Cheeses made from raw milk have higher flavour intensity than pasteurized milk cheeses (Buchin et al. Reference Buchin, Delague, Duboz, Berdague, Beuvier, Pochet and Grappin1998). Raw milk microflora, with its diversity of species and strains, plays a key role in the sensory properties of raw milk cheeses (Montel et al. Reference Montel, Buchin, Mallet, Delbes-Paus, Vuitton, Desmasures and Berthier2014). However, the microbiological content of raw milk is usually highly variable, which makes achieving relative uniformity in the final product particularly complex (Verdier-Metz et al. Reference Verdier-Metz, Michel, Delbès and Montel2009; Montel et al. Reference Montel, Buchin, Mallet, Delbes-Paus, Vuitton, Desmasures and Berthier2014). A wide variety of genera and species of the family Enterobacteriaceae were isolated from milk and other dairy products in previous studies (Kongo et al. Reference Kongo, Gomes and Malcata2008; Maifreni et al. Reference Maifreni, Frigo, Bartolomeoli, Innocente, Biasutti and Marino2013; Ordiales et al. Reference Ordiales, Benito, Martín, Casquete, Serradilla, de Guía and órdoba2013; Torracca, et al. Reference Torracca, Pedonese, Turchi, Fratini and Nuvoloni2018). Enterobacteriaceae can be related to contamination of faecal origin, and therefore are considered as an indicator of poor hygienic practice during milking and cheese-making (Verdier-Metz et al. Reference Verdier-Metz, Michel, Delbès and Montel2009). Even though Enterobacteriaceae are recognized as a part of the natural microbiota of milk and dairy products, their presence is of concern because of their associated negative role in cheese quality. Many Enterobacteriaceae species have the ability to decarboxylate both lysine and ornithine, which leads to the production of several biogenic amines, like cadaverine and putrescine (Pircher et al. Reference Pircher, Bauer and Paulsen2007; Torracca et al. Reference Torracca, Pedonese, Turchi, Fratini and Nuvoloni2018), which are regarded not only as toxic compounds, but also as responsible for faecal and putrid off-flavours (Westling et al. Reference Westling, Danielsson-Tham, Jass, Nilsen, Öström and Tham2016). In addition, gas production capability of many coliform species can lead to early blowing in cheese. When conditions are favourable, CO2 or H2 production within the cheese is manifested by the appearance of eyes, fissures or gas in the packaging (Guggisberg et al. Reference Guggisberg, Schuetz, Winkler, Amrein, Jakob, Fröhlich-Wyder and Wechsler2015). Cheese eyes are characteristic in some cheese varieties, being generally produced by starter cultures, like propionic acid bacteria or heterofermentative lactic acid bacteria. However, gas holes are considered a defect in many others cheeses, especially when it is produced by coliforms, yeast or clostridia (Fox et al. Reference Fox, Guinee, Cogan, McSweeney and Fox2000; Sheehan, Reference Sheehan and McSweeney2007). Early blowing occurs when unwanted gas holes appear during moulding, salting, or even the first week of cheese ripening, and it is usually caused by coliforms or yeast. Contrastingly, late blowing appears after 1 or 2 months of ripening and is produced by clostridia. In general, a minimum population is necessary before early blowing occurs (Martley & Crow, Reference Martley and Crow1996; Alichanidis, Reference Alichanidis and McSweeney2007). Nevertheless, the spoilage risk is also determined by the species present. Enterobacter aerogenes, Escherichia coli, Klebsiella aerogenes and some strains of the genera Citrobacter and Serratia are considered responsible for the appearance of early blowing in cheese (Lück & Dunkeld, Reference Lück and Dunkeld1981; Gaya et al. Reference Gaya, Medina and Núñez1983; Tornadijo et al. Reference Tornadijo, Fresno, Carballo and Martín-Sarmiento1993; Bintsis & Papademas, Reference Bintsis and Papademas2002). In a previous study, the technological characterization of the isolated strains (gas production) and their presence at different ripening stages were used to determine their potential capacity to spoil ewe milk cheese (Tabla et al. Reference Tabla, Gómez, Simancas, Rebollo, Molina and Roa2016).
To the best of our knowledge, no published studies exist comparing levels of Enterobacteriaceae microflora present in defective and non-defective cheeses during manufacture and ripening. Cheeses with similar initial Enterobacteriaceae levels, but different number of eyes in mass, were compared in order to determine the main Enterobacteriaceae species responsible for early gas blowing during curdling or ripening.
Material and methods
Cheese manufacture and sample taking
Samples of raw goats’ milk and curd from the same batch were taken and analysed weekly during two months. Two of these batches were selected for further evaluation, one of them showed early blowing within the first 24 h of ripening (batch A) while the other was considered normal (batch B). Samples of cheese 7, 15, 30 and 60 d of ripening were taken from each batch and kept refrigerated for no longer than 8 h before analysis.
