Background
Fasciolosis is a parasitic zoonotic disease caused by digenetic liver flukes Fasciola hepatica (Linnaeus, 1758) and Fasciola gigantica (Cobbold, 1856) (Valero et al., Reference Valero, Darce, Panova and Mas-Coma2001; Mas-Coma, Reference Mas-Coma2004; Bargues et al., Reference Bargues, Artigas, Mera y Sierra, Pointier and Mas-Coma2007; Dung et al., Reference Dung, Doanh, The, Loan, Losson and Caron2013; Sabourin et al., Reference Sabourin, Alda, Vázquez, Hurtrez-Boussès and Vittecoq2018; Alemu, Reference Alemu2019). The disease affects a wide range of domesticated and wild ruminants (Correa et al., Reference Correa, Escobar, Durand, Renaud, David, Jarne, Pointier and Hurtrez-Boussès2010; Beesley et al., Reference Beesley, Caminade and Charlier2018; Sabourin et al., Reference Sabourin, Alda, Vázquez, Hurtrez-Boussès and Vittecoq2018; Alemu, Reference Alemu2019) and occasionally humans as accidental hosts (Magalhães et al., Reference Magalhães, Passos and dos Santos Carvalho2004; Correa et al., Reference Correa, Escobar, Durand, Renaud, David, Jarne, Pointier and Hurtrez-Boussès2010; Beesley et al., Reference Beesley, Caminade and Charlier2018; Alemu, Reference Alemu2019). This parasitic infection has been well recognized and documented for its veterinary importance throughout the world (Mas-Coma, Reference Mas-Coma2004; Bargues et al., Reference Bargues, Artigas, Mera y Sierra, Pointier and Mas-Coma2007). The occurrences of human infections have been reported to be on the rise recently, documented in five continents except Antarctica (Mas-Coma, Reference Mas-Coma2004; Alemu, Reference Alemu2019).
Previous research indicated that the epidemiology of fasciolosis is highly linked to the ecological characteristics of the snail vector involved in the transmission (Mas-Coma, Reference Mas-Coma2004; Bargues et al., Reference Bargues, Gayo, Sanchis, Artigas, Khoubbane, Birriel and Mas-Coma2011), and the susceptibility of these snail intermediate hosts (IHs) to these Fasciola species may differ (Alemu, Reference Alemu2009) depending on variations in the immunological responses of the IHs (Beesley et al., Reference Beesley, Caminade and Charlier2018). Due to the wide range and distribution of IHs, F. hepatica has been documented as the most common and widely distributed liver fluke, particularly in temperate zones of Australia, Europe and the Americas (Dung et al., Reference Dung, Doanh, The, Loan, Losson and Caron2013; Admassu et al., Reference Admassu, Shite and Kinfe2015; Sabourin et al., Reference Sabourin, Alda, Vázquez, Hurtrez-Boussès and Vittecoq2018; Alemu, Reference Alemu2019). The transmission of this Fasciola species is globally linked to Lymnaeidae species, including Lymnaea tomentosa (Pfeiffer, 1855), Lymnaea bulimoides (Pilsbry & Ferriss, 1906), Lymnaea viator (d'Orbigny, 1835), Pseudosuccinea columella (Say, 1817), Lymnaea humilis (Say, 1822), Lymnaea diaphena (Evans & Shumard, 1856) (Vorster & Mapham, Reference Vorster and Mapham2008; Alemu, Reference Alemu2019; Leka, Reference Leka2019), Lymnaea cubensis (Pfeiffer, 1839) (Bargues et al., Reference Bargues, Artigas, Mera y Sierra, Pointier and Mas-Coma2007; Pointier et al., Reference Pointier, Noya, Alarcón de Noya and Théron2009) and Lymnaea neotropica (Bargues, Artigas, Mera y Sierra, Pointier and Mas-Coma, Reference Bargues, Artigas, Mera y Sierra, Pointier and Mas-Coma2007) (Bargues et al., Reference Bargues, Artigas, Mera y Sierra, Pointier and Mas-Coma2007; Sanabria et al., Reference Sanabria, Mouzet, Courtioux, Vignoles, Rondelaud, Dreyfuss, Cabaret and Romero2012; Bargues et al., Reference Bargues, González, Artigas and Mas-Coma2017). However, the main snail IH of F. hepatica in most regions of the world, particularly in Africa, South America, Europe and in some parts of Asia (Alemu, Reference Alemu2019), is Galba truncatula (Müller, 1774) (Caron et al., Reference Caron, Rondelaud and Losson2008; Alemu, Reference Alemu2019).
Fasciola gigantica is limited to the tropical and/or subtropical regions of Africa, Asia and the Far East (Correa et al., Reference Correa, Escobar, Durand, Renaud, David, Jarne, Pointier and Hurtrez-Boussès2010; Mochankana & Robertson., Reference Mochankana and Robertson2018; Alemu, Reference Alemu2019). According to Mas-Coma (Reference Mas-Coma2005), limitations in the geographical distribution of this species is due to the slow spread of species from the genus Radix that have been implicated in the transmission of F. gigantica. These are species belonging to the Radix auricularia (Linnaeus, 1758) superspecies complex by Hubendick (Reference Hubendick1951), which comprises Radix rubiginosa (Minchelin, 1831) in Asia and Radix natalensis (Krauss, 1848) in Africa (Brown, Reference Brown1994). Alemu (Reference Alemu2019) named five Lymnaea (Radix) species involved in the transmission of F. gigantica, however, the author also reported R. auricularia and R. natalensis as the most important IHs of this Fasciola species.
