For the majority of children, language acquisition is an early, rapid, and seemingly effortless process. However, a sizable group of children struggles to acquire their native language despite the absence of apparent sensory (e.g., hearing) deficits, general cognitive (e.g., nonverbal intelligence) impairments, and other known psychiatric, genetic, and neurodevelopmental conditions (e.g., autism spectrum disorder or epilepsy). The prevalence of this communication disorder, termed developmental language disorder (DLD), has been estimated to be around 7% among preschoolers (Tomblin et al., Reference Tomblin, Records, Buckwalter, Zhang, Smith and O'Brien1997), thus rendering it one of the most prevalent neurodevelopmental disorders.Footnote 1 Despite the relatively high prevalence of DLD in the general population, its developmental continuity into adolescence and adulthood (Poll, Betz, & Miller, Reference Poll, Betz and Miller2010; Stothard, Snowling, Bishop, Chipchase, & Kaplan, Reference Stothard, Snowling, Bishop, Chipchase and Kaplan1998), and its significant negative impact on children's academic, socioemotional, and occupational outcomes (Conti-Ramsden & Botting, Reference Conti-Ramsden and Botting2008; Conti-Ramsden & Durkin, Reference Conti-Ramsden and Durkin2008; Durkin & Conti-Ramsden, Reference Durkin and Conti-Ramsden2007; Durkin, Conti-Ramsden, & Simkin, Reference Durkin, Conti-Ramsden and Simkin2012; Wadman, Botting, Durkin, & Conti-Ramsden, Reference Wadman, Botting, Durkin and Conti-Ramsden2011; Wadman, Durkin, & Conti-Ramsden, Reference Wadman, Durkin and Conti-Ramsden2008), little is known about the cognitive, neural, and genetic etiologies of DLD.
Children with DLD are a heterogeneous population, and they show deficits in the development and functioning of multiple domains of spoken language, in both production and comprehension. Morphosyntactic deficits, manifested by failure to acquire and/or efficiently use grammar, have been proposed as a hallmark of the disorder. Children with DLD have documented deficits in expressive morphology (e.g., omissions or incorrect use of morphological forms in a sentence; Bedore & Leonard, Reference Bedore and Leonard2001; Dromi, Leonard, Adam, & Zadunaisky-Ehrlich, Reference Dromi, Leonard, Adam and Zadunaisky-Ehrlich1999; Leonard & Eyer, Reference Leonard, Eyer, Morgan and Demuth1996) and comprehension and production of complex syntactic structures such as wh-questions (Friedmann & Novogrodsky, Reference Friedmann and Novogrodsky2011), verbal passives (Marshall, Marinis, & van der Lely, Reference Marshall, Marinis and van der Lely2007), and relative clauses (Stavrakaki, Reference Stavrakaki2001).
Lexical and phonological development deficits in DLD are frequently less severe than morphosyntactic deficits and, arguably, have been less frequently studied than the latter. Thus, children with DLD have been shown to have atypical or less detailed phonological representations and abnormal phonological processing compared to their typically developing (TD) peers (Claessen, Leitao, Kane, & Williams, Reference Claessen, Leitao, Kane and Williams2013; Gray, Reiser, & Brinkley, Reference Gray, Reiser and Brinkley2012; Haake, Kob, Wilmes, & Domahs, Reference Haake, Kob, Wilmes and Domahs2013), as well as a markedly reduced phonological working memory capacity (for a meta-analysis, see Estes, Evans, & Else-Quest, Reference Estes, Evans and Else-Quest2007). In the lexical–semantic domain, empirical studies found deficits in learning and retaining new lexical items by children with DLD (Ellis Weismer & Hesketh, Reference Ellis Weismer and Hesketh1996), deficits in the size and depth of their vocabularies and semantic knowledge (Brackenbury & Pye, Reference Brackenbury and Pye2005; McGregor, Oleson, Bahnsen, & Duff, Reference McGregor, Oleson, Bahnsen and Duff2013; Sheng, Pena, Bedore, & Fiestas, Reference Sheng, Pena, Bedore and Fiestas2013), and abnormal dynamics of spoken-word recognition (McMurray, Samelson, Lee, & Tomblin, Reference McMurray, Samelson, Lee and Tomblin2010).
Atypicalities in the domains of lexical and phonological development in DLD contribute to the behavioral heterogeneity of the disorder but also have important implications for our understanding of both typical and atypical language development, especially in the context of developmental neuroplasticity. Because deficits in these domains are frequently less severe than grammatical deficits in DLD, they have been conceptualized as representing relatively spared domains of language development and functioning in DLD, or domains in which deficits are secondary to deficits in other linguistic and general cognitive systems, or both.
In general, as the development of language unfolds in time and its facets (i.e., phonological, lexical, and morphosyntactic) do not mature simultaneously, it is plausible that they do not manifest similar degrees of deficits and that their brain correlates also do not manifest similar degrees of atypicalities. This reasoning corresponds to the general hypothesis of neuroplasticity, where the development of language has a significant impact on the development of the brain (Bishop, Reference Bishop2013). Thus, those facets of language development that are impaired lead to more, rather than fewer, pronounced atypicalities in the brain response to language stimuli.
Specifically, under the residual normality view, language development in children with DLD is relatively selectively affected in the domain of morphosyntax. For example, Fonteneau and van der Lely (Reference Fonteneau and van der Lely2008) investigated neural responses of children with grammatical DLD (grammatical specific language impairment) to both syntactic and semantic violations. They showed that semantic violations (i.e., a noun that violated the verb's semantic [animacy] selection restrictions in auditorily presented sentences) produced a predicted robust electrophysiological response (N400; see below) in children with DLD, as well as TD children. Violations that relied on structural syntactic dependencies produced a robust early left anterior negativity (ELAN) component in TD children, postulated to index early automatic processing of structural dependencies. The ELAN component was not present in the data obtained from children with DLD, who instead displayed a later N400 in response to these violations (the absence of the ELAN nearly perfectly classified individual children as having DLD). Fonteneau and van der Lely (Reference Fonteneau and van der Lely2008) suggested that these results support the presence of selective grammatical deficits in children with grammatical DLD with the appearance of the N400 indexing “a relative strength in semantic processing” (p. 4). Note that under this view, children's morphosyntactic deficits are functionally decoupled from their language ability in other (i.e., lexical) domains.
Moreover, the emergence of the N400 in children with DLD in response to syntactic violations can be viewed as the result of a neuroplastic change of the language-processing system in DLD, instead of (or in addition to) being indicative of residual normality of lexical–semantic processing in DLD. In this case, the apparent application of semantic processing strategies to syntactic violations indexes the developmental coupling of syntactic and lexical–semantic processing in the context of disordered language development. Although the disorder might seemingly selectively affect children's grammar, it has profound effects on the efficiency of the language system as a whole, resulting in plastic changes to its organization. For example, it has been proposed that children with DLD have deficits in the neural systems underlying procedural memory, whereas the declarative memory system (supporting the development of the lexical and semantic knowledge) is relatively intact (Ullman & Pierpont, Reference Ullman and Pierpont2005). According to this procedural deficit hypothesis account of DLD, some observed declarative deficits in DLD may be largely attributed to high demands placed on cognitive systems that partially overlap with the procedural memory system, such as working memory. Although specifying a relatively selective impairment in the procedural memory (and grammar) in DLD, this account also suggests that declarative memory might at least partially compensate for functions that rely on procedural memory (Lum, Conti-Ramsden, Page, & Ullman, Reference Lum, Conti-Ramsden, Page and Ullman2012). This compensatory view is partially supported by a recent behavioral study that showed that children with DLD are more susceptible to lexical–semantic priming than are their TD peers (Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011) and suggested that the overactivation of semantic associates by children with DLD is due to compensatory reliance on lexical semantics in the face of grammatical deficits.Footnote 2
The complex pattern of postulated developmental links between different domains of language impairments in DLD is likely driven by the extreme heterogeneity of DLD as a population that exists at least at three different yet related levels: behavioral, cognitive, and etiological. In her recent review of the limited number of studies of lexical learning and processing in DLD, Nation (Reference Nation2014) emphasized the importance of studying the role of lexical deficits in the etiology of DLD, for example, establishing whether lexical deficits are a consequence of deficits in other domains of linguistic and cognitive development. From our perspective, at the current stage of the development of the field, a prerequisite task is establishing the place of lexical–semantic deficits in the overall syndromic DLD profile and providing a detailed characterization of these deficits, that is, describing them at the level of the underlying neurobiology and relating them to deficits in other domains both correlationally and in the context of experimental manipulations that explicate interplays between different domains of language development (e.g., lexical and phonological). The study reported in this paper capitalizes on the multidimensional approaches to studying DLD while using neural endophenotypes of lexical processing and maintaining the neuroplasticity assumption that specifics of this processing characteristic of children with DLD shape the specifics of the brain's response to the corresponding stimuli. We will now touch upon both of these general ideas prior to reviewing psychophysiological studies of lexical processing in DLD published to date and describing the goals of the current study.
