Introduction
Many people presenting to memory clinics do not ultimately receive diagnoses of the neurodegenerative diseases the clinics were established to identify and treat.Reference Bell, Harkness, Dickson and Blackburn 1 , Reference Menon and Larner 2 Although subjective cognitive symptoms might herald future dementia in a minority, many patients with subjective or mild cognitive impairment (MCI) might alternatively be positively identified as having functional cognitive disorders (FCD).Reference Ball, McWhirter and Ballard 3
FCD have been described as a heterogeneous but overlapping set of clinical presentations which produce genuine cognitive symptoms that are internally inconsistent and not the direct result of brain disease; including memory symptoms in anxiety or depression; excessive attentional focus on everyday memory problems; health anxiety about dementia; and memory symptoms as part of another functional disorder.Reference Teodoro, Edwards and Isaacs 4 , Reference Stone, Pal, Blackburn, Reuber, Thekkumpurath and Carson 5
Meta-analysis of memory clinic populations suggests that 24% of patients are likely to have FCD.Reference McWhirter, Ritchie, Stone and Carson 6 Our clinical experience also tells us that patients with functional neurological disorders (FND) complain bitterly of troublesome cognitive symptoms. But despite the frequency of FCD in both clinical environments, research into functional cognitive symptoms has lagged behind that of other FND domains, and has been largely absent from the neurodegenerative disease arena.
Defining positive clinical signs for FND has improved patient care and invigorated research into mechanisms of and treatments; these are no longer diagnoses of exclusion but can now be accurately identified and therefore studied and treated.Reference Espay, Aybek and Carson 7 There is a pressing need for similar well-evidenced clinical signs to aid accurate diagnosis of FCD and therein improve management.
We now know that large numbers of individuals with FCD present to memory clinics; but in the absence of trials of treatment there remains almost no evidence for the best course of treatment or follow-up. More accurate diagnostic methods, along with recent proposed diagnostic criteria,Reference Ball, McWhirter and Ballard 3 will facilitate much-needed clinical trials of treatments for FCD. Second, there is a risk that patients with FCD are incorrectly described as having preclinical Alzheimer’s disease. As researchers aim to identify, and therefore modify, disease at the earliest stages, it is important to identify not only neurodegenerative disease, but also those individuals with FCD, whose symptom trajectories may obscure trial outcomes and lead to potentially harmful interventions.
Previous studies examining potential FCD diagnostic features have reported that patients with FCD are more likely to attend clinic alone, to report “poor” or “fair” memory on a Likert scale, and to bring a written list of symptoms than those with neurodegenerative disease.Reference Bharambe and Larner 8 - Reference Randall and Larner 11 Others have pointed to impaired metacognition as a potential mechanism and marker of FCD.Reference Bhome, McWilliams, Huntley, Fleming and Howard 12 Reuber et al have analyzed language and interaction during the clinical consultation, finding that patients with FCD provide more linguistically complex accounts of symptoms than those with established diagnoses of neurodegenerative disease.Reference Reuber, Blackburn and Elsey 13 , Reference Jones, Drew and Elsey 14 But these interactional features have primarily been tested against a definition of FCD in which there is an absence of “objective” cognitive impairment, and not in those who struggle with cognitive tests, or in unselected patients typically encountered in memory clinics.
This study aimed to address the question of how we might confidently and accurately diagnose FCD in an unselected sample of patients presenting with cognitive symptoms and complaints but not dementia.
Methods
Participants of all ages were recruited directly from an older-adults memory clinic, neurology and neuropsychiatry clinics, and a county-wide register of people assessed in the memory clinic who had consented to be contacted about research (The Scottish Brain Health Register).
Participants had already been clinically assessed by a consultant old-age psychiatrist, neuropsychiatrist, or neurologist as a part of usual clinical care. Subjects met inclusion criteria who had presented for assessment of predominantly cognitive symptoms, but were not severely cognitively impaired or assessed as having probable Alzheimer’s type dementia (according to current consensus diagnostic criteriaReference McKhann, Knopman and Chertkow 15), or another dementia syndrome. Exclusion criteria, established from case notes and referrer assessment, were: non-English speakers (due to English-language validated measures), age <18, learning disability, psychotic disorder, severe personality disorder, active suicidal ideation, or suspicion of factitious disorder or malingering.