The occurrence of overlapping distribution of both Fasciola spp. has been reported in some tropical regions of Asian and African countries (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005; Dung et al., Reference Dung, Doanh, The, Loan, Losson and Caron2013; Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019) where co-infections in the definitive hosts have been documented (Chen et al., Reference Chen, Chen and Ai2013; Sabourin et al., Reference Sabourin, Alda, Vázquez, Hurtrez-Boussès and Vittecoq2018). According to Mas-Coma et al. (Reference Mas-Coma, Valero and Bargues2009), these overlaps occur in areas with climatic conditions that favour the existence of the intermediate hosts of both F. hepatica and F. gigantica. The overlap may also be caused by the presence of P. columella, the invasive snail originating from Central America, the Caribbean and the southern parts of North America (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). This species succeeded in being one of the most widely distributed freshwater snails in some countries (Prepelitchi et al., Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011), where it plays an important role in the transmission of fasciolosis (Zarco et al., Reference Zarco, Fantozzi and Cuervo2011). According to Mas-Coma (Reference Mas-Coma2005), this invasive snail contributes to the secondary transmission of F. hepatica and has been shown to transmit F. gigantica in South Africa (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019) and Egypt (Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014). In countries such as South Africa, P. columella is presumed to transmit both Fasciola species, due to the observed increased prevalence in infection rate of both trematodes, which coincided with the introduction of P. columella in the country (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019). Therefore, this article reviewed the geographical distribution of P. columella and its implications in the transmission of F. hepatica and F. gigantica worldwide. The knowledge on the global distribution and role played by P. columella in the epidemiology and transmission of Fasciola spp. is crucial in predicting the potential veterinary and public health risk and burden of fasciolosis.
Methodology
Searching strategy
A systematic search of literature was conducted on the electronic databases Google Scholar, JSTOR and PubMed. A literature search was limited to peer-reviewed articles, written in English, and conducted and published between 1990 and 2020 (30 years). The search was performed using the following search terms and Boolean operators (OR, AND): Pseudosuccinea columella AND Fasciola spp., Fasciola hepatica OR Fasciola gigantica AND P. columella, P. columella AND Fasciola infection. Additional articles were identified by screening through the reference lists of selected articles (snowballing). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed during the conduction and reporting of the systematic review.
Inclusion and exclusion criteria
Articles were included if they were published in peer-reviewed journals and explicitly reported on (1) the distribution and ecological preferences of P. columella, and (2) infections (natural and/or experimental) of P. columella with either F. hepatica or F. gigantica or both, globally.
The review excluded studies reporting on infections of Fasciola spp. in gastropods other than P. columella, those focusing on the distribution of Fasciola spp. with no link to P. columella and studies that did not identify P. columella up to species level.
Results
A literature search from the three databases yielded a total of 827 studies (fig. 1). An additional 26 articles were obtained through bibliographic searches from relevant articles. Thirty articles were removed because they were duplicated. A total of 719 were excluded after screening their titles and abstracts. The full texts of 89 articles were downloaded and screened for eligibility, and 33 studies were deemed ineligible since they did not explicitly report on the distribution of P. columella and the role it plays in the transmission of F. hepatica and F. gigantica globally, but reported on Fasciola species infections exclusively on definitive hosts and other Lymnaeidae snail species. A total of 56 studied were retained and used in this systematic review.

Fig. 1. PRISMA diagram.
The distribution of studies which fulfilled the inclusion criteria on a geographical scale and scope varied across continents. Of the 56 articles reviewed, 19 were from the African continent (table 1), 17 from South America (table 2), 11 from North America and the Caribbeans (table 3), eight from Europe (table 4) and Oceania had one article documented in Australia by Molloy & Anderson (Reference Molloy and Anderson2006). Thirty-nine of these articles were field studies and 17 were experimental studies. Africa had the highest number of field-based studies, followed by South America, with 16 and 14 articles, respectively. Twenty-three studies assessed Fasciola infections in P. columella, while 33 articles reported solely on the distribution of P. columella.
Table 1. Summary of studies included in the distribution of Pseudosuccinea columella and its role in the transmission of Fasciola spp. in Africa for a period of 30 years (1990–2020).