Cheeses were made according to the PDO ‘Ibores’ regulation (OJEU, 2004). The starter cultures (MO-1 and LHB02 from Chr. Hansen, Horsholm, Denmark) were added 10 min before milk coagulation by the addition of calf rennet at 28–32 °C. Curd was cut, placed in moulds and pressed. Cheeses were salted in brine (12°B at 10 °C for 19 h) and ripened at 8–12 °C and 80% relative humidity for at least 60 d.
Physicochemical analysis
The pH was measured directly in milk, curd and cheeses using a Crison 52–32 electrode (Crison Instruments, Barcelona, Spain). Total solid (TS), salt and fat content were analysed by standard procedures (ISO 2004, 2006, 2008). All the physicochemical analysis were performed in duplicate.
Microbiological analysis
Ten grams of each cheese sample were homogenized (Stomacher Type 400; Seward, London, UK) for 5 min at 45 °C with 90 ml of a sterile solution of 2% (w/v) sodium citrate (Panreac, Barcelona, Spain). Serial dilutions were prepared in 0·1% (w/v) sterile peptone water and plated on the specific media. Total mesophilic bacteria were enumerated on Plate Count Agar (Cultimed, Spain) at 30 °C for 72 h. Enterobacteriaceae and coliforms were grown on Violet Red Bile Glucose agar (Cultimed) and on E. coli – coliforms Chromogenic Medium (Pronadisa, Spain) at 37 °C for 24 h, respectively.
Isolation and identification of Enterobacteriaceae
After incubation, 15–20 colonies per sample were randomly picked from the countable VRBG plates. A total of 170 Gram-negative, oxidase-negative and catalase-positive isolates were stored in LB broth containing 30% (v/v) glycerol at −80 °C until identification.
The isolates were characterized with the aid of the EnteroPluri-Test System (Liofilchem®, Italy) and identified according to the criteria of Brenner & Farmer (Reference Brenner, Farmer, Brenner, Krieg, Staley and Garrity2005). The following test were carried out on each isolate: indole, H2S, acetoin (Voges-Proskauer) and yellow pigment production; methyl red reaction; fermentation of D-glucose, adonitol, lactose, arabinose, D-sorbitol, dulcitol and citrate; gas production from D-glucose and lactose; phenylalanine deaminase; lysine and ornithine decarboxylases; urea hydrolysis; motility.
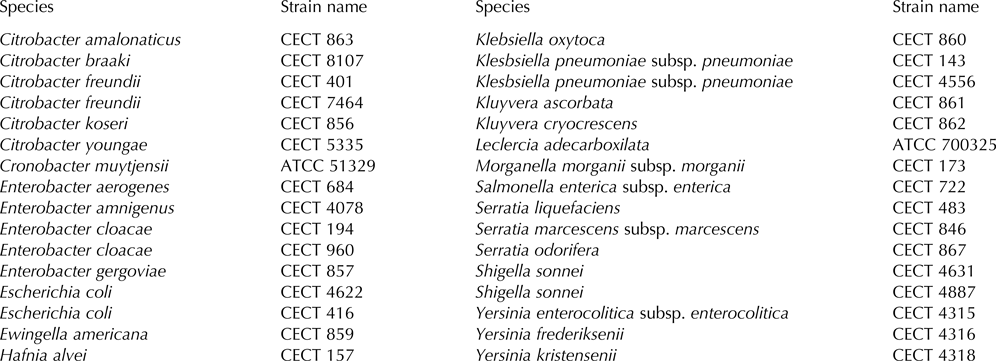
Moreover, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) of whole-cell proteins was performed for further characterization and identification. Reference strains (Table 1) were also included in the analysis. Protein samples were prepared according to Jackman (Reference Jackman, Colwell and Grigorova1987) and analysed by SDS-PAGE (10% acrylamide (w/v) in the resolving gel and 4% acrylamide (w/v) in the stacking gel) by the method of Laemmli (Reference Laemmli1970). Whole-cell sample from Hafnia alvei was used for internal calibration. Gels were digitalized using a densitometer (Bio-Rad GS800) and were analysed with the aid of Phoretix 1D Pro software (Nonlinear Dynamics, Newcastle, United Kingdom). The similarity among digitized profiles was calculated using Pearson correlation and an average linkage (UPGMA) dendrogram was derived from similarity matrix.
Table 1. Reference strain used for identification of Enterobacteriaceae isolates by protein fingerprinting.
