Feathers are unique to birds among extant vertebrates. The major structural proteins of feathers, β-keratins (also referred to as corneous beta-proteins) (Alibardi Reference Alibardi2013, Reference Alibardi2017), are resistant to degradation, which is considered to be a reason why feathers are relatively common in the fossil record (Davis & Briggs Reference Davis and Briggs1995; Schweitzer Reference Schweitzer2011; Kamarudin et al. Reference Kamarudin, Sharma, Gupta, Kee, Chik and Gupta2017; Schweitzer et al. Reference Schweitzer, Zheng, Moyer, Sjövall and Lindgren2018; Benton et al. Reference Benton, Dhouailly, Jiang and McNamara2019). Discoveries of feathers in non-avian dinosaurs indicate that feathers originated long before birds (Ji & Ji Reference Ji and Ji1996; Xu & Guo Reference Xu and Guo2009; Xu et al. Reference Xu, Zhou, Dudley, Mackem, Chuong, Erickson and Varricchio2014; Benton et al. Reference Benton, Dhouailly, Jiang and McNamara2019). Fossil feathers have played an important role in our understanding of evolution of feathers and flight (Longrich et al. Reference Longrich, Vinther, Meng, Li and Russell2012; Zheng et al. Reference Zheng, Zhou, Wang, Zhang, Zhang, Wang, Wei, Wang and Xu2013; Foth et al. Reference Foth, Tischlinger and Rauhut2014; Xu et al. Reference Xu, Zhou, Dudley, Mackem, Chuong, Erickson and Varricchio2014; Feo et al. Reference Feo, Field and Prum2015; Benton et al. Reference Benton, Dhouailly, Jiang and McNamara2019; Pan et al. Reference Pan, Zheng, Sawyer, Pennington, Zheng, Wang, Wang, Hu, O'Connor, Zhao, Li, Schroeter, Wu, Xu, Zhou and Schweitzer2019). Often, fossil feathers are the only evidence for their source animals (Davis & Briggs Reference Davis and Briggs1995; Xing et al. Reference Xing, Cockx and McKellar2020). To date, reported fossil feathers are mainly preserved as compressions (e.g., Davis & Briggs Reference Davis and Briggs1995; Zhang et al. Reference Zhang, Zhou and Dyke2006; Xu & Guo Reference Xu and Guo2009), impressions (Longrich et al. Reference Longrich, Vinther, Meng, Li and Russell2012; Foth et al. Reference Foth, Tischlinger and Rauhut2014), or in amber (e.g., Perrichot et al. Reference Perrichot, Marion, Néraudeau, Vullo and Tafforeau2008; McKellar et al. Reference McKellar, Chatterton, Wolfe and Currie2011; Xing et al. Reference Xing, Cockx and McKellar2020). Less common are feathers preserved in coprolites (Wetmore Reference Wetmore1943) and in hot spring deposits (Channing et al. Reference Channing, Schweitzer, Horner and McEneaney2005).
Ancient siliceous hot spring deposits (sinters) have been demonstrated to be highly productive for the recovery of exceptionally preserved fossils, which may even include cellular details (e.g., Channing & Edwards Reference Channing and Edwards2013; Massini et al. Reference Massini, Escapa, Guido and Channing2016; Dunlop & Garwood Reference Dunlop and Garwood2018; Garwood et al. Reference Garwood, Oliver and Spencer2020). For example, the preservation of the cellular details of the lycopsid Asteroxylon mackiei from the Devonian Rhynie chert allows the identification of the oldest meristems of rooting axes (Hetherington & Dolan Reference Hetherington and Dolan2018). However, internal silicification of feathers and fine details at the cellular level were not observed in the only avian carcass so far discovered in ancient siliceous hot spring deposits of Yellowstone National Park (Channing et al. Reference Channing, Schweitzer, Horner and McEneaney2005).
Though there is only one report of feathers from ancient hot spring deposits (Channing et al. Reference Channing, Schweitzer, Horner and McEneaney2005), feathers are found to be common in modern hot springs (Jones & Renaut Reference Jones and Renaut2003). To determine whether feathers can be internally silicified, here we analyse feathers sampled from a modern hot spring – Champagne Pool – in the Waiotapu geothermal area, Taupo Volcanic Zone, New Zealand. Our results have implications for understanding the preservation potential of feathers in ancient hot spring deposits.
1. Material and methods
1.1. Material
Feathers preserved separately or in association with plants (Fig. 1, referred to as samples 1–3) were collected from the margin of Champagne Pool in 2018. The pool water is slightly acidic (pH 4.9–5.68) with a temperature of about 75 °C (Mountain et al. Reference Mountain, Benning and Boerema2003; Pope et al. Reference Pope, McConchie, Clark and Brown2004; Pope & Brown Reference Pope and Brown2014). The water contains 362–430 mg kg−1 silicon dioxide, which is over-saturated with respect to amorphous silica (Mountain et al. Reference Mountain, Benning and Boerema2003; Pope et al. Reference Pope, McConchie, Clark and Brown2004).

