INTRODUCTION
The species of the genus Paguristes Dana, 1851 was primarily described as having 13 pairs of gills. Later it was found that in several Paguristes species the gill number was reduced to 12 (Rahayu, Reference Rahayu2005). Rahayu (Reference Rahayu2005) reinstated the genus Stratiotes, originally established by Thomson (Reference Thomson1899), because the type species had only 12 pairs of gills. In recent studies, species having this character and previously assigned to Paguristes were transferred to Stratiotes and several new Stratiotes species were also described (Rahayu, Reference Rahayu2005, Reference Rahayu2007; Parente & Hendrickx, Reference Parente and Hendrickx2006; Komai, Reference Komai2009).
In August 2004, a new representative of warm-water hermit crabs was found in Peter the Great Bay (Russian waters of the Sea of Japan) and it was identified as Paguristes ortmanni Miyake, 1978 (see Petryashov & Kornienko, Reference Petryashov and Kornienko2006). Subsequent to this publication we sent some specimens of this hermit crab to Dr Tomoyuki Komai (Natural History Museum and Institute, Chiba, Japan) for confirmation of the identification. As a result, it was found to be actually a new species—Stratiotes nigroapiculus Komai, Reference Komai2009 occurring also from Hokkaido to Sagami Bay and to Akita Prefecture in Japan, subtidal to 140 m (Komai, Reference Komai2009). The name of the genus Stratiotes is now replaced by Areopaguristes by Rahayu & McLaughlin (Reference Rahayu and McLaughlin2010).
At present, the genus Areopaguristes is represented by 22 species (Komai, Reference Komai2009). Among these species the larvae of only A. abbreviatus (Dechancé, Reference Dechancé1963) were previously described (see Dechancé, Reference Dechancé1963). However, this species has a direct larval development; they hatch as megalopal stage.
The aim of this study is to give a description of the complete larval development of A. nigroapiculus based on the laboratory reared material. It is the first illustration of non-direct larval development in species of the genus Areopaguristes. As the genera Areopaguristes and Paguristes are closely related to each other, we have tried to compare the larval characters of Areopaguristes nigroapiculus and Paguristes species.
MATERIALS AND METHODS
An ovigerous female of Areopaguristes nigroapiculus was collected from Vostok Bay (inner bay of Peter the Great Bay, Sea of Japan) in July 2004, at a depth of about 3 m. The female was maintained in an aquarium with aerated seawater until hatching of the larvae. Hatching larvae were concentrated at the edge of aquarium using a point-light source and transferred to 1-l glass vessels with filtered and UV-sterilized seawater and reared to the megalopal stage at temperatures of 22–25°C and a salinity of 32‰. The density of the larvae was about 100 specimens per litre. The larvae were fed newly hatched nauplii of Artemia sp. The water in the vessels was changed daily. The larval development was evaluated twice a day.
The larvae were fixed in 4% formaldehyde for light microscopic studies. The fixed larvae were dissected under a MBS-10 stereomicroscope using fine entomological needles. The outlines of the larvae and their appendages of different developmental stages were drawn using a camera lucida attached to a binocular Ergaval microscope (Carl Zeiss Jena). Respective measurements were made with an ocular micrometer. Methods of measurements, descriptions of setal arrangements and terminology followed that of Clark et al. (Reference Clark, Calazans and Pohle1998) and Konishi & Shikatani (Reference Konishi and Shikatani1998). The setal arrangements are listed from protopod or coxal endite to exopod, from proximal to distal segments, and from anterior to posterior pleonal somites. The carapace length (CL) was measured from the tip of the rostrum to the posterior midpoint of the carapace.
Specimens of larval stages and the spent female are deposited in the Museum of the Institute of Marine Biology, Russian Academy of Sciences, Vladivostok (accession number 22933).
The seasonal occurrence of larvae of A. nigroapiculus in Peter the Great Bay was studied using the material of plankton surveys, twice a month from April to October 2007 and 2008. A Juday net with a ring diameter of 38 cm and a filtering cone made of a 168 mm mesh was used. Samples were taken at 66 stations across the entire water column from the bottom to the surface at depths ranging from 2 to 75 m. Simultaneously the temperature was measured at the water surface.