ATCC, American Type Culture Collection, Rockville; EEUU, CECT, Colección Española de Cultivos Tipo, Universidad de Valencia, Spain
Representative isolates from each cluster (>20%) were analysed with the Biolog Microbial ID System (Biolog, USA), according to the manufacturer's instructions. Identification was carried out with the aid of the Biolog software, which provides the following information: (i) Probability, as an estimate of the reliability of the identification. (ii) Similarity index value (SIM) which assesses how well the sample is identified. (iii) Distance (DIST) that indicates the approximate number of mismatches between the MicroPlate Test Panel and the database pattern for that species. In the present study, only ‘excellent identification’ profiles (SIM ≥ 0·5 and DIST ≥ 2) were accepted.
Gas production capacity and cheese hole formation
Gas production of the isolates from lactose was determined as previously described (Tabla et al. Reference Tabla, Gómez, Simancas, Rebollo, Molina and Roa2016).
Image analysis was used to quantify gas hole formation in cheese during ripening. Cheeses were cut in half at 7 and 60 d of ripening. The inner surface was digitalized using a densitometer (Bio–Rad GS800) and analysed using Fiji software (Schindelin et al. Reference Schindelin, Arganda-Carreras, Frise, Kaynig, Longair, Pietzsch and Cardona2012). If present, mechanical holes were visually identified and erased.
Statistical analysis
Physicochemical data were subjected to analysis of variance (ANOVA) using the SPSS statistic software version 17.0. Mean values and standard error of mean are reported.
Results
Changes in physico-chemical and microbiological parameters during manufacturing and ripening
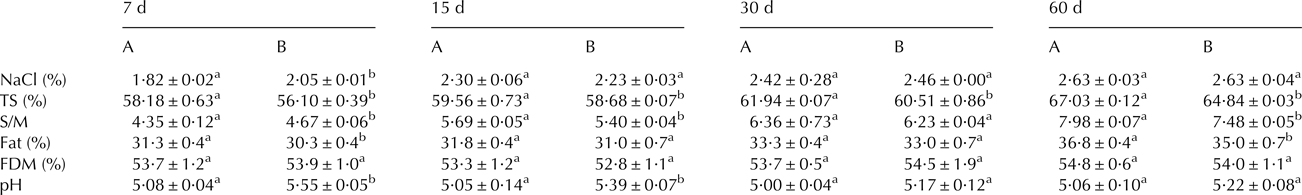