Figure 1 Photos of sampled feathers from a modern hot spring – Champagne Pool – in Waiotapu, New Zealand. (A) Sample 1. (B) Sample 2. (C) Sample 3. Scale bars = 1 cm.
1.2. Methods
Observations and elemental analysis were performed using a Tescan MAIA3 field emission scanning electron microscope (SEM) equipped with energy dispersive X-ray spectrometry (EDS). To determine the mineralisation condition of these samples, elemental mapping was performed on two thin sections (sample 1 and sample 2) and a fracture surface (sample 3) using the SEM-EDS. Samples were coated with platinum before SEM observations and analysis.
1.3. Structure of feathers
Feathers comprise hierarchical branches of rachis, barbs, and barbules (Fig. 2a, b). The barbs consist of a cortex and a central medulla (Fig. 2c). The barb cortex is laminated and composed of keratin fibrils (Fig. 2c–e). The medulla is composed of vacuolated medullary cells (Fig. 2c, e) with fibrous membranes (Fig. 2e). In the cytoplasmic voids of the medullary cells, there are some fibrils that bridge the membranes (Fig. 2e) (Kulp et al. Reference Kulp, D'Alba, Shawkey and Clarke2018).
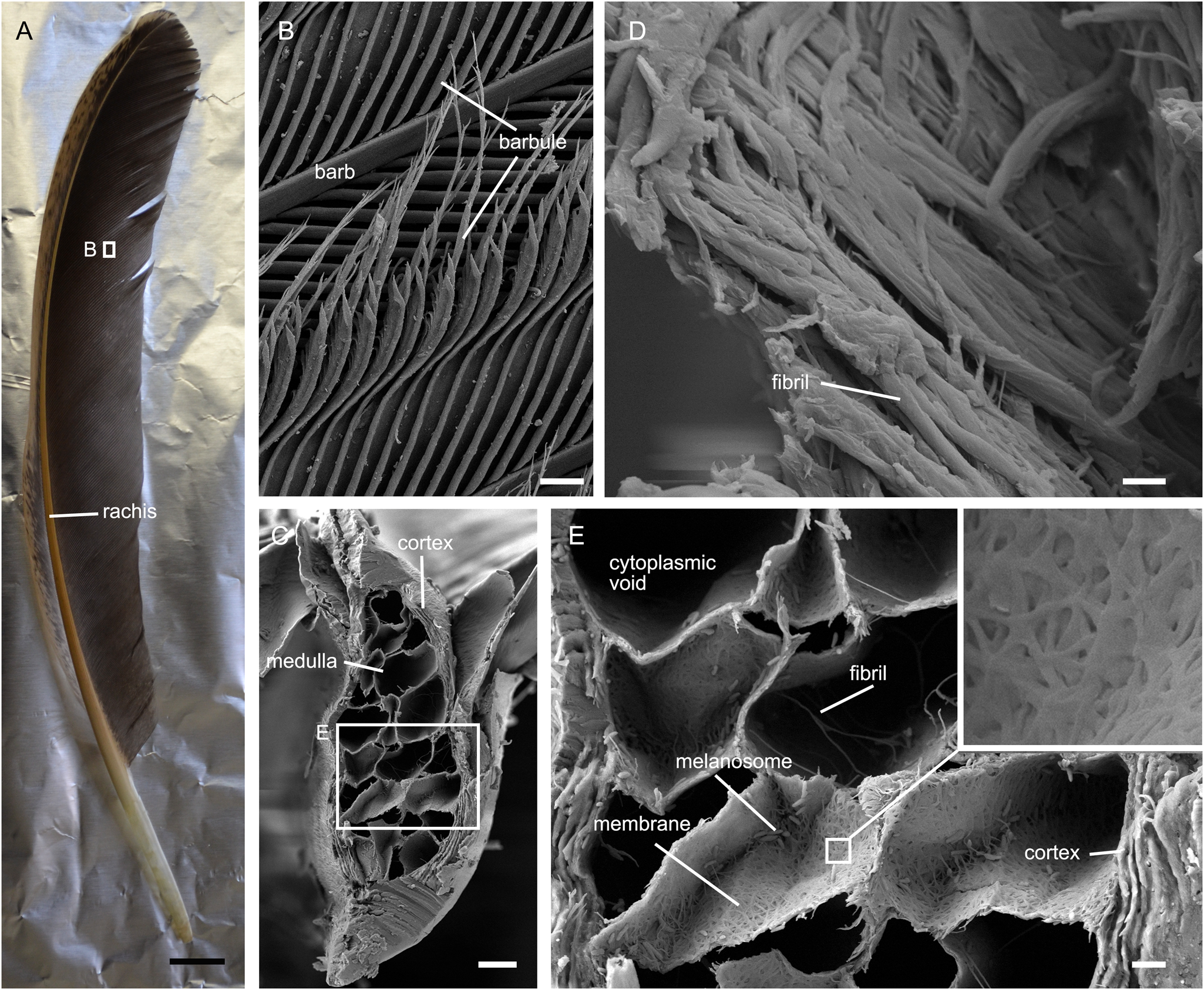
Figure 2 Photo and SEM image of chicken feathers. (A) Photo of a chicken feather. (B) Barb and barbules. (C) Cross section of a barb shows the cortex and medulla. (D) Fractured barb shows the fibrils that make up the barb cortex. (E) Cross section of a barb shows details of the medullary cells, and the laminated barb cortex. Inset in (E) shows the fibrous membrane of a medullary cell. Scale bars = 1 cm (A); 40 μm (B); 10 μm (C); 1 μm (D); 2 μm (E).
