher, Reference Seilacher1999; Mángano & Buatois, Reference Mángano and Buatois2017; Gougeon et al. Reference Gougeon, Mángano, Buatois, Narbonne and Laing2018). Whether bioturbation is a cause or consequence of the Cambrian explosion ultimately depends on the drivers of infaunalization. In turn, the elucidation of these drivers will assist in understanding the selective pressures at this time in Earth’s evolution.
Using primarily body-fossil data, supplemented by ichnologic data, palaeontologists have previously conducted ecospace analyses for the Ediacaran and Cambrian periods (Bambach et al. Reference Bambach, Bush and Erwin2007; Bush & Bambach, Reference Bush and Bambach2011; Bush et al. Reference Bush, Bambach, Erwin, Laflamme, Schiffbauer and Dornbos2011; Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013; Knope et al. Reference Knope, Heim, Frishkoff and Payne2015). In these analyses a few trends are evident. A large amount of ecospace remains unoccupied in the Ediacaran, which is in stark contrast to Cambrian ecospace occupation. First, the advent of diverse groups of swimming and floating animals in the Cambrian Period marks an expansion into the pelagic realm that, with the lone possible exception of jellyfish, was largely unexplored in Ediacaran seas (Gold, Reference Gold2018). Additionally, Cambrian bioturbators begin to exploit the deep-infaunal realm. Finally, the osmotrophic or ‘other’ feeding styles of the Ediacaran Period become rare, replaced by predation, deposit-feeding and suspension-feeding (but see Rahman et al. Reference Rahman, Darroch, Racicot and Laflamme2015; Darroch et al. Reference Darroch, Rahman, Gibson, Racicot and Laflamme2017).
Due to the paucity of the body-fossil record in the Fortunian Stage, the illumination of the transition between these seemingly disparate faunas inevitably will rely on trace-fossil data. While body fossils are excellent sources to help reconstruct phylogeny, their utility to reveal behavioural information is more limited. For this, researchers must turn to ichnology, which provides an independent line of evidence to track not only the appearance of new body plans, but also the establishment of a Phanerozoic benthic ecosystem.
The lowermost Cambrian boundary section at Fortune Head, Newfoundland, Canada, as well as equivalent strata at Grand Bank Head, provides a reasonably continuous 1 km thick record through the late Ediacaran and early Cambrian periods (Myrow & Hiscott, Reference Myrow and Hiscott1993). This is recorded by the five informal members of the Chapel Island Formation (CIF). Member 1 and the first 2.4 m of member 2 are Ediacaran in age, while the Fortunian Stage is documented by the remainder of member 2 and the whole of member 3. The remaining members 4 and 5 are Cambrian Stage 2 in age (Landing, Reference Landing1989). The CIF contains remnants of the Ediacaran matground ecology in Fortunian strata (Buatois et al. Reference Buatois, Narbonne, Mángano, Carmona and Myrow2014) and only becomes truly Cambrian in aspect with the onset of the mixed sediment layer in the lower Cambrian Stage 2 (Gougeon et al. Reference Gougeon, Mángano, Buatois, Narbonne and Laing2018). The appearance of penetrative bioturbation at the section is evidenced by the Treptichnus pedum Ichnofossil Assemblage Zone (IAZ). This zone is delineated by the probing sub-horizontal index fossil Treptichnus pedum, demarcating the beginning of the Cambrian Period (Narbonne et al. Reference Narbonne, Myrow, Landing and Anderson1987; Landing, Reference Landing1994; Buatois, Reference Buatois2018). While it is generally accepted that the ichnofauna of the Treptichnus pedum IAZ represents a higher-diversity benthos with novel feeding strategies (Narbonne et al. Reference Narbonne, Myrow, Landing and Anderson1987; Buatois et al. Reference Buatois, Narbonne, Mángano, Carmona and Myrow2014; Herringshaw et al. Reference Herringshaw, Callow, McIlroy, Brasier, McIlroy and McLoughlin2017), few systematic analyses of this ichnofauna exist (Crimes & Anderson, Reference Crimes and Anderson1985).