First, we note that the field of DLD is slowly transitioning toward much-needed dimensional approaches to understanding the nature of the disorder. Thus, the focus is shifting from trying to uncover the single “core” deficit that would explain the multitude of behavioral manifestations of DLD toward providing a functional characterization of the cognitive systems underlying different deficit domains and the relationships between them. For example, a recent study by Ramus, Marshall, Rosen, and van der Lely (Reference Ramus, Marshall, Rosen and van der Lely2013) established that in a mixed sample of children with DLD and reading disability, phonological skills (i.e., phonological awareness, rapid naming, and phonological memory) were partially independent of nonphonological language skills (i.e., lexical and morphosyntactic). This study demonstrated that among children with DLD, only a subset of children had phonological deficits, and their profile of phonological deficits was different from that of children with reading disability. It also showed that language skills in these subgroups were likely dissociable given the absence of grammatical but not lexical deficits in children with reading disability. Together, these results support a multidimensional model for both the relationship between different components of phonological and nonphonological skills and the overlap between DLD and reading disability. These conclusions were also corroborated in a recent study by Rakhlin, Cardoso-Martins, Kornilov, and Grigorenko (Reference Rakhlin, Cardoso-Martins, Kornilov and Grigorenko2013), who suggested the co-occurrence of grammatical and phonological deficits in DLD is partial and probabilistic rather than deterministic, as suggested by accounts that attribute the former to the latter. Of note is that the conclusions of both studies were based on a careful examination of sources of variance in the performance of groups of children with language-based neurodevelopmental disorders and the acknowledgment of the interactive nature of language as a developmental system (Karmiloff-Smith, Reference Karmiloff-Smith1998). Such examinations permit inference regarding the degree of the overlap between multiple domains of language development and functioning, highlighting the constellations of more and less interdependent deficits.
Second, the acknowledgment of the behavioral heterogeneity in DLD, coupled with inconsistent findings from studies of lexical–semantic development and processing that employ offline methods (Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011), suggest that using online methods of studying language and cognitive processing, in particular neuroimaging and psychophysiological (i.e., electroencephalography [EEG] and event-related potentials [ERPs]) methods, might be a productive approach to investigating the cognitive profiles of DLD and their brain correlates. Neurophysiological methods provide online measures of processing with high temporal resolution (up to 1 ms). In addition, neurophysiological measures have been proposed as endophenotypes (Cannon & Keller, Reference Cannon and Keller2006; Roeske et al., Reference Roeske, Ludwig, Neuhoff, Becker, Bartling and Bruder2011) of developmental disorders, that is, intermediate phenotypes between the levels of observable phenotypic deficits and genetic etiology. Thus, endophenotypes provide a more sensitive and detailed characterization of representational and processing deficits at the level likely more proximal to the etiology of the disorder (i.e., neurocognitive) than end-point behavioral performance measures. To date, this approach has been mostly applied to studying neurophysiological endophenotypes of auditory and phonological processing in DLD (Addis et al., Reference Addis, Friederici, Kotz, Sabisch, Barry and Richter2010; Bishop, Reference Bishop2007). However, obtaining a more comprehensive picture of linguistic and cognitive deficits in DLD requires expanding the spectrum of linguistic endophenotypes of the disorder. Lexical processing is an especially interesting candidate for psychophysiological studies of DLD for several reasons. First, as noted above, lexical–semantic deficits have been proposed as a cause and a consequence of deficits in other linguistic and cognitive domains in DLD. Second, they have been postulated to represent both the area of residual normality and the result of compensatory plastic changes in language development, subserved by the corresponding neuroplastic changes in the brain. Third, behavioral studies have yielded a mixed pattern of results with respect to the specifics of the atypicalities in lexical–semantic processing in DLD.
ERP studies of lexical–semantic processing primarily logically focus on the N400 component, a negative deflection in the scalp-recorded electric activity of the brain that occurs approximately 400 ms after the presentation of the stimulus and has a prominent centro-parietal topographic distribution. The N400 component is reliably elicited by semantic violations and other expectation violations at the word level and is sensitive to a number of factors, including word frequency, predictability, and expectancy (Kutas & Federmeier, Reference Kutas and Federmeier2000, Reference Kutas and Federmeier2011).Footnote 3 Broadly, it is thought to index stimulus-induced semantic activity that occurs against the background of both long-term knowledge and recent experiences (e.g., experimentally generated top-down expectations) that influence the activation of lexical and semantic categories. The unique sensitivity of the N400 to the interplay between different cognitive systems and processes make it both one of the most studied components in cognitive psychophysiology and potentially an interesting target for examining the dynamics of lexical processing in DLD.
To date, only four studies (excluding Fonteneau and van der Lely's study described above) have examined lexical–semantic processing in DLD using neurophysiological methods. Using visually presented sentences that varied with respect to the semantic appropriateness of the final word (anomalous vs. nonanomalous), Neville, Coffey, Holcomb, and Tallal (Reference Neville, Coffey, Holcomb and Tallal1993) found that children with combined DLD and reading disability showed a larger N400 in response to both anomalous and nonanomalous sentence-final words; moreover, the amplitude of the difference waveform (anomalous–nonanomalous) was larger in children with DLD compared to TD children. The findings were interpreted by the authors as indicative of greater compensatory effort required by children with DLD for successful integration of words with context. A similar pattern of results was observed for parents (especially fathers) of children with DLD, who also showed an abnormally large N400 in response to sentence-final semantically anomalous, as well as nonanomalous words (Ors et al., Reference Ors, Lindgren, Berglund, Hagglund, Rosen and Biennow2001). In that study, the amplitude of the difference N400 waveform was smaller in parents of children with DLD than in parents of TD children, suggesting a lesser degree of differentiation between congruous and incongruous sentential endings by parents of children with DLD potentially driven by their need to engage in a significant thematic (re)integration in both situations. Ors et al. (Reference Ors, Lindgren, Berglund, Hagglund, Rosen and Biennow2001) suggested that this need for additional thematic integration in the congruous condition might be driven by the abnormal activation of semantic categories (via associative links) in long-term memory during reading in parents of children with DLD. The presence of N400 abnormalities in parents of children with DLD also tentatively supports the idea that the N400 amplitude might be a heritable endophenotype for the disorder and a marker for residual DLD in adults (although no behavioral language functioning data was reported in the manuscript for the adults).
Two more recently published studies are not fully consistent with the results reported by Ors et al. (Reference Ors, Lindgren, Berglund, Hagglund, Rosen and Biennow2001) and Neville et al. (Reference Neville, Coffey, Holcomb and Tallal1993). Friedrich and Friederici (Reference Friedrich and Friederici2006) investigated the N400 effect at 19 months in two groups of children: TD and children classified as being at risk for expressive DLD based on behavioral measures of language development at 30 months of age. They found that children not at risk for DLD showed a significant N400 effect in response to auditorily presented words that were incongruous with respect to the visually presented picture in a cross-modal picture–word paradigm and to phonotactically legal pseudowords (compared to a baseline when the pictured object's name was presented). In contrast, the group of children at risk for DLD did not display the N400 in either of these conditions. However, both groups showed a significant phonological–lexical priming effect manifested in an enhanced early negativity in response to congruous compared to incongruous words, although in children at risk for DLD this effect was more broadly temporally and spatially distributed. Because this early effect requires the activation of corresponding lexical elements to be robustly elicited, the reduced N400 in children at risk for DLD could not be attributed to their insufficient lexical knowledge per se. Thus, the N400 deficits in this group were likely tapping into the delayed maturation of processes of lexical activation; the altered dynamics of the lexical activation, in turn, could be driven by a number of deficits, most likely in the degree of specification and robustness of phonological and semantic features in lexical knowledge, and the extent of their integration or coherence (thus indexing the quality of their lexical representations; see Perfetti & Hart, Reference Perfetti, Hart, Verhoeven, Elbro and Reitsma2002). Consistent with this hypothesis, Friedrich and Friederici (Reference Friedrich and Friederici2006) also suggested that the reduced N400 effect in children at risk for DLD might be related not to the delayed maturation of the processes involved in the generation of the N400 per se, but rather to their ability to apply these processes to weak or unstable semantic representations.
Finally, Malins et al. (Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013) investigated ERP responses to words presented in a cross-modal picture–word paradigm to children with and without DLD. Their study involved an experimental manipulation not only of the degree of the semantic congruency of the presented word but also of the degree of the phonological overlap between the match and the mismatch words. In their study, both groups of children displayed significant N400 effects in response to words that were both semantically and phonologically unrelated to the target match word (e.g., see SHELL, hear “mug”), and an enhanced N400 effect to cohort mismatches (e.g., see DOLL, hear “dog”) that overlapped with the target word initially. In addition, both groups showed a similar earlier phonological mapping negativity (PMN) effect, suggesting that children with DLD are capable of developing online phonological expectations (congruent with the priming effect observed by Friederich and Friederici, 2006) and detecting violations of these expectations. However, only TD children displayed a significant attenuation of the N400 effect in response to rhyme mismatches (e.g., see CONE, hear “bone”). The lack of this rhyme attenuation effect in the DLD children led the authors to suggest that children with DLD are either not as sensitive to rhyming as TD children (potentially due to problems with establishing robust phonological representations) or are not efficient at suppressing lexical alternatives during spoken-word recognition.
In sum, ERP studies of lexical and semantic processing in children with DLD have resulted in a complex landscape of findings that suggests, at the minimum, atypical organization of lexical–semantic processing in DLD. Indirect evidence also suggests that these atypicalities might be related to deficits in other domains of language development in DLD (e.g., given reduced rhyme sensitivity in the phonological domain; e.g., Desroches, Newman, & Joanisse, Reference Desroches, Newman and Joanisse2009: Malins et al., Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013; or by the apparent compensatory reorganization of the system to rely more heavily on semantic associations in the context of morphosyntactic deficits; Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011).