Participants were visited at home (unless they preferred to attend clinic) by a researcher (L.M.), who was blind to the previous clinical assessment. The research interview opened with an open question: “Tell me about the problems you have been having?,” following which the researcher used an electronic timer to measure the duration in seconds of the initial response; allowing the participant to speak without interruption and stopping the timer when the participant came to a natural stop. The researcher recorded, using a structured proforma, a summary of the response, the number of discrete cognitive complaints (word-finding difficulties and forgetting appointments would be recorded as two complaints); and the number and degree of detail of each example of cognitive failure described. The interview examined awareness and engagement with current news, television or film, reading (books, magazines or newspapers), description of typical daily activities, and a compound question: “Where are you from, and what did you/do you do for a job?”
The interview included questions about the duration and perception of memory and thinking problems: “Did your problems start after an event, injury, or illness?”; “Do you think other people are more worried about your memory and thinking than you? Or are you more worried than other people?”; a 5-point Likert scale: “In general, how would you rate your memory?”Reference Larner 10 , Reference Paradise, Glozier, Naismith and Davenport 16; “What did your memory used to be like?”; “What do you think is the cause of any memory or thinking problems you have been having?”; and “Do you think that your memory or thinking problems are most likely to: Get better/worse/stay the same/come and go.” Participants were asked about dementia in a close family member or previous “daily contact or caring responsibility” for a person with dementia.
Brief examination of gait (short observed walk, turn, and heel-to-toe walk) and coordination (finger-nose test) was followed by cognitive tests: Montreal Cognitive Assessment (MoCA, with responses timed using an electronic timer), Luria 3-step test, interlocking finger test,Reference Moo, Slotnick, Tesoro, Zee and Hart 17 digit spans, and the Medical Symptom Validity Test (MSVT),Reference Green 18 and questionnaires: Patient Health Questionnaire 15 (PHQ-15), Hospital Anxiety and Depression Scale (HADS), the Pittsburgh Sleep Quality Inventory (PSQI), and Multifactorial Memory Questionnaire (MMQ; consisting of three scales; MMQ-Satisfaction—overall satisfaction with memory [scale 0-72], MMA-Ability—perception of memory ability, via experience of 20 common memory mistakes [0-80], and MMQ-Strategy—use of memory strategies and aids [0-76]).Reference Troyer and Rich 19 The assessment concluded with the Mini International Neuropsychiatric Interview (MINI; English version 7.0.2 for Diagnostic and Statistical Manual of Mental Disorders, fifth edition [DSM-5]).
Ethical approval was obtained from the South East Scotland Research Ethics Committee. The protocol was preregistered (https://doi.org/10.17504/protocols.io.z97f99n).
Establishing the reference diagnoses
Reference diagnoses were established during meetings of the senior authors (a consultant neurologist [J.S.], consultant neuropsychiatrist [A.C.], and consultant of psychiatry of ageing [C.R.]). All information from the prestudy clinical assessment (clinical notes from the memory, neurology or neuropsychiatry clinic assessment, electronic medical records, and results of neuroimaging and other investigations), not including information collected during the research assessment, was presented to the panel. Panel members independently recorded their opinion on (a) the most appropriate diagnosis(es) to account for the cognitive symptoms and (b) the contribution of various etiological factors (Supplementary Figure S1). Consensus opinion allowed diagnostic ratings in parallel domains: FCD, neurodegenerative disease, medical or pharmacological cause of cognitive symptoms, and primary psychiatric disorder, recognizing that cognitive symptoms often have overlapping etiologies. Discrepancy in opinions triggered discussion and review of information until consensus was reached. For the purposes of identifying predictors of a functional disorder, a score of “Probable” or “Possible likely” for functional cognitive symptoms indicated presence of FCD (regardless of other contributory factors), whereas “Possible unlikely” or “Unlikely” indicated absence of FCD.
Statistical methods
Excel (v2101) and R (v3.6.0) were used for analyses.
A prestudy sample size calculation suggested that a sample size of 115 would be required for a diagnostic risk prediction accuracy of 90% sensitivity and 90% specificity in a group with a 30% prevalence of FCD.