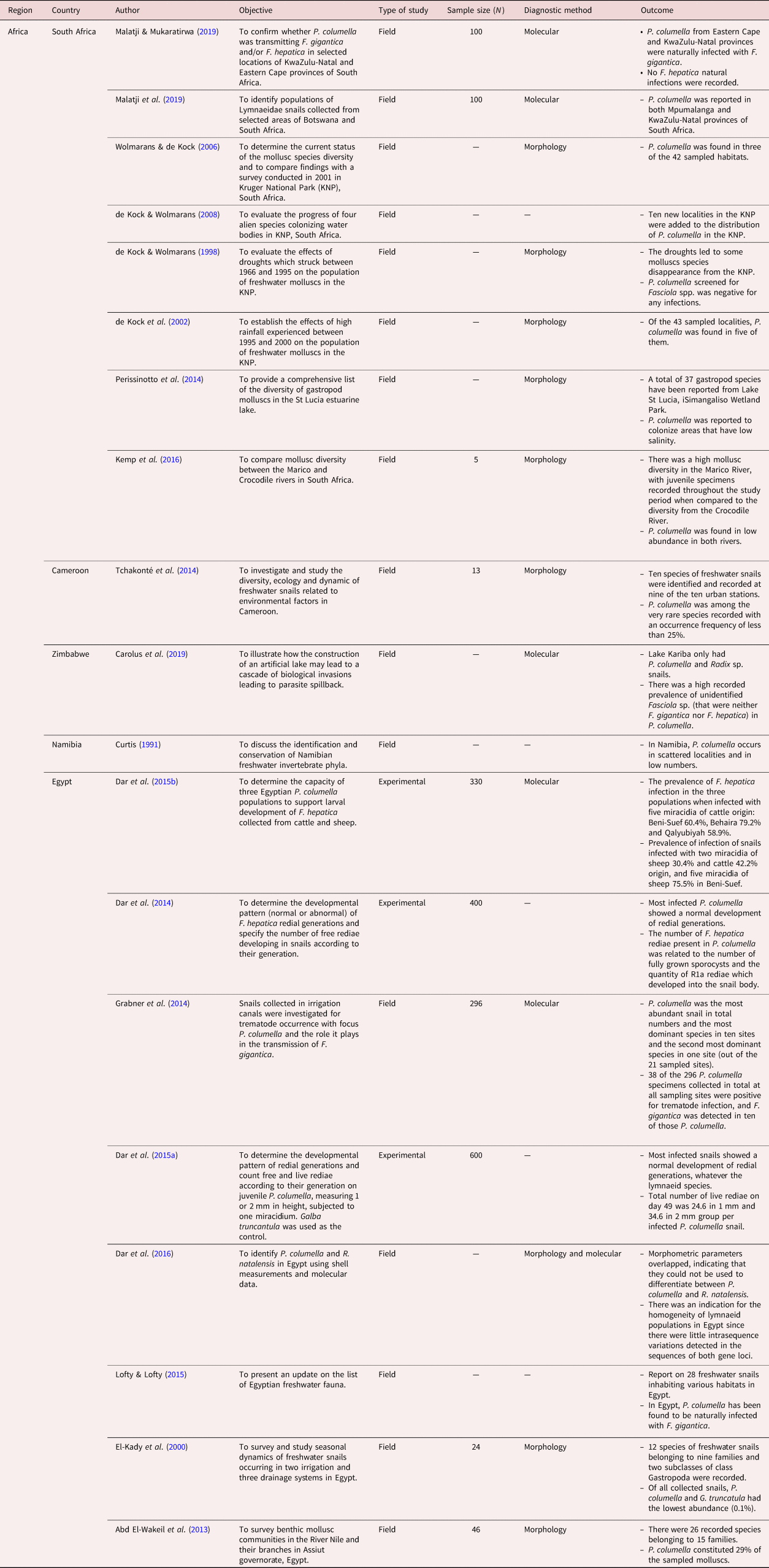
Table 2. Summary of studies included in the distribution of Pseudosuccinea columella and its role in the transmission of Fasciola spp. in South America for a period of 30 years (1990–2020).
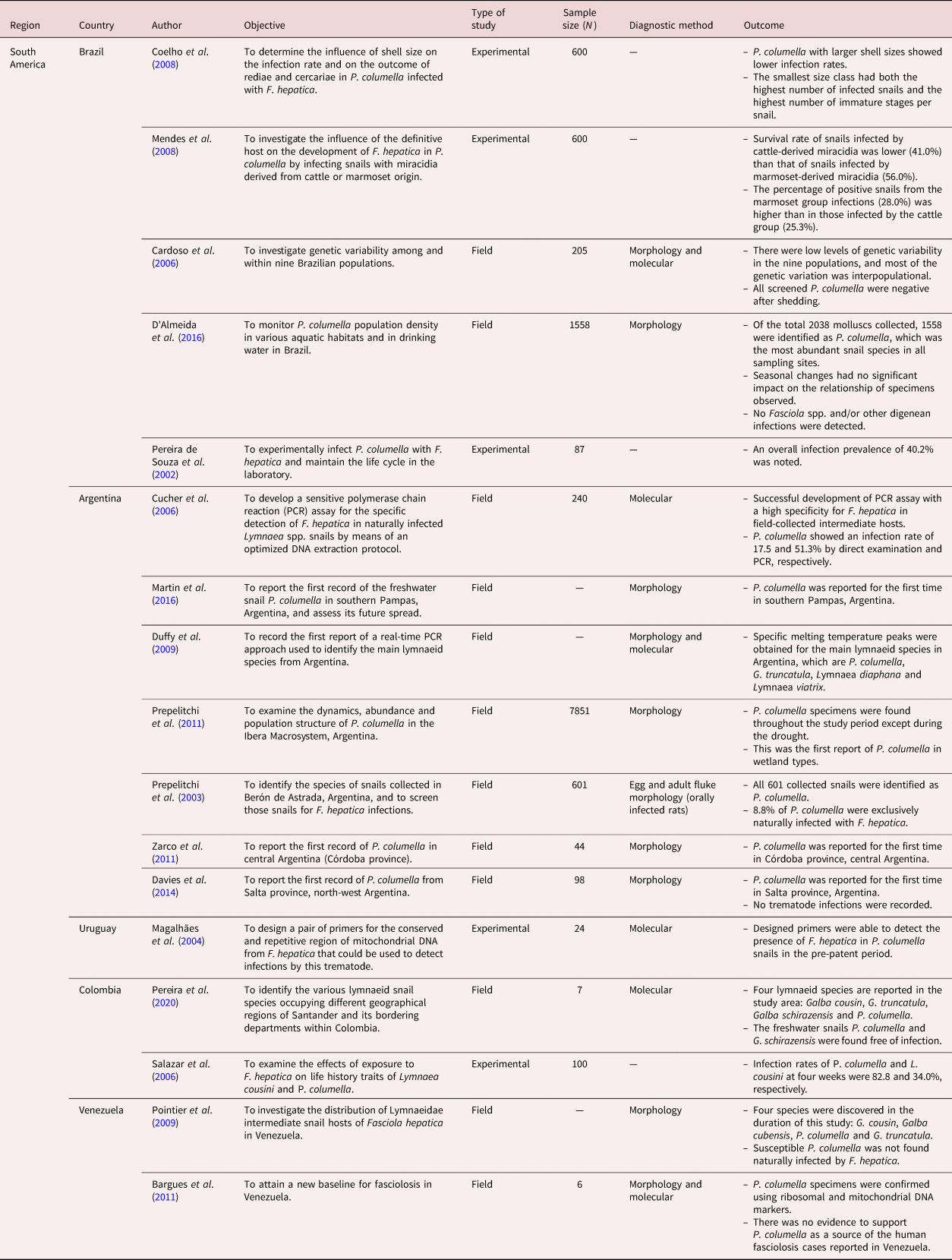
Table 3. Summary of studies included in the distribution of Pseudosuccinea columella and its role in the transmission of Fasciola spp. in North America and the Caribbeans for a period of 30 years (1990–2020).

Table 4. Summary of studies included in the distribution of Pseudosuccinea columella and its role in the transmission of Fasciola spp. in Europe for a period of 30 years (1990–2020).

Global distribution and abundance of P. columella
Pseudosuccinea columella was documented in 22 countries from Africa (table 1), South America (table 2), North America and the Caribbeans (table 3), Europe (table 4) and Oceania. However, the species was widely reported in African (n = 18) and South American (n = 16) countries. In the African continent, P. columella was documented in South Africa, Egypt, Madagascar, Cameroon, La Reunion, Zimbabwe, Namibia and Mayotte. However, the results also showed that this snail species was mostly reported and distributed in South Africa and Egypt. In South America, P. columella has been reported in Argentina, Uruguay, Brazil, Venezuela, Paraguay, Peru and Colombia. In this region, however, P. columella was shown to have a wide distribution in Argentina (n = 8), followed by Brazil (n = 5). In North America and the Caribbeans, P. columella has been documented in the USA, including Kansas and Hawaii states, Cuba and Guadeloupe. In Europe, P. columella has been recorded in France, Romania and Italy. Of all the continents, this freshwater snail is least distributed in Oceania, where it has only been reported in Australia and French Polynesia.