The goals of the current study were twofold. First, we examined the dynamics of lexical processing in children with DLD in a cross-modal picture–word matching paradigm that manipulated the degree of the phonological and semantic similarities between the word corresponding to the pictured object and words presented in other conditions (similar to that employed by Desroches et al., Reference Desroches, Newman and Joanisse2009; and Malins et al., Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013). Specifically, we investigated the neural responses of children with and without DLD to words that were related to the pictured word phonologically (i.e., phonologically overlapped with it initially or finally) or semantically (i.e., as semantic associates), or were semantically and phonologically unrelated to the pictured word. Note that although it is difficult to draw predictions regarding the relative size of the N400 effect in these conditions, given Friedrich and Friderici's (2006) findings for infants at risk for DLD, one can speculate that for older children, the semantically associated condition might better reflect difficulties in processing because their lexical development might be sufficient for eliciting the basic N400 in response to words that are neither phonologically nor semantically related to the target word.
Second, to the best of our knowledge, no study has directly related behavioral indices of language development and neurophysiological indices of lexical processing in DLD.Footnote 4 Therefore, we took an individual differences approach to examine the links between phonological, morphosyntactic, lexical, and semantic/pragmatic development, on one hand, and phonological and lexical processing ERPs, on the other hand. Thus, if lexical deficits in DLD are related to deficits in phonological development, we would expect behavioral indices of phonological development to be related to the amplitudes of the PMN and the N400 in all experimental conditions, and particularly phonologically sensitive conditions (e.g., the final phonological overlap condition). Similarly, if lexical processing abnormalities in DLD are compensatory in nature, the N400 amplitudes should be related to the degree of the grammatical impairment in DLD.
Method
Participants
The participants came from a small geographically isolated Russian-speaking population (AZ; Rakhlin, Kornilov, et al., 2013) characterized by an unusually high prevalence of DLD. On average, 23% to 40% of children and adults exhibit atypical language development despite the absence of apparent neurobiological or sensory pathology, compared to 9% in the comparison rural population. Children with DLD from the AZ population have been characterized behaviorally as having expressive deficits (see Rakhlin, Kornilov, et al., Reference Rakhlin, Kornilov and Grigorenko2013, for a detailed description of the procedure), receptive language deficits (Kornilov, Rakhlin, & Grigorenko, Reference Kornilov, Rakhlin, Grigorenko, Zinchenko and Petrenko2012; Rakhlin, Kornilov, & Grigorenko, Reference Rakhlin, Kornilov and Grigorenko2014), and deficits in phonological working-memory capacity (Kavitskaya, Babyonyshev, Walls, & Grigorenko, Reference Kavitskaya, Babyonyshev, Walls and Grigorenko2011) and the development of literacy (Rakhlin, Cardoso-Martins, et al., Reference Rakhlin, Cardoso-Martins, Kornilov and Grigorenko2013). Together, these characterizations of the population are consistent with the behavioral profile of children with DLD documented in other languages (Ebbels, Dockrell, & van der Lely, Reference Ebbels, Dockrell and van der Lely2012; Graf Estes, Evans, & Else-Quest, Reference Graf Estes, Evans and Else-Quest2007; Hick, Joseph, Conti-Ramsden, Serratrice, & Faragher, Reference Hick, Joseph, Conti-Ramsden, Serratrice and Faragher2002; Marton, Abramoff, & Rosenzweig, Reference Marton, Abramoff and Rosenzweig2005; Robertson & Joanisse, Reference Robertson and Joanisse2010).
Thirty-nine children from the AZ population aged 7.17 to 15.83 years (M = 10.54, SD = 2.34; 23 boys) participated in the study. Of these, 23 were classified as DLD (M = 10.12, SD = 2.40; 16 boys) and 16 were classified as typically developing (TD; M = 11.14, SD = 2.18; 7 boys). The language status classification was based on a set of expressive and receptive language indices obtained using two standardized language development measures (the classification scheme is described below). The two groups did not differ significantly with respect to either gender distribution, χ2 (1) = 2.60, p = .107, or age, t (37) = 1.35, p = .186.
The Yale University Internal Review Board and the appropriate institutions in Russia approved the study protocol. Informed consents were obtained from the children's parents and oral assents were obtained from the children at the time of the data collection. In addition to the 39 children who participated in the study, 3 children also provided EEG data but did not provide behavioral data due to logistical reasons and were excluded from the analysis.
Language and cognitive development measures
Children's language development was assessed using two diagnostic tools: a standardized elicited narrative task developed for establishing the language development status in rural Russian populations (Rakhlin, Kornilov, et al., Reference Rakhlin, Kornilov and Grigorenko2013) and the Assessment of the Development of Russian (Kornilov et al., Reference Kornilov, Rakhlin, Grigorenko, Zinchenko and Petrenko2012).
Narrative task
Expressive language development was assessed using two wordless storybooks: for children under 13, these were Frog, Where Are You? and One Frog Too Many (Meyer, Reference Meyer1969); for those over 13, these were Free Fall (Wiesner, Reference Wiesner2008) and Tuesday (Wiesner, Reference Wiesner1997). The audio and the transcripts of the elicited speech samples were analyzed by two native-Russian linguists and rated on a number of characteristics in phonological, syntactic, and semantic/pragmatic domains, combined to form the following measures: phonetic/prosodic development (i.e., phonological simplifications and omissions, misarticulations and prosodic abnormality); wellformedness (frequency of lexical and grammatical errors and false starts adjusted for the length of the narrative); number of complex structures (e.g., embedded and conjoined clauses, passives, and participial constructions); mean length of utterance in words (MLUw); number of semantic/pragmatic errors; and lexical richness (i.e., number of distinct lexemes/100 words). Robust age-adjusted Z scores were calculated for each measure using data from a comparison rural population that resides in the same administrative region and is similar to the AZ population on a set of demographic and socioeconomic variables (population size, rate of unemployment, occupational structure, income, etc.; see Rakhlin, Kornilov, et al., Reference Rakhlin, Kornilov, Palejev, Koposov, Chang and Grigorenko2013).
Assessment of the development of the Russian language
Children were also administered ORRIA (Kornilov et al., Reference Kornilov, Rakhlin, Grigorenko, Zinchenko and Petrenko2012), a standardized Russian language development test comparable to the Clinical Evaluation of Language Fundamentals (Semel, Wiig, & Secord, Reference Semel, Wiig and Secord1995), the Test of Language Development (Newcomer & Hammill, Reference Newcomer and Hammill1982), and the Comprehensive Assessment of Spoken Language (Carrow-Woolfolk, Reference Carrow-Woolfolk1999). ORRIA is aimed at comprehensively assessing language development in the areas of morphology, syntax, compositional semantics, and lexicon in both receptive and expressive domains. Standardized age-adjusted scores for overall language development (M = 100, SD = 15) were calculated using an external sample (n = 484) representative of the general population of Russian children using five ORRIA subtests (passive vocabulary, linguistic operators, sentence structure, and word structure).
Diagnostic criteria for establishing language group status
Language impairment status (DLD vs. TD) was determined by using the cutoff criterion of a Z score at or below –1.25 on at least two of the six narrative scales listed above or an overall ORRIA score corresponding to this criterion (i.e., a score below 82, roughly corresponding to the epiSLI criterion; Tomblin et al., Reference Tomblin, Records, Buckwalter, Zhang, Smith and O'Brien1997). Predictably, given that these measures were used for grouping purposes, children with DLD significantly underperformed on almost all of the language measures, with the effect sizes (Cohen d) for the significant differences ranging from moderate (0.62) to large (1.29).
Nonverbal intelligence
Scale 2 of the Culture–Fair Intelligence Test (Cattell & Cattell, Reference Cattell and Cattell1973), a standardized measure of nonverbal intelligence, was used to assess nonverbal cognitive functioning of the sample. All children scored above the cutoff score for intellectual disability (IQ > 70). Children with DLD did not significantly differ from TD children with respect to nonverbal intelligence, t (37) = 1.47, p = .150.
Verbal short-term and working memory
We also administered a 32-item digit span task (backward and forward) modeled after the Wechsler Intelligence Scale for Children—Fourth Edition (Wechsler, Reference Wechsler2003) and a 21-item word span task to measure verbal short-term and working-memory capacity. Children with DLD displayed significantly lower scores on digit span, t (37) = 2.35, p = .024, d = 0.77, and word span, t (37) = 2.66, p = .011, d = 0.87, consistent with the established pattern of short-term memory deficits in DLD (Estes et al., Reference Estes, Evans and Else-Quest2007).
Hearing screening
All children were administered a bilateral hearing screening with a Beltone 119 (Beltone New England) portable audiometer at 500, 1000, 2000, and 4000 Hz. All children demonstrated normal hearing acuity by passing the screening at 25 dB.
Experimental stimuli and procedure
We used a set of high-frequency mono- and polysyllabic Russian words paired with color stock photographs in a picture–word matching paradigm to elicit the PMN and the N400 components. In this paradigm, modeled after Desroches, Newman, and Joanisse (Reference Desroches, Newman and Joanisse2009), participants are presented with a picture and a set of spoken words; they are then asked to judge whether the word matches the picture.
The experiment consisted of 40 blocks with 8 trials per block. At the beginning of each block, a fixation cross was presented on the screen for 1000 ms. After that, the fixation cross was replaced by a picture, which remained on the screen throughout the whole block. After 1500 ms of preview time, a set of spoken words were presented with stimulus onset asynchrony of 2000 ms. A total of eight words were presented with each picture, split into five conditions. In the match condition, the word matched the picture (e.g., hear /tort/ “cake”—see tort “cake”). There were also four mismatch conditions: in the initial phonological overlap (IPO) condition, the (semantically unrelated) word matched the beginning of the name of the pictured object (e.g., hear /tors/ “trunk”—see tort “cake”); in the final phonological overlap (FPO) condition, the word matched the ending of the name of the pictured object (e.g., hear /bort/ “board”—see tort “cake”); in the semantically associated (SA) condition, the word did not overlap with the name of the pictured object phonologically but instead was semantically associated with it (e.g., hear /t∫ai / “tea”—see tort “cake”); in the phonologically and semantically unrelated (UR) condition, the word was not related to the name of the pictured object phonologically or semantically (e.g., hear /sat/ “garden”—see tort “cake”). For each picture, the match word was presented four times, and each of the mismatching words was presented once in a randomized order. The order of blocks was randomized across participants.