There were no significant differences in the physico-chemical parameters analysed in milk and curd between different batches. The average pH value of milk (6·70 ± 0·05) was slightly higher than the value for curd (6·65 ± 0·05). Table 2 summarizes the results obtained for the different cheese batches during ripening. The defective cheeses (batch A) showed significantly lower moisture content than the normal cheeses (batch B) throughout the whole ripening period. Likewise, acidification was faster in cheeses from batch A, showing significant differences in pH during the first two weeks of ripening. Additionally, defective cheese showed significantly lower (P < 0·05) content of salt-in-moisture (S/M) during the first week of ripening.
Table 2. Physicochemical composition of raw goats’ milk cheeses from batch A (defective) and B (normal) during the different ripening stages.
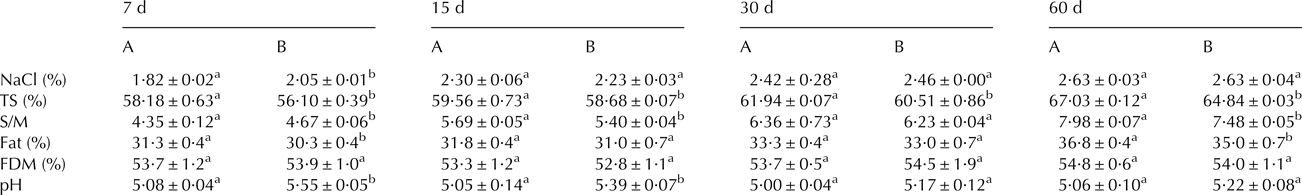
FDM, fat in dry matter
a,bDifferent letter in the same row indicates significant differences between cheese batches at the same day of ripening (P < 0·05)
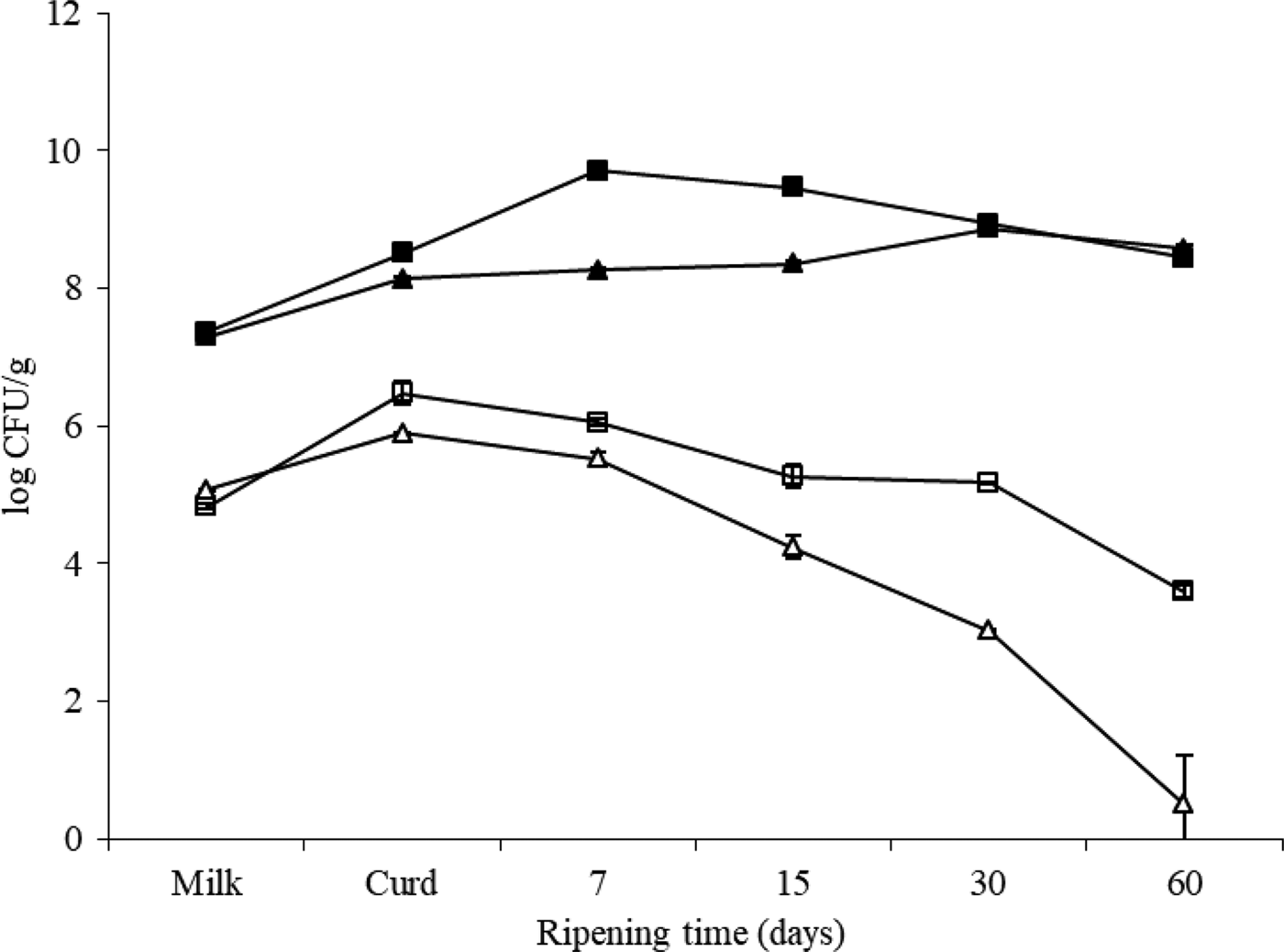
Changes of mesophilic bacteria and Enterobacteriaceae counts throughout the manufacture and ripening are shown in Fig. 1. The aerobic mesophilic flora counts of the milk used in the two batches of Ibores cheese after starter culture addition were 7·32 ± 0·06 log cfu/ml. Although initial microbial levels were quite similar for both cheese types (defective and non-defective), mesophilic levels were higher in defective cheese during the first two weeks of ripening. Maximum levels were attained on day 7 for cheese A and day 30 for cheese B. Similarly, Enterobacteriaceae counts were higher in the defective cheese than in ‘non-defective cheese’. Once the maximum level of Enterobacteriaceae was achieved, their plate counts decreased to the end of ripening in both defective and non-defective cheeses. However, their survival was lower in B cheeses. At the end of the ripening period there were 3 log units difference between batches.
