Introduction
Death assemblages (i.e., the taxonomically identifiable dead or discarded organic remains present in the surficial mixed layer of a landscape [Kidwell Reference Kidwell2013]) are temporally coarse accumulations in which individuals belonging to past and present generations co-occur. These are dynamic open systems, where dead individuals are removed by the action of diverse postmortem processes while newly dead individuals are constantly incorporated (Kidwell and Tomašových Reference Kidwell and Tomašových2013). As a result of taphonomic biases and time-averaging, the ecological and environmental signals stored by the original living community can become distorted to some degree in death assemblages (Tomašových and Kidwell Reference Tomašových and Kidwell2009a). Understanding the extent of this distortion constitutes a topic of primary interest in paleoecological and paleoenvironmental research, because it allows discriminating taphonomic from biologically meaningful changes in fossil assemblages’ diversity and composition (Kidwell and Bosence Reference Kidwell and Bosence1991; Behrensmeyer et al. Reference Behrensmeyer, Kidwell and Gastaldo2000; Tomašových and Kidwell Reference Tomašových and Kidwell2009a). Moreover, the recognition of death assemblages as historical archives of community and ecosystem composition has extended the relevance of live-dead studies to the fields of ecology and conservation biology (Kidwell Reference Kidwell2013; Kidwell and Tomašových Reference Kidwell and Tomašových2013).
Live-dead comparisons are the most common actualistic methods of evaluating the preservation potential of ecological information (Kidwell Reference Kidwell2013). They have mostly focused on compositional fidelity (i.e., preservation of composition, richness and abundance [Tomašových and Kidwell Reference Tomašových and Kidwell2009a, Reference Tomašových and Kidwell2011]), whereas works on environmental fidelity (i.e., the ability of death assemblages to detect environmental gradients) have been particularly scarce (e.g., Stanton Reference Stanton1976; Overpeck et al. Reference Overpeck, Webb and Prentice1985; Miller Reference Miller1988; Gavin et al. Reference Gavin, Oswald, Wahl and Williams2003; Hassan et al. Reference Hassan, Espinosa and Isla2008; Tomašových and Kidwell Reference Tomašových and Kidwell2009b). Most of these have been studies of macroscopic organisms, such as mollusks (see Kidwell Reference Kidwell2001, Reference Kidwell2002a,Reference Kidwellb; Kidwell Reference Kidwell2013 for review), corals (e.g., Pandolfi and Minchin Reference Pandolfi and Minchin1996; Greenstein and Pandolfi Reference Greenstein and Pandolfi1997; Edinger et al. Reference Edinger, Pandolfi and Kelley2001), brachiopods (e.g., Carroll et al. Reference Carroll, Kowalewski, Simões and Goodfriend2003; Tomašových Reference Tomašových2004; Krause et al. Reference Krause, Barbour, Kowalewski, Kaufman, Romanek, Simões and Wehmiller2010), mammals (e.g. Western and Behrensmeyer Reference Western and Behrensmeyer2009; Terry Reference Terry2010a,Reference Terryb), and higher plants (e.g., Sugita Reference Sugita1994; Zhao et al. Reference Zhao, Sayer, Birks, Hughes and Peglar2006; Sims and Cassara Reference Sims and Cassara2009). Works on microorganisms have been mostly restricted to foraminifera (e.g., Scott and Medioli Reference Scott and Medioli1980; Bernhard Reference Bernhard2000; Murray and Pudsey Reference Murray and Pudsey2004) and ostracods (e.g., Park et al. Reference Park, Cohen, Martens and Bralek2003; Alin and Cohen Reference Alin and Cohen2009; Michelson and Park Reference Michelson and Park2013). Only a few works have been conducted in to assess live-dead agreement in sedimentary diatoms (Wilson and Holmes Reference Wilson and Holmes1981; Oppenheim Reference Oppenheim1987; Sawai Reference Sawai2001; Hassan et al. Reference Hassan, Espinosa and Isla2008). As a consequence, little is known about the nature and magnitude of the taphonomic biases affecting live-dead agreement of diatom assemblages, despite their extensive application as modern and fossil bioindicators in paleoecological and paleoenvironmental studies (see Smol and Stoermer Reference Smol and Stoermer2010 for a review).
The influence of taphonomic processes on the preservation of diatoms has been largely recognized (e.g., Lewin Reference Lewin1961; Barker Reference Barker1992; Flower Reference Flower1993; Sawai Reference Sawai2001; Ryves et al. Reference Ryves, Battarbee and Fritz2009). Postmortem processes such as mixing, dissolution, and breakage can significantly bias the composition of the assemblages, and thus affect quantitative and qualitative paleoenvironmental inferences (Haberyan Reference Haberyan1985; Flower Reference Flower1993; Ryves et al. Reference Ryves, Battarbee, Juggins, Fritz and Anderson2006, Reference Ryves, Battarbee and Fritz2009, Reference Ryves, Anderson, Flower and Rippey2013). Severe valve breakage has been associated with high-energy environments (Beyens and Denys Reference Beyens and Denys1982; Flower Reference Flower1993; Sawai et al. Reference Sawai, Jankaew, Martin, Prendergast, Choowong and Charoentitirat2009), grazing effects (Haberyan Reference Haberyan1985; Hamm et al. Reference Hamm, Merkel, Springer, Jurkoic, Maier, Prechtel and Smetacek2003; Austin et al. Reference Austin, Austin and Paterson2005), and compaction and drying of sediments (Flower Reference Flower1993). Valve dissolution occurs when diatom remains are exposed to water with low concentrations of dissolved silica, and it is strongly related to the salinity, pH and temperature of the surrounding medium (Flower and Ryves Reference Flower and Ryves2009). Because diatoms occupy a variety of habitats within lentic environments, their preservation potential is also related to their life form; for example, free-living planktonic taxa are expected to be more susceptible to transport and dissolution than periphytic taxa attached to rocks or plants (Sawai Reference Sawai2001). Moreover, because diatom dissolution and breakage are strongly dependent on the thickness, ornamentation, and surface-area-to-volume ratio of the valves (Lewin Reference Lewin1961; Haberyan Reference Haberyan1985; Van Cappellen et al. Reference Van Cappellen, Dixit and van Beusekom2002), differential preservation can exert significant bias on the composition of diatom assemblages by destroying fragile species (Ryves et al. Reference Ryves, Jewson, Sturm, Battarbee, Flower, Mackay and Granin2003, Reference Ryves, Anderson, Flower and Rippey2013).