The children sat in front of a PC laptop and were instructed to listen carefully to the words and indicate whether each word matched the picture by either pressing a mouse button (for “yes”) or providing no response (for “no”). Prior to testing, all children were familiarized with the visual stimuli and were asked to name each picture. In two instances, when a child provided an incorrect name for the picture, the response was corrected and the picture was presented again after the rest of the stimuli.
The words were recorded by an adult male native Russian speaker using PRAAT audio software (Boersma & Weenink, Reference Boersma and Weenink2009) with 16-bit resolution at a sampling rate of 44100 Hz and presented binaurally at 70 dB (SPL) via Etymotic insert headphones (Etymotic Research, Inc.). Forty photographs were selected from a commercial stock photo database. Two hundred highly imageable and frequent words were selected from a frequency dictionary of Russian (Sharoff, Reference Sharoff2001). Most (63%) of the verbal stimuli were disyllabic (15% were monosyllabic, 19% were trisyllabic, and 3% contained four syllables). The words across five experimental conditions did not differ in either frequency or length (ps > .05). A panel of native Russian speakers (S.K., N.R., and E.L.G.) reviewed the verbal and the visual stimuli to ensure their appropriateness for the experiment (including but not limited to child appropriateness, semantic and phonological relatedness, and imageability for verbal stimuli, and visual clarity and concept relevance for visual stimuli). For the IPO condition, the onset of the mismatch relative to the target word happened on average at 324 ms poststimulus onset. For the FPO condition, the onset of the final phonological overlap with the match word had an average latency of 232 ms.
EEG recording, processing, and analysis
The EEG signal was recorded using a BioSemi ActiveTwo system (BioSemi, Inc.) with 64 sintered Ag/AgCl electrodes mounted using electrolyte gel (SignaGel, Parker Laboratories, Inc.) in an elastic cap approximating the standard 10–20 system. An additional 7 electrodes were used to record the electric activity at the two mastoids and nose tip (data from these 3 electrodes were not used in the analyses reported in this manuscript), and to record the vertical electrooculogram (electrodes placed above and below the left eye) and the horizontal electrooculogram (electrodes positioned lateral to the outer canthi of both eyes). All impedances were kept below 25 kΩ.
The EEG signal was sampled at 1024 Hz and average referenced offline. The preprocessing of the data and the averaging were carried out using EMSE Suite 5.5 (Source Signal Imaging, Inc.). For each participant, EEG channels identified as containing a high amount of technical artifacts (i.e., excessive AC power line noise and/or loss of contact) on the basis of visual inspection were reconstructed using a spline interpolation procedure. Then, the signal was filtered using a digital IIR bandpass filter of 0.50 to 30 Hz. To correct for eye movement artifacts and blinks, a data-driven spatial ocular artifact correction algorithm (Pflieger, Reference Pflieger2001) was applied to the signal.
The EEG was epoched from –200 to 700 ms relative to stimulus onset using a 200-ms prestimulus baseline correction. We only analyzed correct trials (i.e., hits in the match condition and correct rejections in the mismatch conditions) in which the EEG activity did not exceed ±115 µV. On average, the analyses included 127/160 match, 33/40 IPO, 31/40 FPO, 33/40 SA, and 33/40 UR trials. There were no significant differences in the number of trials included for TD and the DLD groups or between mismatch conditions (all ps > .05).
The waveforms were averaged separately for each condition. The conventional peak identification analysis was guided by a combination of visual inspection of averaged waveforms and prior literature regarding the timing and spatial topography of the potentials. The N400 component typically has a pronounced centroparietal scalp distribution (Kutas & Federmeier, Reference Kutas and Federmeier2000), while the PMN shows a more central scalp distribution (Desroches et al., Reference Desroches, Newman and Joanisse2009). However, in our preliminary analysis, both components showed a prominent parietal scalp distribution. Therefore, in our main analyses, we chose to focus on the parietal electrode clusters. Given that children with DLD have been shown to display atypical lateralization of auditory ERPs (Bishop, Reference Bishop2007; Friedrich & Friederici, Reference Friedrich and Friederici2006), we examined three clusters of electrodes to account for potential group differences in the lateralization of the components of interest: left parietal: P5, P3, and PO7; midline parietal: Pz, PO3, PO4, POz, and Oz; right parietal: P4, P6, and PO8.
Following the procedures used by Desroches et al. (Reference Desroches, Newman and Joanisse2009) and Malins et al. (Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013), the time windows were defined as 210–310 ms poststimulus for PMN, 310–410 ms for early N400, and 410–600 ms for late N400 based on the visual analyses of the raw waveforms for all five conditions, as well as difference waveforms for the four mismatch conditions. The N400 time window was split into two to account for the absence of the early N400 in the IPO condition (given the timing of the onset of the mismatch) and for the analyses to be comparable to those reported by Malins et al. (Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013). The ERPs' amplitudes were quantified as average amplitudes in these time windows, and we also obtained fractional (50%) latency estimates for each of the potentials. In addition to this conventional peak-identification based analysis, to ensure that our analyses focused on the parietal region in specific time windows were not missing potential group differences in other scalp regions and other time windows, we performed an exploratory temporospatial principal component analysis (PCA) following Dien's (Reference Dien2010, Reference Dien2012) guidelines.
The amplitude and latency estimates for each of the components were subjected to a set of mixed 5 (condition: match, IPO, FPO, SA, or UR) × 3 (electrode cluster: left, midline, or right) × 2 (language group: DLD or TD) analyses of variance (ANOVAs) with Greenhouse–Geisser corrections applied to the p values whenever appropriate.
Results
Behavioral data
We did not find statistically significant differences between children with DLD and TD children in either number of hits in the match condition or number of correct rejections in any of the four mismatch conditions (for a set of independent t tests, all ps > .27). In the match condition, children with DLD also showed (log-transformed) reaction times similar to those of TD children, Welch t (29.08) = –1.60, p = .12. Thus, overall, children with DLD displayed behavioral performance on the picture–word matching task similar to that of TD children. This result was expected due to the easy nature of the mismatch detection task (also manifested in the ceiling performance displayed by both groups of children, as is evident from Table 1).
Table 1. Behavioral performance of children with DLD and TD children on the picture–word matching task

Note: DLD, Developmental language disorder; TD, typically developing; IPO, initial phonological overlap; FPO, final phonological overlap; SA, semantically associated; UR, semantically and phonologically unrelated.
Group differences in lexical–semantic and phonological processing ERP indices
The ERP waveforms and the topographic distributions for the components of interest for the two groups of children are plotted in Figures 1 and 2, respectively. Children with DLD displayed a trend toward significantly less negative amplitudes in the PMN time window across the five conditions, F (1, 37) = 3.97, p = .054, η2p = 0.097. No group-related interactions were significant (all ps > .159). Thus, this borderline-significant main effect of language group suggests that children with DLD display deficits not in the processes involved in the detection of violations of phonological expectations per se, but rather in relatively early neural responses to auditorily presented words in general. Across the two groups of children, we found a significant main effect of condition, F (4, 148) = 10.07, p < .001, η2p = 0.214. Post hoc analyses with Bonferroni corrections revealed significantly more negative amplitudes in the FPO (p = .006), SA, and UR (both ps < .001) conditions, compared to the match condition, indicating the presence of robust negativity in the 210- to 310-ms time window for these conditions. Predictably, given the initial phonological overlap between the IPO and the match condition, no significant PMN effect was observed for the IPO condition (p = 1.00). The effect of electrode cluster was also significant, F (2, 74) = 5.77, p = .006, η2p = 0.135, with more positive amplitudes observed in the right parietal cluster compared to the midline (p = .020) and the right parietal (p = .004) clusters. However, this effect did not differ with respect to experimental conditions, as indicated by the nonsignificant interaction between electrode cluster and condition, F (8, 296) = 0.72, p = .640, η2p = 0.019. No other effects reached statistical significance (all ps > .160). We did not find any significant effects for the analyses of latency estimates in the PMN time window (all ps > .060).

Figure 1. (Color online) Sample average event-related potential (ERP) waveforms at three parietal electrode clusters (left, midline, right) in two groups of children, developmental language disorder (DLD) and typically developing (TD). (a) Sample waveforms for match, initial phonological overlap (IPO), and final phonological overlap (FPO) conditions; (b) sample waveforms for match, semantically associated (SA), and phonologically and semantically unrelated (UR) conditions; and (c) average difference waveforms for the four mismatch conditions (IPO, FPO, SR, UR) at the parietal midline. Negative is plotted downward.