2. Results
2.1. Elemental mapping
SEM-EDS elemental mapping shows that the feathers in the three analysed samples are silicified to different degrees (Fig. 3). The feather in sample 3 has the highest degree of silicification; silicon (Fig. 3k, o) and oxygen (Fig. 3l, p) are widespread in the barb cortex, the membranes of the medullary cells, and the barbules. Carbon is still present in the feather in sample 3 (Fig. 3j, n). As for the feathers in sample 1 and sample 2, the abundance of silicon is higher in the barbules, the membrane of medullary cells, and the outer part of the barb cortex (arrows in Fig. 3c, g) than in the inner part of the barb cortex. The abundance of carbon is lower in barbules than in the barb cortex in sample 1 (Fig. 3b). In sample 2, the abundance of carbon is lower in the barbules and the outer part of the barb cortex than in the inner part of the barb cortex (Fig. 3f). Silica is present in the medulla in all three samples (Fig. 3c, g, k).
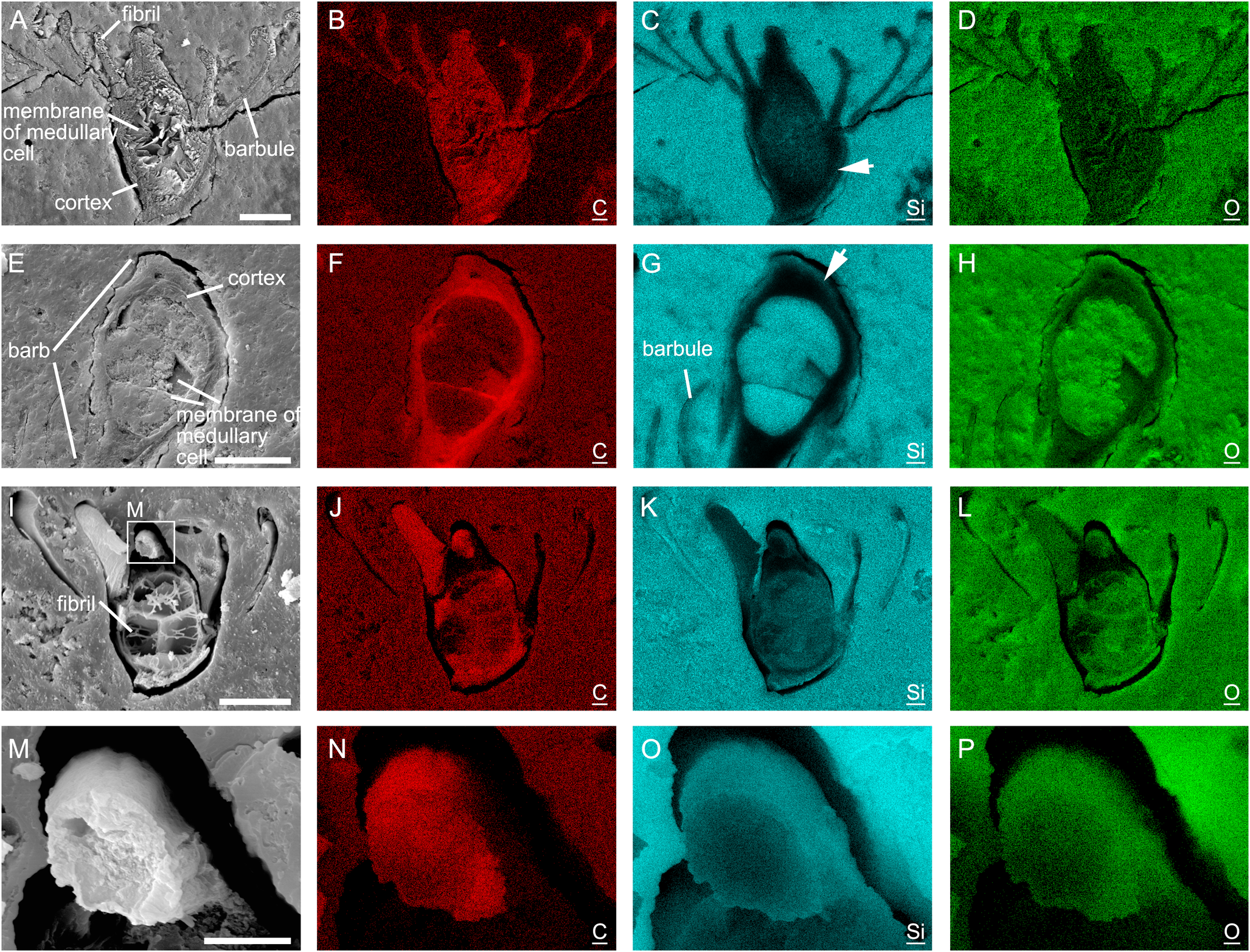
Figure 3 SEM-EDS elemental mapping of samples 1 (A–D), 2 (E–H), and 3 (I–P). Arrows in (C) and (G) point to outer part of the barb cortex where the abundance of silicon is high. C, Si, and O refer to carbon, silicon, and oxygen, respectively. Scale bars = 25 μm (A, E, I); 5 μm (M).