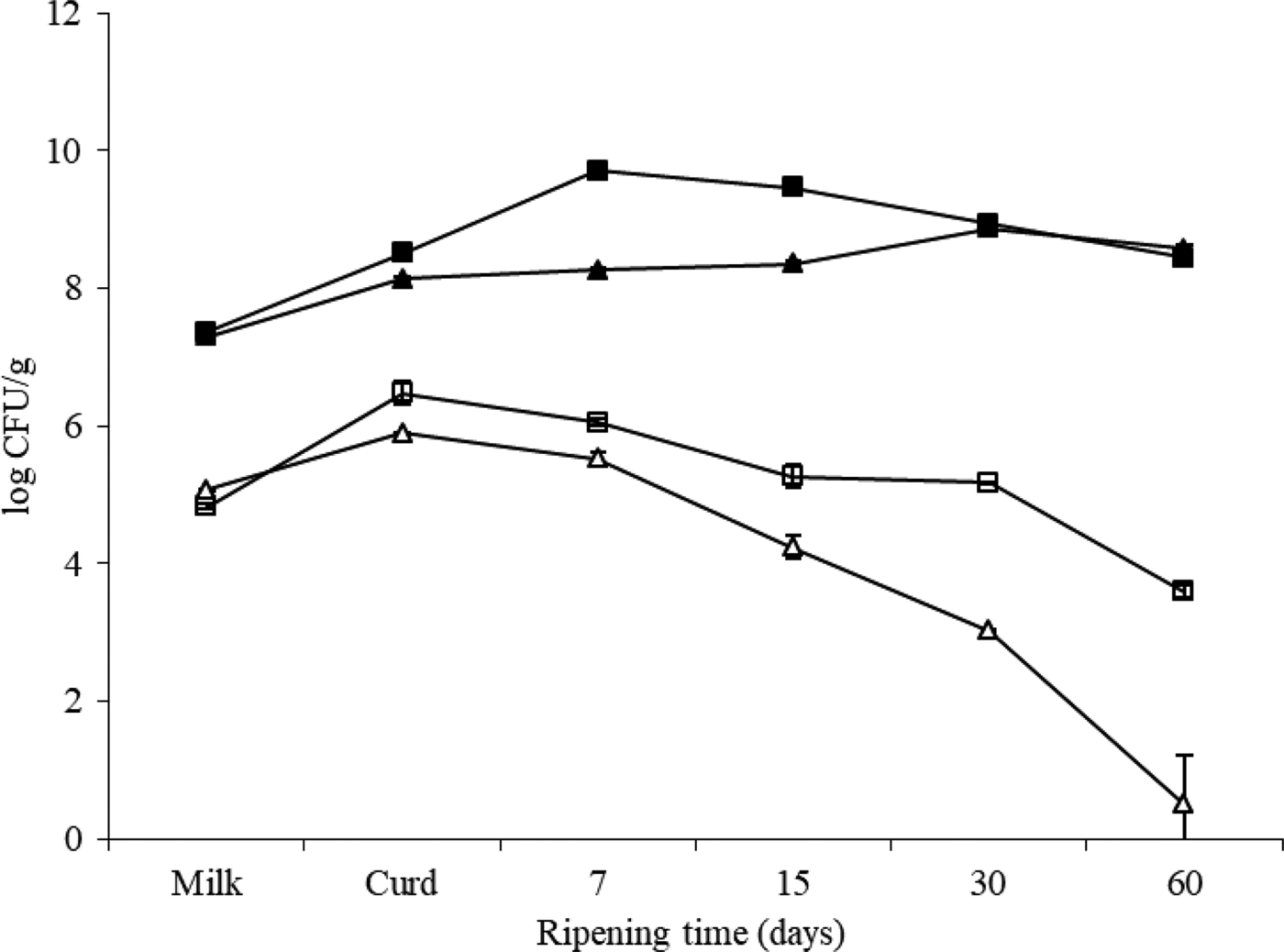
Fig. 1. Changes in total mesophilic bacteria (solid symbols) and enterobacterial (open symbols) counts during cheese making and ripening. Batch A: defective cheese (□); batch B: normal cheese (Δ).
Enterobacteriacea isolates during manufacture and ripening
Among the isolates obtained from VRBG plates, 170 were considered Enterobacteriacea and identified as described above. As a result of this procedure, 9 different species were detected, although numerical analysis of the digitized SDS-PAGE profiles revealed a total of 19 distinct clusters at the 80% (S) similarity level (data not shown). Figure 2 shows the distribution of Enterobacteriacea species isolated in milk, curd and cheese at different stages of ripening. Interestingly Pantoea agglomerans was the most abundant species in both milk batches; although it was not detected in any cheese. K. oxytoca and E. cloacae were the main species in curd for cheese A, while Buttiauxela spp. was the most abundant for cheese B. On the other hand, Hafnia alvei was the dominant isolated species for both batches of cheese throughout the whole ripening process (72·1%), despite the fact that it is barely represented in milk (3·3%) or curd (10·0%). E. coli was also detected in E. coli – coliforms Chromogenic Medium (data not showed) although their levels were 2 or 3 log units lower than the Enterobacteriaceae isolated.

Fig. 2. Changes in species of Enterobacteriaceae during manufacturing and ripening stages of cheeses from batch A and B (defective and normal cheese respectively). N: total number of isolates from each species.
Image analysis of gas holes in cheese and gas production capacity of the isolates
The analysis of the digitalized images showed that more than a half of the eyes formed in both cheeses were small (<3 mm2). The effect of microbial gas production was more intense in cheese with the early blowing defect, resulting in 113 holes after 7 d of ripening, whose area represented the 4·8% of the cut surface (Fig. 3). However, in normal cheese not only were there fewer eyes (19), but also their size was smaller, resulting merely in a 0·3% of surface occupation. By the end of ripening, both cheeses doubled their eyes number, although the surface occupied by holes in defective cheese exceeded the 12% while in non-defective cheese remained under the 3%.