2. Concepts and methods
The palaeoecological concept of ecospace describes the ecological space (i.e. mode of life) that an organism occupies or may theoretically occupy (Bambach et al. Reference Bambach, Bush and Erwin2007). The time-averaged nature of the fossil record, and other taphonomic loss of information, cause discernible ecological parameters to be limited. Instead, palaeontologists rely heavily on functional morphology to glean insights on the lifestyles of ancient organisms (Bambach et al. Reference Bambach, Bush and Erwin2007).
The guild concept, originally introduced by Root (Reference Root1967) and subsequently adopted for palaeobiology by Bambach (Reference Bambach, Tevesz and McCall1983), draws on this, and is a framework for classifying fossil taxa and the niches they occupy by using discernible ecological parameters. Bromley (Reference Bromley1990; Reference Bromley1996) modified the Bambachian guild concept to better suit ichnological data and proposed the ichnoguild concept
The life habits of organisms were subsequently categorized into theoretical modes of life based on three ecologic parameters that can be reasonably defined with fossil data: tiering, motility and feeding (Bambach et al. Reference Bambach, Bush and Erwin2007; Bush et al. Reference Bush, Bambach and Daley2007). Each parameter was divided into six subcategories, and used to construct a 6 by 6 by 6 matrix. Each axis within the matrix represents an ecological property. The subcategories are represented by rows and columns of cubes, and modes of life by individual cubes. This framework was adapted solely for ichnological data by Minter et al. (Reference Minter, Buatois, Mángano, Mángano and Buatois2016 a) and has been useful in examining behavioural innovations and the role of bioturbation through time (Minter et al. Reference Minter, Buatois, Mángano, Macnaughton, Davies, Mángano and Buatois2016 b, Reference Minter, Buatois, Mángano, Davies, Gibling, Macnaughton and Labandeira2017). However, two disparate schemes for body fossils and ichnofossils may inhibit collaboration between the two bodies of evidence. In turn, both the original ecospace occupation framework of Bush et al. Reference Bush, Bambach and Daley2007 and the ecospace analysis of Bambach et al. (Reference Bambach, Bush and Erwin2007) took ichnofossils into account in an effort to include this pivotal body of evidence. Herein, a slightly modified version of the ecospace occupation framework of Bush et al. Reference Bush, Bambach and Daley2007 is employed (Fig. 1).