Data were tested for normality using the Shapiro–Wilkes test. Multiple t-tests, Mann–Whitney tests, Chi-square, and Fishers exact tests were used to compare variables between patients with a reference diagnosis of FCD (“probable” or “possible likely” FCD) and those without (“unlikely” or “possible unlikely” FCD). Significance was adjusted for multiple comparisons using the Holm–Bonferroni method. Variables which were significantly (P < .05) different between groups were entered as covariates in a multivariable logistic regression model, and covariates removed iteratively to optimize the model.
Results
Forty-nine participants were recruited: 26 from memory clinic, 10 from neurology clinic, 6 from neuropsychiatry clinic, and 7 from the Scottish Brain Health Register. Forty-six were visited at home and 3 attended the research facility. Recruitment ended early, in March 2020, because of COVID-19.
Demographic and baseline clinical data are described in Tables 1 and 2. Table 1 also describes the proportion of participants who had undergone brain imaging and/or Addenbrooke’s Cognitive Examination iii prior to recruitment. Table 3 describes results of the key research measures.
Table 1. Demographic and Clinical Characteristics of FCD and Non-FCD cognitive Disorder Participants

Probable or possible AD (n = 4), probable or possible cerebrovascular disease (n = 3), probable or possible mixed AD/cerebrovascular disease (n = 5), alcohol-related cognitive impairment (n = 1), hearing or visual impairment (n = 2), normal ageing (n = 3). Psychiatric comorbidities: anxiety (n = 2), depression (n = 3), and adjustment disorder (n = 1).
Abbreviation: FCD, functional cognitive disorders; SD, standard deviation.
a Non-FCD group reference diagnoses.
Table 2. Diagnoses as Reported in Clinic Letter Prestudy Recruitment

Table 3. Research Measures in FCD and Non-FCD Participants

Abbreviations: HADS-A, Hospital Anxiety and Depression Scale—Anxiety; HADS-D, Hospital Anxiety and Depression Scale—Depression; MINI, Mini-International Neuropsychiatric Interview; MMQ, Multifactorial Memory Questionnaire; MoCA, Montreal Cognitive Assessment; PHQ-15, Patient Health Questionnaire 15; SD, standard deviation.
bold text indicates p < 0.05
Thirty-one participants received a reference diagnosis of FCD. Participants with FCD were significantly younger than those without FCD (P < .01), but there was no significant difference in sex (P = .5), or years of education (P = .9).
Memory symptom self-report
Two non-FCD participants denied memory problems; no FCD participants denied memory problems. FCD participants reported a longer duration of symptoms than patients without FCD (median 3 years [IQR 1-5] vs 1.75 years [IQR 1-2.5]).
Similar proportions of FCD (25 [19%]) and non-FCD (14 [22%]) groups met criteria for Subjective Memory Complaint as ascertained by a rating of “poor” or “fair” on a 5-point Likert scale (SMC Likert) in response to: “In general, how would you rate your memory?” SMC Likert scores inversely correlated with age in the whole group (Spearman test, rho = −0.54, P = <.01) and in the FCD group, but not in the non-FCD group (Spearman test, rho = 0.05, P = .9).
Similar proportions of FCD and non-FCD groups (11/31 [35%] vs 4/18 [22%]) reported previously having an “excellent” memory responding to: “What did your memory used to be like?”
More FCD participants related the start of symptoms to a specific event, injury, or illness (49% FCD vs 11% non-FCD). In the FCD group, adjustment to retirement, a stressful personal event, a bereavement, a fall, an anxiety disorder or another functional disorder (n = 2), serious illness, medication, and elective medical/surgical treatment (n = 2). In the non-FCD group, stroke and medical illness.
More FCD than non-FCD participants reported that others were more worried than they were about their memory (45% [14/31] vs 33% [6/18]), and more FCD than non-FCD participants believed their symptoms would get worse over time (45% [14/31] vs 33% [6/18]); neither difference was statistically significant after Bonferroni–Holm correction.
More FCD participants reported having a close family member with dementia (20 [65%] vs 5 [28%]), or previous caring responsibilities or daily contact with a person with dementia (11 [35%] vs 2 [11%]); neither difference reached significance after Bonferroni–Holm correction.