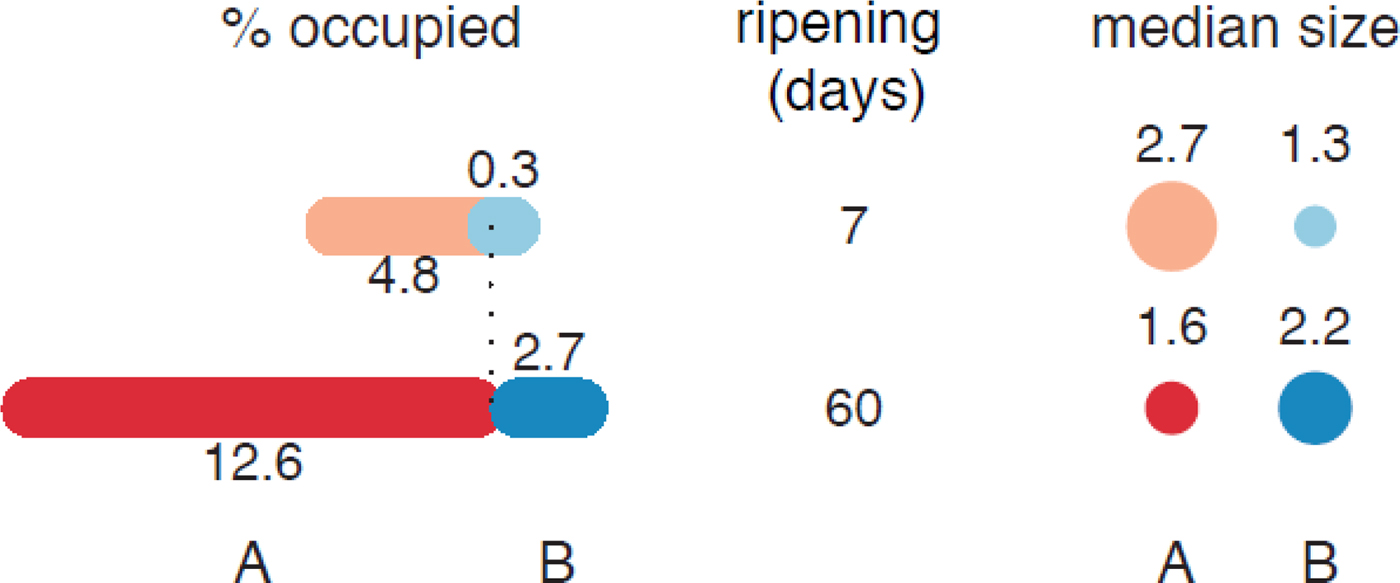
Fig. 3. Mass surface occupied by gas holes and median of the eye size in cheeses (mm2) from batch A and B at 7 and 60 d of ripening.
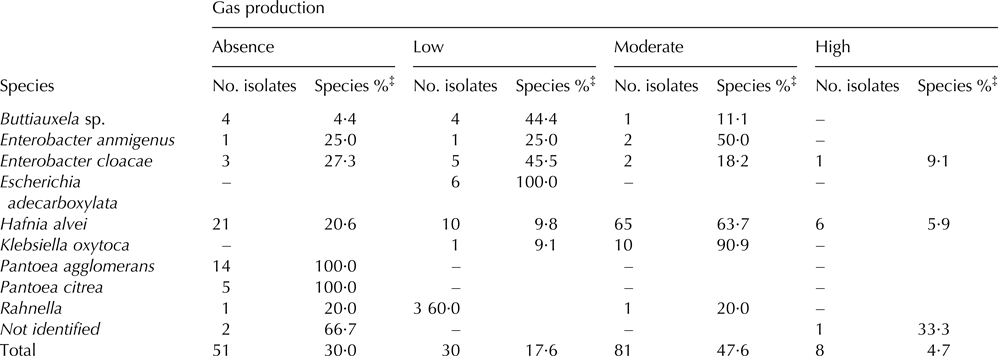
The gas production ability of the 170 isolates is shown in Table 3. Whereas the majority of milk isolates produced none or little gas, the predominant species in curd showed low and moderate gas production. Less than 5% of the isolated strains were considered as high gas producers. Remarkably, the species with the greatest potential for gas production were Klebsiella oxytoca and Hafnia alvei, and thus 90·9 and 63·7% of their isolates respectively were classified as moderate gas producers.
Table 3. Gas production of Enterobacteriaceae isolates from raw goats’ milk and cheese. (Absence: 0–0·1 cm; Low: 0·2–0·9 cm; Moderate: 1–1·9 cm; High: 2–3·5 cm†).