RESULTS
Description
Areopaguristes nigroapiculus hatched at a prezoea, which lasted about half an hour before moulting to the zoea I. It then passed three zoeal stages. The time required to reach the megalopal stage was 6–9 days at temperatures of 22–25°C.
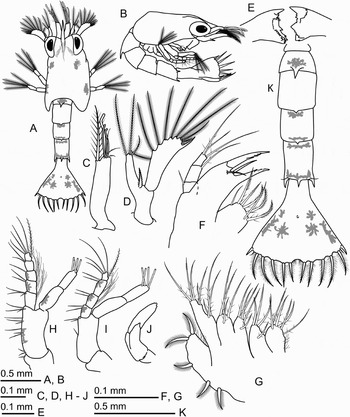
Fig. 1. Areopaguristes nigroapiculus, zoea I. (A) Dorsal view; (B) lateral view; (C) antennule; (D), antenna; (E) mandibles; (F) maxillule; (G) maxilla; (H) maxilliped I; (I) maxilliped II; (J) maxilliped III; (K) pleon, dorsal view.

Fig. 2. Areopaguristes nigroapiculus, zoea II. (A) Dorsal view; (B) lateral view; (C) antennule; (D) antenna; (E) mandibles; (F) maxillule; (G) maxilla; (H) maxilliped I; (I) maxilliped II; (J) maxilliped III; (K) pereiopods; (L) telson, dorsal view.
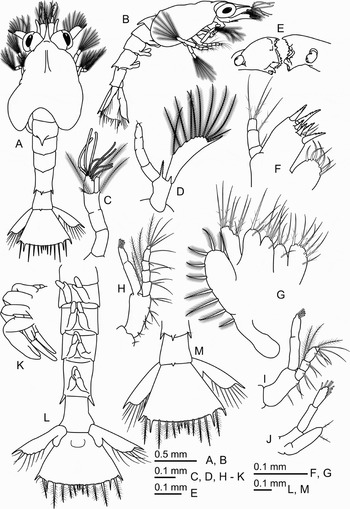
Fig. 3. Areopaguristes nigroapiculus, zoea III. (A) Dorsal view; (B) lateral view; (C) antennule; (D) antenna; (E) mandibles; (F) maxillule; (G) maxilla; (H) maxilliped I; (I) maxilliped II; (J) maxilliped III; (K) pereiopods; (L) pleon, ventral view; (M), telson, dorsal view.

Fig. 4. Areopaguristes nigroapiculus, megalop. (A) Dorsal view; (B) lateral view; (C) antennule; (C′) details of antennular endopod; (C″) details of antennular exopod; (D) antenna; (E) mandibles; (F) maxillule; (G) maxilla; (H) maxilliped I; (I) maxilliped II; (J) maxilliped III.

Fig. 5. Areopaguristes nigroapiculus, megalop. (A) Left cheliped, mesial view; (A′) left chela, dorsal view; (A″) left chela, ventral view; (B) right cheliped, mesial view; (C) pereiopod II; (D) pereiopod III; (E) pereiopod IV; (E′) details of pereiopod IV; (F) pereiopod V; (F′) details of pereiopod V; (G) pleopod I; (H) pleon, dorsal view; (H′) telson and uropods, dorsal view.
ZOEA I
Duration: 2–3 days.
Size: CL = 0.90 ± 0.04 mm (0.84–0.94 mm, N = 13).
Carapace ( Figure 1A, B): no setae on surface, low keel anteriorly in dorsal midline; rostrum short, tapering, not exceeding 1/3 length of carapace, nearly equal in length to antennular exopod but shorter than antennal scaphocerite; posterolateral margins rounded; eyes sessile.
Antennule ( Figure 1C): uniramous, with 3 terminal aesthetascs of different lengths, 3 short terminal sparsely plumose setae, and 1 long subterminal densely plumose seta.
Antenna ( Figure 1D): biramous; endopod fused with protopod, bearing 3 (2 long and 1 short) terminal plumose setae; protopod with 1 strong serrate spine near junction with endopod; scaphocerite longer than endopod and about 4 times longer than wide, with a distal strong spine, 9 long plumose setae and 1 short distal simple seta.