The results showed that P. columella inhabits a wide variety of natural and man-made freshwater systems (table 5). These freshwater systems included riverbanks, ponds (some in botanical gardens), canals, irrigation systems, ditches, ravines, lakes, dams, drain channels, streams, areas of estuarine lake that have low salinity, wetlands and a thermal lake. Although rivers/riverbanks were the most common habitat for P. columella throughout the world, this lymnaeid snail typically inhabits a wide variety of habitats.
Pseudosuccinea columella were collected during all seasons, both in winter (dry) and summer (rainy), or only in summer (table 5). There were no studies that were conducted during the winter season only. Reviewed studies showed that P. columella snails were collected in abundance during the summer (rainy) season (Cardoso et al., Reference Cardoso, Caldiero, Lovato, Coelho, Berne, Muller and Carvalho2006; Bargues et al., Reference Bargues, Gayo, Sanchis, Artigas, Khoubbane, Birriel and Mas-Coma2011; Prepelitchi et al., Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011; D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016) as compared to the winter season (Bargues et al., Reference Bargues, Gayo, Sanchis, Artigas, Khoubbane, Birriel and Mas-Coma2011; Prepelitchi et al., Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011; D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016). Results also showed that South American countries recorded the highest number of collected P. columella specimens globally involving field studies. The highest number of P. columella were collected in the wetlands of Argentina (n = 7851) over a period of two years and four months (tables 2 and 5) (Prepelitchi et al., Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011). Lounnas et al. (Reference Lounnas, Correa and Vazquez2017) collected 1509 P. columella individuals over 16 years in 14 countries (tables 4 and 5). The lowest number of sampled P. columella (n = 5) was recorded in South Africa during the dry and rainy season of 2011 (table 5) (Kemp et al., Reference Kemp, de Kock, Zaayman and Wolmarans2016).