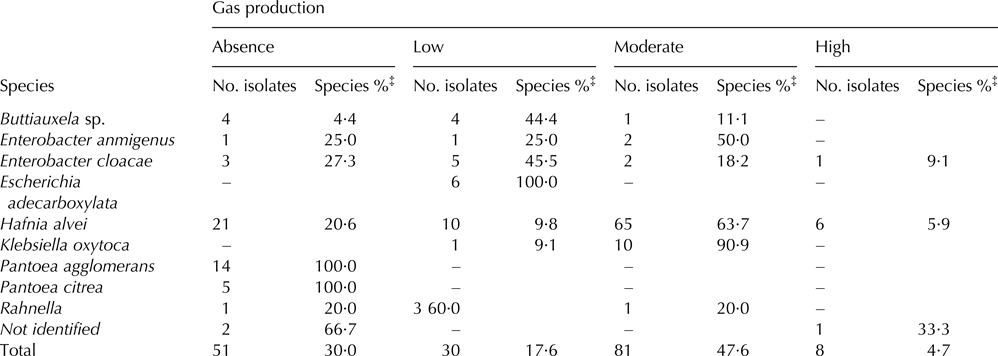
‒, not detected.
† cm of gas trapped inside the Durham tube.
‡ the species percentage was calculated with respect to the total number of isolates from each species.
Discussion
Early blowing in cheese appears to occur when the initial number of microorganisms capable of producing gas is relatively large (>105 CFU mL−1 in milk). The image analysis of cheese mass showed that both the timing of hole formation and the size of the eyes were consistent with early blowing produced by coliforms (Alichanidis, Reference Alichanidis and McSweeney2007). The Enterobacteriaceae counts for both milk batches in this study (~105 CFU mL−1) were in line with a previous study (Mas et al. Reference Mas, Tabla, Moriche, Roa, Gonzalez, Rebollo and Cáceres2002). According to Bester (Reference Bester1976), a population of Enterobacter aerogenes in milk of 2 × 105 CFU mL−1 caused early blowing in cheese. Consequently, the level recorded in the milk in this study could have been high enough to trigger the early blowing in both cheese batches based on levels found in this study. However only cheese from batch A developed early blowing. Despite of having similar initial microbial levels, plate counts during manufacture and ripening reveal a different microbial evolution between the batches, especially for Enterobacteriaceae counts. In addition, disappearance of Enterobacteriaceae during ripening was slower in the defective cheese than in non-defective cheese, which may have contributed to the increased alteration level in defective cheeses.
Too slow or too low acidification are considered as typical causes of early gas blowing in raw milk cheeses, since high pH conditions do no inhibit the growth of the microorganisms associated with this defect. However, slow acidification is unlikely to be the main factor responsible for early blowing in this cheese variety because the defective cheese showed the fastest acidification rate. Furthermore, the highest Enterobacteriaceae levels did not correlate with the lowest pH. Similar findings were reported in previous studies (Tornadijo et al. Reference Tornadijo, Fresno, Carballo and Martín-Sarmiento1993, Reference Tornadijo, García, Fresno and Carballo2001; Psoni et al. Reference Psoni, Tzanetakis and Litopoulou-Tzanetaki2003; Tabla et al. Reference Tabla, Gómez, Simancas, Rebollo, Molina and Roa2016). Higher Enterobacteriaceae counts in defective cheese could also be related to the lower salt-in-moisture content detected at the beginning of the ripening (Melilli et al. Reference Melilli, Barbano, Caccamo, Calvo, Schembari and Licitra2004). However, differences in Enterobacteriaceae population were already detected in curd, prior to salting.
The Enterobacteriaceae species identified in the present work are frequently found in cheese (Tornadijo et al. Reference Tornadijo, Fresno, Carballo and Martín-Sarmiento1993; Kongo et al. Reference Kongo, Gomes and Malcata2008; Maifreni et al. Reference Maifreni, Frigo, Bartolomeoli, Innocente, Biasutti and Marino2013; Ordiales et al. Reference Ordiales, Benito, Martín, Casquete, Serradilla, de Guía and órdoba2013; Tabla et al. Reference Tabla, Gómez, Simancas, Rebollo, Molina and Roa2016). The predominant species in milk, Pantoea agglomerans and P. citrea, are psychrotrophic Enterobacteriaceae commonly isolated from refrigerated milk (Giannino et al. Reference Giannino, Marzotto, Dellaglio and Feligini2009). No gas production was detected in the present work for Pantoea ssp. isolates. This fact together with the difference of Pantoea ssp. population in milk (40·0% from batch A milk against 80·0% from batch B) could have contributed to the lack of gas holes in cheese from batch B. Environmental conditions during cheese making and ripening were not favourable for Pantoea ssp., as they were not detected in curd or cheese. Pantoea was rapidly overcome by other species during curdling, probably due to the rise of temperature.