Interaction and language
Only eight participants were accompanied by another adult during the research assessment (four FCD and four non-FCD), these predominantly being home visits. However, significantly more FCD participants had attended their prerecruitment clinical appointment alone (15/31 [48%] vs 2/18 [11%] of non-FCD participants; Chi-square P < .01).
FCD participants, when asked: “Tell me about the problems you have been having?” Spoke without interruption for a median time of 124 seconds (IQR 80-168), significantly longer than non-FCD participants who spoke for a median time of 42 seconds (IQR 28-55) (Mann–Whitney test, corrected P < 0.01; Figure 1). FCD participants described a mean of three cognitive complaints/symptoms compared with one in the non-FCD group (P < .01), and were more likely to describe one or more specific examples of cognitive failure than non-FCD participants (P = .01) (Box 1 for examples). There was no significant difference between the rate of successfully answering both parts of a compound question between FCD and non-FCD participants (26/31 [84%] vs 11/18 [61%]).
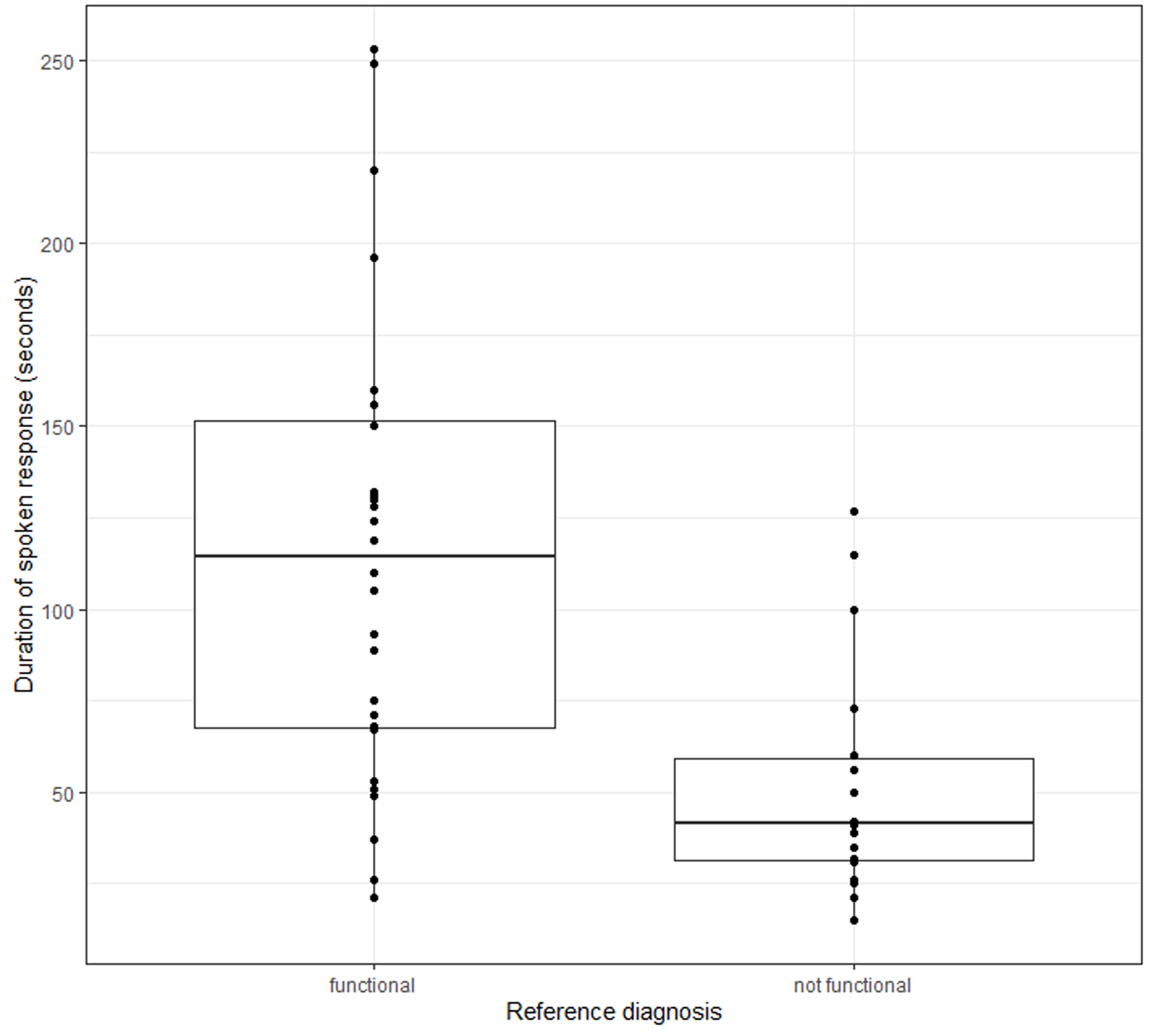
Figure 1. Duration of response: “Tell me about the problem you’ve been having” (three outlier [functional, >500 seconds] removed for plot).