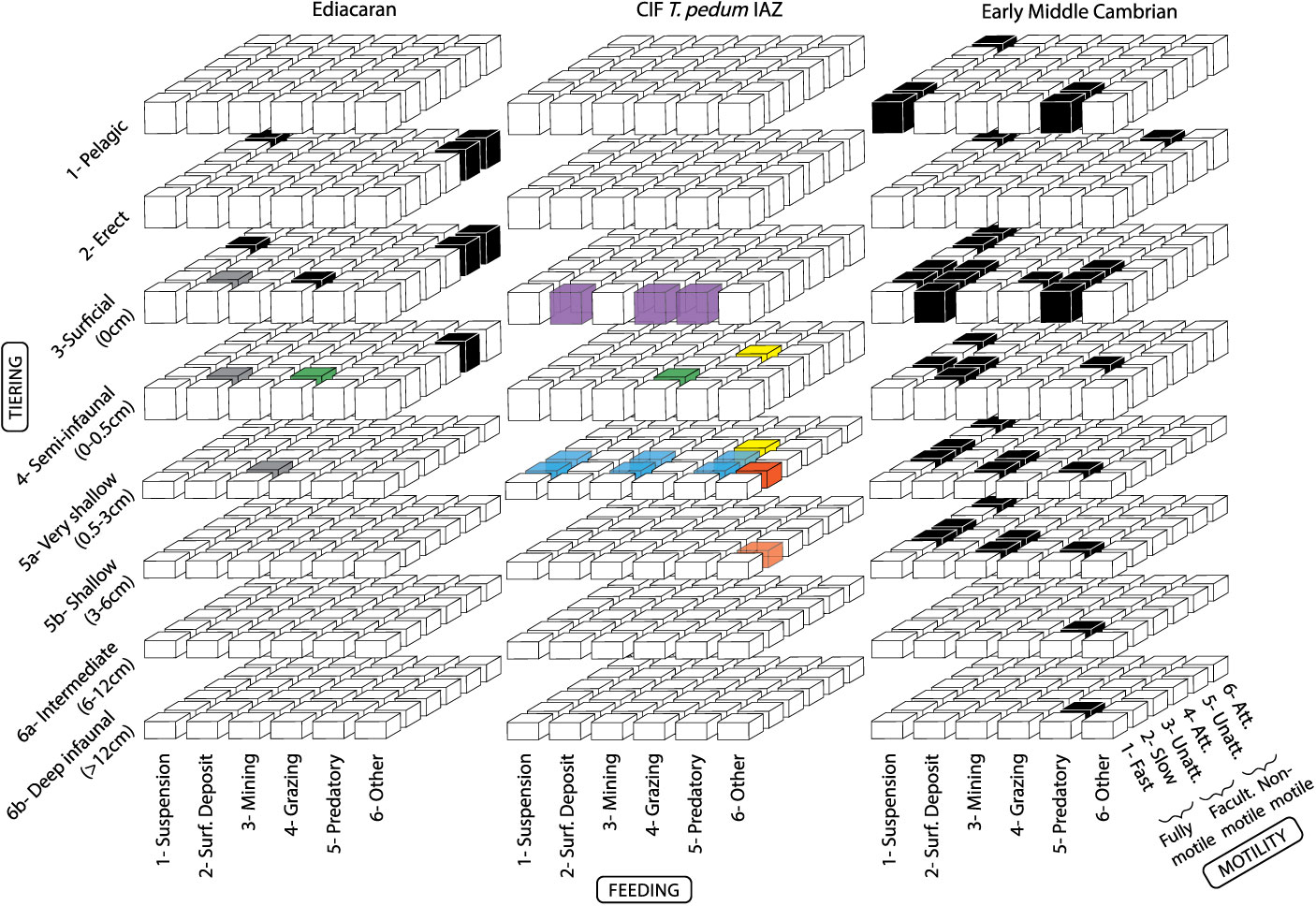
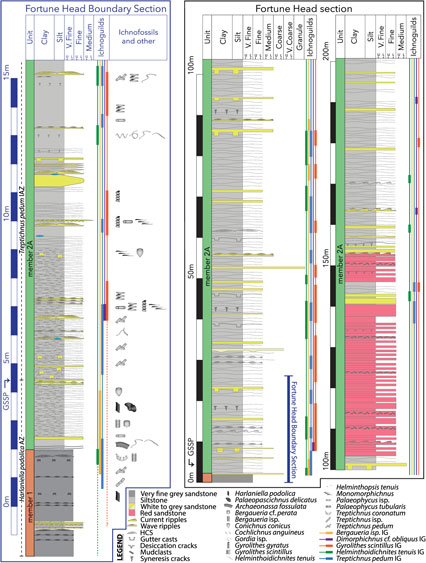
Fig. 1. Ecospace analysis for the Ediacaran Period to early-middle Cambrian. Black boxes represent modes of life occupied by body fossils globally, and grey boxes represent modes of life occupied by trace fossils globally (data from Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013). Coloured boxes represent modes of life occupied by CIF T. pedum IAZ ichnoguilds (IG). Green = Helminthoidichnites IG. Blue = Treptichnus IG. Yellow = Bergaueria IG. Orange = Gyrolithes IG. Purple = Dimorphichnus IG. Transparent boxes indicate modes of life with several possible tiers, feeding styles or motility levels.
In order to achieve a greater resolution of ecological changes, particularly as it pertains to ichnofossils, some tiering ecologic subcategories have been subdivided herein, as per Minter et al. (Reference Minter, Buatois, Mángano, Mángano and Buatois2016 a). For example, a classification of ‘shallow infaunal’ as living in the top 5 cm of sediment is broad, and may dilute evolutionary signals. In this case, all modes-of-life cubes for that subcategory were divided in half, to represent two subdivisions of the ‘shallow infaunal tier’ subcategory. These divided subcategories rows are represented in Figure 1 as half-cubes. When Ediacaran body fossils with a generalized tiering depth or height (e.g. a body fossil broadly occupying ‘5-shallow infaunal’) were combined into this new subdivided ecospace framework, both halves (e.g. both ‘5a- very shallow’ and ‘5b- shallow’) of the original mode-of-life cube were occupied. Likewise, in cases where ichnological affinity was uncertain (in this case, usually within the feeding parameter) all possible modes of life were occupied in semi-transparent colour. In this manner all possible modes of life are shown, with uncertainty demonstrated through the use of semi-transparent boxes.