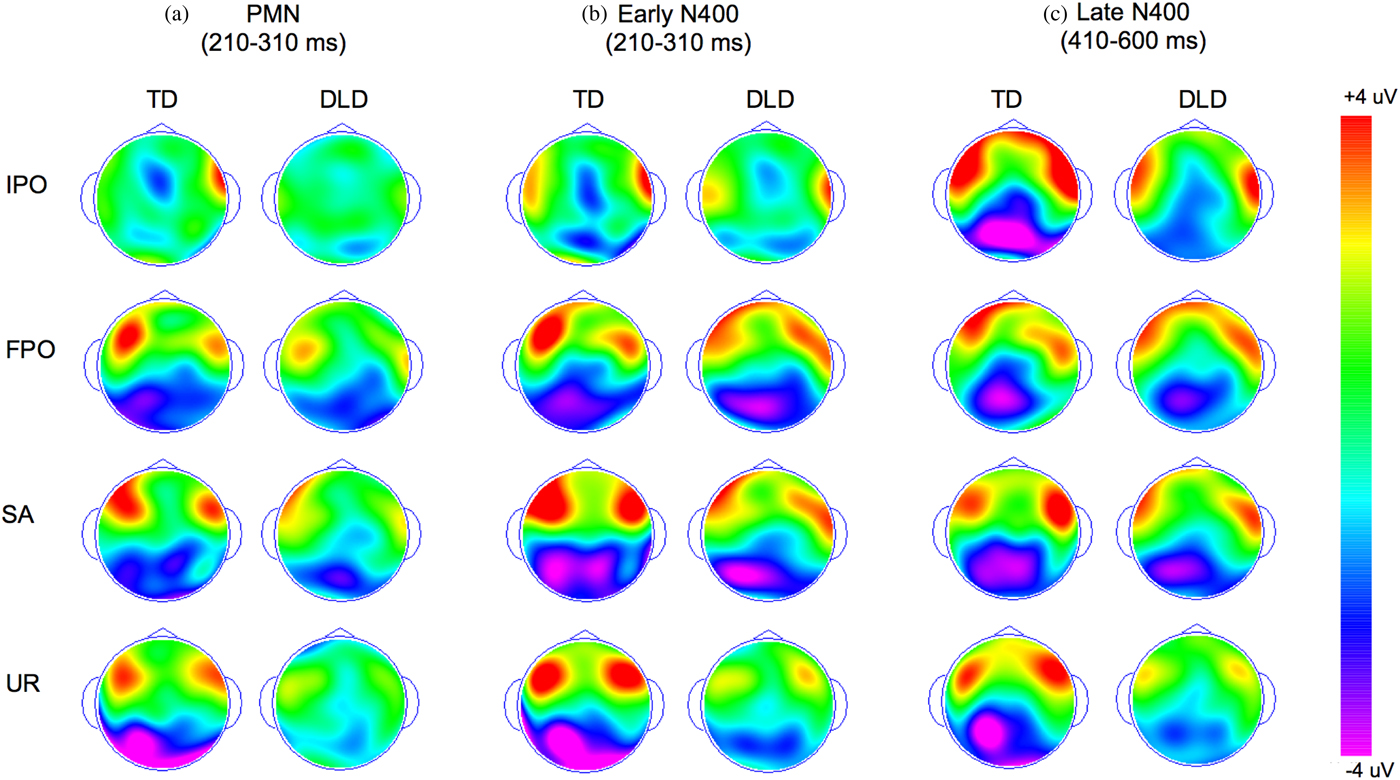
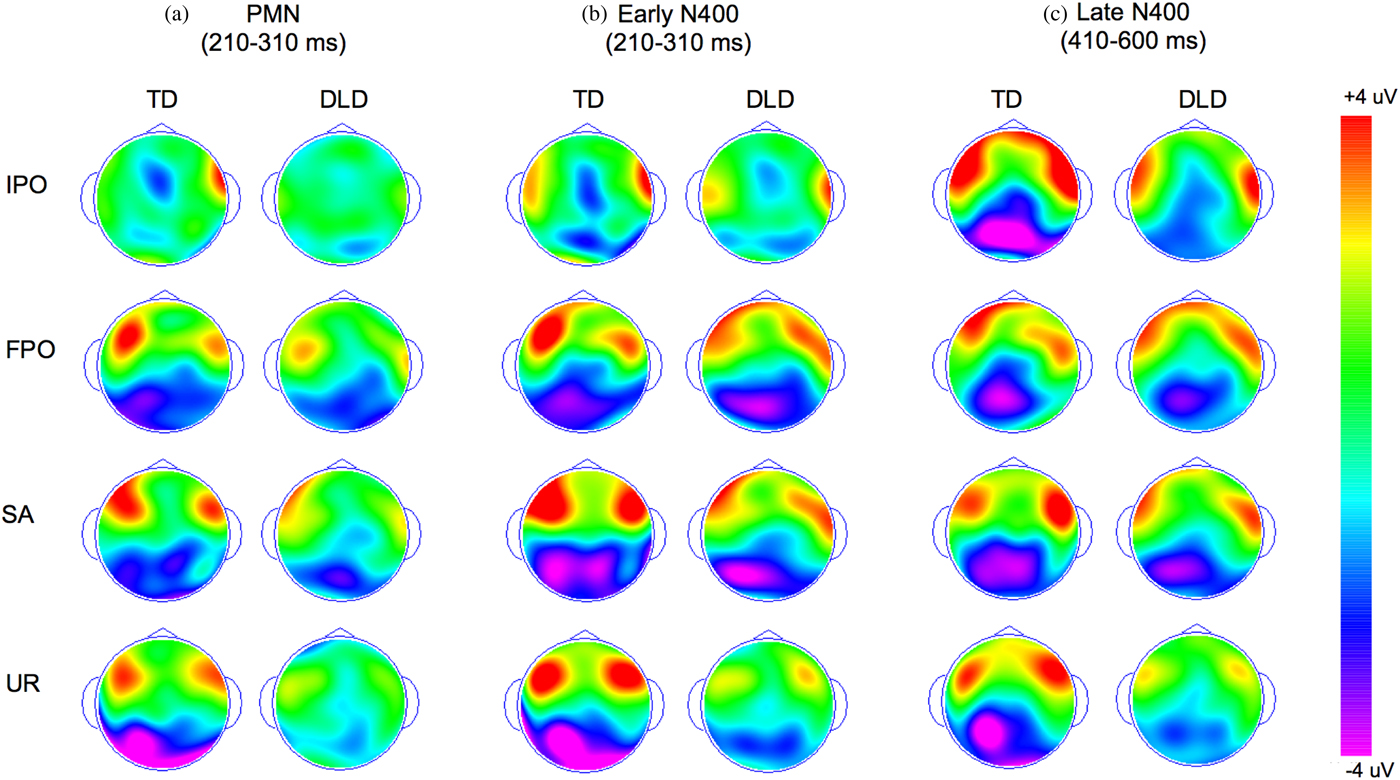
Figure 2. (Color online) Topographic maps for components of interest (based on difference waveforms) in two groups of children, developmental language disorder (DLD) and typically developing (TD). (a) Average in the phonological mapping negativity (PMN) time window, (b) average in the early N400 time window, and (c) average in the late N400 window. IPO, Initial phonological overlap; FPO, final phonological overlap; SA, semantically associated; UR, phonologically and semantically unrelated.
Overall, in the early N400 time window, children with DLD displayed N400 amplitudes similar to those of TD children, F (1, 37) = 0.30, p = .590, η2p = 0.008. The main effect of condition was significant, F (4, 148) = 18.36, p < .001, η2p = 0.332, with the pattern of differences resembling that for the PMN: markedly more negative amplitudes were observed for the FPO, SA, and UR conditions (all ps < .001), but not the IPO condition (p = 1.00). These results indicate the presence of a robust N400 effect in all conditions but the with the initial phonological overlap with the match word (likely because the response in the early window is generated within the period where the IPO items overlap with the expected word, that is, during the period prior to expectation violation). The early N400 amplitude was similar in the FPO, SA, and UR conditions (all ps = 1.00). We also obtained a significant main effect of electrode cluster, F (2, 74) = 11.71, p < .001, η2p = 0.240; post hoc pairwise comparisons indicated that the early N400 was more prominent in the midline compared to the right parietal (p < .001) cluster (no other pairwise comparisons were significant). The analysis of early N400 latency also revealed that the N400 had an earlier latency in the right parietal compared to the left parietal cluster, F (2, 74) = 5.09, p = .009, η2p = 0.121 (p = .022 for the pairwise comparison); no other effects were significant (all ps > .133).
The analysis of the amplitudes in the late N400 time window, however, revealed statistically significant differences between children with DLD and TD children. Across the two groups, there was a main effect of condition, F (4, 148) = 15.69, p < .001, η2p = 0.298, with significantly more negative amplitudes in the four mismatch conditions compared to the match condition (all ps < .001) but no significant differences between the four mismatch conditions. Although the main effect of language group was not significant, F (1, 37) = 1.39, p = .246, η2p = 0.036, the interaction between group and condition was significant, F (4, 148) = 3.04, p = .026, η2p = 0.076, indicating that the two groups of children demonstrated different late N400 patterns across conditions. We investigated this interaction by comparing the average amplitudes of the difference waveforms (mismatch–match) separately for each condition in a set of 2 (language group) × 3 (electrode cluster) mixed ANOVAs. These analyses revealed that the interaction between language group and experimental condition is likely driven by marked differences between children with DLD and TD children in the size of the late N400 in the IPO condition, F (1, 37) = 12.31, p = .001, η2p = 0.247, and the UR condition, F (1, 37) = 5.50, p = .024, η2p = 0.129, but not the FPO or the SA conditions (all ps > .408; see Figure 1b). Additional analyses also revealed that these effects are unlikely to be driven by an enhanced N400 in the match condition, because the two groups did not differ with respect to the amplitude of the N400 when this condition was analyzed separately, F (1, 37) = 2.21, p = .145, η2p = 0.056. We discuss possible explanations for these differences in the Discussion.