2.2. SEM observations
The surfaces of the opal-A materials that coat the feathers are porous, containing large numbers of microorganisms, including fungi and rod-shaped bacteria (Fig. 4a, b) (see Jones et al. Reference Jones, Renaut and Rosen1999a, Reference Jones, Renaut and Rosenb, Reference Jones, Renaut and Rosen2001, Reference Jones, Renaut and Rosen2003 for more information about microorganisms in the hot springs in the Waiotapu geothermal area). The opal-A materials below the surfaces are denser; they are granular or nonporous (Fig. 4c–e). Microorganisms are visible in nonporous opal-A on the fracture surface of sample 3 (Fig. 4e), but difficult to identify in the thin sections of samples 1 and 2 (Fig. 4c, d).
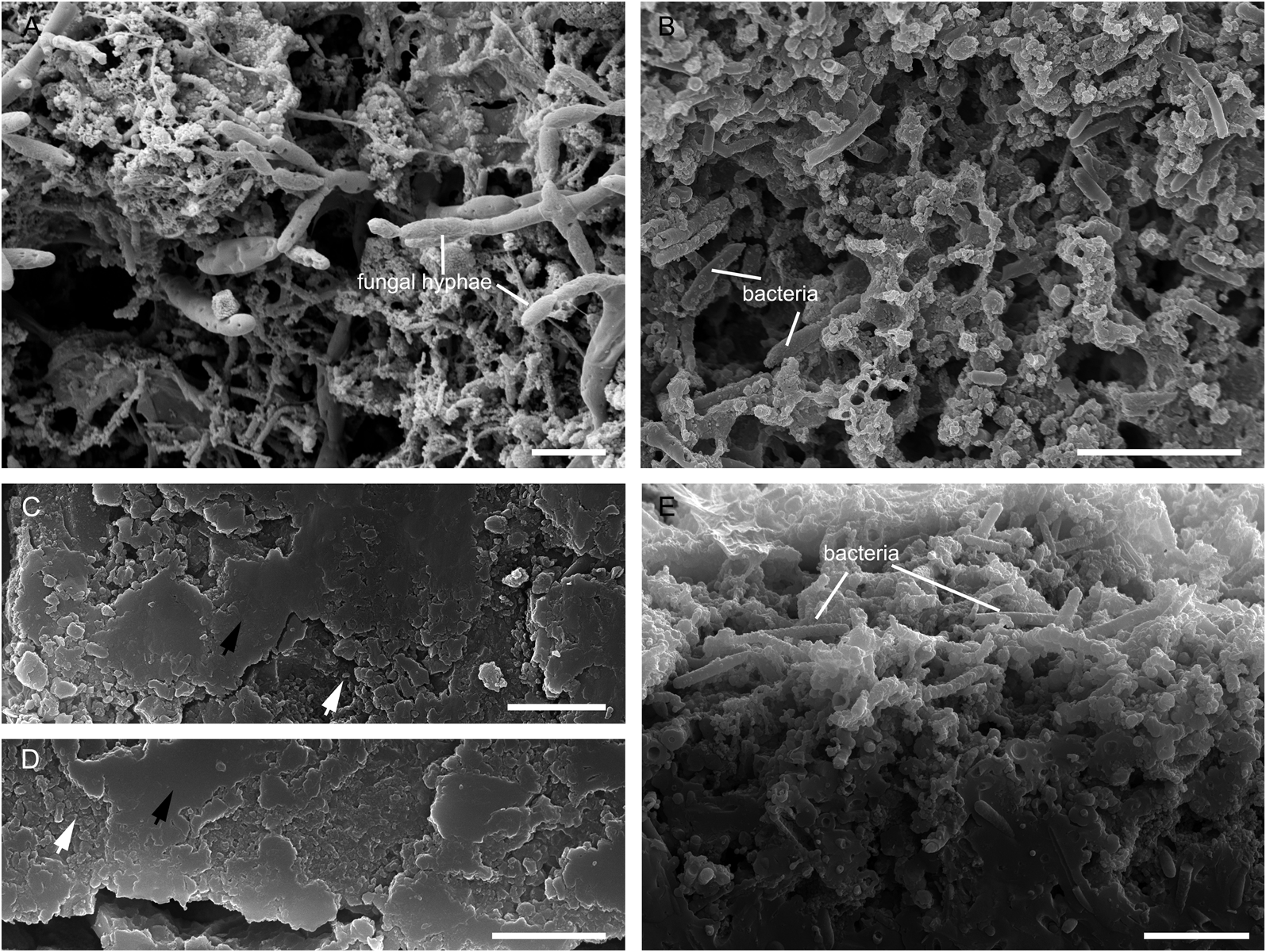
Figure 4 Opal-A fabrics of samples 1–3. (A) Surface of opal-A in sample 1. (B) Surface of opal-A in sample 3. (C) Opal-A close to feather in sample 1. (D) Opal-A close to feather in sample 2. (E) Rim of the fracture surface of opal-A in sample 3 shows the transition of the opal-A fabrics from porous to nonporous. White arrows represent granular opal-A, while black arrows represent nonporous opal-A. Scale bars = 5 μm (A, B, E); 2 μm (C, D).
The gaps between the feathers and the surrounding sinters indicate that these feathers shrank after being encrusted by opal-A (Fig. 5a, c, e). Fracture of the outer part of the barb cortex was involved during the shrinkage (Fig. 5d, e). Fibrils of the barbules and barb cortex are evident in sample 1 (Fig. 5a). Silica particles are present within the open spaces of the barb cortex of the feather in sample 1 (arrow in Fig. 5b). Fibrils are visible in the outer part of barb