Ichnologic data were collected from member 2 of the CIF, located at Fortune Head and Grand Bank Head, Newfoundland. Stratigraphic sections were measured, and ichnofossil occurrences documented and photographed when possible.
3. Ichnoguild analysis
3.a. Bergaueria ichnoguild
The Bergaueria ichnoguild consists of Bergaueria isp., Bergaueria perata and Conichnus conicus. All three are plug-shaped burrows, likely produced by sea anemones (Alpert, Reference Alpert1973; Pemberton et al. Reference Pemberton, Frey and Bromley1988), and therefore these ichnotaxa are interpreted as reflecting the work of predators. Bergaueria perata, Bergaueria isp. and Conichnus conicus are very shallow, semi-infaunal burrows, and Bergaueria isp. demonstrates burrow adjustment with sedimentation (Fig. 2p, q). In turn, the producer actively wedged within the sediment, and in the case of Bergaueria isp. demonstrated motility with sedimentation. Therefore, they are classified as attached, facultative motile, semi-infaunal burrowers which likely fed through predation.

Fig. 2. Ichnotaxa from member 1 and the T. pedum IAZ of member 2 of the Chapel Island Formation. All scale bars are 1 cm. (a) Archaeonassa fossulata, preserved in epirelief (FH 2.6 m). (b) Helminthopsis tenuis, preserved in positive hyporelief (FH 87 m). (c) Gordia isp., preserved in positive hyporelief (FH 142 m). (d) Helminthoidichnites tenuis, preserved in positive and negative hyporelief. (e) Cochlichnus anguineus, preserved in negative epirelief (FH 12 m). (f) Palaeophycus tubularis, preserved in negative epirelief and full relief (FH 20.9 m). (g) Palaeophycus isp. preserved in full relief (FH 69 m). (h) Gyrolithes gyratus, preserved in full relief (FH 14.8 m). (i) Gyrolithes scintillus, preserved in full relief (FH 7.7 m). (j) Treptichnus coronatus, preserved in positive hyporelief (GBH6 12 m). (k) Treptichnus pedum, preserved in positive hyporelief (FH 1.35 m). (l) Monomorphichnus isp. C preserved in positive hyporelief (GBH6 11.1 m). (m) Monomorphichnus isp. B preserved in positive hyporelief (GBH6 11.1 m). (n) Monomorphichnus isp. A preserved in positive hyporelief (GBH6 11.1 m). (o) Trichichnus cf. simplex preserved in full relief (FH 29 m). (p) White arrows: Bergaueria isp. Black arrow: Bergaueria perata. Both preserved in full relief (FH 4.5 m). (q) White arrow: Conichnus conicus. Black arrow: Bergaueria isp. Both preserved in full relief (FH 87 m). (r) Dimorphichnus cf. obliquus, preserved in positive hyporelief. White arrows denote pushers, while black arrows denote rakers (GBH6 12 m). Box outline corresponds with the ichnotaxon’s ichnoguild. Green boxes (a, b, c, d and e) are the Helminthoidichnites ichnoguild. Blue boxes (f, g, j and k) are the Treptichnus ichnoguild. Orange boxes (h, i and o) are the Gyrolithes ichnoguild. Purple boxes (l, m, n and r) are the Dimorphichnus ichnoguild. Yellow boxes (p and q) are the Bergaueria ichnoguild. FH = Fortune Head, GBH6 = Grand Bank Head site 6 (47.108, −55.770).