Given these findings, diatom assemblages subjected to such a wide array of taphonomic biases might be expected to exhibit low live-dead compositional fidelity. In fact, if the variability introduced by taphonomic processes exceeds the original biological variability, then the capability of death assemblages to preserve the ecological information of the living community might be completely obliterated. Likewise, these taphonomic processes might shift the record of the responses of diatom assemblages to environmental gradients, thus testing the assumptions of paleoecological and paleoenvironmental analyses. However, if living communities exhibit structural redundancy (i.e., more than one mutually exclusive subset of species significantly captures community structure based on the full set of species [Clarke and Warwick Reference Clarke and Warwick1998]), live-dead agreement in the response of assemblages to environmental gradients can be preserved even under conditions of low compositional fidelity (Tomašových and Kidwell Reference Tomašových and Kidwell2009b). Given their high richness and diversity, most diatom data sets are likely to display structural redundancy, thus allowing hypotheses about a decoupling between community response to environmental gradients and community composition. The ability to assess such a decoupling therefore becomes a key issue in live-dead research.
In this contribution, I analyzed three live-dead data sets in order to evaluate the compositional and environmental fidelity exhibited by diatom death assemblages in shallow lakes. Three main points are addressed: (1) the ability of the death assemblages to preserve the composition of the original living community (compositional fidelity); (2) the ability of the death assemblages to preserve the original responses of living communities to environmental gradients (environmental fidelity); and (3) the presence of structural redundancy in the living communities and its relationship to compositional and environmental fidelity. I use these results to examine whether there is decoupling between compositional and environmental fidelity in diatom death assemblages and then discuss the implications for paleoenvironmental reconstructions.
Material and Methods
Field and Laboratory Methods
Sampling Strategy
The study was conducted in three shallow lakes located in the southeastern Pampa plain (Buenos Aires province, Argentina), close to the Atlantic coast: (1) Los Carpinchos (LC, 37°3′34″S; 57°19′56″W), (2) Las Mostazas (LM, 37°9′57″S; 57°14′50″W), and (3) Nahuel Rucá (NR, 37°37′21″S; 57°25′42″W). The climate is temperate humid or sub-humid with a mean annual temperature of 15°C and a mean annual precipitation of 1100 mm (Feijoó and Lombardo Reference Feijoó and Lombardo2007). The studied lakes have a surface of approximately 250 ha and maximum depths of 1.5–2 m. They are located along a north-south gradient covering a distance of approximately 60 km (Fig. 1). The three lakes are located in private farms, and the watersheds have been subjected to agricultural use for the past several decades. In each lake, sampling was carried out on a seasonal basis over a year. The sampling was designed in order to allow comparison between the two main habitats represented in each lake: the highly vegetated littoral zone (LIT) and the open waters area (OW). During each visit, six surface sediment samples were randomly collected with a piston core from both the LIT and the OW areas. Given the very low water levels, Las Mostazas became very muddy during the summer, preventing access to the sampling area. This was mostly a consequence of the settling of suspended sediments in its extensive and very shallow littoral area during the dry season. Hence, the LM data set included data from only three fieldtrips. Hence, the final data set comprised a total of 132 surface sediment samples (LC: 48; NR: 48; LM: 36) each one representing a live-dead pair. The top 1cm of each core was sliced in the field and preserved with alcohol.

Figure 1 Location of the studied lakes: Nahuel Rucá (NR), Los Carpinchos (LC) and Las Mostazas (LM).
Each sample was mounted on Brunel aquatic mounting medium and examined under a light microscope (1000× magnification) in order to determine relative abundances of diatom cells with intact chloroplasts (i.e., Living Assemblages, LA) and empty valves (i.e., Death Assemblages, DA), as well as the taxonomic composition of each assemblage. Estimation of the abundances of living diatoms for live-dead comparisons and bioassessment studies has largely relied on counts of cells containing intact chloroplasts (e.g., Owen et al. Reference Owen, Afzal and Cody1979; Pryfogle and Lowe Reference Pryfogle and Lowe1979; Wilson and Holmes Reference Wilson and Holmes1981; Oppenheim Reference Oppenheim1987; Sawai Reference Sawai2001; Hassan et al. Reference Hassan, Espinosa and Isla2008; Gillett et al. Reference Gillett, Pan and Parker2009). Although the criteria for “intact” chloroplasts are rather subjective, it is not difficult to distinguish cells with full, apparently intact chloroplasts from those in various stages of condensation or breakdown (i.e., sparse, small green droplets scattered within the cell [Stockner and Lund Reference Stockner and Lund1970]). This methodology is based on the assumption that upon death, the nonsiliceous components of a diatom deteriorate, leaving empty frustules, as diatom protoplasm degrades rapidly under typical biostratinomic conditions (Pryfogle and Lowe Reference Pryfogle and Lowe1979). Nonetheless, it is impossible to equate robust chloroplasts with a living cell without culturing individual cells (Stockner and Lund Reference Stockner and Lund1970), which I did not do in this study. However, in cases where the preservation of cytoplasmic structures in the fossil record is exceptional—often as a result of modern displacement along the sedimentary column due to invertebrate burrowing activity (Stockner and Lund Reference Stockner and Lund1970)—the chloroplast are usually condensed (Wolfe et al Reference Wolfe, Edlund, Sweet and Creighton2006; Tanimura et al. Reference Tanimura, Kato, Fukusawa, Mayama and Yokoyama2006). Moreover, good agreement between chloroplast-bearing cell counts and cultures in some freshwater lakes indicated that at least in these lakes the chloroplast counts are a realistic measure of algal viability in recent sediments (Stockner and Lund Reference Stockner and Lund1970). As a consequence, although exceptional preservation may lead to an overestimation of diatom viability in this study, this error should be minimal because only intact chloroplasts were considered.