cortex of the feather in sample 2 (Fig. 5d), where the abundance of silicon is high (Fig. 3g). The cytoplasmic voids of the medullary cells in sample 2 are filled with silica aggregates (Fig. 5c), while those in sample 1 (Fig. 5a) and sample 3 are not (Fig. 5e). The barb cortex of the feather in sample 3 has degraded to become hollow (arrow in Fig. 5e). The widespread distribution of silica particles in the cross section of the barb cortex also indicates the degradation of fibrils that make up the barb cortex (Fig. 5f). The membranes of the vacuolated medullary cells and the fibrils within the cytoplasmic voids in sample 3 are densely covered by silica particles (Fig. 5g).
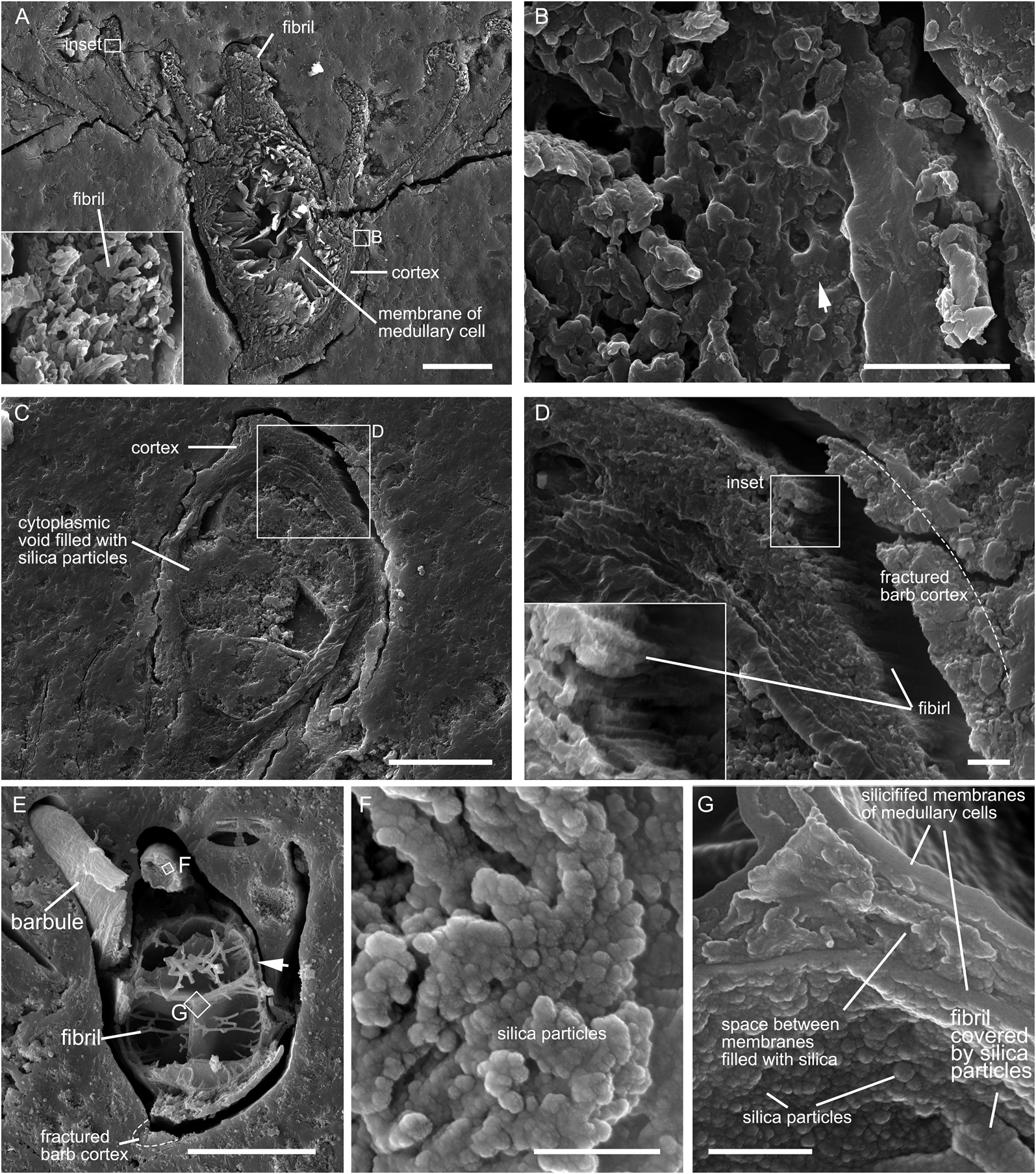
Figure 5 Morphological details of the feathers in samples 1 (A, B), 2 (C, D), and 3 (E–G). (A) Cross section of a barb and barbules shows the retention of fibrils in sample 1. Inset in (A) shows the fibrils that make up the barbule. (B) Cross section of the barb cortex in sample 1 shows the deposition of silica particles (arrow) within the open spaces within the barb cortex. (C) Cross section of a barb in sample 2 shows that the cytoplasmic voids of medullary cells are filled with silica particles. (D) Fractured part of the barb in (C) shows the retention of fibrils. (E) Fracture surface of a barb and barbules in sample 3. (F) Close-up of the barb cortex shows that silica particles largely replace the fibrils. (G) Silicified membranes of the medullary cells in sample 3. Scale bars = 20 μm (A, C); 2 μm (B, D); 25 μm (E); 500 nm (F); 1 μm (G).
3. Discussion
3.1. Silicification of feathers