Susceptibility of P. columella to Fasciola species infection
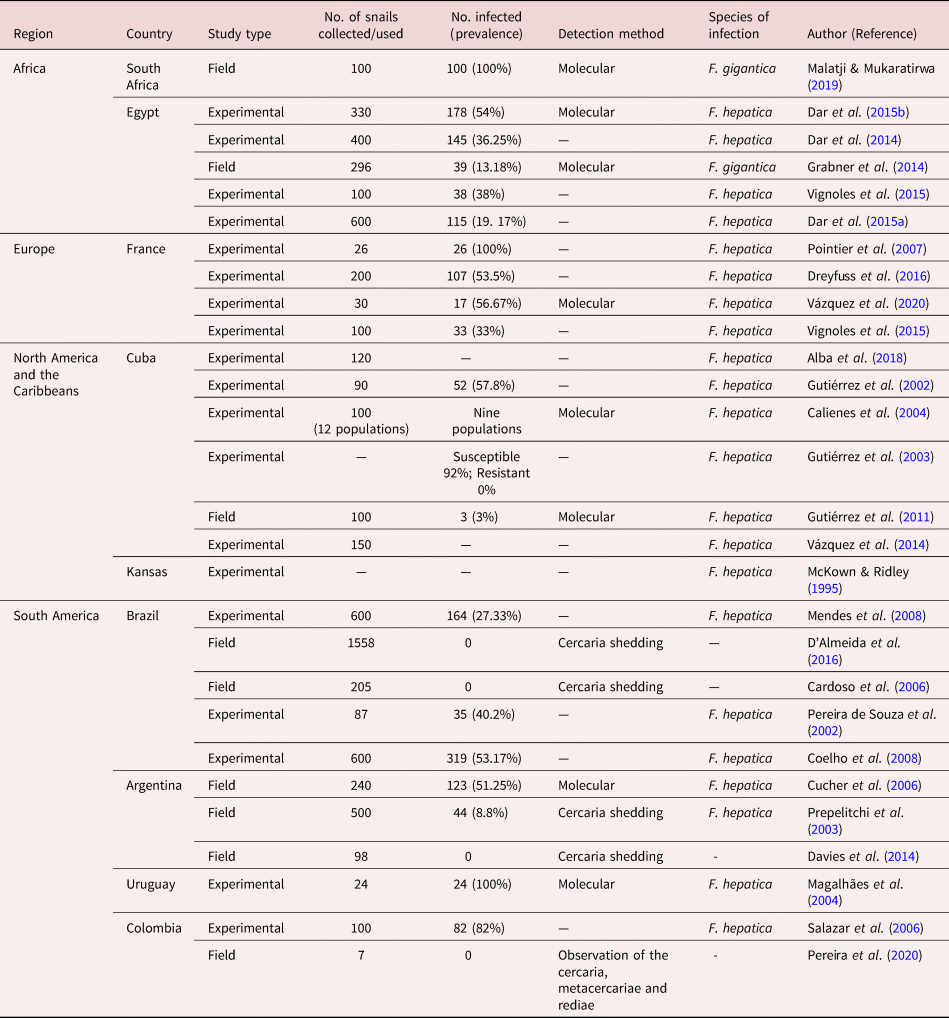
From the 56 reviewed studies, 28 assessed the epidemiological role played by P. columella in the transmission of Fasciola species, and these studies were reported in South America (tables 2 and 6), North America and the Caribbeans (tables 3 and 6), in Africa (tables 1 and 6) and Europe (tables 4 and 6). No studies reported on the infection of P. columella with Fasciola spp. in Oceania. Of these 28 studies, most studies (n = 19) assessed the susceptibility of P. columella to Fasciola spp. infection experimentally in all reviewed countries, with the exception of South Africa where natural infections were reported. Only nine studies assessed natural infections of Fasciola species in P. columella, and these studies were from Africa (South Africa and Egypt), South America (Argentina, Brazil and Columbia) and North America and the Caribbeans (Cuba).
Table 5. Global distribution of Pseudosuccinea columella between 1990 and 2020 (30 years).

The results showed that P. columella/F. hepatica infections were more prevalent, reported in 22 studies, as compared to P. columella/F. gigantica infections, which were reported in two studies (table 6). Pseudosuccinea columella populations in Egypt were experimentally susceptible to both F. hepatica and F. gigantica. No P. columella populations have been found naturally infected with F. hepatica in the field in the African continent (table 6). Experimental studies from Cuba (n = 2) showed populations of P. columella resistant to F. hepatica infections. Furthermore, field studies from Brazil and Argentina also did not report natural F. hepatica infections in P. columella populations collected from F. hepatica-endemic areas.
Prevalence of Fasciola species infection intra-P. columella populations
Although most studies did not indicate the methods they used to check for infection, 13 studies checked snail infections using either cercarial shedding (n = 4), molecular techniques (n = 8) or checking for any of the developmental stages of Fasciola inside the IH tissue under the microscope (n = 1) (table 6).