Mandibles ( Figure 1E): asymmetrically dentate; incisor process with a single strong tooth and few small teeth; molar process with serrate ridges and small acute denticles; no palp buds.
Maxillule ( Figure 1F): coxal endite with 7 (5 marginal and 2 submarginal) plumodenticulate setae; basial endite with 2 marginal, elongated spine-like teeth bearing small denticles and with 2 submarginal simple setae; endopod 3-segmented, proximal segment with 1 short simple seta, median segment with 1 long plumodenticulate seta, distal segment with 3 long terminal plumodenticulate setae.
Maxilla ( Figure 1G): coxal and basial endites each bilobed; coxal endite with 5 or 6 marginal and 1 submarginal setae on proximal lobe and with 3 marginal and 1 submarginal setae on distal lobe; basial endite with 4 marginal and 1 submarginal setae on proximal lobe and 3 marginal and 1 submarginal setae on distal lobe; all setae on coxal and basial endites plumodenticulate; endopod unsegmented, with 8 plumodenticulate setae in 3 tiers (3 + 2 + 3); scaphognathite fused with protopod in proximal part, distal lobe with 5 marginal plumose setae.
Maxilliped I ( Figure 1H): coxa with 1 plumodenticulate seta; basis with setal formula 2, 3, 3, 3; endopod 5-segmented, setal formula progressing distally 3, 2, 1, 2, 4 + I plus numerous thin setae on first to third segments; exopod 2-segmented, with 4 terminal plumose natatory setae.
Maxilliped II ( Figure 1I): coxa without setae; basis with setal formula 1, 2; endopod 4-segmented, setal formula progressing distally 2, 2, 2, 4 + I, numerous thin setae on second and third segments; exopod as in first maxilliped.
Maxilliped III ( Figure 1J): biramous, without setae.
Pereiopods: visible as small buds, first pereiopod bilobed.
Pleon ( Figure 1A, K): consisting of 5 somites and telson. Somite 2 with 1 prominent mediodorsal spine; somites 3 and 4 each with 1 short mediodorsal spine; somite 5 with 1 prominent mediodorsal and 1 pair of lateral spines, 1 fine seta present at base of each lateral spine.
Telson ( Figure 1A, K): fan-shaped, nearly as long as wide; posterior margin with median cleft and with 7 + 7 processes: first (outermost) process showing a short smooth articulated spine, second process a short plumose anomuran hair, third to seventh processes plumodenticulate articulated setae, fourth process longest; posterior margin also covered with short setules, few long setules in median cleft; small anal spine present.
Coloration in life: body generally translucent; dorsal surface of carapace and pleon with red-black chromatophores (as shown in Figure 1A); dorsal surface of telson with violet chromatophores (as shown in Figure 1K), ventral surface with yellow-brown chromatophores; basis of maxilliped I with red-brown chromatophore, endopod and exopod each with red-brown chromatophores; endopod and exopod of maxilliped II with red-brown chromatophores (as shown in Figure 1H, I); ventral surface of body with red chromatophores at base of mandibles.
ZOEA II
Duration: 2–3 days.
Size: CL = 0.99 ± 0.04 mm (0.90–1.05 mm, N = 10).
Carapace ( Figure 2A, B): eyes stalked, otherwise as in zoea I.
Antennule ( Figure 2C): biramous; protopod with 1 short seta at distal end; endopod and exopod clearly separated from protopod; endopod with 1 long terminal plumose seta; exopod with 6 or 7 terminal aesthetascs and 3 terminal plumose setae.
Antenna ( Figure 2D): biramous; protopod with 2 spines (1 strong serrate, 1 short smooth) on distal margin; endopod separated from protopod; scaphocerite as in zoea I.
Mandibles ( Figure 2E): palps present as buds.
Maxillule ( Figure 2F): coxal endite and endopod as in zoea I; basial endite with 4 marginal elongated spine-like teeth bearing small denticles and with 2 submarginal simple setae.
Maxilla ( Figure 2G): coxal and basial endites, endopod as in zoea I; scaphognathite with 7 marginal plumose setae.
Maxilliped I ( Figure 2H): coxa and basis as in zoea I; endopod setal formula progressing distally 3 + I, 2 + I, 1 + I, 2, 4 + I; exopod with 7 terminal plumose natatory setae.