In order to avoid sample size effects, valves were counted until 200 valves of both LAs and DAs were reached. Taxa were identified to the lowest possible taxonomic category following standard floras (see Hassan Reference Hassan2013).
In some cases, because the samples had not been chemically cleaned and were mounted in aquatic mounting medium, it was not possible to distinguish morphologically similar species, particularly in the LAs, where cytoplasmic contents were present. This methodological problem can lead to artificially introduced differences in richness and diversity between DA and LA. In order to prevent this effect and allow a direct comparison of DA and LA, valves of some problematic taxa (such as certain species belonging to the genera Nitzschia and Navicula) were grouped at the generic level in both LAs and DAs.
Environmental Variables
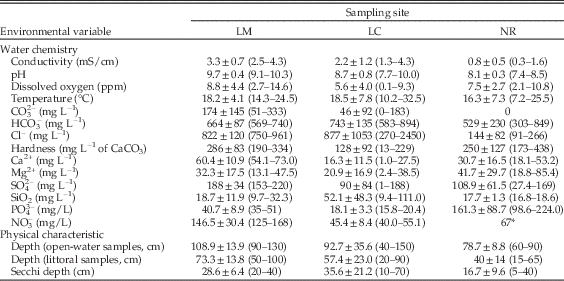
Six replicate measurements of conductivity, temperature, oxygen, pH and Secchi disk transparency (an estimative of turbidity, Wetzel Reference Wetzel2001) were taken seasonally at each lake with portable instruments. Water depth was measured with a meter stick at each sampling point (both littoral and open-water). Additionally, one subsurface water sample (1L) was taken in the center of each lake in each survey in order to determine chemical parameters through standard methods (APHA 1992). Water samples were collected in polyethylene bottles and stored in ice until they were transported to the laboratory. Water samples were analyzed within 15 days of collection for concentrations of nitrate (NO3−), sulfate (SO42−), chloride (Cl−), fluoride (F−), phosphate (PO43−), carbonate (CO3−2), bicarbonate (HCO3−), magnesium (Mg2+), calcium (Ca2+), silica (SiO2), and hardness (mg L−1 CaCO3; Table 1).
Table 1 Summary of environmental information. Values are mean±SD; minimum and maximum values are given in parentheses. LM, Las Mostazas; LC, Los Carpinchos; NR, Nahuel Rucá.

*Only one measurement available.
Data Analyses
Compositional Fidelity
In order to evaluate the live-dead agreement in species composition, I analyzed four different aspects of life and death diatom assemblages:
1. Fidelity in richness and diversity: The raw number of taxa (S; standardized at a sample size of n=200 individuals) and the Shannon-Wiener (H’) and Simpson (1−D) diversity indices were calculated for LAs and DAs in each sample using the package “vegan” version 2.0–7 (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, O’Hara, Simpson, Solymos, Stevens and Wagner2013) in R version 3.0.1 (R Development Core Team 2013). Differences in richness and diversity between both assemblages were evaluated with two-sample permutation tests using the permTS function in “perm” package version 1.0–0.0 (Fay and Shaw Reference Fay and Shaw2010), which allowed testing the null hypothesis of no difference between means of diversity in LAs and DAs.
2. Fidelity in species composition (pairwise similarity): Two similarity measures were calculated in order to capture different components of species turnover among pairs of LAs and DAs: (1) Bray-Curtis similarity (BC) based on untransformed proportional data, which is sensitive to changes in dominant taxa; and (2) Sorensen index (S) based on presence/absence data, which gives equal weights to abundant and rare taxa (Tomašových and Kidwell Reference Tomašových and Kidwell2009a). In order to analyze the patterns of distribution of the main diatom taxa across LAs and DAs, I plotted the relative abundances of dominant taxa (i.e., taxa which reached 20% in at least one sample and occurred in at least 50% of the samples) in LAs against the relative abundances in DAs. The significance of the differences between relative abundance in LAs and DAs in taxa that appreciably deviate from the 1:1 line was analyzed using Kruskal-Wallis tests, using the program PAST version 1.78 (Hammer et al. Reference Hammer, Harper and Ryan2001).
3. Live-dead differences in multivariate dispersions: I used a modified analysis of homogeneity in multivariate dispersions (HMD; Tomašových and Kidwell Reference Tomašových and Kidwell2011) in order to test for differences in live-dead multivariate dispersions in the total assemblage and between LIT and OW sites separately. This approach allows differentiating between the effects of premortem and postmortem processes on time-averaged assemblages. The analysis was based on two dissimilarity measurements: (1) Jaccard dissimilarity based on presence/absence data, which reflects the probability that two randomly chosen species from two assemblages do not belong to any species shared by both assemblages, and (2) Horn-Morisita dissimilarity, based on untransformed proportional abundances, which reflects the probability that two randomly drawn individuals from two assemblages do not belong to the same species (Tomašových and Kidwell Reference Tomašových and Kidwell2011). The HMD approach developed by Tomašových and Kidwell (Reference Tomašových and Kidwell2011) was applied using R version 3.0.1 (R Development Core Team 2013). In order to evaluate the degree of overlap between the multivariate spaces occupied by LAs and DAs, Principal Coordinates Analyses (PCoA) were applied to the different data sets using both Jaccard and Horn-Morisita indices as similarity measures through the program Past v 1.78 (Hammer et al. Reference Hammer, Harper and Ryan2001). Bivariate plots of dissimilarity between LAs and their centroid vs. dissimilarity between DAs and the centroid of LAs were constructed in order to evaluate the magnitude of the deviation of each data set from the expected line of good agreement (Tomašových and Kidwell Reference Tomašových and Kidwell2011).