Box 1. “Tell Me about the Problems You have been Having?”
Participants without FCD reference diagnoses:
“I don’t know. I have a bad memory. I always check with [my husband]”
(77-year-old woman)
“My daughter says I don’t remember her shifts. Other than that, my memory’s fine.”
(79-year-old woman)
Participants with FCD reference diagnoses:
“It’s forgetfulness. For example, I forgot the name of the doctor I saw in clinic—Dr [X]—I had to check his name. It is frustrating. I will watch a film and think ‘who is that actor?’ For example, I was watching a film called ‘Pimpernel Smith’ and I couldn’t remember the actor in it—it’s Lesley Howard of course! I can remember things from 40-50 years ago or even 4-5 years ago. Sometimes I struggle with finding words. The other day I went out to meet a pal—I took my jacket off and thought I had lost my wallet—but I had just put it on the side.”
(74-year-old man)
“I wonder around the house trying to remember what I’m looking for. I’m bad on names, even with people I know well. I have difficulty calculating in recipes, for example to make a recipe for 4 for 8 people. And yesterday my son asked where the nearest ATM and I couldn’t remember but it came back to me later. Things often come back later on. I went to collect the Christmas tree at Christmas time and when I reached a fork in the road I couldn’t visualise which way to go…”
(80-year-old woman)
Report of cognitively engaging activities
When asked to describe typical daily activities, 13/31 (42%) FCD participants and 2/18 (11%) non-FCD participants described cognitively engaging activities (Fisher test, corrected P = .45): office work, reading, academic study, and administrative tasks in the FCD group; reading and playing piano in the non-FCD group.
There was no difference between FCD and non-FCD participants in ability to recall details from a recently watched specific television program or movie (8/31 [26%] vs 3/18 [17%]), or in detailed recall of books, magazines, or newspapers (7/31 [23%] vs 3/18 [17%]). Non-FCD participants were more often unable to recall the name of a book they were currently reading (10/18 [56%] vs 7/31 [23%] FCD participants) but this was not significant (Fisher test, corrected P = .45).
When asked “can you tell me what has been happening in the news?,” FCD participants tended to describe events with evidence of some cognitive engagement (rather than just broad naming of topics), compared with non-FCD participants, but this was not statistically significant (11/31 [35%] vs 2/18 [11%]). Similar proportions of FCD and non-FCD participants reported no awareness at all of current news (5/31 [16%] vs 5/18 [28%]).
Multifactorial memory questionnaire
FCD participants had significantly lower MMQ-Satisfaction scores and MMQ-Ability scores than non-FCD participants, and reported greater use of memory strategies, but only for MMQ-Ability was this difference significant after correction for multiple comparisons.
Cognitive tests
Mean MoCA score was 22 in the FCD group and 20 in the non-FCD group (t-test corrected P = 1). Differences in orientation score and time taken to draw a wire cube were no longer significant after correction for multiple comparisons. FCD and non-FCD participants achieved similar scores in Luria 3-step and Interlocking fingers tests.
Exploratory analyses were performed to identify patterns of internal inconsistency within cognitive tests. Individual participants tended to score similarly across the board; ie, those who performed well performed well in all tests; those who performed in the impaired range did so throughout, regardless of reference diagnosis. Perseverations on verbal fluency were more frequent in non-FCD participants (7/31 and 8/18), not reaching significance. No participant scored better on delayed recall than registration.