The overall prevalence of P. columella infected with Fasciola spp. varied from 0 to 100%, the lowest (0%) recorded in Brazil (Cardoso et al., Reference Cardoso, Caldiero, Lovato, Coelho, Berne, Muller and Carvalho2006; D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016), Argentina (Davies et al., Reference Davies, Nieva, Choke, Issa, Pujadas and Prepelitchi2014) and Colombia (Pereira et al., Reference Pereira, Uribe and Pointier2020), and the highest (100%) recorded in South Africa (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019), Uruguay (Magalhães et al., Reference Magalhães, Passos and dos Santos Carvalho2004) and France (Pointier et al., Reference Pointier, Coustau, Rondelaud and Théron2007). Pseudosuccinea columella/Fasciola spp. natural infections prevalence ranged from 0 to 100% (table 6). Experimental P. columella/F. hepatica infections showed prevalence ranging from 19.7% in Egypt (Dar et al., Reference Dar, Rondelaud, Vignoles and Dreyfuss2015a) to 100% in France (Pointier et al., Reference Pointier, Coustau, Rondelaud and Théron2007) and Uruguay (Magalhães et al., Reference Magalhães, Passos and dos Santos Carvalho2004) (table 6). No studies are reported on experimental F. gigantica intra-P. columella infections. Pseudosuccinea columella naturally infected with F. hepatica had a prevalence between 0 and 51.25%, with no infections documented in Brazil (Cardoso et al., Reference Cardoso, Caldiero, Lovato, Coelho, Berne, Muller and Carvalho2006; D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016), Argentina (Davies et al., Reference Davies, Nieva, Choke, Issa, Pujadas and Prepelitchi2014) and Colombia (Pereira et al., Reference Pereira, Uribe and Pointier2020), and a high infection rate of 51.25% was documented in Argentina (Cucher et al., Reference Cucher, Carnevale, Prepelitchi, Labbé and Wisnivesky-Colli2006). The prevalence range for F. gigantica intra-P. columella natural infections was reported to be between 13.18 and 100% in Egypt (Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014) and South Africa (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019), respectively. The highest naturally occurring infections of P. columella with F. hepatica and F. gigantica were documented in Argentina (51.25%) (Cucher et al., Reference Cucher, Carnevale, Prepelitchi, Labbé and Wisnivesky-Colli2006) and South Africa (100%) (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019).
Discussion
Pseudosuccinea columella is thought to have originated from Central America, the Caribbean and the southern part of North America (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005), and this review has shown that this species has been successfully introduced and established in other continents with varying environmental and ecological conditions. The results from this review showed that in addition to its native regions (Dar et al., Reference Dar, Rondelaud, Vignoles and Dreyfuss2014; Martin et al., Reference Martin, Ovando and Seuffert2016; Lounnas et al., Reference Lounnas, Correa and Vazquez2017; Alba et al., Reference Alba, Vázquez, Sánchez, Duval, Hernández, Sabourin, Vittecoq, Hurtrez-Boussés and Gourbal2018; Vignoles et al., Reference Vignoles, Dreyfuss and Rondelaud2018; Carolus et al., Reference Carolus, Muzarabani, Hammoud, Schols, Volckaert, Barson and Huyse2019), P. columella has been documented in Africa, Europe, South America (Martin et al., Reference Martin, Ovando and Seuffert2016; Lounnas et al., Reference Lounnas, Correa and Vazquez2017; Alba et al., Reference Alba, Vázquez, Sánchez, Duval, Hernández, Sabourin, Vittecoq, Hurtrez-Boussés and Gourbal2018) and Oceania (Martin et al., Reference Martin, Ovando and Seuffert2016; Lounnas et al., Reference Lounnas, Correa and Vazquez2017; Alba et al., Reference Alba, Vázquez, Sánchez, Duval, Hernández, Sabourin, Vittecoq, Hurtrez-Boussés and Gourbal2018; Vignoles et al., Reference Vignoles, Dreyfuss and Rondelaud2018; Alba et al., Reference Alba, Vázquez, Sánchez, Lounnas, Pointier, Hurtrez-Boussè and Gourbal2019b). The results further indicate that from these four newly invaded continents, this invasive freshwater snail has been reported in 19 countries, but is now well established and widely distributed in Africa and South America, and is least distributed in Oceania (Lounnas et al., Reference Lounnas, Correa and Vazquez2017). However, abundance varied with localities and various environmental factors, such as the availability of suitable habitats and seasonal changes, amongst other factors. Although the reviewed studies showed that P. columella specimens were collected in abundance in native Argentina (Prepelitchi et al., Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011) and Brazil (D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016), this invasive snail is considered the most abundant lymnaeid species in Egypt (Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014), Brazil (D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016) and in Kansas, USA (McKown & Ridley, Reference McKown and Ridley1995), and the second most abundant species in Zimbabwe (Carolus et al., Reference Carolus, Muzarabani, Hammoud, Schols, Volckaert, Barson and Huyse2019) in comparison to other freshwater lymnaeids.
According to de Kock et al. (Reference de Kock, Wolmarans and du Preez2002), the availability of suitable water habitats largely influences the distribution of freshwater snails. Although an inspection of the frequency of habitats in reviewed studies showed that P. columella is commonly found on riverbanks, the results also showed that P. columella thrives in diverse freshwater habitats ranging from man-made, to natural, temporal and permanent habitats. In addition to freshwater habitats, this lymnaeid species has been found in ditches of acid soils that have water with low levels of calcium (Vignoles et al., Reference Vignoles, Dreyfuss and Rondelaud2018) and areas with low salinity (Perissinotto et al., Reference Perissinotto, Miranda, Raw and Peer2014). Furthermore, it has been documented to occur in places with high altitudes and low temperatures (Bardales-Valdivia et al., Reference Bardales-Valdivia, Bargues, Hoban-Vergara, Bardales-Bardales, Goicochea-Portal, Bazán-Zurita, Del Valle-Mendoza, Ortiz and Mas-Coma2021). The ability of P. columella to adapt to and inhabit almost all types of freshwater bodies, including thermal lakes and acidic soils, and tolerate a wide range of climatic conditions are some of the factors that make this snail such a successful invader (Prepelitchi et al., Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011; Vignoles et al., Reference Vignoles, Dreyfuss and Rondelaud2018) and the most widely distributed invasive freshwater snail species globally (Pointier et al., Reference Pointier, Noya, Alarcón de Noya and Théron2009; Lounnas et al., Reference Lounnas, Correa and Vazquez2017).