Environmental Fidelity
I used two different approaches to evaluate the degree to which DAs capture the original environmental patterns affecting the structure of LAs:
1. Within-lake environmental fidelity: Assessing the ability of death assemblages to capture the intrinsic environmental compartmentalization (littoral vs. open waters) of each lake involved three approaches. First, differences in diversity and richness between littoral and open-water LAs and DAs were tested with two-sample permutation tests using the permTS function in “perm” package version 1.0–0.0 (Fay and Shaw Reference Fay and Shaw2010), which allowed testing the null hypothesis of no difference between means of diversity in littoral and open-water LAs and between littoral and open-water DAs. Second, to test for differences in the dispersion between littoral and open waters, I used tests of homogeneity of multivariate dispersions (Anderson Reference Anderson2006), analyzing living and dead assemblages separately. Dispersions are represented by distances of samples to their environment centroid (i.e., centroid of littoral and open-water sites) in a Euclidean space, calculated with PCoA. Dissimilarity matrices for life and death assemblages were constructed based on the Horn-Morisita index and tested under 999 permutations using the betadisper function in the package “vegan” version 2.0–7 (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, O’Hara, Simpson, Solymos, Stevens and Wagner2013). Finally, I used gradient analysis to assess the ability of life and death assemblages to capture environmental gradients in depth and vegetation coverage. Relative frequencies of taxa were square-root-transformed prior to gradient analyses in order to stabilize their variances. Compositional gradient length of the species data was estimated by means of Detrended Correspondence Analysis (DCA, detrending by segments, rare taxa down-weighted) in order to determine whether ordination techniques assuming linear or unimodal species distributions were most appropriate. In general, if the gradient length is greater than 2 standard deviation (SD) units, a unimodal response model is considered more appropriate. As gradient lengths resulted long enough (>2 SD, Supplementary Table 5), I used Canonical Correspondence Analysis (CCA, focused on interspecies distances, rare taxa down-weighted) with forward selection option to assess and compare the degree to which the variation in proportional abundance in life and death assemblages is explained by these environmental variables. The statistical significance was assessed by unrestricted ANOVA-like permutation tests (full model) involving 199 permutations. All ordinations were performed using the package “vegan” version 2.0–7 (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, O’Hara, Simpson, Solymos, Stevens and Wagner2013).
2. Between-lake environmental fidelity: In order to evaluate the ability of diatom death assemblages to preserve the environmental gradients captured by life assemblages at a regional scale, LAs and DAs from the three lakes were combined and their relationship to environmental variables examined. Only open-water samples (for which measurements of conductivity, pH, and Secchi depth were taken) were included in the data set. Two CCA analyses (one for LAs and the other for DAs) were performed on the combined data set in order to assess and compare the percentage of variance explained by major environmental variables. Partial CCAs (run with one explanatory variable at each time) were applied in order to calculate the percentage of variance explained by each environmental parameter in LAs and DAs. All analyses were performed as detailed in the previous section using the package “vegan” version 2.0–7 (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, O’Hara, Simpson, Solymos, Stevens and Wagner2013).
Structural Redundancy
I used the stepwise procedure BVSTEP (Clarke and Warwick Reference Clarke and Warwick1998) in order to analyze the structural redundancy within living communities. The procedure allows an “analytical peeling” of the data set, which involves selecting the smallest subset of species for which the Spearman rank correlation (ρ) with sample similarities for the full species set exceeds a predetermined value. I selected a threshold of ρ=0.75, given the moderate species richness found (63–85 species), following Tomašových and Kidwell (Reference Tomašových and Kidwell2009b). Once the first species subset was identified, I evaluated further subsets of species that could replicate the full community pattern, by excluding the first subset unit and rerunning the algorithm for the reduced species matrix against the full set. BVSTEP was run using 50 random starts and an initial number of trial species of 30, and the significance of rank correlations were estimated with permutation tests (999 random permutations) using the PRIMER v. 6.0 package (Clarke Reference Clarke1993).
Results
Compositional Fidelity
Fidelity in Richness and Diversity
Shannon-Wiener and Simpson indices, comparing total (littoral+open waters) LAs and DAs, showed significant differences both in Los Carpinchos (LC) and Nahuel Rucá (NR), but were not significant in assemblages from Las Mostazas (LM; Supplementary Table 1). Similar results were found when LAs and DAs were compared separately for littoral and open waters, which showed significant differences in all indices both in LC and NR, but only between Simpson indices in LM (Supplementary Table 1). Boxplots showed that richness and Shannon diversity were higher in LC and NR than in LM (Fig. 2). In these two lakes, richness and diversity were higher in DAs than in LAs in both littoral and open waters (Fig. 2). The ratio of dead to live species ranged from 0.63 to 1.5 in LM, from 0.87 to 2.9 in LC, and from 0.87 and 2.23 in NR. Mean values were 1.1 in LM and 1.4 in LC and NR, meaning that in these two lakes death assemblages were on average 40% more species rich than their corresponding living communities.

Figure 2 Boxplots of raw richness (at n=200 individuals), and Shannon-Wiener and Simpson indices comparing open-water (OW) and littoral (LIT) living (−L) and death (−D) assemblages.
Fidelity in Pairwise Similarity
Samples from LM exhibited a significantly higher fidelity in LAs-DAs pairwise distances both in species abundance (Bray-Curtis) and in presence/absence (Sørensen), when compared with NR and LC (p<0.001; Fig. 3). Several taxa showed noticeable deviation from the 1:1 line when proportional abundances of the dominant species in the LAs were plotted against proportional abundances of the same species in the DAs, both in littoral and in open-water samples, although biases were more evident in NR and LC than in LM (Fig. 4). Most of the taxa from LM showed similar proportional abundances in LAs and DAs, fitting the 1:1 line of the plot relatively well (Fig. 4). The only two exceptions were the epiphytic Cocconeis placentula, which had significantly higher abundances in DAs, from both littoral and open waters (p<0.001), and the benthic Nitzschia amphibia, which had significantly higher abundances in littoral LAs (p<0.05).