3.b. Dimorphichnus ichnoguild
The Dimorphichnus ichnoguild consists of Dimorphichnus cf. obliquus, Monomorphichnus isp. A, Monomorphichnus isp. B and Monomorphichnus isp. C (Fig. 2r, n, m, l, respectively), all representing animals with articulated appendages able to produce ‘scratch marks’ (i.e. striae). They are all the result of fast fully motile arthropods walking on, or scratching, a sediment surface and as such occupied the surficial tier (Seilacher, Reference Seilacher, Schindewolf and Seilacher1955; Crimes, Reference Crimes1970; Fillion & Pickerill, Reference Fillion and Pickerill1990). The feeding habits of these primitive arthropods are still a debated topic among researchers. Dimorphichnus is generally regarded as a ‘grazing’ trace (Seilacher, Reference Seilacher, Schindewolf and Seilacher1955, Reference Seilacher and Said1990); however, it is not clear whether or not this organism grazed on microbial mats, fed on organic detritus, or preyed on meiofauna or small macrofauna. In fact, there is growing evidence that small epibenthic organisms may have constituted a significant part of the diet of many Cambrian arthropods (Vannier, Reference Vannier2012; Zacai et al. Reference Zacaï, Vannier and Lerosey-Aubril2016). Minter et al. (Reference Minter, Mángano and Caron2012) interpreted large, arthropod trackways attributed to a Tegopeltid arthropod as recording predation on meiofauna and small macrobenthic elements. As a result, all three possible feeding modes of life are shown as occupied in Figure 1. Therefore, they are classified as fast freely motile, surficial organisms, with a variety of feeding styles.
3.c. Gyrolithes ichnoguild
The Gyrolithes ichnoguild consists of Gyrolithes gyratus, Gyrolithes scintillus and Trichichnus cf. simplex (Fig. 2h, i, o, respectively). These are all vertical burrows, attaining a maximum depth of 3 cm, representing very shallow tier burrowers. They were probably originally deeper however, as all burrows have been truncated by erosion. The Gyrolithes ichnospecies were likely constructed by a vermiform organism (Laing et al. Reference Laing, Buatois, Mángano, Narbonne and Gougeon2018), while Trichichnus has been hypothesized as formed by large bacterial colonies (Kędzierski et al. Reference Kędzierski, Uchman, Sawlowicz and Briguglio2015; although see McBride & Picard, Reference McBride and Picard1991 and Gingras & Pickerill, Reference Gingras, Pickerill and Pemberton2002 for alternative possible producers). They are both slow, fully motile organisms. The Trichichnus producer is suspected to have relied on chemosynthesis, while Gyrolithes scintillus and G. gyratus likely fed through microbial gardening. Therefore, they are classified as slow fully motile organisms, occupying the very shallow tier, with ‘other’ feeding styles.
3.d. Helminthoidichnites ichnoguild
The Helminthoidichnites ichnoguild consists of Archaeonassa fossulata, Cochlichnus anguineus, Gordia isp., Helminthoidichnites tenuis and Helminthopsis tenuis (Fig. 2a, e, c, d, b respectively). These are all simple, horizontal trails, occupying the uppermost 0.5 cm of sediment (semi-infaunal). Their common association with microbially induced sedimentary structures suggests they all fed on microbial mats, implying a grazing feeding style (Buatois & Mángano, Reference Buatois and Mángano2003; Buatois et al. Reference Buatois, Narbonne, Mángano, Carmona and Myrow2014; Carbone & Narbonne, Reference Carbone and Narbonne2014). While Helminthoidichnites has been suggested as recording opportunistic scavenging of Ediacaran organisms in Australia (Gehling & Droser, Reference Gehling and Droser2018), this behaviour has not been documented in the CIF specimens. Consequently, these ichnofossils are essentially interpreted as pascichnial trails, combining feeding and locomotion, and as a result were likely slow fully motile semi-infaunal, grazers.