Differences in the Enterobacteriaceae species were detected in curd and early ripening stages between defective and non-defective cheese. The predominant species in curd from batch A was K. oxytoca while in batch B curd was Buttiauxela ssp. Based on levels found in this study, the former can be considered as moderate gas producer while Buttiauxela’s gas production was low or undetected in most cases. The relative proportion of K. oxytoca isolated from defective and non-defective cheese curd was 5 : 1. The real difference in K. oxytoca population between batches could have been higher if we considered that Enterobacteriaceae counts were 0·6 log units greater in curd A than in curd B. Previous studies reported that K. oxytoca was able to yield almost three times more gas holes and larger eye size in cheese than some other Enterobacteriaceae species (Tabla et al. Reference Tabla, Gómez, Simancas, Rebollo, Molina and Roa2016). In addition to the total number of microorganisms capable to produce gas, the proportion of moderate and high gas producer species in curd and cheese could be a major cause for the early blowing observed in this study.
H. alvei increased as time elapsed, representing about the 90% of the isolates in both cheese batches, after 7 d of ripening. Similar results were previously reported for different cheese varieties (Tornadijo et al. Reference Tornadijo, García, Fresno and Carballo2001; Abriouel et al. Reference Abriouel, Martín-Platero, Maqueda, Valdivia and Martínez-Bueno2008; Ordiales et al. Reference Ordiales, Benito, Martín, Casquete, Serradilla, de Guía and órdoba2013; Tabla et al. Reference Tabla, Gómez, Simancas, Rebollo, Molina and Roa2016; Westling et al. Reference Westling, Danielsson-Tham, Jass, Nilsen, Öström and Tham2016). This fact can be a consequence of the biodiversity decrease that takes place in the cheese core during ripening (Montel et al. Reference Montel, Buchin, Mallet, Delbes-Paus, Vuitton, Desmasures and Berthier2014). H. alvei resistance during cheese ripening has been related to its stress-tolerant character (Maifreni et al. Reference Maifreni, Frigo, Bartolomeoli, Innocente, Biasutti and Marino2013). In contrast, other studies found other Enterobacteriaceae species dominant throughout ripening (Tornadijo et al. Reference Tornadijo, Fresno, Carballo and Martín-Sarmiento1993; Tavaria & Malcata, Reference Tavaria and Malcata1998). Most of H. alvei isolated showed moderate gas production. This fact, together with its high prevalence, reveals its role in gas production during ripening, as reflected by the increase in cheese eyes by the end of the maturation period. In the defective cheese, although the average eye size diminished, fused eyes and even fissures appeared, increasing the whole area occupied by eyes. Fissures can be due to proteolysis, which makes the cheese body brittle, and thereby potentially exacerbating the formation of cracks in the later ripening stage (Guggisberg et al. Reference Guggisberg, Schuetz, Winkler, Amrein, Jakob, Fröhlich-Wyder and Wechsler2015). Presence of H. alvei during cheese ripening has been related with stronger aromatic intensities, even inhibition of pathogen microorganisms (Delbès-Paus et al. Reference Delbès-Paus, Irlinger and Coton2011, Reference Delbès-Paus, Pochet, Helinck, Veisseire, Bord, Lebecque, Coton, Desmasures, Coton, Irlinger and Montel2012; Irlinger et al. Reference Irlinger, In Yung, Sarthou, Delbès-Paus, Montel, Coton, Coton and Helinck2012). However, H. alvei has also been related to off-flavours like ‘bitter’ or ‘manure’ (Westling et al. Reference Westling, Danielsson-Tham, Jass, Nilsen, Öström and Tham2016) and biogenic amine production in dairy products (Benkerroum, Reference Benkerroum2016). According to these observations, high levels of H. alvei during ripening can be considered as a threat for cheese quality.
Conclusions
This study highlighted the initial Enterobacteriaceae biodiversity within the complex microbial flora of raw goat milk and cheese, their evolution during ripening and its role in the early blowing of cheese. The initial Enterobacteriaceae population can be a valuable indicator of bacteriological quality, however, since gas production is species, even strain related; a better knowledge of Enterobacteriaceae composition of milk is also required to estimate the risk of early blowing.
K. oxytoca during the first stages of cheese making and ripening and later H. alvei were the main Enterobacteriaceae species involved in cheese eye formation. Moreover, cheese blowing by Enterobacteriaceae species can be extended beyond the first week of maturation. Since cheese acidification or salting seems to be insufficient to control the development and the effect of these spoilage bacteria, other biocontrol strategies should be considered, like the development of specific bacteriophages or starter cultures against these microorganisms.
This work has been funded by the Autonomous Community of Extremadura and European Regional Development Fund (PCJ1007 and GR15065 grants).