Maxilliped II ( Figure 2I): coxa and basis as in zoea I; endopod setal formula progressing distally 2, 2 + I, 2 + I, 4 + I; exopod with 7 terminal plumose natatory setae.
Maxilliped III ( Figure 2J): biramous; endopod with 2 or 3 plumodenticulate setae; exopod incompletely 2-segmented, with 6 or 7 terminal plumose natatory setae.
Pereiopods ( Figure 2K): unsegmented, distinctly chelate.
Pleon ( Figure 2A, B): as in zoea I.
Telson ( Figure 2A, L): median cleft absent; few long setules replaced by a small spine; posterior margin covered with short setules, with 8 + 1 + 8 processes; anal spine inconspicuous.
ZOEA III
Duration: 2–3 days.
Size: CL = 1.11 ± 0.04 mm (1.03–1.14 mm, N = 12).
Carapace ( Figure 3A, B): as in zoea II.
Antennule ( Figure 3C): protopod incompletely 3-segmented, with 2 long plumose setae and 3 short simple setae at distal margin; endopod with 1 terminal plumose seta; exopod with 8 aesthetascs in 2 tiers and 3 short terminal setae.
Antenna ( Figure 3D): protopod as in zoea II; endopod incompletely segmented, with 2 terminal short setae; scaphocerite with 10 plumose setae.
Mandibles ( Figure 3E): palp buds increasing in size.
Maxillule ( Figure 3F): coxal endite with 9 marginal plumodenticulate setae; basial endite with 5 or 6 marginal and submarginal spine-like elongated teeth bearing small denticles and with 2 submarginal simple setae; endopod as in previous stages.
Maxilla ( Figure 3G): coxal and basial endites, endopod usually as in zoea II (number of setae on proximal lobe of coxal endite sometimes increasing up to 8); proximal lobe of scaphognathite without setae, now well separated from protopod, distal lobe with 8 or 9 marginal plumose setae.
Maxilliped I ( Figure 3H): unchanged from zoea II.
Maxilliped II ( Figure 3I): unchanged from zoea II.
Maxilliped III ( Figure 3J): exopod with 7 terminal plumose natatory setae; otherwise as in zoea II.
Pereiopods ( Figure 3K): increasing in size but incompletely segmented; first pereiopod chelate.
Pleon ( Figure 3A, L): sixth somite now delineated, with small mediodorsal spine; second to fifth somites each with a pair of small, biramous pleopod buds.
Uropod ( Figure 3L, M): endopod as a small bud; exopod fused with protopod, with 8 plumose setae on inner margin and a smooth, long terminal spine.
Telson ( Figure 3M): posterior margin with 9 + 9 processes; fourth process comparatively shorter than in zoea II, fused to telson; marginal setules present.
MEGALOP
Duration: undetermined.
Size: CL = 0.65 ± 0.02 mm (0.61–0.68 mm, N = 12).
Carapace ( Figure 4A, B): dorsal surface covered with sparse simple setae, with 7 setae along each posterolateral margin; rostrum small, inconspicuous; ocular acicles absent; ocular peduncles longer than antennular peduncles, corneas subequal in width to ocular peduncles.
Antennule ( Figure 4C, C′, C″): biramous; peduncle 3-segmented, with proximal segment bearing 6 or 7 (4 or 5 plumose) setae and 1 small spine on distal margin, penultimate segment with 4 or 5 setae, ultimate segment with 3 setae; endopod 2-segmented, proximal segment with 1 simple seta, distal segment with 7 simple setae; exopod 2-segmented; proximal segment without setae; distal segment with 5 or 6 subterminal aesthetascs, 1 long terminal and 7 or 8 short subterminal simple setae.
Antenna ( Figure 4D): shorter than cheliped; peduncle 3-segmented, with 4 or 5, 1 or 2, 2 or 3 simple setae; flagellum 4-segmented; first segment without setae; second segment with 0–2 short simple setae; third segment with 6 long simple setae, fourth segment with 8 or 10 long simple setae; antennal acicle terminating acutely, with 2 subterminal spines and 3 subterminal simple setae.
Mandibles ( Figure 4E): simplified, palps 3-segmented, terminal segment with 4 or 5 short setae.