The abundance of freshwater snails varies throughout the year with seasonal changes such as temperature, rainfall and water levels (Kleiman et al., Reference Kleiman, Pietrokovsky, Prepelitchi, Carbajo and Wisnivesky-Colli2007). According to D'Almeida et al. (Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016), an increase in rainfall and temperature favours an increase in mollusc populations. This is consistent with the results from this review, as P. columella was noticeably more abundant during rainy seasons, and this was easily observed in studies that collected snails during all seasons of the year (de Kock et al., Reference de Kock, Wolmarans and du Preez2002; Abd El-Wakeil et al., Reference Abd El-Wakeil, Obuid-Allah, Mohamed and Abd El-Aziz2013; Tchakonté et al., Reference Tchakonté, Ajeagah, Diomandé, Camara and Ngassam2014; D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016; Dar et al., Reference Dar, Amer, Eddine and Dreyfuss2016). Studies in Egypt (El-Kady et al., Reference El-Kady, Shoukry, Reda and El-Badri2000) and Argentina (Kleiman et al., Reference Kleiman, Pietrokovsky, Prepelitchi, Carbajo and Wisnivesky-Colli2007) showed that the best time to collect snails when they are in their highest numbers was in spring and summer due to favourable temperature conditions and the availability of plenty of vegetation cover in the aquatic habitats. However, Prepelitchi et al. (Reference Prepelitchi, Pietrokovsky, Kleiman, Rubel, Issia, Moriena, Racioppi, Álvarez and Wisnivesky-Colli2011) noted that the highest number and largest size of specimens of P. columella populations in the north of Corrientes province (Argentina) were found during winter. This is, however, inconsistent with Charlier et al. (Reference Charlier, Soenen, De Roeck, Hantson, Ducheyne, Van Coillie, De Wulf, Hendrickx and Vercruysse2014) and Beesley et al. (Reference Beesley, Caminade and Charlier2018), who stated that adult snails are known to be abundant in summer and spring seasons, while juvenile snails are mainly found in autumn. These results show that although P. columella populations can be found throughout the year and in different seasons, the season in which P. columella may be found in abundance differs between and within countries due to varying habitats and climatic conditions.
The epidemiological importance of P. columella as an intermediate host for both F. gigantica and F. hepatica has been documented (de Kock et al., Reference de Kock, Joubert and Pretorius1989; Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014; Alba et al., Reference Alba, Tetreau, Chaparro, Sánchez, Vazquez and Gourbal2019a; Carolus et al., Reference Carolus, Muzarabani, Hammoud, Schols, Volckaert, Barson and Huyse2019; Malatji et al., Reference Malatji, Lamb and Mukaratirwa2019). From the review, infections were commonly detected through shedding of cercariae from snails collected from their natural environments, observation of different developmental stages of the parasites after crushing or dissecting snails (Magalhães et al., Reference Magalhães, Passos and dos Santos Carvalho2004; Caron et al., Reference Caron, Rondelaud and Losson2008; Beesley et al., Reference Beesley, Caminade and Charlier2018) and through molecular techniques for better sensitivity (Magalhães et al., Reference Magalhães, Passos and dos Santos Carvalho2004; Cucher et al., Reference Cucher, Carnevale, Prepelitchi, Labbé and Wisnivesky-Colli2006; Beesley et al., Reference Beesley, Caminade and Charlier2018). Reviewed studies showed that P. columella infections with F. gigantica have only been reported in Egypt (Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014) and South Africa (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019). Despite the high natural infections reported by Malatji & Mukaratirwa (Reference Malatji and Mukaratirwa2019) in South Africa, Grabner et al. (Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014) still concluded that the maintenance of the natural life cycle of F. gigantica in P. columella remains uncertain. This conclusion may have been attributed to the low infection rate (13.18%) of F. gigantica observed in Egypt (Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014). The limited information on the role played by P. columella in the transmission of F. gigantica in the invaded countries highlights the need for more field and experimental infection studies in these areas. The high prevalence of F. gigantica intra-P. columella infection (100%) reported in South Africa by Malatji & Mukaratirwa (Reference Malatji and Mukaratirwa2019) could be due to livestock drinking from seasonal ponds/small dams with no other source of drinking water and thereby snails getting exposed to heavy infections from hatching miracidia. There are plenty of such scenarios in the rural areas of South Africa and elsewhere in southern Africa, where animals drink and graze around these areas which are heavily contaminated by Fasciola spp. cercariae (Malatji & Mukaratirwa, pers. comm.).
The results for this review show that F. hepatica infections in P. columella were the most documented in five continents. This is not surprising, as according to Mas-Coma et al. (Reference Mas-Coma, Bargues and Valero2005), this invasive lymnaeid species is responsible for the secondary spread of F. hepatica. The results also show that most F. hepatica intra-P. columella infection studies were conducted in the laboratory (experimental infections), as compared to field reports, which is a great concern as this does not give a full representation of what happens in the field. Additionally, the recorded infection rate in the reviewed studies was generally low in natural/field infection studies (Prepelitchi et al., Reference Prepelitchi, Kleiman, Pietrokovsky, Moriena, Racioppi, Alvarez and Wisnivesky-Colli2003; Gutiérrez et al., Reference Gutierrez, Vázquez, Hevia, Sánchez, Correa, Hurtrez-Boussès, Pointier and Théron2011) as compared to experimental studies, with exception to that reported by Cucher et al. (Reference Cucher, Carnevale, Prepelitchi, Labbé and Wisnivesky-Colli2006) in Argentina. Although the observed high experimental infection rates of P. columella with F. hepatica show the importance of this invasive snail as one of the vectors responsible for the transmission of fascioliasis (Dar et al., Reference Dar, Vignoles, Rondelaud and Dreyfuss2015b), there is still a need for field-based studies to further determine not only the prevalence of Fasciola spp., but also the competence of different populations of P. columella in maintaining and transmitting both Fasciola species globally.