There was no significant difference in overall failure rate or in the proportion of either “invalid” or “severe impairment/dementia” profiles on the MSVT (Figure 2). That is, participants in both FCD and non-FCD groups had invalid profiles, and participants in both groups had “severe impairment/dementia” profiles.

Figure 2. Medical Symptom Validity Test (MSVT) performance.
Psychiatric symptoms and diagnoses
FCD participants had significantly higher scores on both anxiety and depression subscales of the HADS. In the MINI diagnostic interview, significantly more FCD participants met criteria for at least one current psychiatric diagnosis (19 [68%] vs 1 [11%], Chi-square test, corrected P < .01).
In the non-FCD group, two participants met criteria for current major depressive disorder.
In the FCD group, 13 met criteria for primary diagnosis of current major depressive disorder, of whom eight also met criteria for an anxiety disorder (panic disorder, social anxiety disorder, and generalized anxiety disorder). Six met criteria for primary diagnosis of an anxiety disorder (panic disorder and generalized anxiety disorder). One reported a previous episode of hypomania. Three endorsed passive suicidal thoughts, but were assessed as being at low risk of suicide.
Two FCD participants became tearful in discussion of bereavements but did not meet criteria for any psychiatric diagnosis. Of note, although no participants met DSM-5 criteria for obsessive–compulsive disorder (OCD), one participant described previous severe OCD and several others were noted by the researcher to describe obsessional thought structures and compulsive cognitive processes which were not detected by the study measures.
Sleep and physical symptoms
FCD participants reported poorer sleep than non-FCD participants, globally and on all subscales of the PSQI except for sleep latency, sleep disturbance, and use of sleep medication; only on the sleep efficiency subscale did this difference remain significant after Bonferroni–Holm correction (Mann–Whitney, corrected P < .01).
FCD participants endorsed more physical symptoms than non-FCD participants on PHQ-15: noteworthy given the younger age of the FCD participants (a mean of 5 vs 2 symptoms in the non-FCD group, t test, corrected P < .01).
Predictive models for FCD reference diagnosis
On multiple logistic regression analysis, decreasing age and increasing duration of spoken response were both associated independently and significantly with FCD (age in years β = −0.23, standard error [SE] = 0.09, odds ratio [OR] = 0.79 [95% CI 0.67-0.95], duration of response in seconds β = 0.03, SE = 0.01, OR = 1.03 [95% CI 1-1.05]). The model explained 74% of the variability in diagnosis (Nagelkerke’s pseudo R 2) with a sensitivity of 90%, specificity of 83%, and accuracy of 80%, and an area under the Receiver-operating characteristic (ROC) curve in the observed data of 0.94. HADS depression and anxiety scores and PHQ-15 scores were no longer significant in multiple regression in this small sample.
Receiver-operating curves comparing the performance of this model with predictive models based solely on age, solely on duration of response, and solely on MoCA score, are illustrated in Figure 3.

Figure 3. Model performance.
An alternative model was calculated with a view to clinical utility, using optimum cut points for age (<74 years) and duration of spoken response (>67 seconds). A logistic regression model using these binary classifiers explained 63% of variability in diagnosis (Nagelkerke’s pseudo R 2), and produced odds ratios favouring diagnosis of FCD of 34.8 for age <74 years (95% CI 29.1-41.5) and 7.48 for duration of spoken response >67 seconds (95% CI 7.31-7.64); this model had a sensitivity of 93%, specificity of 78%, accuracy of 88%, and area under the ROC curve of 0.91 in the observed data.
Discussion
In this study, a robust expert panel consensus process identified probable FCD in 63% of the 49 patients with cognitive symptoms recruited to the study. This sample of “borderline” cases, excluding those with dementia, may not be representative of all new cognitive presentations in the population. Nevertheless, the proportion of probable FCD diagnoses was consistent with prevalence figures identified in our previous meta-analysis of memory clinic patients (in which, of the 47% of 12 000 patients who did not receive diagnoses of dementia, 51% received diagnoses in keeping with FCD, and 28% descriptive diagnoses of MCI).Reference McWhirter, Ritchie, Stone and Carson 6 Functional cognitive disorder appears to be a common cause of cognitive symptoms.