Maxillule ( Figure 4F): coxal endite with 3 short setae and 3 or 5 minute teeth; basial endite with 7 marginal teeth, 3 or 4 plumodenticulate and 3 simple setae; endopod reduced, unsegmented, without setae.
Maxilla ( Figure 4G): coxal and basial endites bilobed; proximal lobe of coxal endite with 3 or 4 setae, distal lobe with 2 or 3 setae; proximal and distal lobes of basial endite with 4 or 5 and 6 setae, respectively; endopod reduced, with 0–1 short seta on distall end; scaphognathite with 22 or 24 marginal plumose setae and 4 simple setae on surface.
Maxilliped I ( Figure 4H): coxa with 3 simple setae; basis with 16 simple or plumodenticulate setae; endopod and exopod reduced; endopod with 1 minute terminal seta or unarmed; exopod 2-segmented, proximal segment with 1 median plumose seta, distal segment with 1 minute terminal seta.
Maxilliped II ( Figure 4I): basis with 2 or 3 simple setae; endopod 4-segmented, with setal formula progressing distally 0–2, 0–1, 1–2, 2–3; exopod incompletely 2-segmented, with proximal part bearing 1 short median simple seta, with distal part bearing 5 or 6 terminal plumose setae.
Maxilliped III ( Figure 4J): basis with 2 or 4 simple setae; endopod 5-segmented, first segment with 2 or 3 simple setae, second segment with 3 simple setae, following three segments with 7, 10 and 7 plumodenticulate or simple setae, respectively; exopod incompletely 2-segmented, proximal part with 1 short median simple seta, distal part with 6 terminal plumose setae.
Pereiopods ( Figure 5A–F′): covered with scattered plumose and simple setae of different lengths. Chelipeds nearly equal; dorsal surfaces of carpus, propodus (palm) and dactylus covered with numerous minute setules; ischium with 1 small spine on ventral margin; merus with 3 spines on ventral margin and 2 or 3 spines on dorsal margin, carpus with 2 spines on dorsodistal margin and 3 spines on dorsomesial margin; propodus with 3 spines on dorsomesial margin; dactylus with 1 proximal spine on mesial surface; dactylus and fixed finger of both chelipeds each with a row of small teeth along cutting edge, terminating in a corneous claw. In pereiopod II (first ambulatory leg), ischium with 1 minute distal spine on both dorsal and ventral margins; merus with 1 minute spine on ventral margin; carpus with 1–3 spines on dorsal margin; propodus with 1 distal spine on ventral margin; dactylus with 3 spine-like setae on ventral margin. In pereiopod III, carpus with 1 distal spine on dorsal margin; propodus with minute distal spine on ventral margin; dactylus with 2 or 3 spine-like setae on ventral margin. Carpi, propodi and dactyli of pereiopods II and III covered with short transverse rows of minute setules. In pereiopod IV, propodus has a rasp of 5 corneous scales on ventral margin; dactylus terminating in a simple small claw. In pereiopod V, propodus has a rasp of 9 corneous scales and 2 or 3 long curved setae; dactylus has a rasp of 3 corneous scales; dactylus articulated with propodus subterminally.
Pleon ( Figure 5H): consisting of six somites and telson; dorsal surface of all somites covered with simple setae as illustrated; somites 2–5 each with biramous pleopods; somite 6 with uropods.
Pleopods ( Figure 5G): biramous; endopods each with 2 small hooks on distal margin; exopods each with 8 marginal plumose setae.
Uropods ( Figure 5H, H′): biramous, nearly symmetrical; endopod and exopod clearly separated from protopod; protopod with 1 long sparsely plumose seta, 1 short simple seta and 1 small spine; endopod with 4 or 5 marginal corneous scales, 2 or 3 long plumose and 3 or 4 short simple setae; exopod marginally with 8–11 corneous scales, 10 or 11 plumose setae, and 3 or 4 simple setae.
Telson ( Figure 5H, H′): nearly symmetrical, dorsal surface with 2 rows of short simple setae along midline; posterior margin rounded, with 10 long plumose setae, lateral margins with some short simple setae.