Literature has shown that geographical variations in Lymnaea species influence their susceptibility to infections by F. hepatica (Gasnier et al., Reference Gasnier, Rondelaud, Abrous, Carreras, Boulard, Diez-Baños and Cabaret2000; Coelho et al., Reference Coelho, Guimaraes and Lima2008). This has been observed by Gutiérrez et al. (Reference Gutierrez, Vázquez, Hevia, Sánchez, Correa, Hurtrez-Boussès, Pointier and Théron2011) and Alba et al. (Reference Alba, Vázquez, Sánchez, Duval, Hernández, Sabourin, Vittecoq, Hurtrez-Boussés and Gourbal2018), who reported on variations within P. columella species that influenced their susceptibility to F. hepatica infections. In Cuba, two different phenotypes of P. columella populations have been identified and reported to show either resistance or susceptibility to F. hepatica infections (Alba et al., Reference Alba, Vázquez, Sánchez, Duval, Hernández, Sabourin, Vittecoq, Hurtrez-Boussés and Gourbal2018). These authors have shown that the resistant field-occurring P. columella phenotypes are characterized by their ability to encapsulate and phagocytize miracidia using their (host's) immune cells (Gutiérrez et al., Reference Gutierrez, Pointier, Yong, Sánchez and Théron2003, Reference Gutierrez, Vázquez, Hevia, Sánchez, Correa, Hurtrez-Boussès, Pointier and Théron2011; Alba et al., Reference Alba, Vázquez, Sánchez, Duval, Hernández, Sabourin, Vittecoq, Hurtrez-Boussés and Gourbal2018).
Although the presence of resistant P. columella phenotypes has not been extensively studied in most countries, the inability of some phenotypes of this invasive snail to be infected and transmit particularly F. hepatica in endemic areas may lead to the assumption that this phenotype is unknowingly widely distributed. Such cases have been reported in Australia (Molloy & Anderson, Reference Molloy and Anderson2006), Venezuela (Pointier et al., Reference Pointier, Noya, Alarcón de Noya and Théron2009; Bargues et al., Reference Bargues, Gayo, Sanchis, Artigas, Khoubbane, Birriel and Mas-Coma2011), Colombia (Pereira et al., Reference Pereira, Uribe and Pointier2020), north-west Argentina (Davies et al., Reference Davies, Nieva, Choke, Issa, Pujadas and Prepelitchi2014) and Brazil (Cardoso et al., Reference Cardoso, Caldiero, Lovato, Coelho, Berne, Muller and Carvalho2006; D'Almeida et al., Reference D'Almeida, Freitas, Carneiro, Camargo, Azevedo and Martins2016), where P. columella phenotypes collected in Fasciola-endemic areas were found with no infections. Additionally, P. columella phenotypes collected in locations in South Africa where both F. hepatica and F. gigantica overlap were only found infected with F. gigantica (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019). This, therefore, raises concerns about the existence and geographical distribution of the different phenotypes of P. columella. As a result, there is still a need to conduct both an experimental and field assessment of the infection of P. columella phenotypes with both Fasciola species, especially in countries where this invasive snail has been reported with no clear epidemiological role in the transmission of Fasciola species, and in areas where both F. hepatica and F. gigantica overlap. This will help determine the geographical expansion of P. columella phenotypes resistant to F. hepatica infections, as well as the competence of the susceptible species in the transmission of both Fasciola species in fasciolosis-endemic areas.
In conclusion, it is evident that intensive research still needs to be conducted focusing on (1) assessing the global distribution and importance of this invasive snail in the transmission of both Fasciola species, especially in the field/natural environment, as there seem to be more countries where the snail is present and yet to be documented; (2) assessing the susceptibility of different P. columella phenotypes to Fasciola spp. populations to differentiate susceptible and resistant phenotypes, in countries where the species has been reported; (3) the experimental infection of P. columella populations not found with infections but collected in fasciolosis-endemic areas; and (4) studies to compare the competence of P. columella in the transmission of fasciolosis with other lymnaeid IHs native to those particular areas where fasciolosis is endemic. Additionally, this review has pointed out the importance of researchers to describe the ecological parameters of habitats where species have been collected from, as this could assist in explaining questionably high rates of documented P. columella naturally infected by either Fasciola species.
A limitation in this review might be that a number of studies published in languages other than English might not have been included and that the study only considered articles published in the last 30 years.
Table 6. Natural and experimental Fasciola infections intra-Pseudosuccinea columella reported between 1990 and 2020 (30 years).
Acknowledgements
The authors would also like to the University of KwaZulu-Natal library for giving us access to full-text reprints of some publications.
Financial support
This study was supported by the National Research Fund (NRF) Grant number: 121371.
Conflicts of interest
None.