In addition, we found tentative evidence for atypical topographic distribution of the late N400 in children with DLD, F (2, 74) = 8.72, p = .051, η2p = 0.078, for the two-way interaction between cluster and group; while the TD children showed a late N400 that was larger in the left and midline clusters compared to the right parietal cluster, F (2, 30) = 9.26, p = .001, η2p = 0.382 (both pairwise ps < .024), children with DLD did not show this effect, displaying similar N400 responses in all three clusters, F (2, 44) = 0.89, p = .423, η2p = 0.038. No significant effects were found for late N400 latencies (all ps > .057).
In retrospect, it might not be surprising that we did not find any N400 diminishment in the SA condition. The picture–word matching paradigm does not require semantic activation for any item aside from the pictured object (and there, shallow activation sufficient to retrieve phonological form is all that is required); rather, comparison of just the first expected and heard phoneme (or first few phonemes, for IPO) would suffice. The absence of an FPO effect (predicted diminished late N400, as in Desroches et al., Reference Desroches, Newman and Joanisse2009; and Malins et al., Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013) may reflect differences in materials; true rhyme pairs conforming to the constraints of the experiment (concrete, imageable, roughly matched on frequency, etc.) are harder to find in Russian than in English, and so we relied upon items matching in two to six final phonemes (M = 3.20). It may be that observing a diminished late N400 depends upon overlap from syllable nucleus onward.
In addition to the amplitude and latency analyses, we analyzed the data using the full set of electrodes and a bottom-up approach via temporospatial PCA (Dien, Reference Dien2012) as implemented in the MATLAB ERP PCA Toolkit (Dien, Reference Dien2010). At the first step, the individual participants' average waveforms for the match and four mismatch conditions were subjected to a temporal PCA with Promax rotation. The scree test suggested that 25 temporal factors (explaining 96% of variance) should be retained. The factor scores from the first step were then subjected to a spatial ICA with Infomax rotation. This analysis identified 7 spatial factors that explained 73% of variance. Thus, the temporospatial PCA revealed a total of 25 (temporal) × 7 (spatial) = 175 temporospatial factors. Only 14 of these 175 factors explained more than 1% of variance and were further analyzed using a set of 5 (condition) × 2 (group) ANOVAs applied to the factor scores converted back into microvolts. These analyses revealed several effects, overall corroborating and even strengthening the pattern of findings presented above for the conventional peak identification analysis.
The first temporospatial factor for which the group differences were obtained was represented by the combination of the first temporal factor (TF1; peaking at 454 ms poststimulus) and the first spatial factor (SF1; characterized by the prominent parietal topography with the most negative amplitudes observed in the Oz electrode; see the online-only supplemental materials for the topographic maps of each of the temporospatial factors). Thus, this temporospatial factor, TF1SF1, can be thought of as overall corresponding to the N400 potential. For TF1SF1 (average factor score differences for the two groups are plotted in Figure 3), we found a statistically significant interaction between language group and condition, F (4, 148) = 2.71, p = .043, η2p = 0.068. The follow-up analysis of this interaction revealed that whereas the TD children displayed robust N400 responses in all four mismatch conditions, F (4, 60) = 11.81, p < .001, η2p = 0.440 (for pairwise comparisons between the match and the four mismatch conditions, all ps < .008), children with DLD showed a different pattern of results. Specifically, while they also displayed a significant main effect of condition, F (4, 88) = 8.61, p < .001, η2p = 0.281, the N400 effect was only robust for the FPO, SA, and UR conditions (all ps < .005), but not the IPO condition (p = .260). Difference scores also revealed that, in addition to a markedly reduced IPO-match N400, t (37) = –2.75, p = .009, d = –0.86, children with DLD showed a reduced N400 in the UR condition, t (37) = –2.16, p = .038, d = –0.72 (all other ps > .472).

Figure 3. Average principal component analysis (PCA) factor scores for three temporospatial factors (with 95% confidence intervals). Difference factor scores for each of the mismatch conditions are plotted for TF1SF1 (top row) and TF4SF1 (bottom row). Raw factor scores for each of the five experimental conditions are plotted for TF2SF1 (middle row). IPO, Initial phonological overlap; FPO, final phonological overlap; SA, semantically associated; UR, phonologically and semantically unrelated; DLD, developmental language disorder, TD, typically developing. Negative is plotted downward.
The second temporospatial factor for which group differences were observed was TF2SF1, which represents a component characterized by a relatively early latency (196 ms poststimulus) and a parieto-occipital distribution similar to that of TF1SF1. This factor corresponds to the PMN/N200 component in the early time window. We did not find significant differences in TF2SF1 amplitudes across the five experimental conditions, F (4, 148) = 2.37, p = .067, η2p = 0.060. However, children with DLD displayed significantly smaller TF2SF1 amplitudes than did children with TD overall, F (1, 37) = 10.83, p = .002, η2p = 0.226. As Figure 3 demonstrates, children with DLD essentially showed TF2SF1 amplitudes close to zero; this result corresponds to the finding of less negative amplitudes in the conventional peak identification analysis of the PMN time window reported above.
Finally, we found additional evidence for atypical neural processing responses between DLD and TD children in the IPO condition manifested in the amplitudes of the TF4SF1, a temporospatial factor characterized by a parietal positivity peaking at 325 ms poststimulus, a latency that corresponds to the onset of the phonological mismatch in the IPO condition. Specifically, we found a significant interaction between condition and group, F (4, 148) = 3.44, p = .019, η2p = 0.085; the follow-up investigation of this interaction revealed that TD children displayed a strong and significant effect of condition for TF4SF1 amplitude, F (4, 60) = 12.01, p < .0001, η2p = 0.445, driven by the markedly more positive amplitudes for the IPO compared to the match condition (p = .009; other ps for the match vs. mismatch comparisons > .076). Children with DLD, however, did not display such an effect, F (4, 88) = 2.41, p = .077, η2p = 0.009 (all ps > .345 for the pairwise comparisons between match and mismatch conditions). Visual inspection of the IPO time course plotted in Figure 1c suggests that the mismatch response in DLD children was delayed until the late N400 window in this condition; again, we discuss possible explanations in the Discussion.
Relating individual differences in ERPs and behavioral measures of linguistic and cognitive development
To further investigate which facets of DLD might be driving the group differences reported above, we performed a correlational analysis. Specifically, we related the ERPs shown to differentiate between groups (i.e., the amplitude of the N400 difference for the IPO and UR conditions from the conventional peak identification analyses, and the estimated difference factor scores for TF1SF1 in the IPO and UR conditions, TF2SF1 across all five conditions, and TF4SF1 in the IPO condition) and children's behavioral indices of verbal memory capacity and language development. To account for the potential effects of demographic characteristics (i.e., age and gender) and nonverbal intelligence, these variables were entered as covariates in the partial correlation analysis.
The correlations between assessment measures and ERP indices of lexical–semantic and phonological processing are presented in Table 2. We found an association between the N400 amplitude in the IPO condition and phonetic/prosodic characteristics (r = –.485, p < .05 for the amplitude difference and r = –.363, p < .05 for TF1SF1 factor score difference); we also found that the TF1SF1 IPO difference was associated with MLUw (r = –.484, p < .05), lexical richness (r = –.480, p < .05), and verbal memory as indexed by Word Span (r = –.346, p < .05). Thus, the size of the TF1SF1/N400 in this condition was related to indices of phonological, grammatical, and lexical development, and the development of verbal short-term memory. When controlled for verbal short-term memory, the pattern of correlations remained essentially the same, suggesting that the amplitude of the N400 in this condition is independently affected by language development and verbal memory. However, the association of the TF1SF1/N400 amplitude with an index of grammatical development, MLUw, lost statistical significance when phonological and lexical development were controlled for (r = –.266, p > .05). In contrast to the TF1SF1 scores in the IPO condition, the TF1SF1 scores for the UR condition only correlated with lexical richness (r = –.331, p < .05) but not measures of development in other linguistic domains or verbal memory. In sum, we found that the reduced amplitude of the N400 in the IPO and UR conditions was associated with lower lexical and phonological development, as well as verbal memory, but not grammatical development.
Table 2. Intercorrelations between ERP indices of lexical and phonological processing and behavioral indices of language and verbal memory development

Note: TF1SF1, First temporal factor and first spatial factor; IPO, initial phonological overlap; FPO, final phonological overlap; SA, semantically related; UR, semantically and phonologically unrelated; MLUw, mean length of utterance in words; ORRIA, a standardized Russian language development test (Kornilov et al., Reference Kornilov, Rakhlin, Grigorenko, Zinchenko and Petrenko2012) comparable to the Clinical Evaluation of Language Fundamentals (Semel, Wiig, & Secord, Reference Semel, Wiig and Secord1995), the Test of Language Development (Newcomer & Hammill, Reference Newcomer and Hammill1982), and the Comprehensive Assessment of Spoken Language (Carrow-Woolfolk, Reference Carrow-Woolfolk1999). For the late N400 IPO and the late N400 UR, amplitude difference measures (IPO/UR – match) at the midline parietal clusters were used. For the N200, the average amplitude across five conditions at the midline were used. For TF1SF1, differences in factor scores (mismatch – match) were used. For TF2SF1, average factor scores across five conditions were used. For TF4SF1, the difference in factor scores (IPO – match) was used.
†p = .06. *p < .05.
In the early PMN window, TF2SF1 amplitude negatively correlated with lexical richness (r = –.363, p < .05). We also found marginally significant correlations between the scores for the TF4SF1, the temporospatial factor characterized by a parietal positivity in the IPO condition for the TD children, and phonetic/prosodic characteristics (r = .312, p = .06).