Coloration in life: carapace with 3 pairs of chromatophores on dorsal surface; ocular peduncle with chromatophore on dorsoproximal part; endopod and exopod of maxilliped II with few chromatophores; telson with 2 pairs of chromatophores on dorsal surface. All chromatophores are yellow-brown.
Seasonal occurrence of larvae in the plankton
In the plankton of Peter the Great Bay, the larvae of Areopaguristes nigroapiculus occasionally occurred over depths ranging from 3 to 45 m, with maximal density of 1 specimens/m3. They were found only in July and August, when surface water temperatures were 18–22°C.
DISCUSSION
The genus Paguristes, which contains about 120 species in the world, is the largest genus in the family Diogenidae (De Grave et al., Reference De Grave, Pentcheff, Ahyong, Chan, Crandall, Dworschak, Felder, Feldmann, Fransen, Goulding, Lemaitre, Low, Martin, Ng, Schweitzer, Tan, Tshudy and Wetzer2009; McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010). However, the descriptions of larval developments are available for only nine species: Paguristes sericeus A. Milne-Edwards, 1880 and P. spinipes A. Milne-Edwards, 1880 from West India; P. eremita (Linnaeus, 1767) = P. oculatus (Fabricius, 1775) from the Mediterranean Sea; P. turgidus (Stimpson, 1857) from the north-eastern Pacific; P. frontalis (H. Milne Edwards, 1836) from southern Australia; P. tortugae Schmitt, 1933 and P. erythrops Holthuis, 1959 from Brasilia; P. digitalis Stimpson, 1858 and P. ortmanni Miyake, 1978 from the north-western Pacific.
Larval development in diogenid hermit crabs is highly variable. Diogenids are well known to have abbreviated larval development (Rabalais & Gore, Reference Rabalais and Gore1989). Most Paguristes species have 3 zoeal and a megalopal stages, viz., P. turgidus (see Hart, Reference Hart1937), P. digitalis (see Kurata, Reference Kurata1968), P. eremita (see Pike & Williamson, Reference Pike and Williamson1960), P. spinipes (see Provenzano, Reference Provenzano1978), P. erythrops (see Hebling & Fransozo, Reference Hebling and Fransozo1982), P. tortugae (see Hebling & Negreiros-Fransozo, Reference Hebling and Negreiros-Fransozo1983) and P. ortmanni (see Quintana & Iwata, Reference Quintana and Iwata1987). Paguristes sericeus has an abbreviated development with only 2 zoeal stages (Rice & Provenzano, Reference Rice and Provenzano1965). In P. frontalis, larvae hatch at the megalopal stage, which indicates a direct larval development (Morgan, Reference Morgan1987). As most members of the genus Paguristes, Areopaguristes nigroapiculus has also 3 zoeal stages.
Based on our data, the larvae of A. nigroapiculus, as with most Paguristes species, attained the megalopal stage approximately within one week after hatching. Only P. spinipes (see Provenzano, Reference Provenzano1978) possessed more prolonged development: the megalopal stage of this species was attained after 3–4 weeks after hatching.
In our studies, the larvae of A. nigroapiculus are rarely encountered in Peter the Great Bay. Probably, this is due to that fact this species is not numerous in this area and its pelagic period is very short compared to other hermit crabs.
The zoea of A. nigroapiculus is smaller than any known Paguristes larvae. Carapace length of zoea I of A. nigroapiculus is 0.9 mm, whereas that of the Paguristes species varies from 1.2 mm in P. digitalis to 1.9 mm in P. erythrops.
In the principal larval characters, A. nigroapiculus agrees well with Paguristes species having 3 zoeal stages. In zoea, these characters include: carapace with low keel anteriorly on the dorsal midline; posterolateral margins of carapace being rounded; short rostrum not extending as far as the antennular exopod and antennal scaphocerite; scaphocerithe with a distal spine; endopod of the maxillula being 3-segmented in all stages; pleonal somites 2–5 with mediodorsal spines of different length (except for P. eremita); somite 5 with posterolateral spines; telson formula being usually 7 + 7, 8 + 1 + 8 and 9 + 9 processes; uropods being nearly equal from right to left, their endopod and exopod being fused to the protopod in zoea III; small anal spine being present. In megalop, the shared characters include: no ocular scales; cornea being narrower than the eyestalk; antennules and antennae being distinctly shorter than the chelipeds; antennular endopod being 2-segmented; chelipeds and uropods being nearly equal from right to left; telson being rounded, with numerous long setae.