The widespread distribution of silicon and oxygen in the cross section of the barb in sample 3 indicates that feathers can be silicified internally as well as externally. This is the first report of internal silicification of feathers, Channing et al. (Reference Channing, Schweitzer, Horner and McEneaney2005) having reported only encrustation of external feather surfaces. The incipient degree of silicification of the silica-encrusted feather in sample 1 suggests that the internal silicification of feathers mainly occurs after being encrusted by opal-A (Fig. 6a). The silica in the medulla (Fig. 3) indicates that silica-bearing fluids can enter the medulla and silicify feathers from the inside outwards as well as from the outside inwards (Fig. 6a). The opal-A surrounding feathers when originally formed is likely to be granular and porous, as suggested by the newly formed opal-A on the sample surfaces (Fig. 4a, b, e). Therefore, silica-rich fluids supply the silica that internally silicify the encrusted feathers by permeating through earlier formed opal-A. Along with the silicification of feathers, silica will also precipitate in pores of earlier formed opal-A, progressively reducing the porosity (Fig. 6a; Cady & Farmer Reference Cady, Farmer, Bock and Goode1996; Jones & Renaut Reference Jones and Renaut2003; Orange et al. Reference Orange, Lalonde and Konhauser2013), which will reduce the permeation of silica-bearing fluids and may leave some empty spaces within feathers unoccupied by silica (Fig. 6a), as seen in sample 3 (Fig. 5e, f). These various features are well documented in the silicification of plants in hot spring waters (e.g., Channing & Edwards Reference Channing and Edwards2004, Reference Channing and Edwards2009).