In sum, we found that although children with DLD were just as accurate and fast on the picture–word matching task as TD children, their neural responses to auditory words presented in this paradigm were markedly different. Specifically, children with DLD displayed atypical ERPs in both the mid- and late latency range. Children with DLD showed significantly reduced amplitudes in the PMN time window across conditions; the PCA estimates of this component were linked to the lexical development of children in the sample. They also did not display a positive component in response to the phonological mismatch in the IPO condition; the absence of this component could be also tentatively linked to their deficits in phonological development. Finally, we found that children with DLD displayed markedly reduced late N400 amplitudes in response to words that acoustically and phonologically initially overlapped with the target word (i.e., in the IPO condition) and to words that were not related to the target word either semantically or phonologically (i.e., in the UR condition). However, the results of the correlational analysis suggested that while the latter was associated with lexical development, the former is linked to both lexical and phonological development.
Discussion
Lexical–semantic and phonological processing deficits in DLD
We investigated lexical and phonological processing in children with DLD using a cross-modal picture–word matching paradigm similar to the one used by Desroches et al. (Reference Desroches, Newman and Joanisse2009) and Malins et al. (Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013).
We found that children with DLD displayed significantly attenuated N400 amplitudes in the late time window compared to TD children in the UR and the IPO conditions. Given the absence of significant differences between the magnitudes of the N400 effects for the four mismatch condition within groups, we must be cautious with any explanations of effects that manifest as Condition × Group interactions. The difference in the IPO condition (similar timing, but reduced amplitude for DLD) could result from reduced activation for the expected (pictured) word. Such a reduction could follow from sluggish lexical activation (consistent with accounts that postulate the presence of generalized processing speed deficits in DLD; Miller, Kail, & Leonard, Reference Miller, Kail and Leonard2001), which in turn could result from overactivation (Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011) or reduced phonological or lexical inhibition (Magnuson et al., Reference Magnuson, Kukona, Braze, Johns, Van Dyke, Tabor, McCardle, Miller, Lee and Tzeng2011); either overactivation or reduced inhibition would result in more words being activated in the early time course, and if we assume word recognition involves competition among activated lexical representations (McClelland & Elman, Reference McClelland and Elman1986), lexical activations will increase more slowly when more words are activated.
The relative depression of the N400 response for children with DLD in the UR condition is more challenging to explain. First, we must assume that any differential activation for the SA and UR conditions (again, relative to TD N400s) indicates that children with DLD do not focus attention at the phonological level, contrary to TD children. This would be testable in future work using other paradigms that normally shift attention away from semantics (e.g., semantic priming is normally reduced in a lexical decision task compared to a task like artifact judgment). Second, we must account for why the N400 is depressed in children with DLD in the UR condition but not in the SA and FPO conditions. We offer two explanations for this pattern of findings. First, it could result from abnormal effects of overlap for SA and FPO, whereby overlap has opposite effects than are normally found. That is, rather than semantic or phonetic relatedness diminishing the N400 response, overlap somehow enhances the N400, perhaps because abnormal connectivity in the DLD lexicon initially leads to increased activation of the expected word and thus to a larger mismatch effect when the heard word finally overcomes the expected word in lexical competition. Second, the other possibility is that the unrelated word has functionally greater consistency with the expected word for children with DLD. This could follow from the overactivation hypothesis (Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011), which postulates that items in the mental lexicon of children with DLD have developed stronger associative links and, overall, the lexicosemantic network is activated more strongly but diffusely in children with DLD than in TD children (due to compensatory reliance on such associations in the face of difficulties at morphological and syntactic levels of processing). On this account, one might speculate that target word activation spreads over more levels of linkage in the lexicon in DLD children during the picture preview period (e.g., rather than activating just directly associated items, e.g., CAKE → TEA, stronger associative links could permit more diffuse activation, e.g., CAKE → PARTY → GARDEN PARTY → GARDEN).Footnote 5
Again, though, we offer these speculative explanations for effects that must be addressed with caution (there were relatively weaker N400 responses for the IPO and UR conditions for DLD children relative to TD children, in the absence of reliable differences between these conditions within the sample). These effects are intriguing and, as we have just discussed, can motivate several testable hypotheses as to differences in language processing in DLD that could be explored in future work. In contrast, we can have much greater confidence in the patterns that emerged over the full sample of children (TD and DLD) in our individual differences analyses, and as we discuss next, our correlational findings are consistent with the Condition × Group interactions.
Once more, children with DLD displayed a significantly attenuated N400 in the IPO condition compared to TD children. In this condition, the bottom-up acoustic/phonetic information is consistent with the match word for a period of approximately 324 ms. Desroches et al. (Reference Desroches, Newman and Joanisse2009) and Malins et al. (Reference Malins, Desroches, Robertson, Newman, Archibald and Joanisse2013) found that this condition elicited the largest N400 in the picture–word priming paradigm in both children and adult samples. This enhancement is driven by the bottom-up reinforcement (from the overlapping onset) of the top-down expectations regarding the identity of the upcoming stimulus. The initial phonological overlap thus strengthens the activation of the target lexical item, and the word-medial mismatch produces a large N400 because the expected target has been so strongly activated; it must be deactivated as the actual auditory target activates. In our data the amplitude of the N400 effect was not statistically different across the four mismatch conditions (although in the combined sample, a trend was observed for larger N400 amplitudes in the IPO compared to the UR and SA conditions with p = .06), the visual analysis of the waveforms presented in Figure 1 suggests that the IPO condition produced the largest N400 in the group of TD children but not children with DLD. Unlike the reduction of the N400 in the UR condition, this reduction of the N400 in the IPO condition correlated with behavioral indices of both lexical and phonological development. Although TF1SF1/N400 amplitude in the IPO condition correlated with MLUw, a measure of grammatical development, in our sample MLUw correlated with both lexical and phonological development. When both lexical and phonological development were controlled for, the TF1SF1 amplitude in the IPO condition was no longer significantly related to MLUw, suggesting that it indexes deficits in lexical and phonological processing and is relatively independent from grammatical or memory deficits in DLD.
We hypothesize that this reduction of the N400 in the IPO condition is thus due to the combination of lexical and phonological processing deficits in children with DLD. One possible explanation we have already discussed is that lexical activation is slower in children with DLD; thus, even given initial phonological overlap, the expected word may not be substantially activated by the time mismatch is encountered word medially. Slower activation could be attributed to less specified phonological features of lexical representations or otherwise altered dynamics of lexical activation (e.g., overactivation or reduced phonological or lexical inhibition, as discussed above), or both. Consistent with this hypothesis, children with DLD have been shown to require more acoustic/phonological information to recognize spoken words than their do TD peers (Dollaghan, Reference Dollaghan1998) and show lower performance on tasks aimed at measuring the precision of phonological representations (such as speech categorization, discrimination, and articulation, as well as nonword repetition; Ramus et al., Reference Ramus, Marshall, Rosen and van der Lely2013).
Alternatively, one could speculate that the N400 is not enhanced in the IPO condition for children with DLD, not because lexical activation is sluggish, but rather because they are less capable of detecting the phonological mismatch that occurs in the middle of the word. This would lead them to essentially accept lexical items in the IPO condition as corresponding to the target match words. Consistent with this hypothesis, children with DLD did not show the same parietal PCA component in response to the onset of the mismatch in the IPO condition as was observed in the TD group. Although the amplitude of the PMN effect (match–mismatch) in other conditions was similar in the two groups, suggesting that children with DLD are capable of detecting violations of phonological expectations per se, it is possible that in the IPO condition, this detection (picked up by the PCA analysis as a separate temporospatial factor) is less robust because it happens further along in the processing stream than in other conditions. However, this hypothesis predicts that the reduction of the N400 in the IPO condition should be linked to the reduction of the TF4SF1 amplitude. Additional analyses revealed that the amplitude of TF4SF1 did not correlate with the TF1SF1/N400 difference amplitude in the IPO condition (r = –.06, p = .725), suggesting that this explanation is not likely. This hypothesis makes two additional predictions not supported by the data. First, it predicts a significant increase in the rate of false alarms in the IPO condition in the group of children with DLD compared to TD children, which was not the case. Second, it predicts that the timing of the N400 in the IPO condition would be atypical/delayed in children with DLD compared to TD children, which was also not the case.
We also found that children with DLD showed overall reduced amplitude of the N200/PMN response in the early time window across conditions. This potential has been linked to both phonological processing (Connolly & Phillips, Reference Connolly and Phillips1994; Lee, Harkrider, & Hedrick, Reference Lee, Harkrider and Hedrick2012) and early semantic processing following the initial phonological analysis of the available (incomplete) information about the word and early lexical selection (van den Brink, Brown, & Hagoort, Reference van den Brink, Brown and Hagoort2001). In our data, the PCA-derived amplitude of this component was positively related to the PCA-derived amplitudes of the N400 effect in both the IPO condition (r = .432, p < .01) and the UR condition (r = .341, p < .05), and also to the behavioral measure of lexical development (r = –.363, p < .05) but not the measure of phonological development (r = .126, p > .05). This pattern of correlations suggests that this component is more likely to represent aspects of lexical rather than phonological processing, and that children with DLD might have deficits in processes involved in early lexical selection, consistent with the general pattern of atypical dynamics of lexical activation in DLD. However, we would like to emphasize that these data should be interpreted cautiously given that the topographic characteristics of this component were more similar to the N400 than in the previously published studies mentioned above.