3.e. Treptichnus ichnoguild
The Treptichnus ichnoguild consists of Palaeophycus isp., Palaeophycus tubularis, Treptichnus coronatum (Fig. 2g, f, j respectively), Treptichnus isp. and Treptichnus pedum (Fig. 2k). These sub-horizontal to horizontal burrows penetrated 0.5–3 cm into the substrate, occupying a very shallow tier. As vermiform burrowers of a possible priapulid-like scalidophoran affinity (Kesidis et al. Reference Kesidis, Slater, Jensen and Budd2019), that likely seldom left their burrows, they are classified as either slow fully motile or facultative motile. The feeding mechanism of these tracemakers is difficult to discern, and it would be premature to create multiple ichnoguilds given this uncertainty. Palaeophycus may be the burrow of a passive predator or suspension feeder (Pemberton & Frey, Reference Pemberton and Frey1982). Treptichnus is often hypothesized to be the result of passive predation (Vannier et al. Reference Vannier, Calandra, Gaillard and Zylinska2010) or undermat mining (Seilacher et al. Reference Seilacher, Buatois and Mángano2005). As a result, only one ichnoguild is currently proposed; however, this may need to be subdivided when more information on the lifestyle of the burrowers is made available. Therefore, they are classified as slow fully motile or facultative motile, very shallow tier burrowers, with a variety of feeding styles (suspension feeding, undermat mining and predation).
4. Discussion
By plotting these ichnoguilds in a stratigraphic (Fig. 3) and ecospace framework (Fig. 1), a few initial trends can be noted. The Helminthoidichnites ichnoguild is documented within the Ediacaran Period (Crimes & Anderson, Reference Crimes and Anderson1985; Narbonne et al. Reference Narbonne, Myrow, Landing and Anderson1987; Landing et al. Reference Landing, Narbonne and Myrow1988). The Treptichnus and Bergaueria ichnoguilds appear just below the Ediacaran–Cambrian boundary, documenting a deeper tier than the stratigraphically older Helminthoidichnites ichnoguild, accompanied by a possible Gyrolithes (Gehling et al. Reference Gehling, Jensen, Droser, Myrow and Narbonne2001). Additionally, Treptichnus as well as Bergaueria isp. document more sophisticated methods of interacting with the substrate. Treptichnus pedum and T. coronatum are the earliest penetrative burrows, while Begaueria isp. may represent the first uncontroversial equilibrium structure. The deeper agrichnial and chemichnial ichnofossils (Gyrolithes ichnoguild) appear in large numbers slightly above (~3 m) the Ediacaran–Cambrian boundary accompanied by ichnofossils indicative of fast, freely motile organisms (Dimorphichnus ichnoguild). These represent the first true vertical burrows as well as the first evidence of limbs recorded in this section and globally.