Despite these consistent empirical observations, functional cognitive disorders remain under-recognized, or under-reported, in real-world clinical practice. Of the 31 FCD participants in this study, only 17 (54.8%) had received a clinical diagnosis of, or descriptive diagnosis in keeping with, a functional disorder. The remaining 14 had been described in clinic letters as having “subjective cognitive impairment” or similar, “MCI,” or described in terms of likely absence of disease.
To describe this as a “missed” FCD diagnosis rate of 45.2%, however, is an oversimplification. We do believe that the opportunity to explain functional disorder etiologies is often missed in this group. However, some people presenting with minor functional cognitive symptoms are reporting “normal” cognitive symptoms and a “disorder” diagnosis may not be appropriate. Careful assessment of the extent and severity of distress and disability associated with the functional cognitive symptoms would be one reasonable way of ascertaining who will benefit from explanation and reassurance, and who will do better with diagnosis and treatment following a medical model.
We suggest that the wide and varied range of diagnostic descriptions used by clinicians for this group of patients with cognitive symptoms but not dementia (Table 2) reflects the inadequacy of research terms such as MCI and subjective cognitive decline (SCD); clinicians quite appropriately look instead to multiaxial formulations in attempts to address issues of multiple etiology, uncertainty, and comorbidity.
This study suggests that not only is FCD a common cause of symptoms, but also can be confidently identified on the basis of positive clinical features of internal inconsistency.
The most striking feature predicting FCD in the research assessment was longer duration and greater degree of detail of participants’ response to an open question. Participants with an FCD reference diagnosis, when asked: “Tell me about the problems you have been having?,” spoke without interruption for on average three times longer than those without FCD. This supports findings of conversation analysis studies,Reference Reuber, Blackburn and Elsey 13 but crucially also demonstrates utility of these factors not only in selected patients with definite FCD but in an unselected “real” clinical cohort. Moreover, our study suggests that these techniques do not require special technology but are accessible as part of simple clinical assessment, supported only by a clock.
“Duration of spoken response” is at core a proxy marker of internal inconsistency between perceived and observed function. While the person with FCD perceives amnestic, severe attentional difficulties and cognitive “struggle,” their detailed and linguistically intact description of their difficulties and past cognitive lapses demonstrates: preserved episodic memory function, ability to maintain attention, and, often, sophisticated use of language and information. That is not to say that people with FCD do not have genuine cognitive difficulties in these areas. Rather, we suggest that the “automatic” nature of the task of relaying their difficulties and experiences allows them to circumvent processes (not yet clearly understood) which cause processes akin to “choking” during more deliberate cognitive tasks. Similar clinical signs of inconsistency are key in the diagnosis of other forms of functional neurological disorder. For example, in Hoover’s sign, leg weakness resolves or improves when attention is shifted to moving the contralateral leg.
Duration of spoken response reflects additional factors likely to increase specificity to FCD. Detailed spoken response requires intact language function, contrasting with early disruption and semantically impoverished language in neurodegenerative diseases,Reference Fraser, Meltzer and Rudzicz 20 and reflects the metacognitive evaluation of a cognitive problem, also reflected in FCD participants’ lower memory satisfaction and ability MMQ scores.
Although internal inconsistency is key to FCD diagnosis, it is important that we look for internal inconsistency in the right places. Internal inconsistency within cognitive tests, including in a forced-choice performance validity test, was less helpful in predicting FCD in this study. Some participants with FCD scored consistently highly and others consistently poorly; cognitive scores did not significantly differ between FCD and non-FCD participants. Another study of neuropsychological test profiles in FCD found subtle deficits and similar performance to healthy controls: suggesting that these researchers examined patients from the former “high-performing” FCD category.Reference Wakefield, Blackburn, Harkness, Khan and Reuber 21 FCD with “objective” cognitive impairment (ie, poor performance on cognitive tests) is poorly described in the FCD literature, and yet consists a group at particular risk of misdiagnosis. Our study suggests that cognitive tests, including performance validity tests, appear largely unhelpful in the diagnosis of FCD.
The other significant predictive variable for FCD in this study was younger age; advancing age being the largest risk factor for neurodegenerative disease.