Implications for understanding neuroplasticity in the context of atypical language development in DLD
Whereas the primary meaning of neuroplasticity, at least in studies of development and psychopathology, relates to the reorganization of the brain in response to implicit learning or targeted intervention, whether medical (Lonka et al., Reference Lonka, Relander-Syrjänen, Johansson, Näätänen, Alho and Kujala2013) or behavioral (Eldar & Bar-Haim, Reference Eldar and Bar-Haim2010; Seppänen, Hämäläinen, Pesonen, & Tervaniemi, Reference Seppänen, Hämäläinen, Pesonen and Tervaniemi2013; Song et al., Reference Song, Sun, Wang, Zhang, Kang and Ma2010; Wild-Wall, Falkenstein, & Gajewski, Reference Wild-Wall, Falkenstein and Gajewski2012), another important aspect of neuroplasticity is how the system develops in the context of both typical (Brandwein et al., Reference Brandwein, Foxe, Russo, Altschuler, Gomes and Molholm2011) and atypical/disordered development (Föcker, Best, Hölig, & Röder, Reference Föcker, Best, Hölig and Röder2012; Mills et al., Reference Mills, Dai, Fishman, Yam, Appelbaum and St. George2013; Spironelli, Bergamaschi, Mondini, Villani, & Angrilli, Reference Spironelli, Bergamaschi, Mondini, Villani and Angrilli2013). In the case of DLD, several promising attempts at finding a specific, single core deficit (e.g., such as generalized slowing, phonological working memory, procedural memory, or deficits circumscribed to grammar) have proved untenable when all of the affected children are considered. The neuroconstructivist perspective of Karmiloff-Smith and colleagues (Elman et al., Reference Elman, Bates, Johnson, Karmiloff-Smith, Parisi and Plunkett1996; Karmiloff-Smith, Reference Karmiloff-Smith1998) provides a more nuanced but theoretically challenging possibility: subtle differences or deficits in one developmental cognitive domain (e.g., memory) can have pervasive effects on distal domains such as language, and the same is true for deficits in different subdomains within the language system. That is, the normal developmental trajectory in a domain that can be descriptively isolated (e.g., morphosyntax or lexical knowledge) depends crucially on its relation to and interactions with disparate domains throughout the course of development.
For children with DLD, word-level strengths and weaknesses have been alternatively attributed to lower level causes (auditory, phonological, or memory; e.g., Ellis Weismer & Hesketh, Reference Ellis Weismer and Hesketh1996; Gathercole, Reference Gathercole2006) or higher level causes such as atypical development of grammar (e.g., Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011). The attraction of such explanations is clear; if the constellation of symptoms that emerge in DLD were fully attributable to a single cause, the search for the etiology of DLD would presumably be greatly simplified. Single-cause or core-deficit hypotheses might at first seem consistent with a neuroconstructivist perspective; however, even if there were originally a very specific deficit underlying DLD, the neuroconstructivist perspective predicts a recursive impact on closely and possibly distantly related domains. That is, grammatical deficits could impair word learning, which could in turn lead to additional compromise of grammatical abilities (e.g., grammatical deficits could increase memory load by requiring a child to hold an utterance in memory in a holistic form rather than in a more compact, syntactically recoded form, impairing the child's ability to encode grammatical or even phonetic details of new words when they are encountered). Thus, even a very specific deficit in one subdomain would be expected to lead to altered development across a wide variety of domains. All the same, we can assess which domains covary, and attempt to rule out hypothesized primary causes of specific deficits.
Along with the accumulation of data on the multidimensionality of DLD, its faceted nature, and the relative concurrent and developmental dissociation of its facets, there has been an increase in the evidence substantiating the remarkable amount of neuroplasticity demonstrated by the brain as it learns to deal with linguistic stimuli experimentally (Chen et al., Reference Chen, Liu, Wang, Larson, Huang and Liu2012; Kung et al., Reference Kung, Hsieh, Liou, Lin, Shaw and Liang2014; Spironelli, Galfano, Umiltà, & Angrilli, Reference Spironelli, Galfano, Umiltà and Angrilli2011) and developmentally by children (Kuhl, Reference Kuhl2010) and adults (Batterink & Neville, Reference Batterink and Neville2013; Fernandez, Tartar, Padron, & Acosta, Reference Fernandez, Tartar, Padron and Acosta2013). This growing literature substantiates the hypothesis that the brain does not shape language unilaterally; rather, language development also structures the brain, so that impaired language development is traceable in the brain's response to linguistic stimuli.
As such, the current study has important implications for understanding the complex pattern of linguistic deficits in DLD and their correlates in the brain. We found that abnormal N400 amplitudes demonstrated by children with DLD could be partially attributed to their lexical abilities (for the IPO and UR conditions) and to their phonological abilities (for the IPO condition). Although the precise mechanisms responsible for the generation of the N400 are not known (but see Lau, Phillips, & Poeppel, Reference Lau, Phillips and Poeppel2008), it is nevertheless reasonable to surmise that if the N400 indexes at least in part the efficiency of lexical–semantic processing, its amplitude should be related to the development of language in domains most proximal to lexical–semantic processing (i.e., such as vocabulary size and ease of lexical access as measured by lexical richness or quality of phonological representations, mostly word level, as measured by phonetic/prosodic characteristics).
Recall that several accounts view the absence of lexical–semantic processing deficits in DLD within the frameworks of residual normality (Fonteneau & van der Lely, Reference Fonteneau and van der Lely2008), and their presence, within the framework of compensatory reorganization of processing (Pizzioli & Schelstraete, Reference Pizzioli and Schelstraete2011), while others attribute evidence for lexical processing difficulties to relatively distal domains (lower or higher level; see above). Crucially, the neurobiological indices of atypical lexical and phonological development were not related to the levels of basic morphosyntactic and complex syntactic development in our sample, suggesting relative independence of lexical/phonological and morphosyntactic development in the context of language disorder. Therefore, instead of being viewed as compensatory, lexical and phonological deficits represent a relatively independent locus of language disorder in DLD, which is best viewed as syndromic and dimensional. We would like to emphasize that deficits in different subdomains of language in DLD are not necessarily fully functionally isolated. For example, it is possible that typical and atypical development in the domains of lexicon and morphosyntax are related, but the close link between the two can only be detected when examined within the critical periods for the acquisition of grammar, lexical knowledge, or both. Correspondingly, a neuroplastic change of the language system that either accommodated morphosyntacitc processing difficulties in a compensatory way or detrimentally affected other language domains could have happened earlier in the development than we were able to capture in this study. In this case, the seeming independence of lexical–semantic, phonological, and grammatical deficits in DLD could be viewed as emergent (Karmiloff-Smith, Reference Karmiloff-Smith2009), mirroring findings from adult patients with acquired language disorders. If this is the case, our findings nevertheless suggest that either this neuroplastic relationship is moderate in strength and does not persist into school age or some other factors play a larger role in the development of either of these domains beyond the “critical overlap” period.
Alternatively, our findings might suggest the presence of multiple loci rather than a single locus of deficits in DLD. Our results suggest certain constraints on the dynamic interplay between the (atypical) development of different facets of language. While such interactions, including the use of compensatory processing strategies capitalizing on developmental plasticity, might occur for certain domains of language development, certain processing deficits (e.g., in lexical processing) might be at least partially separable from other deficits (i.e., grammatical deficits).
Overall, the results of our study suggest that children with DLD have deficits in lexical processing that are related to their levels of lexical and phonological but not grammatical development. These results, in addition to providing further evidence for the separation of grammatical and lexical domains in typical development, indicate that these domains also represent relatively independent domains of deficits in DLD, while highlighting the links between phonological and lexical development abnormalities in DLD. We found atypical amplitudes of ERPs in children with DLD at different stages of processing, implicating initial phonological analysis, early lexical access, and, presumably, semantic processing in the lexical processing deficits in DLD. Further studies should focus more closely on the interplay between phonological and lexical development and processing in DLD, for example, by interrelating neurophysiological (rather than behavioral) indices of language development or by examining which specific characteristics of lexical and phonological development might be driving deficits in lexical processing in DLD.
Finally, note that the ERP study reported in this manuscript is part of a larger epidemiological study of the genetic and neurocognitive bases of DLD in a geographically isolated Russian-speaking population (described by Rakhlin, Kornilov, et al., Reference Rakhlin, Kornilov, Reich, Babyonyshev, Koposov and Grigorenko2013). The population is characterized by a high prevalence of DLD, a high degree of environmental homogeneity, and reduced genetic variability. Thus, the sample recruited for this study is inherently more homogenous than typical referral-based samples of children with DLD. As part of the larger study, we have been examining various ERP indices of cognitive and language processing in the same sample that we report on in this manuscript. Two other ERP studies (Kornilov et al., Reference Kornilov, Landi, Rakhlin, Fang, Grigorenko and Magnuson2014) found that children with DLD from the same population displayed atypical attentional auditory processing (manifested in a decreased P3b amplitude) but not preattentive auditory processing (manifested in intact mismatch negativity amplitudes and latencies). In individual differences analyses, deficits in attentional auditory processing were linked to the development of complex syntax, vocabulary, and verbal working memory. Thus, contrary to the hypotheses that the locus of deficits in DLD lies in low-level phonological representations and/or processing, our work so far suggests that in our sample, the deficits are related to more complex aspects of processing. Our goal in this study was to explore to what degree ERP measures indicate that lexical representations and/or processing are atypical in this sample, and whether atypical lexical processing could be related to individual differences in specific aspects of linguistic or cognitive ability or, even broader, viewed as an indicator of a particular neuroplastic trajectory, capturing the dynamic relationships between the development of language and that of its key substrate: the brain.
Supplementary Materials
The supplementary materials for this article can be found online at http://journals.cambridg.org/dpp.