Figure 3 Boxplots of Sørensen (top) and Bray-Curtis (bottom) similarities between living and death assemblages in lakes Las Mostazas (LM), Los Carpinchos (LC), and Nahuel Rucá (NR). Identical letters indicate no statistically significant differences between similarities at α=0.05.

Figure 4 Bivariate plots of species’ proportional abundances in living assemblages (x-axis) vs. death assemblages (y-axis).
DAs from LC showed significantly higher abundances in Cocconeis placentula (OW and LIT: p<0.0001); and Navicula peregrina (OW: p<0.01; LIT: p<0.0001). On the other hand, two taxa were significantly more abundant in LAs in this lake: Aulacoseira granulata var. angustissima (OW and LIT: p<0.0001); and Pseudostaurosira subsalina (LIT: p<0.05).
In Nahuel Rucá there was significant deviation of the 1:1 line. Three species were more abundant in both littoral and open-water DAs: C. placentula (OW and LIT: p<0.0001), Navicula peregrina (OW: p<0.05; LIT: p<0.0001), ) and Surirella spp. (OW: p<0.01; LIT: p<0.05). Cyclotella meneghiniana and Aulacoseira granulata were more abundant in littoral waters (p<0.001 for both species). Other taxa had significantly higher proportional abundances in LAs, such as Aulacoseira granulata var. angustissima (OW: p<0.05; LIT: p<0.0001) and Pseudostaurosira subsalina (OW and LIT: p<0.05; Supplementary Table 2).
Live-dead Differences in Multivariate Dispersions
The HMD tests indicated that total LD variation was significantly larger than premortem variation in the three lakes when based on presence-absence data (Jaccard) and in two of the three lakes when based on relative abundances (Horn-Morisita), suggesting that DAs do not occupy the same portions of multivariate space, and thus differ from LAs. Overall, DAs were significantly over-dispersed (i.e., the variance from the centroid of LAs was larger among DAs than among LAs, Supplementary Table 3).
Live-dead agreement in multivariate dispersions was higher in Las Mostazas lake, where marginally significant differences were found when the Jaccard index was used (p=0.03, Supplementary Table 3), but not with Horn-Morisita (p=0.21, Supplementary Table 3). Although DAs were significantly over-dispersed, the magnitude of postmortem variation was relatively low (0.05). The magnitude of LD variation explained by premortem variation was higher for analyses using Jaccard (0.83; Fig. 5) than for those based on Horn-Morisita (0.74; Fig. 6) (Supplementary Table 3). Similar results were obtained when LD comparisons were performed separately between Las Mostazas LIT and OW habitats; over-dispersions were significant when based on Jaccard index (LIT p=0.001; OW p=0.03) but not with Horn-Morisita index (LIT p=0.22; OW p=0.1; Supplementary Table 3). Premortem variation explained a high percentage of total LD variation both when the Jaccard index (LIT ratio=0.77; OW ratio=0.76; Fig. 6, Supplementary Table 3) and when the Horn-Morisita index (LIT ratio=0.80; OW ratio=0.63; Supplementary Table 3, Fig. 7) was used.

Figure 5 First two axes of Principal Coordinates Analyses (PCoA) based on Jaccard (presence-absence) dissimilarities comparing multivariate ordinations of living (white circles) and death (black squares) assemblages in total, littoral, and open-water samples from the three lakes.

Figure 6 First two axes of Principal Coordinates Analyses (PCoA) based on Horn-Morisita (proportional abundance) dissimilarities comparing multivariate ordinations of living (white circles) and death (black squares) assemblages in total, littoral, and open-water samples from the three lakes.

Figure 7 Relationship between premortem variation among LAs (x-axis) and total live-dead variation (y-axis) when using Jaccard (black squares) and Horn-Morisita (white circles) dissimilarities. Light gray diagonal band in the plot defines the magnitude of change in assemblage composition caused by within-habitat time-averaging of LAs (sensu Tomašových and Kidwell Reference Tomašových and Kidwell2011).
In Los Carpinchos the magnitude of LD change in the composition of the diatom assemblages was higher than in Las Mostazas. DAs were significantly over-dispersed both in Jaccard and in Horn-Morisita analyses (p=0.001, Supplementary Table 3). The magnitude of postmortem dispersion was relatively low (0.06 based on Jaccard index and 0.14 based on Horn-Morisita index), and the magnitude of total LD variation explained by premortem variation was higher for Jaccard (0.79) than Horn-Morisita analyses (0.58). Similarly, when analyses were performed on LIT and OW assemblages separately, the composition was over-dispersed in all cases (Supplementary Table 3). Postmortem variation was equivalent to that found on the total data set, for both types of analyses (Jaccard: LIT ratio=0.07; OW ratio=0.08; Horn-Morisita: LIT ratio=0.14; OW ratio=0.13). Premortem variation was better able to explain total LD variation when the Jaccard index was used (LIT ratio=0.77; OW ratio=0.74; Fig. 6, Supplementary Table 3) than when the Horn-Morisita index was used (LIT ratio=0.57; OW ratio=0.54; Fig. 7, Supplementary Table 3).
In Nahuel Rucá deviations in LD compositions were also significant (Supplementary Table 3). In this lake, DAs were significantly over-dispersed when total and LIT assemblages were considered, but not when analyses were based on OW samples (Supplementary Table 3). Overall, analyses based on Jaccard index yielded very similar results, with postmortem biases ranging between 0.05 and 0.09 and ratios of premortem to total LD variation between 0.72 and 0.86 (Supplementary Table 3). Results of analyses based on Horn-Morisita yielded postmortem biases ranged between 0.05 and 0.16 and ratios of premortem to total LD variation between 0.47 and 0.91 (Supplementary Table 3).