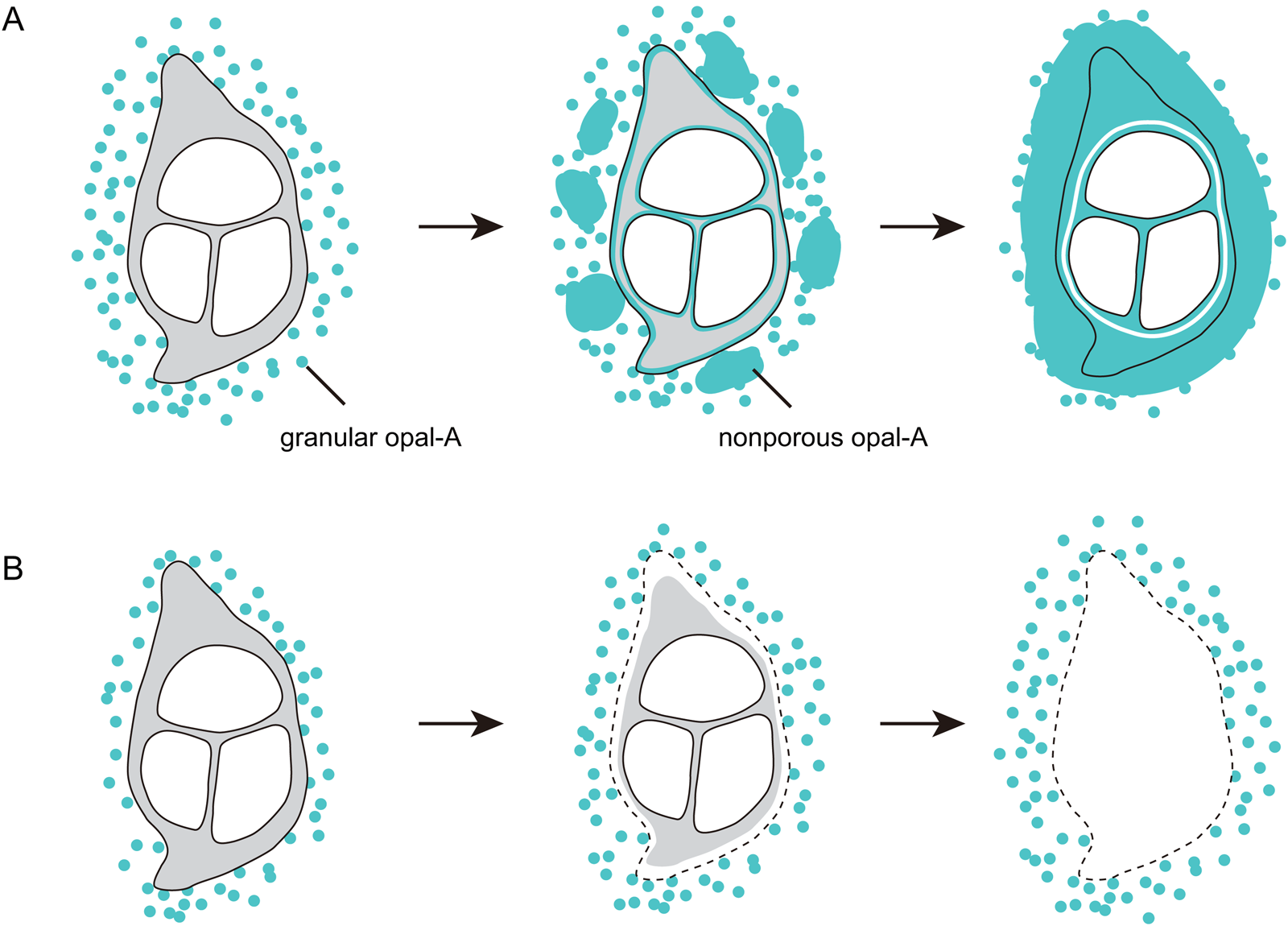
Figure 6 Schematic diagrams of the silicification of feathers. (A) A feather is silicified when the supply of silica is sufficient. (B) A feather is not silicified when the supply of silica is reduced.
Elemental mapping (Fig. 3) and SEM observations (Fig. 5) indicate that degradation is involved in the silicification of feathers. The abundance of carbon decreased in the barbules in sample 1, and the barbules and the outer part of the barb cortex in sample 2 (Fig. 3). The barb cortex became hollow in sample 3 because of the loss of the keratin fibrils that make up the barb cortex (Fig. 5). Degradation can create empty spaces where silica can be deposited, and facilitate the permeation of silica-supersaturated fluids (Channing & Edwards Reference Channing and Edwards2004). The presence of carbon in the highly silicified feather in sample 3 suggests that silicification may not necessarily lead to the total replacement of the original organic material by silica. The slight degradation of the feather in sample 1 indicates that degradation mainly occurs after being encrusted by opal-A. Though the hot spring water contains abundant microorganisms, the microorganisms near the feathers were silicified during the formation of the external opal-A encrustation. The dense opal-A encrustation can protect the feathers from decomposition by microorganisms, as well as reduce the permeation of silica-bearing fluids. It is thus likely that the degradation of feathers mainly resulted from hydrolysis. Taphonomic experiments show that entombment within silica can limit the molecular degradation of microorganisms (Toporski et al. Reference Toporski, Steele, Westall, Thomas-Keprta and McKay2002; Alleon et al. Reference Alleon, Bernard, Le Guillou, Daval, Skouri-Panet, Pont, Delbes and Robert2016), which may also apply to feathers.