Many authors believe that the anterolateral carapace spines and posterolateral spines on the fifth pleonal somite are the characters diagnostic of Paguristes zoeas (Rice & Provenzano, Reference Rice and Provenzano1965; Provenzano, Reference Provenzano1978; Quintana & Iwata, Reference Quintana and Iwata1987). It should be pointed out that zoea of A. nigroapiculus also has posterolateral spines on the fifth pleonal somite, but lacks anterolateral carapace spines. Thus, the absence of any carapace spines can be treated as a possible larval character for Areopaguristes species. It is pertinent to note that Diogenes larvae also possess both features mentioned above (Korn et al., Reference Korn, Kornienko and Komai2008). Areopaguristes zoea shares many morphological features with the larvae not only of Paguristes but also of Diogenes and other diogenid genera. As noted by Provenzano (Reference Provenzano1968), only a combination of characters allows distinguishing larvae of various diogenid genera from each other.
A very important character diagnostic to anomuran larvae is the armature of telson (McLaughlin et al., Reference McLaughlin, Crain and Gore1992, Reference McLaughlin, Siddiqui and Crain1993). Based on the differences in the formation of the fourth telson process beyond the second zoeal stage, four informal groups of species are recognized in Diogenidae (Bartilotti et al., Reference Bartilotti, Calado and dos Santos2008). Zoea of the genus Paguristes is referred to group 4 where the telson processes are represented by setae in all the larval stages. Our data revealed that the fourth telson process of A. nigroapiculus in zoea III is shorter than that in the previous stage, fused to the telson, and lacks setules. Areopaguristes larvae should be most likely placed in group 2, including Clibanarius species, based on the fourth telson process being either a well developed or a reduced fused spine.
Only two Paguristes species (P. ortmanni and P. digitalis) occur in the Sea of Japan from Kyushi northward to Hokkaido (Asakura, Reference Asakura2006), so their larvae and the larvae of A. nigroapiculus, may be simultaneously found in the plankton sample in this region. The description of P. digitalis larvae by Kurata (Reference Kurata1968) is not fully detailed. A comparison of our material of A. nigroapiculus with the larvae of P. ortmanni (see Quintana & Iwata, Reference Quintana and Iwata1987) indicated that the setation of appendages of both species are nearly identical in zoea I and have slight differences in later zoeal stages. Considerable distinctions are noted in the megalopal stage, but they correspond to the usual differences between the larvae within one genus.
Since the larvae of A. nigroapiculus, like Paguristes, have only 3 zoeal stages, they represent accelerated development in comparison with the prolonged development of other hermit crabs which comprise 4 or more zoeal stages. In A. nigroapiculus, the protopod of the antennule and endopod of the antenna in zoea III are incompletely 3-segmented; the mandibular palp appears in zoea II; and pleopods appear in zoea III. It is interesting to note that although P. eremita has also 3 zoeal stages (Pike & Williamson, Reference Pike and Williamson1960), certain larval characters appear at an earlier stage in this species than in the rest of Paguristes having 3 zoeal stages. The rudiments of pleopods are already present in zoea I; the final numbers of telson processes (9 + 9) are attained in zoea II; and the uropods are fully segmented on their emergence in zoea III (endopod and exopod being articulated to the protopod).
Unfortunately, the lack of larval information about most Areopaguristes species does not allow us to provide the diagnosis of this genus and to summarize the larval characters distinguishing Areopaguristes from Paguristes. The absence of any carapace spines and fused fourth telson process beyond zoea II are the possible zoeal characters diagnostic of the genus Areopaguristes. In the remaining larval characters, A. nigroapiculus agree well with Paguristes species having 3 zoeal stages. The found differences between larvae of these two genera lend support to the separation of Areopaguristes from Paguristes.
ACKNOWLEDGEMENTS
We are greatly indebted Dr Tomoyuki Komai (Natural History Museum and Institute, Chiba, Japan) for the identification of Areopaguristes nigroapiculus females. This work was supported by the Russian Foundation for Fundamental Researches (grant number 08-04-00929).