Bivariate plots (Fig. 7) showed that sites from Las Mostazas fall along the expected line of correlation for good agreement, or slightly above it, as expected from within-habitat time-averaging (Tomašových and Kidwell Reference Tomašových and Kidwell2011). A few sites are located above this expected line, indicating that they are over-dispersed—because the variance from the centroid of LAs is larger among DAs than among LAs—and are subjected to significant postmortem biases and/or between-habitat time-averaging. This effect was more notable when sites of Los Carpinchos and Nahuel Rucá were plotted (Fig. 7), as a high number of sites fell above the expected line of agreement. In these lakes, postmortem processes have significantly affected the composition of DAs, both when presence/absence and when abundance data are considered. Additionally, a number of sites fell into the lower triangular area plus the dark gray shaded band above the line of unity, indicating that live-dead differences could be explained entirely by within habitat time-averaging (Fig. 7).
Environmental Fidelity
Within-lake Environmental Fidelity
Richness of LAs showed no significant differences between LIT and OW in any of the lakes (Supplementary Table 1). In contrast, both diversity indices showed significantly higher values in open-water than in littoral LAs in Las Mostazas, but no significant differences in LAs from the other two lakes (Fig. 2, Supplementary Table 1). For DAs, on the other hand, there were significantly higher values or richness and diversity in littoral assemblages than in open-water assemblages for Nahuel Rucá and Los Carpinchos, but not for Las Mostazas (Fig. 2, Supplementary Table 1).
The test for homogeneity of multivariate dispersions showed a general similarity between the dispersions of LIT and OW assemblages, both in LAs and in DAs of the three lakes. Non-significant results were obtained in all tests, except for LAs in Las Mostazas, where marginally significant results were obtained (F=4.24; p=0.047; Supplementary Table 4).
According to CCA ordinations, depth and vegetation cover have similar effects on the composition of living and death diatom assemblages, as the proportion of compositional variation explained by these environmental parameters and their significance were similar for both DAs and LAs (Supplementary Table 5). Correlations between species and environmental variables were also well preserved in DAs (Supplementary Table 5). The ordination plots show similar segregation of LIT and OW sites both in DAs and in LAs, as well as similar lengths and directions of the depth and vegetation cover gradients (Fig. 8).

Figure 8 Canonical correspondence analysis biplots of littoral (black squares) and open-water (white circles) samples from living assemblages (left column) and death assemblages (right column) against environmental variables in the three studied lakes. Veg=vegetation cover.
Between-lake Environmental Fidelity
Results of CCA analyses indicated that death assemblages capture environmental gradients better than life assemblages. The proportion of compositional variation explained by the environmental variables was higher in DAs (20.6%) than in LAs (16.1%, Supplementary Table 6).
Correlations between species and environmental variables were high and well preserved in DAs, and ordinations were significant in both cases (Supplementary Table 6). Partial CCAs indicated that conductivity was the strongest variable, explaining 9.85% of the variance in LAs, followed by depth (4.66%), Secchi depth (2.20%), and pH (2.14%; Supplementary Table 7). The percentage of variance explained by these environmental variables and their corresponding significance increased noticeably in DAs: although conductivity remained the strongest explanatory variable (12.26%), it was followed by depth (10.66%) and pH (10.66%), whereas Secchi depth explained only 3.55% of the variance (Supplementary Table 7). Hence, DAs captured environmental gradients better than LAs, particularly for the pH gradient, which explained a higher percentage of variance in DAs with respect to LAs. The ordination plots showed similar segregations of sites from the three lakes both in DAs and in LAs (Fig. 9).

Figure 9 Canonical correspondence analysis biplots of living (left) and death (right) assemblages from Las Mostazas (black squares), Los Carpinchos (gray pentagons), and Nahuel Rucá (white circles) against environmental variables.
Structural Redundancy
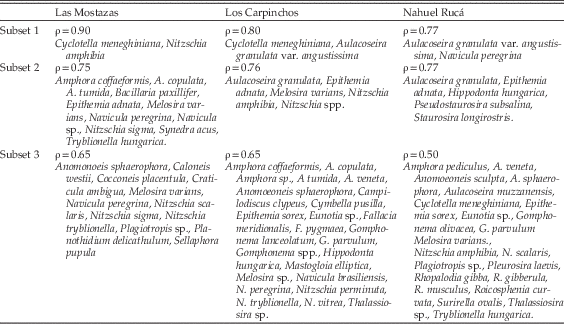
The application of “analytical peeling” to the data sets of the three lakes showed that living diatom assemblages present structural redundancy; at least two different subsets of species significantly replicated the community structure of the full assemblage in each lake at ρ=0.75 (Table 2).
Table 2 Taxonomic composition of species subsets from the peeling procedure for the three lakes studied. Spearman rank-correlation values (ρ) are given. All correlations significant at p<0.001.

In Las Mostazas, the first subset comprised only two taxa, the planktonic Cyclotella meneghiniana and the benthic Nitzschia amphibia, and achieved a high correlation (ρ=0.90, p<0.001). The second subset also reached the correlation threshold (ρ=0.75, p<0.001) and comprised 11 taxa, which included planktonic (i.e., Melosira varians), benthic (i.e., Amphora coffaeformis, A. copulata, A. tumida, Bacillaria paxillifer, Navicula peregrina, Navicula sp., Nitzschia sigma, Tryblionella hungarica), and epiphytic taxa (i.e., Epithemia adnata, Synedra acus). The correlation of the third subset, comprising 12 taxa, was significant but below the correlation threshold (ρ=0.65, p<0.001, Table 2).
The first subset from Los Carpinchos data set comprised two planktonic taxa (Cyclotella meneghiniana and Aulacoseira granulata var. angustissima), which attained high rank correlation to the total assemblage (ρ=0.80, p<0.001). The second subset also presented a correlation above the threshold (ρ=0.76, p<0.001), and included five taxa: the planktonic Aulacoseira granulata and Melosira varians, the epiphytic Epithemia adnata, and the benthic Nitzschia amphibia and Nitzschia spp. The third subset was composed of 24 taxa, and presented a significant but low correlation with the total assemblage (ρ=0.65, p<0.001).