The mouldic preservation of the feathers reported from the Yellowstone hot spring deposits (Channing et al. Reference Channing, Schweitzer, Horner and McEneaney2005) suggests that during degradation of these feathers, a steady supply of silica was lacking – hence, these feathers were not internally silicified though being encrusted by silica deposits (Fig. 6b). The preservation of the gross morphology of a coot (Fulica americana) indicates that the rate of silica precipitation was high, and this aquatic bird is preserved in a hot spring vent pool or an associated high-temperature sinter terrace pool (Channing et al. Reference Channing, Schweitzer, Horner and McEneaney2005). Similarly, the feathers studied in the present study are also from a hot spring vent pool – Champagne Pool – in New Zealand (Campbell et al. Reference Campbell, Guido, Gautret, Foucher, Ramboz and Westall2015). However, a key difference between the feathers from the Yellowstone hot spring deposits and the feathers in the present study is that the feathers from the Yellowstone hot spring deposits were attached to a carcass, while the feathers in the present study are isolated. The plumage of many aquatic birds is specialised to avoid wetting the skin and losing heat during swimming and diving (Yang et al. Reference Yang, Xu and Zhang2006). It is likely that the opal-A encrustation surrounding the whole bird and the arrangement of the feathers limited the permeation of silica-bearing fluids, which resulted in the mouldic preservation.
3.2. Implications for exploration of silicified feathers in ancient siliceous hot spring deposits
As feathers are a common component of the biodebris in modern hot springs (Jones & Renaut Reference Jones and Renaut2003), silicified feathers are unlikely to be rare in the Jurassic and post-Jurassic hot spring deposits. The fossil record shows that feathers appeared in the Jurassic (Foth et al. Reference Foth, Tischlinger and Rauhut2014; Xu et al. Reference Xu, Zhou, Dudley, Mackem, Chuong, Erickson and Varricchio2014; Benton et al. Reference Benton, Dhouailly, Jiang and McNamara2019). The Middle to Late Jurassic hot spring deposits of the Deseado Massif (Patagonia, Argentina) are the only Mesozoic hot spring deposits having been studied in detail (Guido et al. Reference Guido, Channing, Campbell and Zamuner2010; Guido & Campbell Reference Guido and Campbell2011; Des Marais & Walter Reference Des Marais and Walter2019), from which silicified plants, animals, and microorganisms have been documented (Massini et al. Reference Massini, Escapa, Guido and Channing2016). Although no feathers have been reported from these hot spring deposits, at the time the deposits were formed, Argentina had diverse dinosaurs (Rauhut et al. Reference Rauhut, Carballido and Pol2015; Rauhut & Pol Reference Rauhut and Pol2019). Discoveries of feather-like structures in ornithischian dinosaurs suggest that feathers may have been present in the earliest dinosaurs (Godefroit et al. Reference Godefroit, Sinitsa, Dhouailly, Bolotsky, Sizov, McNamara, Benton and Spagna2014; Xu et al. Reference Xu, Zhou, Dudley, Mackem, Chuong, Erickson and Varricchio2014). It seems worthwhile to increase efforts in exploring these hot spring deposits for feathers or feather-like structures in order to understand the evolution of feathers and dinosaurs (and see Channing & Edwards Reference Channing and Edwards2013, table 2). In comparison, Cenozoic siliceous hot spring deposits (Channing & Edwards Reference Channing and Edwards2013, table 1) are more common and usually subject to less diagenetic alterations because of their younger ages (Guidry & Chafetz Reference Guidry and Chafetz2003; Hinman & Walter Reference Hinman and Walter2005; Campbell et al. Reference Campbell, Guido, John, Vikre, Rhys and Hamilton2019), which means that more feathers in better preservation states may be discovered in Cenozoic hot spring deposits.
4. Conclusions
Our results show for the first time that internal silicification of feathers at the cellular level is possible in hot spring vent pools, the water of which is over-saturated with respect to amorphous silica. The SEM-EDS elemental mapping shows that the feathers collected from Champagne Pool are internally silicified to varying degrees and one of them is pervasively silicified. In this highly silicified example, the silicon and oxygen are pervasive in the barb cortex, the membranes of the medullary cells, and barbules, where the abundance of carbon is reduced. By integrating the results from less silicified samples, we establish a sequential process of silicification illustrated graphically in Figure 6.
5. Acknowledgements
We are grateful to Jingjing Tang for photographing the samples and Yan Fang for assistance during SEM observations. The research was supported by the National Natural Science Foundation of China (grant numbers 41902013, 41872016, 41922011, 41688103) and the Strategic Priority Research Program of Chinese Academy of Sciences (grant number XDB26000000).