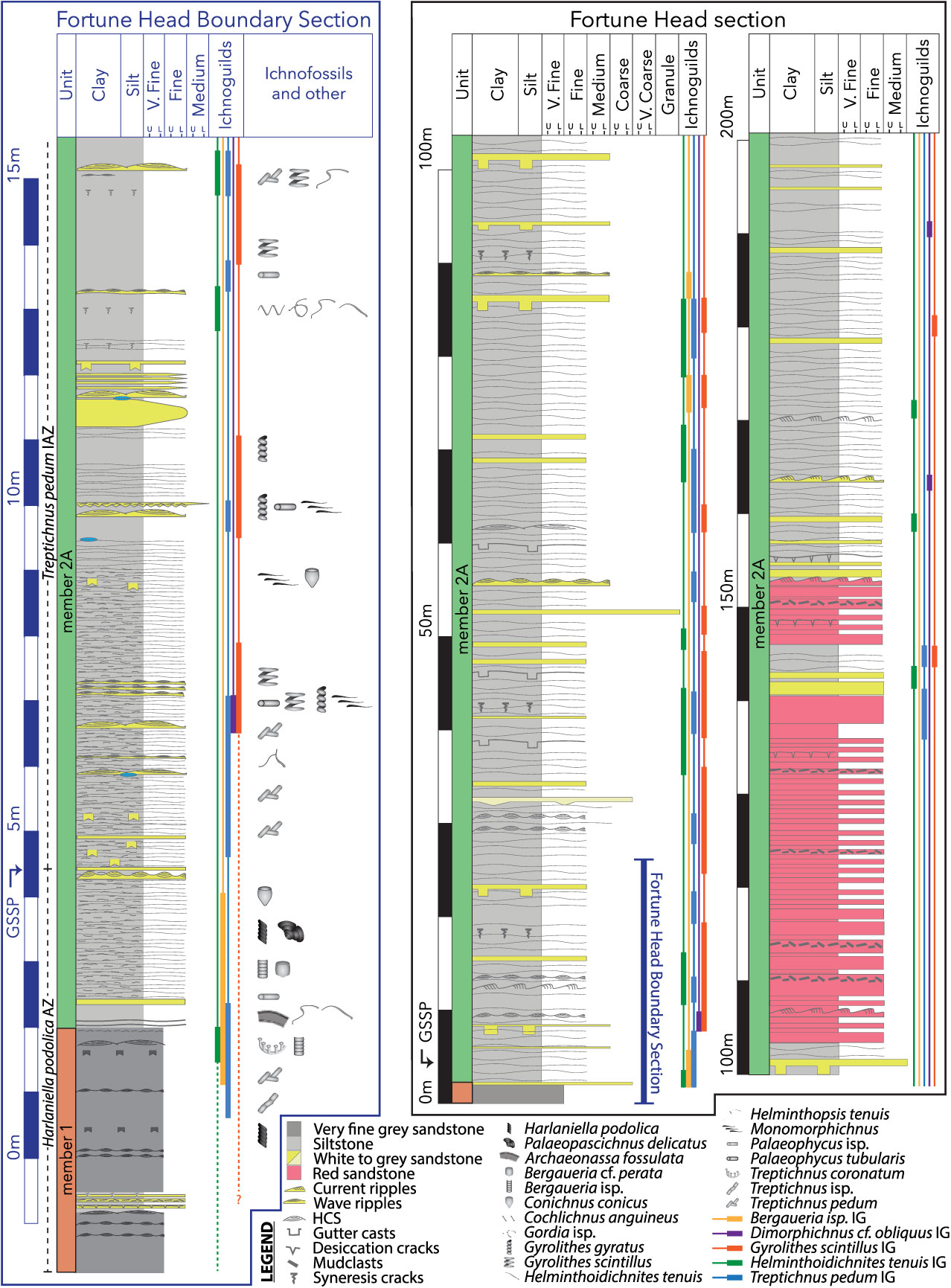
Fig. 3. Stratigraphic sections of the Chapel Island Formation at Fortune Head, Newfoundland. The rightmost two sections (surrounded by a black box) document the sedimentology and ichnoguild appearances of the last 4 m of member 1 and the T. pedum IAZ of member 2. In this section, the region indicated by a blue bar corresponds to the leftmost section (surrounded by the blue box). This section encompasses the Ediacaran–Cambrian boundary, and documents the sedimentology, ichnoguilds and ichnofauna therein.
The interval documented by the T. pedum IAZ is notoriously body-fossil poor, and ecospace analyses have been restricted to the Ediacaran and early-middle Cambrian (Bambach et al. Reference Bambach, Bush and Erwin2007; Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013). Fortunian ichnofossils therefore provide an important window between these two disparate faunas. The CIF trace fossils offer a local view into this critical time period. When contrasted with the modes of life hypothesized for Ediacaran fauna and early-middle Cambrian fauna (Fig. 1), a few initial trends can be detected. First, a transition in feeding styles can be seen. Hypothesized feeding styles for Ediacaran forms range from mat grazing (Buatois et al. Reference Buatois, Narbonne, Mángano, Carmona and Myrow2014; Gehling et al. Reference Gehling, Runnegar and Droser2014), mat digesting (Sperling & Vinther, Reference Sperling and Vinther2010), osmothrophy (Laflamme et al. Reference Laflamme, Xiao and Kowalewski2009) and chemosynthesis (Burzynski et al. Reference Burzynski, Narbonne, Dececchi and Dalrymple2017) (for a review see Droser & Gehling, Reference Droser and Gehling2015). While the Ediacaran fauna is dominated by ‘other’ and grazing feeding styles, early-middle Cambrian feeding types are similar to those recorded in modern benthic ecosystems, notably predation and deposit-feeding. Interestingly, the CIF ichnofauna documents a transition between the two. The Gyrolithes and Helminthoidichnites ichnoguilds show affinities with Ediacaran-like feeding-styles, such as chemosynthesis and mat grazing. However, the Cambrian-like predatory and deposit-feeding modes of life are documented by the three remaining ichnoguilds (Bergaueria, Dimorphichnus and Treptichnus ichnoguilds).