For the Nahuel Rucá data set two subsets were identically correlated with the total data set (ρ=0.77, p<0.001). One subset comprised two taxa: the planktonic Aulacoseira granulata var. angustissima and the benthic Navicula peregrina. The second subset comprised five taxa: the planktonic Aulacoseira granulata, the benthic Hippodonta hungarica, and the periphytic Epithemia adnata, Pseudostaurosira subsalina and Staurosira longirostris. The third subset yielded a low but significant correlation (ρ=0.50, p<0.001) and was composed of 22 taxa (Table 2).
Discussion
Low Compositional Fidelity of Death Diatom Assemblages
The different estimates of compositional fidelity indicate a broadly unfaithful representation of living diatom communities by death assemblages: LAs and DAs were significantly different in richness, diversity, and multivariate dispersions in two of the three shallow lakes analyzed (NR and LC). Furthermore, for several taxa the proportional abundances were significantly different between LAs and DAs, becoming either under- or overrepresented in the latter.
Biases in the fidelity of richness and diversity were related to significantly higher numbers of taxa and higher values of Shannon and Simpson indices in DAs than in LAs. These results are in agreement with live-dead studies focused on mollusks, which demonstrated that sedimentary death assemblages contain far more species than the local living communities (see Kidwell Reference Kidwell2002a and references therein). Excess of richness in DAs could be an artifact of insufficient sampling of the LA: given the patchy or ephemeral nature of some living populations, they might be excluded from the data set when the sampling period is short or the environmental heterogeneity is not fully covered (Kidwell Reference Kidwell2002a). Even when I sampled living diatom communities on a seasonal basis, the samples collected in each lake would represent only snapshots of the full seasonal variability, which can be high in diatoms given their very short generation times (on the order of hours to a few days, Baars Reference Baars1981). DAs, on the other hand, constitute time-averaged representations of living communities and integrate dead-valve inputs over long periods of time, capturing demographic and environmental stochasticity and leading to time-averaged species richness (Kidwell Reference Kidwell2002a; Tomašových and Kidwell Reference Tomašových and Kidwell2011). Enrichment of death assemblages can also be related to the input of valves from lake sub-environments not included in the sampling strategy, such as epiphytic taxa living upon macrophytes (e.g., Cocconeis placentula). Hence, it is expected that, given longer sampling periods and more intensive sampling of all lake sub-environments, pooled living community richness and diversity values would more closely approximate those of the DAs.
HMD indicated that species composition of DAs significantly differed from that of LAs in the three lakes when based on presence-absence data (Jaccard) and in two of the three lakes when based on proportional abundances (Horn-Morisita). Although a small proportion of this over-dispersion can arise from within-habitat time averaging (Tomašových and Kidwell Reference Tomašových and Kidwell2011), many samples were significantly affected by postmortem biases in species composition. This additional variation in assemblage composition can be created by (1) time-averaging within one or multiple successive habitats (environmental condensation), (2) differential turnover and preservation among species, and (3) human modification of the living community within the window of time-averaging (Tomašových and Kidwell Reference Tomašových and Kidwell2011; Kidwell Reference Kidwell2013). By examining the differences in the proportional abundances of taxa between living and death assemblages I was able to identify a group of species that were significantly under- or overrepresented in death assemblages. Most of the taxa whose proportional abundances were higher in DAs than in LAs (i.e., Navicula peregrina, Surirella striatula, Aulacoseira granulata, Cyclotella meneghiniana) are characterized by highly silicified and robust valves (Barker Reference Barker1992). On the other hand, valves of taxa with significantly lower abundances in DAs than in LAs (i.e., Nitzschia amphibia, Pseudostaurosira subsalina, Aulacoseira granulata var. angustissima) are weakly silicified and more prone to fragmentation given their elongated shape and denser areolation patterns (Haberyan Reference Haberyan1985). These live-dead differences in abundance, linked to strong differences in the intrinsic durability of species, are consistent with preservational bias as opposed to other possible bias sources. Moreover, given their very shallow nature, the lake floors are likely subject to significant reworking of sediments as a consequence of wind action. The resulting turbulence would favor breakage and subsequent dissolution of valves (Ryves et al. Reference Ryves, Battarbee, Juggins, Fritz and Anderson2006). Because the probability of a diatom valve’s breaking is strongly related to its length/width ratio and pore row frequency (Haberyan Reference Haberyan1985), differential breakage of taxa in these shallow lakes is probably responsible for the observed deviations in proportional abundances. Moreover, variation in the fidelity exhibited by the three lakes may also be explained by the differential breakage of dominant taxa: the greater fidelity found in Las Mostazas could be related to lower proportional abundance of the fragile Aulacoseira granulata var. angustissima in the LAs than either Nahuel Rucá or Los Carpinchos.
Live-dead mismatches in marine mollusk assemblages have also been related to human impact: if anthropogenic stressors have altered LA composition during the window of time-averaging, a significant live-dead difference might arise if the DA retains memory of pristine environmental conditions (Kidwell Reference Kidwell2013). The three studied lakes lie in the Argentinian Pampas, an area of intensive agricultural activity during the past 30 years. Although diatom analyses of Holocene sedimentary sequences in pampean lakes have described evidence of increased nutrient loading in recent sediments (Hassan Reference Hassan2013), the high sedimentation rates and very short generation times of diatoms raise doubts about the ability of surface-sediment death assemblages to represent pre-impact conditions. According to previous paleoenvironmental research, the topmost 10–20 cm of the cores record modern lake conditions; pre-historical assemblages are buried below this core depth (Hassan Reference Hassan2013). Moreover, the taxonomic differences between LAs and DAs do not indicate a selective replacement of taxa according to their nutrient preferences. Although more testing is needed, it appears that pre-impact diatom DAs in this study would be outside the window of time-averaging for surface-sediment DAs in this study; the live-dead mismatch is more probably related to differential preservation of taxa than to anthropogenic effects.