Presence of symptoms of anxiety and depression, and DSM-5 psychiatric diagnoses, were associated with FCD in this study, but were not significantly predictive on multiple regression, being strongly inversely related to age. Symptoms of depression and anxiety are recognized associations with FCD and SCD,Reference McWhirter, Ritchie, Stone and Carson 6 but are also common features of neurodegenerative disease.Reference Diniz, Butters, Albert, Dew and Reynolds 22 , Reference Becker, Lorena and Rios 23 Our findings support a recommendation that diagnosis of FCD should not rest solely on presence of anxiety or depression in the absence of crucial diagnostic features of internal inconsistency.
However, more detailed phenomenological inquiry into the nature of the experience of cognitive failure in FCD may be a fruitful avenue for future research. For example, we observed descriptions of obsessive–compulsive patterns of thinking in FCD participants who did not satisfy DSM-5 criteria for diagnosis of OCD. Better description and measurement of these phenomena may help both in diagnosis and in generating accurate models of mechanism of cognitive impairment in FCD.
Some previously suggested predictors did not emerge from this study as we might have expected. Recruitment was cut short by the COVID-19 pandemic, and it seems likely that small sample size will have led to false negative errors in some comparison variables. For example, we did not find statistically significant differences between those reporting a prior excellent memory, those reporting that others were more worried than themselves, who had previous contact with a person with dementia, reporting detail of television watching, or being able to respond to a compound question.
The design of this study emphasized positive detection of FCD, but we recognize the possibility of functional symptoms existing alongside symptoms of neurodegenerative disease (functional “overlay”) or occurring as part of a neurodegenerative prodrome. These possibilities will be important to bear in mind in longitudinal studies of FCD. Acknowledging this complexity will help us to better understand prodromal states in neurodegenerative disease. On a practical clinical basis, insightless amnestic deficits, new impairment of praxis, and new impairment of social cognition might be considered examples of “red flags” of prodromal neurodegenerative disease, meriting careful follow-up.
Strengths of this study include the rich dataset, painstaking reference diagnosis process, and engagement with the “real world” problem of how to distinguish FCD not from clear-cut dementia but from the “gray area” of prodromal neurodegenerative disease and other causes of mild and subjective cognitive symptoms. We acknowledge the possibility that reference diagnoses may have been influenced by clinical features overlapping with research measures interrogated for diagnostic specificity, although this was avoided as far as practicable with blinding. Longitudinal follow-up and replication are important next steps.
In conclusion, we suggest that the predictive methods described in this study are an important move toward parity of esteem for FCD: an important differential diagnosis in the investigation of possible neurodegenerative disease, and a definable target for clinical trials.
Acknowledgments
The authors thank the study participants who generously gave up their time to take part. The authors also thank the medical and nursing staff of the City of Edinburgh Memory Assessment and Treatment Service and the Neurology and Neuropsychiatry Departments of the Edinburgh Department of Clinical Neurosciences for their help with recruitment.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S1092852921000845.
Author contributions
L.M. was responsible for study conception and design. A.C., J.S., and C.R. reviewed clinical data and as a panel established consensus diagnoses for the study. LM collected all data, analyzed the data, and wrote the final manuscript, with advisory input from A.C., J.S., and C.R. A.C., J.S., and C.R. contributed equally to study design and subsequent review and revision of the manuscript.
Disclosures
Laura McWhirter, Craig Ritchie, Jon Stone, and Alan Carson have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare that: L.M. is funded by a University of Edinburgh Clinical Research Fellowship funded philanthropically by Baillie Gifford. L.M. provides independent medical testimony in court cases regarding patients with functional disorders, is a member of the board of directors of the British Neuropsychiatry Association, and is a trainee editor with the British Journal of Psychiatry. A.C. is a director of a limited personal services company that provides independent medical testimony in Court Cases on a range of neuropsychiatric topics on a 50% pursuer 50% defender basis, is an associate editor of the Journal of Neurology Neurosurgery and Psychiatry, and is the treasurer of the International Functional Neurological Disorder Society. J.S. reports personal fees from UptoDate, outside the submitted work, runs a self-help website for patients with functional neurological symptoms (www.neurosymptoms.org) which is free and has no advertising, provides independent medical testimony in personal injury and negligence cases regarding patients with functional disorders, and is secretary of the International Functional Neurological Disorder Society. JS is a Chief Scientists Office NHS Research Scotland Career Researcher. C.R. declares no conflicts of interest.