Second, burrowers (slow fully motile organisms) begin to explore deeper tiers than previously documented. Ediacaran tiering was restricted to benthic organisms attached to the sea-floor with no definitely pelagic or nektonic organisms (Clapham & Narbonne, Reference Clapham and Narbonne2002; Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013; Gold, Reference Gold2018). Documented burrowers existed in the latest Ediacaran Period, although they were restricted to the shallow infaunal realm and there is no discernible infaunal tiering (Jensen et al. Reference Jensen, Saylor, Gehling and Germs2000, Reference Jensen, Droser and Gehling2005, Reference Jensen, Droser, Gehling, Xiao and Kaufman2006; Jensen & Runnegar, Reference Jensen and Runnegar2005; Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013; Buatois & Mángano, Reference Buatois, Mángano, Olea and Wilson2016; Buatois et al. Reference Buatois, Mángano, Olea and Wilson2016, Reference Buatois, Almond, Mángano, Jensen and Germs2018). In the Fortunian of the CIF however, true vertical burrows represented by the Gyrolithes ichnoguild (Gyrolithes gyratus, G. scintillus and Trichichnus cf. simplex) attained a depth of at least 3 cm (Laing et al. Reference Laing, Buatois, Mángano, Narbonne and Gougeon2018). In turn, sub-horizontal burrows (Treptichnus and Palaeophycus) as well as sea anemone resting burrows (Begaueria isp.) occupied a greater proportion of the shallow infaunal tier than in the Ediacaran. While not well developed, the Gyrolithes, Treptichnus, Bergaueria and Helminthoidichnites ichnoguilds together demonstrate an infaunal tiering structure which was non-existent in the Ediacaran.
Finally, motile modes of life became more common. Arthropod striae of the Dimorphichnus ichnoguild mark the appearance of fast freely motile organisms. This evidences the appearance of limbed organisms and likely reflects the diversification and ecological success of the arthropod body plan. In turn, the burrow Bergaueria isp. demonstrates an ability to adjust with sedimentation, and may be the first equilibrium burrow recorded.
5. Conclusions
The Fortunian ichnofauna within the Treptichnus pedum IAZ at the basal Cambrian GSSP (Global Boundary Stratotype Section and Point) documents a wide variety of innovations characteristic of the Cambrian. Within 3 m of the basal Cambrian boundary, the ichnofossils document: (1) early penetrative burrows, first with the Treptichnus ichnoguild, then with the deeper Gyrolithes ichnoguild, (2) fast freely motile (limbed) organisms, with the Dimorphichnus ichnoguild, (3) uncontroversial equilibrium structures, with Bergaueria isp., and (4) the appearance of possible predators, with the Bergaueria, Dimorphichnus and Treptichnus ichnoguilds. In addition to these innovations, the ichnofossils document ecologic strategies characteristic of the Ediacaran, such as (5) chemosynthetic or ‘other’ feeding styles with the Gyrolithes ichnoguild and (6) grazing feeding styles, with the Helminthoidichnites ichnoguild. The CIF demonstrates that the ichnofauna right above the boundary in Newfoundland employed strategies which mirrored their transitioning ecosystems, utilizing both Ediacaran strategies and Cambrian strategies to survive.
Author ORCIDs
Brittany A. Laing 0000-0002-0874-8879
Acknowledgements
We thank Richard Thomas for facilitating our work in the Fortune Head Ecological Reserve under a Scientific Research Permit from Parks and Natural Areas, Newfoundland and Labrador. The manuscript has been improved thanks to the comments by Sören Jensen and an anonymous reviewer. Funding: This work was supported by Natural Sciences and Engineering Research Council (NSERC) Discovery Grants to G.M.N., L.A.B. and M.G.M. (05561-2014, 311726-13 and 311727-15 respectively), a Queen’s University Research Chair to G.M.N., and a 2016 Student Research Grant from the Society for Sedimentary Geology and a 2016 Research Grant from the Geological Society of America to B.A.L.
Declaration of interest
Brittany A. Laing, M. Gabriela Mángano, Luis A. Buatois, Guy M. Narbonne and Romain C. Gougeon certify that they have no conflict of interests.