Environmental Fidelity
Living diatom assemblages varied along environmental gradients in the studied lakes. Results of CCA indicated that environmental variables exert significant influence on the community composition of LAs at both local and regional scales. Remarkably, despite the low compositional fidelity of DAs to LAs, DAs were able to capture the same environmental gradients as LAs with high significance. What is more, the percentage of variance in diatom composition explained by the environmental variables was higher in DAs than in LAs, both in analyses based on single lakes and those using total data sets, indicating that DAs were more sensitive to environmental gradients than were living assemblages. This better performance of DAs over LAs has also been mentioned in mollusk-based environmental fidelity studies, and has been related to the capability of DAs to capture community composition (especially rare species) over longer temporal durations, given the homogenization of demographic and environmental stochasticity, which can blur composition of living assemblages over short durations (see Tomašových and Kidwell Reference Tomašových and Kidwell2009b and references therein). Overall, this good agreement implies that past environmental conditions inferred from fossil diatom assemblages would be even more accurate than those based on a single sampling of the original living community, thus supporting one of the main assumptions of diatom-based paleoenvironmental reconstructions.
The surprising result of low live-dead agreement in assemblage composition and yet good live-dead agreement in assemblage response to gradients can be explained by the presence of structural redundancy in living diatom communities. This redundancy was inferred from the existence of at least two mutually exclusive subsets of species that significantly captured compositional dissimilarities based on the full set of species in the three lakes (at ρ=0.75). Such redundancy in environmental information implies that the between-sample relationships of living assemblages can be significantly preserved by DAs even if some taxa are randomly removed by taphonomic processes or are missed during sampling (Tomašových and Kidwell Reference Tomašových and Kidwell2009b). Preservation of environmental gradients does not require good preservation of all living taxa, because structural redundancy can compensate for compositional fidelity that is lost through postmortem biases in the diatom data set. For example, the underrepresentation of Aulacoseira granulata var. angustissima in DAs of Nahuel Rucá is compensated by the overrepresentation of A. granulata in the same assemblages, as both taxa are grouped in two subsets with identical significant correlations to the total data set (Table 2). Nevertheless, this structural redundancy does not necessarily imply functional redundancy (Clarke and Warwick Reference Clarke and Warwick1998): the different subsets might respond identically to some environmental gradients (those tested) but differently to others (Tomašových and Kidwell Reference Tomašových and Kidwell2009b). The good preservation of environmental gradients observed in diatom assemblages here thus does not imply similar results for environmental characteristics that weren’t considered in this study, such as nutrient status or longer conductivity and pH gradients.
Conclusions
Analysis of compositional and environmental fidelity of three diatom data sets from shallow lakes revealed that death assemblages do differ significantly in their taxonomic composition from living assemblages. This difference is probably mainly a consequence of (1) differences in the temporal resolution between time-averaged DAs and non-averaged LAs, and (2) differential preservation of diatom taxa related to the intrinsic properties of their valves, specifically the overrepresentation of robust species and underrepresentation of fragile ones. Because diatom assemblages are characterized by a wide variability of cell sizes, valve forms, and ornamentation, effects of differential preservation would be variable and highly dependent on the taxonomic composition of the local community. The significant effects of taphonomic constraints on compositional fidelity found here enhance the importance of considering the relative preservation potential of diatom taxa when conducting paleoenvironmental studies. What is more, the role of fragmentation in structuring sedimentary diatom assemblages in these wind-stressed shallow lakes requires further investigation.
Compositional dissimilarities notwithstanding, DAs were able to preserve environmental gradients with high fidelity, mainly as a consequence of the structural redundancy exhibited by diatom LAs. This is good news for diatom-based paleoenvironmental studies, as it implies that multi-species diatom assemblages would be able to preserve environmental information even in situations of low compositional fidelity. However, because redundancy is an emergent property of communities, the decoupling of compositional and environmental fidelity would not favor paleoenvironmental inferences based on interpretation of autecological (i.e., ecology of single taxa) data. In contrast, statistical inference models derived from contemporary species-environment relationships would be more likely to benefit from multi-species redundancy. Overall, knowledge on the taphonomic constraints on the sedimentary record of each diatom species is crucial for understanding the usefulness and limitations of these taxa as archives of past environmental conditions.
The scope of the conclusions of this study is somewhat limited by the number and nature of the sites included in the data set. Because diatom preservation is strongly linked to pH, temperature, ionic strength, sediment reworking, and resuspension (e.g., Lewin Reference Lewin1961; Flower and Nicholson Reference Flower and Nicholson1987; Flower Reference Flower1993; Barker Reference Barker1992; Ryves et al. Reference Ryves, Battarbee, Juggins, Fritz and Anderson2006), different physico-chemical conditions may lead to different patterns of live-dead agreement. This work focused on diatoms from alkaline, fresh- to brackish eutrophic shallow lakes, which are numerous in the South American Pampas and constitute the primary source of paleoenvironmental information and, consequently, of modern-analogue data sets (García-Rodriguez et al. Reference García-Rodríguez, Piovano, del Puerto, Inda, Stutz, Bracco, Panario, Córdoba, Sylvestre and Ariztegui2009). Hence, whether the results or conclusions apply to a wider range of lakes of different physical and chemical characteristics requires further study. Unfortunately, the dearth of diatom live-dead calibration studies in freshwater settings prevents discussion of these results in a wider context, and thus enhances the need for more studies on diatom fidelity in continental settings worldwide.
Acknowledgments
Financial support for this study was provided by the National Council of Scientific and Technical Research of Argentina (CONICET, PIP 0063/10) and University of Mar del Plata (EXA 587/12). I am indebted to P. Urrutia, H. Sanabria, and S. González Aguilar for permission to sample on the lakes, and to D. Martínez and G. Bernava for water chemistry analyses. C. G. De Francesco, E. Tietze, P. Cristini, L. Ferrero, and M. A. González-Sagrario helped with fieldwork. A. Tomašových made very useful suggestions on statistical analyses. C. G. De Francesco, E. Tietze, S. Kidwell, and an anonymous reviewer made helpful comments on earlier versions of this manuscript. G. Hassan is a member of the Scientific Research Career of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Supplementary Material
Supplemental materials deposited at Dryad: doi:10.5061/dryad.313dq